Abstract
This study first investigated whether common complex diseases mediate the associations between self-rated health and cognitive abilities. Second, the genetic and environmental mediation of these relationships was explored using bivariate quantitative genetic analyses. Slight evidence was found that associations between self-rated health and cognitive test scores were mediated by chronic diseases. In the younger age group (< 67 years) associations between self-rated health and spatial reasoning and perceptual speed were mediated by both genetic and environmental factors. In the oldest age group (≥ 67 years), associations between self-rated health and verbal ability, spatial reasoning, perceptual speed and visual memory were entirely due to genetic factors.
Keywords: self-rated health, twins, cognitive abilities, genes, environment
A very important aspect of health perception and quality of life in the elderly is intact cognitive functioning (Waldstein, 2000). It is well known that deficits in cognitive performance are associated with the normal aging process (Salthouse, Babcock, & Shaw, 1991). However, despite its normality, cognitive slowing with age does not necessarily pass unnoticed by the individual and may be reflected in self reports of health. Studies have shown that respondents who report poorer self-rated health have lower cognitive test scores (Hultsch, Hammer, & Small, 1993), but the mechanisms behind this relationship still remain to be explored.
Self-rated health, especially in the oldest ages, might reflect feelings of slowing and memory loss (Earles, Connor, Smith, & Park, 1997). Elderly who report poorer self-rated health also complain more about memory problems (Bazargan & Barbre, 1994). Conversely some studies have also suggested that health factors may contribute to age-related changes in memory performance. Results, however, on the relationship of self-rated health and recall tasks have been mixed (Jelicic, Jonker, & Deeg, 1999; Perlmutter & Nyquist, 1990; Salthouse, Kausler, & Saults, 1990). Earles (Earles et al., 1997) found that self-rated health is correlated with speed (r=0.29) better than it correlated with memory (r=0.20). Wahlin and coauthors (Wahlin, Maitland, Backman, & Dixon, 2003) investigated whether self-rated health and episodic memory are related in persons aged 75-84 years. They concluded that the cross-sectional relationship was non-significant, although longitudinal change in self-rated health over a 3-year time period was related to change in episodic memory performance. Rosnick and collaborators (Rosnick, Small, Graves, & Mortimer, 2004) suggest that health status is associated to a greater extent with lower order cognitive processes, like processing speed, rather than with higher order cognitive processes, such as memory.
It has been suggested that decrements in cognition not only are due to primary biological aging processes but also to systemic medical diseases, such as cardiovascular disease, that are common in older adults (Waldstein, 2000). Several studies have shown that various forms of vascular disease, such as atherosclerosis and cerebrovascular disease are associated with lower levels of performance, in particular for psychomotor speed (Elias, Elias, & Elias, 1990). Cardiovascular symptoms were found to predict performance on tests of episodic memory and visuospatial skill in a Swedish sample aged 75 to 96 years (Fahlander et al., 2000). Presence of stroke and poorer health ratings predicted poorer cognitive performance in a US nationally representative sample aged 70 to 103 years (Zelinski, Crimmins, Reynolds, & Seeman, 1998). Long duration of Type 2 diabetes mellitus has also shown to be related to lower test performance across several cognitive domains, but not for short-term memory (Hassing et al., 2004; Zelinski et al., 1998). In addition, hypertension and Type 2 diabetes mellitus combined were also associated with detectable cognitive decrements in persons less than 60 years of age (Knopman et al., 2001; Pavlik, Hyman, & Doody, 2005). Neurological problems such as Parkinson’s disease and multiple sclerosis have shown to be related to decrements in cognition (Achiron & Barak, 2003; Albrecht et al., 1994). It has also been reported that cancer- or rather cancer treatment- is related to decrements in cognitive functioning (Ahles & Saykin, 2001). Chronic pain is strongly associated with poor self-rated health (Mantyselka, Turunen, Ahonen, & Kumpusalo, 2003). It may also be that pain has some impact on cognitive performance. Apkarian and co-authors found support for impairment on an emotional decision-making task (Iowa Gambling task) although not for general intelligence, short-term memory or attention (Apkarian et al., 2004). Poor self-rated health is highly correlated with number of medical diagnoses, physical symptoms and morbidity, such as diabetes, arthritis, cancer (Bjorner et al., 1996; Idler, 1993) and cardiovascular disease (Svardh, Isacson, & Pedersen, 1998). It is therefore also possible and of interest to investigate if the self-rated health-cognition relationship is mediated through common complex diseases.
To our knowledge there are no studies exploring the genetic and environmental mediation of the relationship between self-rated health and cognitive abilities. However, preliminary analyses from the Swedish Adoption/Twin Study of Aging (SATSA) sample showed a small association between number of chronic illness and cognition and a larger association between functional abilities such as general motor function, upper body strength and performance and cognition (Harris & Pedersen, 1994). Age differences in the etiology of the relationship between functional and cognitive abilities showed environmental effects to be more important than genetic effects with greater age. Other preliminary results from the SATSA data showed an association between self-rated health and the speed of processing measure (Pedersen & Harris, 1992). In twin studies, estimates of genetic and environmental influences are obtained by biometrical model-fitting methods. This has been proven to be a powerful method for exploring individual differences. Cognitive abilities are moderately heritable (for speed h2 = .65, and for memory h2 = .40) (Pedersen, Plomin, Nesselroade, & McClearn, 1992), self-rated health less so (h2 = .40), with genetic variance decreasing across earlier born cohorts (Svedberg, Lichtenstein, & Pedersen, 2001). Thus, both genetic and environmental influences in common to self-rated health and cognition may mediate the association.
In the present study, we examined the association between self-rated health and cognitive abilities in the SATSA study in two age groups (<67 years, ≥67 years). Measures reflecting verbal ability, spatial reasoning, perceptual speed, working memory and visual memory were assessed during in person testing sessions. Based on previous findings in the literature of a correlation between self-rated health and both perceptual speed and memory, we hypothesized that these correlations would be stronger in the older age group (67 years and older). We then examined if chronic illness conditions affect the self-rated health-cognition relationship. We expected that chronic illnesses would explain the self-rated health – cognition relationship to some extent. Finally, the relationship between self-rated health and cognitive measures was analyzed using bivariate models that yield estimates of the degree to which genes and environments mediate the self-rated health-cognition relationship in the two age groups. We hypothesized that genes that influence self-rated health also influence cognitive abilities.
METHOD
Participants
Ascertainment procedures for SATSA have been described previously (Finkel & Pedersen, 2004). In brief, the sample is a subset of twins from the population-based Swedish Twin Registry (Lichtenstein et al., 2002). The base population comprises all pairs of twins who indicated that they had been separated before the age of 11 and reared apart, and a sample of twins reared together matched on the basis of gender and date and county of birth (Pedersen, Friberg, Floderus-Myrhed, McClearn, & Plomin, 1984). Twins were first mailed questionnaires in 1984, and a sub-sample of those pairs in which both twins responded and were 50 years and older was invited to participate in an in-person test (IPT) entailing examination of health and cognitive abilities (Finkel & Pedersen, 2004; Pedersen et al., 1991). In-person testing took place in a location convenient to the twins, such as district nurses’ offices, health-care schools, and long-term care clinics. Testing was completed during a single 4-hour visit. At the first occasion (IPT1), no self-rated health questions were asked. A second wave of in-person testing (IPT2) occurred three years later and a third wave of in-person testing (IPT3) was conducted after an additional three-year interval.
The sample used in the present study was selected on the following basis; IPT2 was chosen as baseline as self-rated health and cognitive information were collected at the same time. For twins who were added to the IPT sample after IPT2 (as they became 50), IPT3 served as the baseline (7 % of the sample). The minimum requirement for inclusion was data on at least one cognitive measure and self-rated health at the baseline test occasion. Mean age of the sample was 67 years (SD=9.3). Both complete and incomplete pair responses (in which cognitive data and/or self-rated health data are available for only one twin in a pair) were included. The sample comprised 640 cognitively intact (i.e. no suspicion of dementia) individuals, including 292 complete twin pairs with known zygosity. As expected from population demographics in this age range, more than half of the sample was female (59 %). When the sample was divided at the mean age into two age groups, the proportion of women in the <67 group was 53.3% while the proportion of women in the ≥67 group was 64%.
Measures
Self-rated health was summed over four questions, one of which asks respondents for a global rating of their health (1. How would you rate your general health status? Response alternatives: Bad, Reasonable, Good) and three items about their health in the context of their own aging experience (2. How would you rate your general health status compared to 5 years ago? Response alternatives: Worse, About the same, Better. 3. How would you rate your health status compared to others in your age group? Response alternatives: Worse, About the same, Better. 4. Do you think your health prevents you from doing things you would like to do? Response alternatives: To a great extent, Partly, Not at all.) This scale is similar to that in the OARS (1978). Following the same procedure as Harris, Pedersen, McClearn, Plomin and Nesselroade (1992) the items were standardized to a mean of 0 and a standard deviation of 1 before summing. In IPT 2 and 3 the items are standardized with the weights from the first measurement occasion in order to maintain the validity of the comparative items. A more favorable self-rating of health is indicated by a high value on the scale. The items included were reasonably homogeneous (Cronbach’s coefficient alpha = .76).
Scales reflecting chronic illnesses were based on respondent self-reports of whether or not he or she had a health condition affecting each major organ system (see Harris et al., 1992, for a more detailed description). The following seven categories were included in our analysis; Cardiovascular disorders include angina pectoris, heart infarct, heart insufficiency, heart attack, high blood pressure, thrombosis, stroke, circulation problems in limbs and claudication. Respiratory disorders include prolonged cough, asthma, emphysema, chronic bronchitis, tuberculosis, and lung problems. Musculoskeletal disorders include back pain, shoulder pain, neck pain, rheumatoid arthritis, arthritis, sciatic problems, osteoporosis, hip, joint and muscle problems. Problems associated with allergic responses include conditions such as hay fever. Central nervous system related disorders include migraine, dizziness, seizures, epilepsy, Parkinson’s disease, multiple sclerosis, speech problems and polio. Metabolic disorders include diabetes, goiter, anemia and gout. Cancer includes leukemia or tumors. Each category served as a single item indicator of whether that category of chronic illness was reported by the respondent as ever present or not.
The SATSA cognitive test battery includes cognitive measures drawn from various sources and chosen to assess different areas of cognitive abilities (Nesselroade, Pedersen, McClearn, Plomin, & Bergeman, 1988; Pedersen et al., 1992). The cognitive tests were selected to provide representation of the domains of verbal ability (crystallized knowledge), spatial reasoning, perceptual speed, working memory and visual memory.
A Swedish version of the WAIS Information subtest (verbal ability) (Jonsson & Molander, 1964) includes 22 items assessing general knowledge (e.g., “What is the population of Sweden?”). Respondents are allowed 20 seconds to answer each question.
Koh’s Block Design is a spatial reasoning test, similar to the WAIS Block Design subtest, in which respondents create designs using colored blocks (Dureman, Kebbon, & Osterberg, 1971). Each of its seven items is scored from 0 to 6 based on the amount of time the respondent takes to correctly complete the design.
In Symbol Digit, respondents verbally report digits that correspond to symbols. They have 45 seconds to complete each of 10 groups of 10 items. Symbol digit measures perceptual speed.
Digit Span Backward (working memory), was scored as the sum of the highest number of digits the respondent was able to repeat correctly backwards (Jonsson & Molander, 1964). Respondents were given two trials of different strings of digits; correct performance on either string was counted toward their final score.
Thurstone’s Picture Memory tests visual memory of 28 drawings of common items such as a truck and a table (Dureman et al., 1971). Respondents are shown each picture for five seconds; their response is not timed.
The reliabilities for these tests range from .82 to .96 (Pedersen et al., 1992). For every test except Block Design, answers were reported orally to the examiner to minimize the effect of motor speed on performance. The cognitive battery was designed to allow analyses for split-halves of some tests to maximize the sample-size by allowing for inclusion of the participants who failed to complete the second portion of the test. First-halves performance was used for the Information test and Symbol Digit. In IPT 2 and 3 the tests were standardized with the weights (means and SD) from the first measurement occasion in order to maintain the comparability of the tests across measurement occasion.
Statistical analyses
Descriptive statistics and correlations were calculated using SAS (SAS/STAT, 1999-2001). Pearson correlations were used to test the association between self-rated health and the different cognitive measures. Partial correlation was used to test the strength of the association between self-rated health and cognitive abilities after controlling for the seven chronic illness indicators. In addition, to test the statistical significance of each chronic illnesses as a potential mediator, the Sobel test of regression coefficients was used (Baron & Kenny, 1986; Preacher, 2001).
Twin intraclass correlations and cross-twin-cross-trait correlations for the different zygosity groups (MZ, DZ) were calculated to give a first impression of the genetic and environmental influences on self-rated health and the cognitive measures in two different age groups (<67 and ≥67). Cross-twin-cross-trait correlations are calculated between twin A’s score on self-rated health and twin B’s score on the different measures of cognitive abilities.
The next step of analysis was biometrical model fitting in which expected patterns of intrapair similarity are fitted to observed variance-covariance matrices using the structural equation model program Mx (Neale & Maes, 2002). Monozygotic twins (MZ) share all their genes while dizygotic twins (DZ) share on average 50 percent of their segregating genes. The expected correlation of the additive genetic factors for MZ twins is therefore 1.0, while it is 0.5 for DZ twins. Greater MZ than DZ similarity is evident for additive genetic effects (A). When nonadditive genetic effects (D) are present, the DZ correlation is 0.25. Environmental effects can be either shared or nonshared by twins. Shared environmental effects (C) are those that contribute to familial similarity regardless of the zygosity of the twinpair, such as rearing environment or contact as adults. Nonshared environmental effects (E) refer to environmental experiences that are unique to the individual and not shared by family members, such as accidents, different peer influences, different teachers and friends. Non-shared environmental effects also include measurement error.
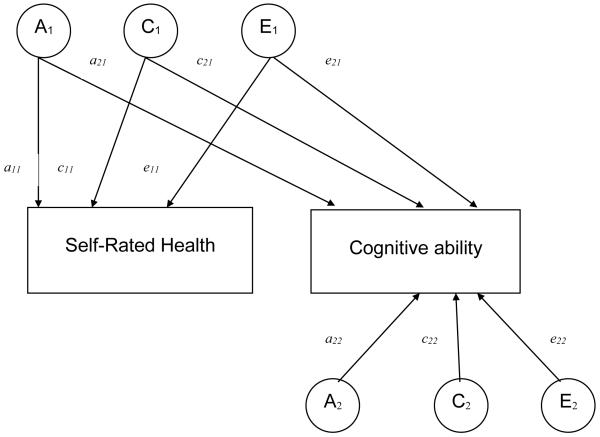
The univariate model can be extended to explore how environmental and genetic factors contribute to the covariation between self-rated health and cognitive abilities. We expect that the influences on variation in self-rated health may also contribute to some extent to variation in cognitive abilities. This model is not causal; it only explores associations within cross-sectional data. Figure 1 is a simplified version of a bivariate Cholesky (ACE) model, including only one twin in a pair. Each single-headed arrow represents the loadings of a latent factor on an observed variable. The first set of latent factors, (A1, C1, E1) are allowed to load on both observed traits (self-rated health and cognitive ability). The second set of latent factors (A2, C2, E2) are allowed to load on only one observed trait and thus represent the portion of the variation in cognitive ability that is not associated with score variance in self-rated health. The a21, c21, and e21 paths are the contribution of latent etiological factors to the covariance of the two observed factors.
Figure 1.
A bivariate Cholesky ACE decomposition model depicting common and unique factors for genetic and environmental sources of variance for self-rated health and cognitive ability.
Note: The figure is simplified and contains only one of the twins in the pair. A denotes genetic factors, a = genetic loadings, E denotes nonshared environmental factors, e = nonshared environmental loadings, C denotes shared environmental factors and c = shared environmental loadings.
We began with fitting a full ACE or ADE model, different for different age groups based on univariate model fitting results, and thereafter we performed tests of sub-models to test significance of genetic and shared environmental influences. C and D cannot be estimated in the same model (Evans, Gillespie, & Martin, 2002). Rearing status was not taken into account as little or no rearing effect was found in univariate analyses of the different cognitive measures and self-rated health (Pedersen et al., 1992; Svedberg, Gatz, Lichtenstein, Sandin, & Pedersen, 2005) . We tested whether a model with genetic and nonshared environmental influences only (AE) gave a significantly worse fit than the full model (ACE or ADE). We then tested whether a model without A (CE or DE) gave a significantly worse fit than the full model (ACE or ADE) and finally, we tested whether individual differences are based solely on nonshared environmental factors (E). The standardized path coefficients from the Cholesky model can be used to estimate how the correlations between self-rated health and the cognitive abilities are mediated, and thereby describe what factors contribute to the phenotypic correlation. The proportions of the phenotypic correlation attributable to a, c and e between self-rated health and the different cognitive abilities for each age group were calculated as a11*a21, c11*c21 and e11*e21, respectively (Plomin & DeFries, 1981). The bivariate modeling was carried out only for those associations that showed phenotypic correlations (rp) ≥ 0.10. All variables were adjusted for age and sex within age group.
RESULTS
Descriptive statistics for the sample on all measures are presented in Table 1. Phenotypic correlations between self-rated health and the different cognitive measures are presented in Table 2. Correlations range from −0.03 to 0.26 with the lowest correlation for the total sample between working memory (Digits backward) and self-rated health and the highest correlation between spatial reasoning (Block design) and self-rated health. Contrary to expectations, the correlations were stronger in the younger age group than in the older age group for spatial reasoning (0.26 vs. 0.13) and perceptual speed (0.18 vs. 0.15), although the difference was not statistically significant. For memory though, we found a significant correlation between self-rated health and visual memory only in the older age group (0.20).
Table 1.
Number (N) of individuals, mean values and standard deviations (SD) for the variables included in the study, by age group.
| Age group | < 67 years | ≥67 years | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % Affected | N | % Affected | |||
| Cardiovascular | 294 | 29.93 | 280 | 53.57 | ||
| Respiratory | 293 | 12.97 | 280 | 19.29 | ||
| Musculoskeletal | 292 | 48.63 | 280 | 54.29 | ||
| Allergy | 293 | 23.89 | 273 | 23.44 | ||
| CNS | 294 | 29.59 | 280 | 28.57 | ||
| Metabolic | 292 | 15.65 | 280 | 23.93 | ||
| Cancer | 294 | 4.08 | 273 | 4.03 | ||
|
| ||||||
| N | Mean (min-max) |
SD | N | Mean (min-max) |
SD | |
|
| ||||||
| Self-rated healtha | 290 | 0.41 (−9.06 - 5.22) |
2.53 | 268 | −0.22 (−9.06 - 5.22) |
2.79 |
| Verbal ability | 297 | 19.79 (2 - 22) |
3.04 | 279 | 18.81 (6 - 22) |
3.72 |
| Spatial reasoning | 294 | 22.12 (3 - 37) |
6.52 | 265 | 16.14 (0 - 34) |
6.65 |
| Perceptual speed | 289 | 19.28 (5 - 35) |
4.85 | 247 | 13.65 (0 - 27) |
5.54 |
| Working memory | 298 | 4.42 (0 - 8) |
1.28 | 275 | 3.86 (0 - 7) |
1.28 |
| Visual memory | 291 | 22.07 (9 - 28) |
4.12 | 252 | 19.88 (7 - 28) |
4.50 |
Note: Self-rated health is summed over four questions (1. How would you rate your general health status? 2. How would you rate your general health status compared to 5 years ago? 3. How would you rate your health status compared to others in your age group? 4. Do you think your health prevents you from doing things you would like to do?) The items were standardized to a mean of 0 and a standard deviation of 1 before summing.
Table 2.
Phenotypica and partial correlations between cognitive abilities and self-rated health controlling for Cardiovascular disorders, Respiratory disorders, Musculoskeletal disorders, Allergic responses, Central nervous system related disorders, Metabolic type disorders and Cancer (Number of included individuals within parenthesis).
| Phenotypic correlations | ||||
|---|---|---|---|---|
| Scale | All | < 67 years | ≥ 67 years | Cognitive ability |
| Information | 0.07 (552) | 0.03 (288) | 0.10 (264) | Verbal ability |
| Block design | 0.20 (535)** | 0.26 (285)** | 0.13 (250)* | Spatial reasoning |
| Symbol digit | 0.16 (515)** | 0.18 (281)* | 0.15 (234)* | Perceptual speed |
| Digits backward | 0.004 (548) | −0.02 (288) | 0.03 (260) | Working memory |
| Thurstone’s picture memory | 0.08 (521) | −0.03 (283) | 0.20 (238)** | Visual memory |
|
| ||||
| Partial correlations | ||||
|
| ||||
| Scale | All (499) |
< 67 years (273) |
≥67 years (226) |
Cognitive ability |
| Information | −0.01 | −0.01 | 0.01 | Verbal ability |
| Block design | 0.17** | 0.22** | 0.11 | Spatial reasoning |
| Symbol digit | 0.12** | 0.16** | 0.10 | Perceptual speed |
| Digits backward | −0.03 | −0.09 | 0.02 | Working memory |
| Thurstone’s picture memory |
0.06 | −0.03 | 0.17** | Visual memory |
Notes: All individuals with data available regardless of pair status.
Controlling for age and sex within age group.
p<.01
p<.05
For a smaller sub sample (N = 437) containing individuals participating in both IPT2 and IPT3 we performed a posthoc analysis to check the correlations between self-rated health measured at IPT2 against the cognitive measures at IPT3. Correlations were somewhat greater than when testing the opposite direction, except for visual memory that was relatively unaffected by the time order.
Partial correlation and mediation test
Inspection of Pearson correlations between specific disease categories and self-rated health and the specific cognitive abilities revealed very few associations in common to health and cognition. Central nervous system related (CNS) disorders and musculoskeletal disorders were associated with both self-rated health and spatial reasoning and perceptual speed in the younger age group, and with self-rated health and verbal ability in the older age group (results not shown). The bottom half of Table 2 shows the results from the partial correlation analysis. The strength of the correlation between self-rated health and spatial reasoning as well as the correlation between self-rated health and perceptual speed was somewhat weaker when we controlled for all of the disorders (Cardiovascular disorders, Respiratory disorders, Musculoskeletal disorders, Problems associated with allergic responses, Central nervous system related disorders, Metabolic type disorders and Cancer) and still significant in the total sample and in the age group less than 67 years. In the older age group, 67 years or older, the correlations became nonsignificant, and only the correlation between self-rated health and visual memory remained significant.
Further testing of mediation using the Sobel test using all individuals regardless of pair status (Baron & Kenny, 1986) indicated only one significant mediation, that between self-rated health, Central nervous system related disorder, and verbal ability (Sobel test statistics = 2.03, p = 0.04) in the older age group. No other test of mediation achieved statistical significance for either age group. Dependencies due to including both members of twin pairs do not bias the results but do lead to slightly smaller than expected standard error.
Intraclass and cross-twin-cross-trait correlations
Intraclass correlations and cross-twin-cross-trait correlations for self-rated health and all cognitive measures are presented by zygosity and age group in Table 3. Greater MZ than DZ correlations for all measures in both age groups suggest that genetic effects contribute to variability. Greater MZ than DZ cross-twin-cross-trait correlations suggest that genetic effects contribute to covariation for verbal ability, spatial reasoning and visual memory in both age groups.
Table 3.
Intraclass and cross-twin-cross-trait correlations for self-rated health and the cognitive scales separately by zygosity and age group (adjusted for sex and age within group).
| Intraclass Correlations | Cross Correlations with self-rated health | ||||
|---|---|---|---|---|---|
|
| |||||
| Age group | Scale | MZ | DZ | MZ | DZ |
| < 67 years old | Self-rated health | 0.29 (50)** | 0.11 (93) | ||
| Verbal ability | 0.64 (51)** | 0.14 (96)* | 0.07 | -0.05 | |
| Spatial reasoning | 0.74 (52)** | 0.28 (92)** | 0.13 | 0.08 | |
| Perceptual speed | 0.66 (50)** | 0.04 (89) | 0.07 | 0.04 | |
| Working memory | 0.49 (52)** | 0 (96) | -0.04 | 0.06 | |
| Visual memory | 0.44 (49)** | 0.07 (92) | 0.21* | -0.01 | |
| ≥ 67 years old | Self-rated health | 0.27 (43)* | 0.23 (85)* | ||
| Verbal ability | 0.64 (47)** | 0.06 (90) | 0.15 | 0.05 | |
| Spatial reasoning | 0.64 (40)** | 0.28 (85)** | 0.08 | 0.03 | |
| Perceptual speed | 0.42 (38)** | 0.37 (71)** | 0.16 | 0.18* | |
| Working memory | 0.48 (46)** | 0.06 (88) | -0.07 | -0.03 | |
| Visual memory | 0.42 (39)* | 0.30 (73)** | 0.20 | 0.09 | |
Note: Number of twin pairs in parentheses.
p<.01
p<.05
A fundamental assumption of quantitative genetic analysis based on twin data is that variances are equal for MZ and DZ twins. Analyses of variance indicated that there were no differences in means between MZ and DZ twins. We found variance differences between MZ and DZ pairs only for the verbal ability subtest (p=0.04). There were no mean or variance differences between twin 1 and 2 in a pair for any of the measures included.
Univariate analyses
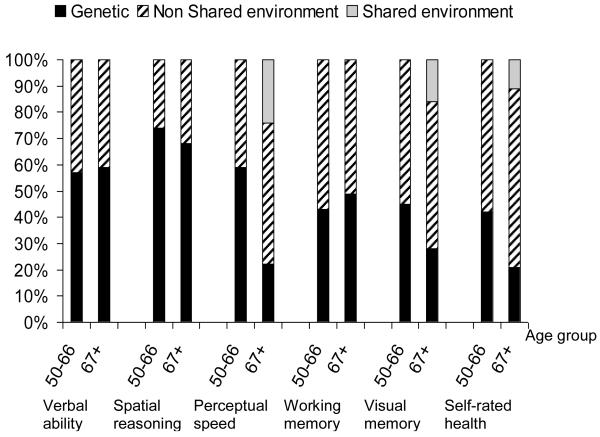
Before conducting bivariate analyses, scales were analyzed separately to determine which influences were important sources of variation for each scale. Using Mx we apportioned total variance into genetic and environmental components of variance. Our estimates presented in Figure 2 match those previously reported in SATSA (Harris, Pedersen, Stacey, McClearn, & Nesselroade, 1992; Pedersen et al., 1992). Both genetic and nonshared environmental factors are important to self-rated health and all cognitive scales. Genetic effects are more important than environmental effects for verbal ability, spatial reasoning and perceptual speed. For self-rated health, visual memory and working memory, the environmental variance is higher than the genetic.
Figure 2.
Proportion of variance explained by genetic and environmental factors for cognitive abilities and self-rated health.
Bivariate analyses
Based on the phenotypic correlations, we performed bivariate analyses only for the measures with correlations ≥ 0.10. In Table 4 we present fit statistics from the bivariate model-fitting procedure for self-rated health and each cognitive scale for both age groups. ADE models fit best, i.e., provide the most parsimonious explanations to data, for the younger age group. For the older age group, ACE models correspond best to the intraclass correlations, while the AE sub-model fit best. Table 4 shows fit statistics for both the full and sub model.
Table 4.
Fit statistics from Bivariate Cholesky models for self-rated health (SRH) and cognitive abilities by age groups.
| Sample | Model | χ 2 | AIC | Df | p | rp | Components of rp | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Genetic component |
Environmental component |
|||||||
|
| ||||||||
| Age group < 67 years old |
Bivariate Cholesky | |||||||
| SRH – Spatial reasoning | Full model (ADE) | 13.48 | −8.52 | 11 | 0.26 | 0.27 | 0.14 | 0.13 |
| SRH – Perceptual speed | Full model (ADE) | 19.32 | −2.68 | 11 | 0.06 | 0.20 | 0.12 | 0.08 |
| SRH – Verbal ability | Full model (ACE) | 18.91 | −3.08 | 11 | 0.06 | |||
| Best fit (AE) a | 19.13 | −10.87 | 15 | 0.21 | 0.13 | 0.13 | - | |
| SRH – Spatial reasoning | Full model (ACE) | 12.29 | −9.71 | 11 | 0.34 | |||
| Best fit (AE)a | 12.98 | −17.02 | 15 | 0.60 | 0.13 | 0.13 | - | |
| SRH – Perceptual speed | Full model (ACE) | 14.38 | −7.62 | 11 | 0.21 | |||
| Best fit (AE) a | 16.89 | −13.11 | 15 | 0.32 | 0.17 | 0.17 | - | |
| SRH – Visual memory | Full model (ACE) | 13.36 | −8.63 | 11 | 0.27 | |||
| Best fit (AE) a | 14.99 | −15.00 | 15 | 0.45 | 0.18 | 0.18 | - | |
Notes: All variables are adjusted for age and sex within age group.
Most parsimonious model according to AIC is the AE-models (no E covariance); hence, genetic and environmental components of the phenotypic correlations (rp) are calculated from best fitting models.
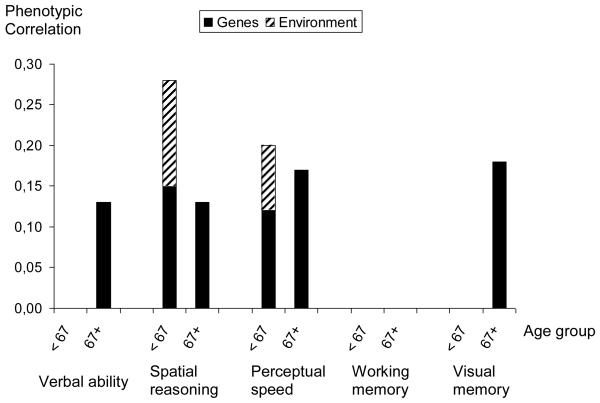
Figure 3 illustrates the genetic and environmental contribution to the phenotypic correlations in both age groups for the models that were estimated. In the age group less than 67 years old, 54% of the correlation (rp=0.27) between self-rated health and spatial reasoning is due to genetic factors in common to both traits while 46% is attributable to non-shared environmental factors. Sixty percent of the correlation (rp=0.20) between self-rated health and perceptual speed is attributable to genetic factors in common to both traits and hence, 40% is due to non-shared environmental factors.
Figure 3.
Genetic and environmental mediation of the association between self-rated health and cognitive abilities by age group.
Note: Shown only where phenotypic correlations ≥ .10.
In the oldest age group (≥ 67 years) 100% of the correlations are due to genetic factors in common to self-rated health and verbal ability (rp=0.13), self-rated health and spatial reasoning (rp=0.13), self-rated health and perceptual speed (rp=0.17) and finally self-rated health and visual memory (rp=0.18). The environmental components found for the younger age group are no longer present.
DISCUSSION
In this study our goals were to examine the association between self-rated health and cognitive abilities in two age groups (<67, ≥67). We tested phenotypically whether these relationships are mediated by self-reported chronic illness conditions, and we used biometrical twin models to examine the extent to which genes and environments mediate the associations found. In line with previous research in this field (i.e. (Earles et al., 1997; Rosnick et al., 2004; Stankov & Anstey, 1997) we found low or modest correlations (rp<0.30) between self-rated health and cognitive abilities. Except for the association between self-rated health and visual memory, we did not find support for our prediction that strength of association between self-rated health and cognition would be stronger for the older age group than for the younger. With respect to explaining the observed correlations, individual chronic illnesses do not seem to explain the associations between self-rated health and cognitive abilities to any great extent. When the associations were examined in bivariate twin models, both genetic and environmental factors mediated the associations found for persons less than 67 years of age. However, genetic factors alone mediate the associations found between self-rated health and the cognitive abilities (verbal ability, spatial reasoning, perceptual speed and visual memory) for adults 67 years or older.
Cognitive and health correlates of self-rated health
As suggested in the literature (Rosnick et al., 2004) we found that self-rated health is associated with perceptual speed and spatial reasoning. Rosnick and co-authors (2004) present correlations similar to what we found in the present study for self-rated health and a variety of different cognitive tests (r=−0.10 to r=0.26). Perlmutter (Perlmutter & Nyquist, 1990) and Rosnick (Rosnick et al., 2004) hypothesized that information processing and speed are more likely than memory to be affected by health. The prediction regarding speed is supported by our results for both age groups, but on the contrary, memory was associated with self-rated health only in the older age group. The association between self-rated health and memory in the older age group is not very surprising given that elderly people are indeed worried about their memory (Anstey & Low, 2004). It is possible that perceiving one’s memory to be poor or to be deteriorating is translated into reporting that one has poor health (see (Bazargan & Barbre, 1994; Wahlin et al., 2003).
Specific chronic illnesses played only a small role in explaining the associations between self-rated health and the cognitive tests. We did not find that any of the seven illness categories included in the study stood out specifically as a mediator. Central nervous system (CNS) related disorders and musculoskeletal problems were important in both age groups. Migraine, dizziness, epilepsy, Parkinson’s disease, multiple sclerosis, different kinds of pain, osteoporosis and muscle problems are all included in these sub groups of illness. It may be that pain is the common factor underlying these relationships. In the working segment of the population, absence of pain is very important. Poor self-rated health is associated with chronic pain (Mantyselka et al., 2003) and there are indications that pain also is associated with cognition (Apkarian et al., 2004). It may also be that particular CNS problems have some direct impact on both health and cognition. Parkinson’s patients may experience subtle cognitive changes that are related to verbal fluency and impaired retrieval of verbal material (Albrecht et al., 1994). Attention and verbal abilities have also shown to be affected among patients with multiple sclerosis (Achiron & Barak, 2003).
Although others have reported that cardiovascular disorders (Elias et al., 1990; Fahlander et al., 2000) and metabolic disorders (Zelinski et al., 1998) are related to poorer cognitive performance and poorer self-rated health, they did not explain the association between self-rated health and cognitive abilities in our sample. It is possible that these relationships are due to something more fine graded like variation in the atherosclerosis process not captured by our broad dichotomy of cardiovascular disorders.
Our findings indicate that other factors than illnesses seem to play a substantial part in the self-rated health – cognition relationship. We conducted posthoc tests of whether either socioeconomic status in childhood or years of education played a significant mediating role and found no significant evidence of mediation.
Genetic and environmental mediation of the associations
Using the twin design, we found that in the younger age group (<67 years), the associations between self-rated health and perceptual speed, and between self-rated health and spatial ability are mediated by both genetic and environmental factors. In contrast, in the oldest age group, 67 years and older, the associations between self-rated health and verbal ability, spatial reasoning, perceptual speed and visual memory are all mediated by genetic influences alone. Environmental mediation could reflect life style changes such as work related stressors as shift work or retirement that are particularly relevant to the younger age group still working, or a general reduction of homeostatic mechanisms and a poorer (DNA) repair that shows up as environment in our models (Finch & Kirkwood, 2000). Genetic mediation may reflect age-related physical changes and chronic illnesses not fully tapped by our indicators of specific chronic conditions, or general slowing processes (Birren & Fisher, 1995).
Strengths and limitations
Our analyses are based on a population based sample that besides measures of self-rated health and cognitive abilities also includes a number of self-reported chronic conditions as covariates. Some of these are reported in the literature previously while others are new to this field, such as allergy and respiratory problems.
One limitation of the study is that the analyses were cross-sectional. Whether health precedes cognitive performance or vice versa is difficult to tell from cross-sectional research. Our posthoc analysis suggested that—except for visual memory—it was largely the case that lower self-rated health preceded lower cognitive scores; however, we did not have a sufficiently large longitudinal sample with the entire set of measures to explore direction of change more completely. Thus, we were unable to measure how change in self-rated health relates to change in cognitive abilities; associations that previously have shown to be significant (Wahlin et al., 2003). A further limitation, also related to limited sample size and modest correlations, was that we could not put self-rated health, cognitive abilities and dichotomously coded diseases in the same genetic model due to power problems. A third limitation is that the measures of chronic conditions were crude and may not have captured more fine graded variation in health.
Conclusions
The present results provide additional insight into the mediation mechanisms behind the associations between self-rated health and various cognitive abilities for middle aged and older adults. Both health and cognitive functioning are important among middle aged and elderly people and as has been shown in this study they are related to some extent. These associations are mediated by both environmental and genetic factors in the younger age group (<67 years), while for the older age group these associations are mediated by genetic factors alone.
ACKNOWLEDGEMENTS
The Swedish Adoption/Twin Study of Aging (SATSA) is supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Aging, the US National Institute on Aging (grants AG-04563, AG-10175) and the Swedish Council for Social Research (97:0147:1B).
REFERENCES
- Achiron A, Barak Y. Cognitive impairment in probable multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:443–446. doi: 10.1136/jnnp.74.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin A. Cognitive effects of standard-dose chemotherapy in patients with cancer. Cancer Investigation. 2001;19:812–820. doi: 10.1081/cnv-100107743. [DOI] [PubMed] [Google Scholar]
- Albrecht NN, Netherton SD, Elias JW, Albrecht JW, Whitfield KE, Hutton JT. Assessment of intellectual functioning of patients with Parkinson’s disease using the Satz-Mogel (1962) short form of the Wechsler Adult Intelligence Scale. Experimental Aging Research. 1994;20:155–172. doi: 10.1080/03610739408253961. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Low LF. Normal cognitive changes in aging. Australian Family Physician. 2004;33:783–787. [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bazargan M, Barbre AR. The effect of depression, health status, and stressful life-events on self-reported memory problems among aged blacks. International Journal of Aging and Human Development. 1994;38:351–362. doi: 10.2190/XUAY-9C0Q-5VDP-MKHE. [DOI] [PubMed] [Google Scholar]
- Birren JE, Fisher LM. Aging and speed of behavior: possible consequences for psychological functioning. Annual Review of Psychology. 1995;46:329–353. doi: 10.1146/annurev.ps.46.020195.001553. [DOI] [PubMed] [Google Scholar]
- Bjorner JB, Søndergaard Kristensen T, Orth-Gomér K, Tibblin G, Sullivan M, Westerholm P. Self-rated health: A useful concept in research, prevention and clinical medicine. Swedish Council for Planning and Coordination of Research (FRN); Stockholm: 1996. [Google Scholar]
- Dureman I, Kebbon L, Osterberg E. Manual till DS-Batteriet [Manual of the DS-Battery] Psykologi Forlaget; Stockholm: 1971. [Google Scholar]
- Earles JL, Connor LT, Smith AD, Park DC. Interrelations of age, self-reported health, speed, and memory. Psychology and Aging. 1997;12:675–683. doi: 10.1037//0882-7974.12.4.675. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias JW, Elias PK. Biological and health influences on behavior. In: Birren JE, Schaie KW, editors. Handbook on the Psychology of Aging. 3rd ed Academic Press; San Diego: 1990. pp. 79–102. [Google Scholar]
- Evans DM, Gillespie NA, Martin NG. Biometrical genetics. Biological Psychology. 2002;61:33–51. doi: 10.1016/s0301-0511(02)00051-0. [DOI] [PubMed] [Google Scholar]
- Fahlander K, Wahlin A, Fastbom J, Grut M, Forsell Y, Hill RD, et al. The relationship between signs of cardiovascular deficiency and cognitive perfromance in old age: a population-based study. Journal of Gerontology: Social Sciences. 2000;55B:259–265. doi: 10.1093/geronb/55.5.p259. [DOI] [PubMed] [Google Scholar]
- Finch CE, Kirkwood TBL. Chance, development, and aging. Oxford University Press; New York: 2000. [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology and Cognition. 2004;11:325–345. [Google Scholar]
- Harris JR, Pedersen NL. 12:e Nordiska kongressen i gerontologi. Vol. 43. Jönköping; Sweden: 1994. How do genes and environments contribute to the relationship between health and cognition? Abstractbook. [Google Scholar]
- Harris JR, Pedersen NL, Stacey C, McClearn GE, Nesselroade JR. Age differences in the etiology of the relationship between life satisfaction and self-rated health. Journal of Aging and Health. 1992;4:349–368. [Google Scholar]
- Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, McClearn G, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age and Ageing. 2004;33:355–361. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hammer M, Small BJ. Age differences in cognitive performance in later life: relationships to self-reported health and activity life style. Journal of Gerontology: Psychological Sciences. 1993;48:1–11. doi: 10.1093/geronj/48.1.p1. [DOI] [PubMed] [Google Scholar]
- Idler EL. Age differences in self-assessments of health: age changes, cohort differences, or survivorship? Journal of Gerontology; Social Sciences. 1993;48:289–300. doi: 10.1093/geronj/48.6.s289. [DOI] [PubMed] [Google Scholar]
- Jelicic M, Jonker C, Deeg DJ. Do health factors affect memory performance in old age? International Journal of Geriatric Psychiatry. 1999;14:572–576. doi: 10.1002/(sici)1099-1166(199907)14:7<572::aid-gps994>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Jonsson C-O, Molander L. Manual till CVB-skalan [Manual of the CVB Scales] Psykologi Forlaget; Stockholm: 1964. [Google Scholar]
- Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Mantyselka PT, Turunen JHO, Ahonen RS, Kumpusalo EA. Chronic Pain and Poor Self-rated Health. The Journal of the American Medical Association. 2003;290:2435–2442. doi: 10.1001/jama.290.18.2435. [DOI] [PubMed] [Google Scholar]
- Neale MC, Maes HHM. Methodology for genetic studies of twins and families. Kluwer Academic Publisher B.V; Dordrecht, The Netherlands: 2002. [Google Scholar]
- Nesselroade JR, Pedersen NL, McClearn GE, Plomin R, Bergeman CS. Factorial and criterion validities of telephone-assessed cognitive ability measures: Age and gender comparisons in adult twins. Research on Aging. 1988;10:220–234. doi: 10.1177/0164027588102004. [DOI] [PubMed] [Google Scholar]
- OARS . Multidimentional functional assessment: The OARS methodology. 2nd ed Duke University; Durham, NC: 1978. [Google Scholar]
- Pavlik VN, Hyman DJ, Doody R. Cardiovascular risk factors and cognitive function adults 30-59 years of age (NHANES III) Neuroepidemiology. 2005;24:42–50. doi: 10.1159/000081049. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Friberg L, Floderus-Myrhed B, McClearn GE, Plomin R. Swedish early separated twins: Identification and characterization. Acta Geneticae Medicae et Gemellologiae (Roma) 1984;33:243–250. doi: 10.1017/s0001566000007285. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Harris JH. Understanding the relationship between functional capacity and cognitive abilities in the elderly: Findings from the Swedish Adoption/Twin Study of Aging. The Gerontologist. 1992;32:196. [Abstract] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, de Faire U. The Swedish Adoption Twin Study of Aging: An update. Acta Geneticae Medicae et Gemellologiae (Roma) 1991;40:7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science. 1992;3:346–353. [Google Scholar]
- Perlmutter M, Nyquist L. Relationships between self-reported physical and mental health and intelligence performance across adulthood. Journal of Gerontology: Psychological Sciences. 1990;45:145–155. doi: 10.1093/geronj/45.4.p145. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC. Twin Research 3: Intelligence, personality, and development. Alan R. Liss, Inc; New York: 1981. Multivariate behavioral genetics and development: Twin studies; pp. 25–33. [PubMed] [Google Scholar]
- Preacher KJ, Leonardelli GJ. Calculation for the Sobel test: An interactive calculation tool for mediation tests [Computer software] 2001 Mar; Available from http://www.unc.edu/~preacher/sobel/sobel.htm.
- Rosnick CB, Small BJ, Graves MB, Mortimer JA. The association between health and cognitive performance in a population-based study of older adults: the Charlott County Healthy Aging Study (CCHAS) Aging, Neuropsychology and Cognition. 2004;11:89–99. [Google Scholar]
- Salthouse TA, Babcock RL, Shaw RJ. Effects of adult age on structural and operational capacities in working memory. Psychology and Aging. 1991;6:118–127. doi: 10.1037//0882-7974.6.1.118. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Kausler DH, Saults JS. Age, self-assessed health status, and cognition. Journal of Gerontology: Psychological Sciences. 1990;45:156–160. doi: 10.1093/geronj/45.4.p156. [DOI] [PubMed] [Google Scholar]
- SAS/STAT Users Guide (Version V8) SAS/STAT. SAS Institute Inc; Cary, NC, USA: 1999-2001. [Google Scholar]
- Stankov L, Anstey K. Health and cognitive ageing: The emerging role of sensorimotor abilities. Australian Journal on Ageing. 1997;16:34–39. [Google Scholar]
- Svardh C, Isacson D, Pedersen NL. Self-rated health among cardiovascular drug users in a study of Swedish twins. Scandinavian Journal of Social Medicine. 1998;26:223–231. doi: 10.1177/14034948980260030101. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Gatz M, Lichtenstein P, Sandin S, Pedersen NL. Self-rated health in a longitudinal perspective: A 9-year follow-up twin study. Journal of Gerontology: Social Sciences. 2005;60B:331–340. doi: 10.1093/geronb/60.6.s331. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Lichtenstein P, Pedersen NL. Age and sex differences in genetic and environmental factors for self- rated health: a twin study. Journal of Gerontology: Social Sciences. 2001;56:171–178. doi: 10.1093/geronb/56.3.s171. [DOI] [PubMed] [Google Scholar]
- Wahlin A, Maitland SB, Backman L, Dixon RA. Interrelations between subjective health and episodic memory change in Swedish and Canadian samples of older adults. International Journal of Aging and Human Development. 2003;57:21–35. doi: 10.2190/9VAA-KMYV-U2HU-PVAW. [DOI] [PubMed] [Google Scholar]
- Waldstein SR. Health effects on cognitive aging. In: Stern, editor. The aging mind. Opportunities in cognitive research. National Academy of Sciences; Washington, D.C.: 2000. pp. 189–217. [Google Scholar]
- Zelinski EM, Crimmins E, Reynolds S, Seeman TE. Do medical conditions affect cognition in older adults? Health Psychology. 1998;17:505–512. doi: 10.1037//0278-6133.17.6.504. [DOI] [PubMed] [Google Scholar]