Abstract
The intensity of bone remodeling is a critical determinant of the decay of cortical and trabecular microstructure after menopause. Denosumab suppresses remodeling more than alendronate, leading to greater gains in areal bone mineral density (aBMD). These greater gains may reflect differing effects of each drug on bone microarchitecture and strength. In a phase 2 double‐blind pilot study, 247 postmenopausal women were randomized to denosumab (60 mg subcutaneous 6 monthly), alendronate (70 mg oral weekly), or placebo for 12 months. All received daily calcium and vitamin D. Morphologic changes were assessed using high‐resolution peripheral quantitative computed tomography (HR‐pQCT) at the distal radius and distal tibia and QCT at the distal radius. Denosumab decreased serum C‐telopeptide more rapidly and markedly than alendronate. In the placebo arm, total, cortical, and trabecular BMD and cortical thickness decreased (−2.1% to −0.8%) at the distal radius after 12 months. Alendronate prevented the decline (−0.6% to 2.4%, p = .051 to <.001 versus placebo), whereas denosumab prevented the decline or improved these variables (0.3% to 3.4%, p < .001 versus placebo). Changes in total and cortical BMD were greater with denosumab than with alendronate (p ≤ .024). Similar changes in these parameters were observed at the tibia. The polar moment of inertia also increased more in the denosumab than alendronate or placebo groups (p < .001). Adverse events did not differ by group. These data suggest that structural decay owing to bone remodeling and progression of bone fragility may be prevented more effectively with denosumab. © 2010 American Society for Bone and Mineral Research
Keywords: denosumab, alendronate, HR‐pQCT, volumetric bone mineral density, cortical thickness
Introduction
The aim of treating patients with osteoporosis is to reduce fracture risk by reversing or preventing the structural deterioration characteristic of this disease. Fracture risk is influenced by differences in morphology, such as bone size and shape; the distribution of its mass as cortical and trabecular bone; cortical thickness and porosity; trabecular number, thickness, and connectivity; and the material properties of the bone tissue.1, 2 Historically, the focus on the pathogenesis of osteoporosis has been on vertebral fractures and trabecular bone loss, with relatively less attention given to nonvertebral fractures and cortical bone loss, even though 80% of fractures are nonvertebral and occur at predominantly cortical sites. Indeed, cortical bone accounts for 80% of skeleton mass, and 90% of the morbidity and mortality associated with fractures are the result of nonvertebral fractures.3, 4, 5
While vertebral fracture risk is reduced by about 50% with most therapeutic agents, nonvertebral fracture risk reduction is uncommon, and when observed, the risk reduction is about 20% to 30%.6, 7, 8 The reasons for the lower efficacy in reducing nonvertebral fractures are incompletely understood. Several factors may contribute, including greater severity of trauma, advanced intracortical porosity (which may be irreversible by the time treatment is initiated), and the degree to which treatments influence the larger volume of cortical bone given its low surface‐to‐volume ratio and so lower accessibility to being remodeled than trabecular bone.9
In preclinical studies, 6 months of treatment with denosumab, a fully human monoclonal antibody to RANK ligand (RANKL), increased cortical and trabecular bone mass and strength of the lumbar vertebra and femur in mice expressing chimeric human/mouse RANKL (huRANKL) and increased cortical density by reducing cortical porosity in ovariectomized cynomolgus monkeys.10, 11 In clinical studies, compared with the bisphosphonate alendronate, denosumab results in more rapid and greater reductions in bone remodeling and correspondingly greater increases in areal bone mineral density (aBMD) at all skeletal sites in untreated postmenopausal women and in women transitioning from alendronate to denosumab.12, 13
Denosumab's greater potency in suppressing bone remodeling and greater effect on aBMD than alendronate, particularly at predominantly cortical sites such as the distal third of the radius, may reflect the differing mechanism of action of these drugs, which, in turn, influence bone microarchitecture.12, 13, 14, 15, 16 Thus the aim of this study was to compare the effects of denosumab and alendronate on cortical and trabecular microarchitecture at the radius and tibia in postmenopausal women using quantitative computed tomography (QCT) and high‐resolution peripheral quantitative computed tomography (HR‐pQCT; XtremeCT, Scanco, Bruttisellen, Switzerland) during a 1‐year placebo‐controlled study.
Methods
This pilot phase 2 international randomized double‐blind double‐dummy active‐controlled parallel‐group study was conducted at nine sites in Argentina, Australia, Canada, France, and the United States between May 2006 and April 2008. Ambulatory postmenopausal women in good health and between 50 and 70 years of age were eligible if they had a lumbar spine or total‐hip T‐score between –2.0 and –3.0 by dual‐energy X‐ray absorptiometry (DXA). Subjects were included provided that HR‐pQCT (XtremeCT) could be performed in at least one wrist. Subjects were excluded if they had a fragility fracture after age 50 or had moderate to severe vertebral deformity using semiquantitative criteria.17 Additional exclusion criteria included vitamin D deficiency {serum 25‐hydroxyvitamin D [25(OH]D] < 12 ng/mL (30 nmol/L)}; conditions affecting bone metabolism; contraindications to alendronate; history of intravenous bisphosphonate, fluoride (except for dental procedures), or strontium ranelate use; cumulative oral bisphosphonate use for 3 months or more, bisphosphonate use for 1 month or more within the past year, or any use within 3 months of randomization; parathyroid hormone (PTH) or PTH derivative administration within the past year; or drugs known to affect bone remodeling or density within 3 months of randomization.
The institutional review board or ethics committee at each site approved the study protocol. The study was conducted according to all appropriate country regulations and the International Conference on Harmonization Good Clinical Practice Guidelines. All subjects provided written informed consent prior to enrollment.
Subjects were randomized 1:1:1 to subcutaneous injection of denosumab 60 mg every 6 months (n = 83), oral alendronate weekly (Fosamax 70 mg, n = 82; Merck, Whitehouse Station, NJ, USA), or placebo (n = 82). The sponsor generated the randomization scheme before the study. Subjects at each study site were randomized to treatment using a central interactive voice‐response system. Subjects and study sites were blinded to the treatment using a double‐dummy technique; subjects in the denosumab group received weekly placebo tablets, subjects in the alendronate group received placebo subcutaneous injections every 6 months, and subjects in the placebo group received both placebos.
The denosumab solution contained 60 mg/mL of denosumab, 5% sorbitol, and 10 mM sodium acetate in water for injection (USP), pH 5.2. The placebo injection solution was identical to the denosumab injection solution except for the protein content. Oral tablets (alendronate or placebo) were presented as matching oval tablets. All subjects received calcium supplements (≥500 mg/day). Daily vitamin D supplementation was based on concentrations of serum 25(OH)D at screening. The dosage of vitamin D was 400 IU or more daily if screening 25(OH)D concentration was greater than 20 ng/mL (>50 nmol/L) or 800 IU or more daily if screening 25(OH)D was 12 ng/mL or more to 20 ng/mL or less (≥30 to ≤50 nmol/L).
Study visits were scheduled at baseline; week 1; months 1, 3, and 6; month 6 + 1 week; and months 7, 9, and 12. At the screening visit, a medical history, physical examination, vital signs, and concomitant medications were documented; fasting serum samples were collected for hematology and chemistry analyses. Vertebral fracture assessment was performed at baseline. Review of concomitant medication, vital signs, and fasting serum samples for laboratory evaluation of turnover markers was done at all study visits. Additional hematology and chemistry evaluations were done at baseline and the month 6 and 12 visits.
HR‐pQCT of the distal radius and distal tibia and QCT of the distal radius were done during baseline, month 6, and month 12 visits. The HR‐pQCT scans were analyzed using standard manufacturer's software (Scanco Medical, Brüttisellen, Switzerland). Radius QCT scans were obtained at a location comparable with that of the HR‐pQCT scans. Total volumetric BMD (vBMD) was assessed using a threshold of 100 mg/cm3 to delineate the periosteal surface, as described previously.18, 19 QCT scans also allowed calculation of a density‐based polar moment of inertia (PMI).
The oral product was dispensed at baseline and the month 3, 6, and 9 visits. Tablet counts were recorded at the month 3, 6, 9, and 12 visits. Subcutaneous injection of denosumab or placebo was administered at the baseline and month 6 visits after all study‐related procedures were completed. Adverse events were collected at all study visits subsequent to baseline.
Statistical analysis
At the time of study protocol development, there was no information regarding the magnitude of expected changes in HR‐pQCT parameters with placebo or therapy. Therefore, formal statistical hypothesis testing was not preplanned for this study; only estimation of treatment effects was planned. p Values for the differences between treatments were calculated post hoc. Efficacy endpoints included the percentage change from baseline in cortical thickness; the percentage changes in total, cortical, and trabecular vBMD; trabecular number, thickness, and separation as measured by HR‐pQCT at the distal radius and tibia; the percentage change in QCT parameters total vBMD and PMI at the distal radial site corresponding to the region scanned with HR‐pQCT; and the changes in bone turnover markers serum C‐telopeptide of type I collagen cross‐links (CTX) and procollagen type 1 N‐terminal propeptide (P1NP). Safety was evaluated by adverse‐event reporting and monitoring changes in laboratory values and vital signs.
Efficacy analyses included all subjects who received at least one dose of investigational product and had a baseline measurement and at least one postbaseline measurement. Safety analyses included all subjects who received at least one dose of investigational product.
The treatment difference in the percentage changes in bone volumetric and geometric parameters derived from HR‐pQCT and QCT were evaluated using an analysis of covariance model (ANCOVA), adjusting for age group and baseline values in addition to the treatment effect. Changes in the biochemical markers of bone turnover had a nonnormal distribution and thus were summarized using medians and interquartile ranges.
Role of the funding source
The study design, conduct, data collection, statistical analysis, and funding were the responsibility of the sponsor. The manuscript was drafted by E Seeman and C Libanati. All other authors participated in collecting data and critical review of drafts and approved the submitted manuscript. Authors had access to all study data. The decision to submit the manuscript was at the discretion of the authors.
Results
Baseline demographics were similar among the groups (Table 1); 96% of women were Caucasian. A total of 247 women were randomized to placebo (n = 82), alendronate (n = 82), or denosumab (n = 83); 217 (88%) completed 12 months of follow‐up (Fig. 1). The main reasons for discontinuation were withdrawal of consent, loss to follow‐up, and adverse events (AEs).
Table 1.
Baseline Demographics and Disease Characteristics
| Placebo (n = 82) | Alendronate 70 mg qw (n = 82) | Denosumab 60 mg q6m (n = 83) | Total (n = 247) | |
|---|---|---|---|---|
| Age, years, mean (SD) | 60.8 (5.2) | 60.7 (5.2) | 60.3 (5.9) | 60.6 (5.4) |
| ≤60 years, n (%) | 39 (48) | 38 (46) | 39 (47) | 116 (47) |
| >60 years, n (%) | 43 (52) | 44 (54) | 44 (53) | 131 (53) |
| Ethnicity/race, n (%) | ||||
| White or Caucasian | 81 (99) | 77 (94) | 79 (95) | 237 (96) |
| Hispanic or Latino | 0 (0) | 1 (1) | 1 (1) | 2 (< 1) |
| Asian or Japanese | 1 (1) | 3 (4) | 3 (4) | 7 (3) |
| Other | 0 (0) | 1 (1) | 0 (0) | 1 (< 1) |
| Geographic location, n (%) | ||||
| Argentina | 58 (71) | 62 (76) | 56 (67) | 176 (71) |
| Canada | 10 (12) | 10 (12) | 12 (14) | 32 (13) |
| France | 8 (10) | 3 (4) | 6 (7) | 17 (7) |
| United States | 5 (6) | 5 (6) | 7 (8) | 17 (7) |
| Australia | 1 (1) | 2 (2) | 2 (2) | 5 (2) |
| Years since menopause, mean (SD) | 12. 8 (6.2) | 13.1 (8.0) | 13.6 (7.6) | 13.2 (7.3) |
| Baseline BMD T‐score, mean (SD) | ||||
| Total hip | −1.1 (0.7) | −1.4 (0.7) | −1.4 (0.8) | −1.3 (0.7) |
| Lumbar spine | −2.4 (0.3) | −2.5 (0.3) | −2.4 (0.4) | −2.4 (0.3) |
Figure 1.
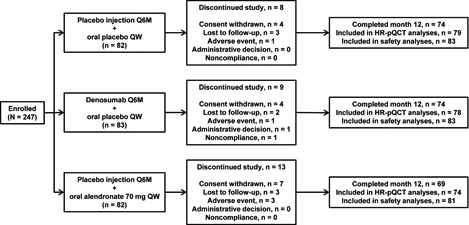
Subject disposition. Although the placebo and alendronate groups had 82 patients each, the safety analyses in these groups included 83 and 81 patients, respectively, because 1 subject in the alendronate group received placebo injection but no oral alendronate treatment; this subject was evaluated for safety in the placebo group. Q6M = every 6 months; QW = every week.
At the distal radius at 12 months, total, cortical, and trabecular vBMD and cortical thickness assessed by HR‐pQCT decreased in the placebo group. Alendronate prevented the decrease in total, cortical, and trabecular vBMD and increased cortical thickness. By contrast, denosumab increased total, cortical, and trabecular vBMD and cortical thickness relative to baseline, producing changes that significantly exceeded those observed with alendronate for total and cortical vBMD but not trabecular vBMD or cortical thickness (Fig. 2). There were no differences between groups for trabecular number, thickness, or separation at the distal radius at 6 or 12 months (data not shown).
Figure 2.
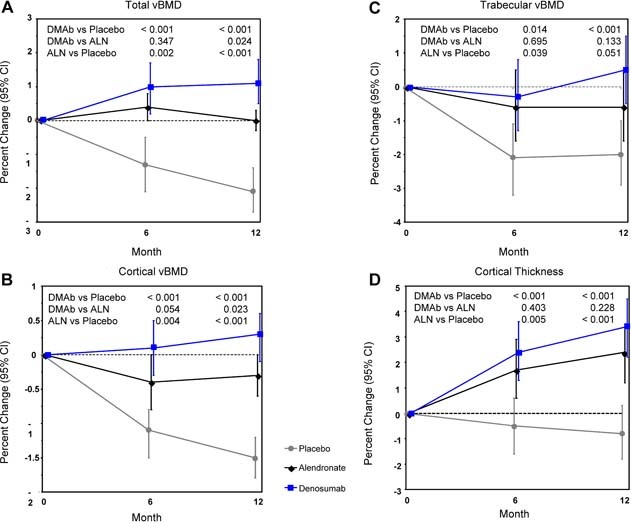
Percent changes by HR‐pQCT at the distal radius: total vBMD (A), cortical vBMD (B), trabecular vBMD (C), and cortical thickness (D). Least‐squares means with 95% CIs based on an ANCOVA model adjusting for baseline, age group, and treatment. Between‐group p values at months 6 and 12 are shown. DMAb = denosumab; ALN = alendronate.
At the distal tibia at 12 months, total, cortical, and trabecular vBMD assessed by HR‐pQCT decreased in the placebo group, whereas cortical thickness increased in the placebo group. Alendronate increased total and trabecular vBMD, maintained cortical vBMD, and increased cortical thickness. By contrast, denosumab increased total, cortical, and trabecular vBMD and cortical thickness and did so to a significantly greater extent than alendronate for total and cortical vBMD, but not for trabecular vBMD and cortical thickness (Fig. 3). No differences were seen between groups for trabecular number, thickness, or separation at the distal tibia at 6 or 12 months (data not shown).
Figure 3.
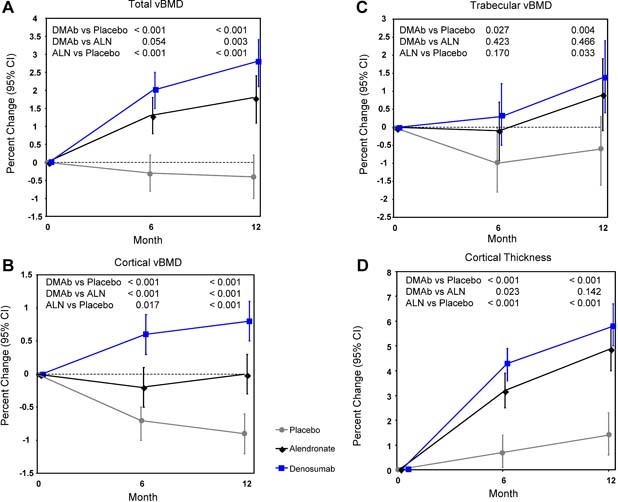
Percent changes by HR‐pQCT at the distal tibia: total vBMD (A), cortical vBMD (B), trabecular vBMD (C), and cortical thickness (D). Least‐squares means with 95% CIs based on an ANCOVA model adjusting for baseline, age group, and treatment. Between‐group p values at months 6 and 12 are shown. DMAb = denosumab; ALN = alendronate.
At the radius at 12 months, total vBMD as assessed using QCT decreased in the placebo group but increased in the alendronate and denosumab groups (Fig. 4 A). Density‐weighted PMI was unchanged at 12 months in the placebo group but increased in the alendronate and denosumab groups (Fig. 4 B). The increases in vBMD and PMI were significantly greater with denosumab than with alendronate; these differences between treatments also were observed at 6 months.
Figure 4.
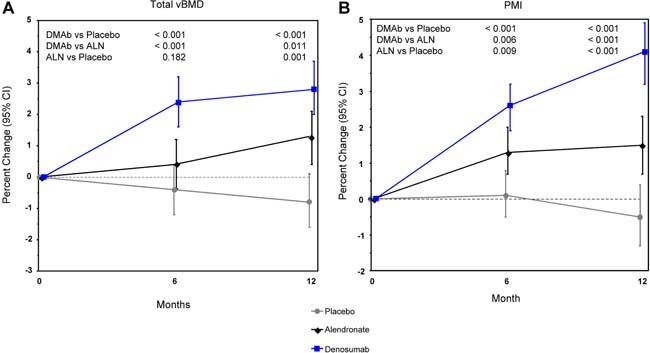
Percent change in total vBMD by QCT (A) and calculated PMI (B) at the distal radius. Least‐squares means with 95% CIs based on an ANCOVA model adjusting for baseline, age group, and treatment. Between‐group p values at months 6 and 12 are shown.
Serum CTX decreased slightly in the placebo group and substantially in the alendronate and denosumab groups (Fig. 5 A). Serum CTX in the alendronate group reached a nadir by 3 months, and this level of reduction remained comparable throughout the 12 months of the study. The reduction in serum CTX occurred more rapidly and was greater with denosumab than with alendronate. Serum CTX decreased within the first week following each denosumab dose, and the level of suppression lessened by the end of the 6‐month dosing interval.
Figure 5.
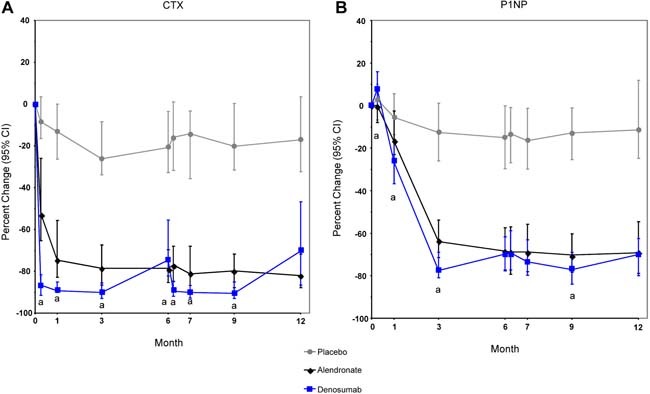
Median percent change in bone turnover markers. (A) Serum C‐telopeptide of type 1 collagen cross‐links (CTX). (B) Procollagen type 1 N‐terminal propeptide (P1NP). aSignificantly different from alendronate (p < .05).
P1NP also decreased slightly in the placebo group and substantially in the alendronate and denosumab groups (Fig. 5 B). For the alendronate group, the maximal reduction in P1NP occurred by month 3 and was maintained throughout 12 months. For the denosumab group, the suppression in P1NP was slower than that of CTX; the nadir occurred by 3 months, and then the suppression lessened by the end of the 6‐month dosing interval.
The incidence of AEs was similar between treatment groups (Table 2). Most AEs were mild. Serious AEs were reported in five subjects in the placebo group, five subjects in the alendronate group, and two subjects in the denosumab group. Infections were reported for 55%, 56%, and 54% of subjects in the placebo, alendronate, and denosumab groups, respectively. Only one serious AE of infection was reported during the study (pneumonia in the placebo group). No trends in serum chemistry or hematology were noted other than mild decreases in albumin‐adjusted serum calcium, phosphorus, and total alkaline phosphatase that were not clinically significant. No subject tested positive for antidenosumab antibodies.
Table 2.
Adverse Events
| Placebo (n = 83) | Alendronate 70 mg qw (n = 81) | Denosumab 60 mg q6m (n = 83) | |
|---|---|---|---|
| Adverse events, n (%) | 78 (94.0) | 77 (95.1) | 76 (91.6) |
| AEs occurring with ≥10% frequency | |||
| Constipation | 12 (14.5) | 13 (16.0) | 15 (18.1) |
| Influenza | 15 (18.1) | 10 (12.3) | 14 (16.9) |
| Pain in extremity | 10 (12.0) | 10 (12.3) | 10 (12.0) |
| Nasopharyngitis | 14 (16.9) | 8 (9.9) | 10 (12.0) |
| Arthralgia | 8 (9.6) | 8 (9.9) | 10 (12.0) |
| Back pain | 10 (12.0) | 6 (7.4) | 10 (12.0) |
| Bronchitis | 11 (13.3) | 11 (13.6) | 9 (10.8) |
| Headache | 9 (10.8) | 12 (14.8) | 6 (7.2) |
| Upper abdominal pain | 8 (9.6) | 10 (12.3) | 5 (6.0) |
| Dyspepsia | 7 (8.4) | 9 (11.1) | 5 (6.0) |
| Diarrhea | 9 (10.8) | 10 (12.3) | 3 (3.6) |
| Abdominal pain | 3 (3.6) | 9 (11.1) | 2 (2.4) |
| Treatment‐related adverse eventsa | 32 (38.6) | 36 (44.4) | 26 (31.3) |
| Serious adverse events, n (%) | 5 (6.0) | 5 (6.2) | 2 (2.4) |
| Acute cholecystitis | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| Loss of consciousness | 0 (0.0) | 1 (1.2) | 1 (1.2)b |
| Hyperglycemia | 0 (0.0) | 0 (0.0) | 1 (1.2)b |
| Breast cancer | 0 (0.0) | 2 (2.5) | 0 (0.0) |
| Adenocarcinoma of the cervix | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Biliary colic | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Cholelithiasis | 2 (2.4) | 0 (0.0) | 0 (0.0) |
| Amnesia | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Confusional state | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Pneumonia | 1 (1.2) | 0 (0.0) | 0 (0.0) |
Assessed by the investigator as being possibly or probably related to investigational product administration without unblinding of treatment.
One subject in the denosumab group had two serious adverse events (hyperglycemia and loss of consciousness), neither of which was considered related to study treatment.
Discussion
This study examined the effects of denosumab and alendronate on cortical and trabecular density and microarchitecture. The response to therapy was treatment‐specific. Bone remodeling, as reflected by serum CTX, an independent predictor of fracture risk,20 was suppressed more rapidly and more markedly with denosumab than with alendronate. P1NP also was more greatly suppressed with denosumab but not more rapidly. This suppression of remodeling was accompanied by changes in bone morphology, as measured using HR‐pQCT and QCT. At the distal radius, the decreases in the placebo arm in total, cortical, and trabecular BMD and associated structural parameters were prevented by both treatments and partly reversed with denosumab. Denosumab increased total BMD, cortical BMD, and PMI relative to baseline within 6 months, and the improvements exceeded those observed with alendronate.
The benefit observed with denosumab over alendronate in total and cortical BMD may be the result of differences in the mechanism of action of these drugs at both the tissue and basic multicellular unit (BMU) levels. At the tissue level, denosumab probably suppresses the birth rate of new remodeling units because it interferes with the synthesis of osteoclasts from their precursors. Thus fewer newly excavated sites appear during denosumab than alendronate therapy, as supported by the greater reduction in serum CTX. The reduction in P1NP also was greater with denosumab than with alendronate but not as rapid as CTX perhaps because remodeling sites present at the onset of treatment complete their formation phase more slowly than the resorption phase. The net effect is a greater rise in BMD with denosumab because filling of the resorption cavities (initiated before treatment began) with new bone is offset by the simultaneous appearance of fewer newly excavated resorption cavities during denosumab than alendronate therapy.2 Further supporting this assumption is the recent report of fewer eroded surfaces determined from bone biopsies obtained from women transitioning from alendronate to denosumab compared with women continuing on alendronate.21
The more rapid reduction in CTX also may result from differences in the mechanism of action of these drugs at the cellular level. Denosumab rapidly binds RANKL, which is essential for the synthesis, activity, and survival of mature osteoclasts. Therefore, denosumab may rapidly reduce resorption at the BMU level as well (ie, reduce resorption in existing resorption cavities). By contrast, the antiresorptive effect of alendronate is believed to be the result of uptake by osteoclasts as they resorb bone mineral containing the bisphosphonate.22 Thus, when alendronate is commenced, existing osteoclasts may continue resorbing bone until they resorb matrix containing the bisphosphonate, a process that may not be immediate nor occur uniformly throughout the skeleton. This effect also may depend on the cumulative bisphosphonate dose and its affinity for bone. The delay in achieving a nadir in serum CTX may reflect the fact that a longer interval is needed for active osteoclasts to stop resorbing with alendronate compared with denosumab.
Thus, during denosumab therapy, reduction in progression of bone fragility is likely to be the result of the appearance of fewer and perhaps smaller excavation cavities.23 We speculate that if treatment with denosumab reduces the volume of bone resorbed, the smaller erosion cavity could be more completely filled with new bone, resulting in a less negative net BMU balance with denosumab. The greater number of newly excavated cavities appearing during alendronate therapy is likely to result in remodeling and erosion of bone, allowing further structural decay.
The greater remodeling suppression with denosumab also may be the result of a greater accessibility of denosumab to the cortical volume, which constitutes 80% of skeletal mass. While denosumab distributes through the skeleton, the penetration of bisphosphonates into the matrix partly depends on their binding affinity to hydroxyapatite and is reported to be less for agents with greater affinity such as alendronate rather than risedronate.22, 24 Although the role of distribution and penetration of treatments in determining remodeling suppression remains unconfirmed, the larger gains in cortical bone with denosumab reported using DXA and observed in this study might be partly accounted for by this mechanism.12, 13, 14, 15, 16 The failure to identify changes between treatments or placebo in indices of trabecular morphology, such as trabecular thickness, number, and separation, in this study may reflect a limitation of the HRpQCT technique in that the 82‐µm resolution did not allow detection of changes in this 1‐year study.
This clinical study demonstrates that reduction in bone remodeling in response to antiresorptive therapies influences cortical thickness. However, this increase may be the result of a change in tissue density in the cortical compartment rather than the result of periosteal or endosteal apposition. Cortical thickness is derived using an annular model where the measured cortical area is divided by the periosteal perimeter.25 The increases in tissue mineral density owing to mineralization of bone that would have been removed by the high remodeling may influence edge detection and so produce an apparent increase in cortical area from which the cortical thickness is derived. Whether completion of bone formation in existing resorption sites on the endocortical or trabecular surfaces produces focal thickening is uncertain because any potential changes also are below the resolution of the technology available for in vivo serial measurements in human subjects. A more tenable explanation is that the increase in cortical thickness results from an improvement in cortical area produced by a reduction in cortical porosity, a mechanism supported by preclinical data in monkeys treated with denosumab.11, 26, 27 That is, in a cross section of cortex, there is mineralized matrix and porosity (canals in longitudinal section). If porosity is reduced by filling or partial filling with mineralized bone, the area that is bone mineral will increase.
An increase in effective cortical area by a reduction in porosity increases compressive strength and resistance to bending. The results observed using forearm QCT are consistent with those using HR‐pQCT and suggest that the changes observed at the distal radius achieve an improvement in bone strength, estimated using PMI. These changes also were greater with denosumab than with alendronate. Overall, the increases in BMD and cortical measurements in the treatment groups relative to placebo are consistent with the known fracture risk reduction reported for both agents.28, 29 Additional study is needed to assess whether the benefits of denosumab on cortical bone compared with alendronate lead to improved fracture outcomes, particularly fractures occurring as a result of structural decay of cortical bone.
In summary, these data advance understanding of the structural consequences of postmenopausal bone loss and highlight potential differences in microarchitectural outcomes of treatment with denosumab and alendronate, two antiresorptive therapies with different mechanisms of action.
Disclosures
ES has received research support from Amgen, Inc., and has served as a consultant for Amgen, Inc., Eli Lilly, Sanofi‐Aventis, Servier, and Merck Sharpe & Dohme. DAH has received research support and served as a consultant and speaker for Amgen, Inc. DS and AMC have received research support from Amgen, Inc. ES has received research support from Amgen, Inc., Eli Lilly, Merck, and Novartis. TT has served as a consultant for and received lecture fees from Amgen, Inc., Eli Lilly, Merck Sharpe & Dohme, Procter & Gamble, Roche, Novartis, and Ipsen. MF and CL are employees of Amgen, Inc., and may own stock or stock options in Amgen, Inc. JZ has received research support and/or served as a consultant for Amgen, Inc., Eli Lilly, Pfizer, and Servier. AK, SB, SB, SM, and CB state that they have no conflicts of interest.
Acknowledgements
We dedicate this paper to the memory of Professor Pierre Delmas, who was instrumental in the development of this protocol. We are indebted to Amy Foreman‐Wykert, PhD, of Amgen, Inc., and Jonathan Latham, PharmD, of PharmaScribe, LLC, for assistance with manuscript formatting and version control, preparation of figures, reference management, and collating the authors' comments during manuscript preparation. We thank Dr Michael Ominsky for his review of the manuscript and valuable comments. Amgen, Inc., supported the study and the development of this manuscript. Clinicaltrials.gov registration: NCT00293813 (registered February 17, 2006).
References
- 1. Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003; 14: S13–18. [DOI] [PubMed] [Google Scholar]
- 2. Seeman E, Delmas PD. Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med. 2006; 354: 2250–2261. [DOI] [PubMed] [Google Scholar]
- 3. Augat P, Schorlemmer S. The role of cortical bone and its microstructure in bone strength. Age Ageing. 2006; 35: S27–31. [DOI] [PubMed] [Google Scholar]
- 4. Kanis JA, Oden A, Johnell O, et al. The components of excess mortality after hip fracture. Bone. 2003; 32: 468–473. [DOI] [PubMed] [Google Scholar]
- 5. Sornay‐Rendu E, Boutroy S, Munoz F, et al. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007; 22: 425–433. [DOI] [PubMed] [Google Scholar]
- 6. Bruyere O, Roux C, Detilleux J, et al. Relationship between bone mineral density changes and fracture risk reduction in patients treated with strontium ranelate. J Clin Endocrinol Metab. 2007; 92: 3076–3081. [DOI] [PubMed] [Google Scholar]
- 7. Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002; 359: 2018–2026. [DOI] [PubMed] [Google Scholar]
- 8. Watts NB, Geusens P, Barton IP, et al. Relationship between changes in BMD and nonvertebral fracture incidence associated with risedronate: reduction in risk of nonvertebral fracture is not related to change in BMD. J Bone Miner Res. 2005; 20: 2097–2104. [DOI] [PubMed] [Google Scholar]
- 9. Zebaze RM, Seeman E. Cortical stability of the femoral neck and hip fracture risk. Lancet. 2005; 366: 1523; author reply 1524–1525. [DOI] [PubMed] [Google Scholar]
- 10. Ominsky MS, Li X, Tan HL, et al. The effects of alendronate or denosumab on cortical and trabecular bone mass, bone strength, and bone mass‐strength relationships in mice. Abstract 1139. J Bone Miner Res. 2008; 23 (Suppl1): S40. [Google Scholar]
- 11. Ominsky MS, Smith SY, Jolette J, et al. Denosumab, a fully human RANKL antibody, reduced bone turnover and increased cancellous and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Calcif Tissue Int. 2008; 82 (Suppl 1): S240. [Google Scholar]
- 12. Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009; 24: 153–161. [DOI] [PubMed] [Google Scholar]
- 13. Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010; 25: 72–81. [DOI] [PubMed] [Google Scholar]
- 14. Lewiecki EM, Miller PD, McClung MR, et al. Two‐year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res. 2007; 22: 1832–1841. [DOI] [PubMed] [Google Scholar]
- 15. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006; 354: 821–831. [DOI] [PubMed] [Google Scholar]
- 16. Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long‐term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008; 43: 222–229. [DOI] [PubMed] [Google Scholar]
- 17. Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993; 8: 1137–1148. [DOI] [PubMed] [Google Scholar]
- 18. Boutroy S, Bouxsein ML, Munoz F, et al. In vivo assessment of trabecular bone microarchitecture by high‐resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005; 90: 6508–6515. [DOI] [PubMed] [Google Scholar]
- 19. MacNeil JA, Boyd SK. Accuracy of high‐resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007; 29: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 20. Garnero P, Sornay‐Rendu E, Claustrat B, et al. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000; 15: 1526–1536. [DOI] [PubMed] [Google Scholar]
- 21. Reid I, Benhamou L, Bolognese M, et al. Effects of denosumab on bone histology and histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2009; 24 (Suppl 1): S9. [DOI] [PubMed] [Google Scholar]
- 22. Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008; 19: 733–759. [DOI] [PubMed] [Google Scholar]
- 23. Vanderoost J, Jaecques S, Van der Perre G, et al. Resorption cavity depth explains strength enhancing effect of antiresorptive agents. J Bone Miner Res. 2009; 24 (Suppl 1): S157. [Google Scholar]
- 24. Reid I, Cornish J, Callon K, et al. Risedronate is preferentially deposited in trabecular bone in rats. J Bone Miner Res. 2009; 24 (Suppl 1): S353. [Google Scholar]
- 25. Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D‐QCT of the human forearm. Technol Health Care. 1998; 6: 329–337. [PubMed] [Google Scholar]
- 26. Ominsky MS, Schroeder J, Smith SY, et al. Denosumab (AMG 162, a fully human RANKL antibody) increases cortical and cancellous bone mass and density in aged ovariectomized cynomologus monkeys. J Bone Miner Res. 2006; 21 (Suppl 1): 1272. [Google Scholar]
- 27. Ominsky MS, Smith SY, Vlasseros F, et al. Transition from alendronate to denosumab in ovariectomized cynomolgus monkeys maintained or improved cortical and trabecular bone mass, without altering the linear relationship between bone mass and bone strength. J Bone Miner Res. 2008; 23 (Suppl 1): S22. [Google Scholar]
- 28. Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000; 85: 4118–4124. [DOI] [PubMed] [Google Scholar]
- 29. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009; 361: 756–765. [DOI] [PubMed] [Google Scholar]


