Abstract
The present experiments were conducted to provide a more detailed behavioral analysis of the dissociable roles of the basolateral (BLA) and central nucleus (CeA) of the amygdala in mediating intra-accumbens (Acb) opioid-induced feeding of a high-fat diet. Confirming previous findings, temporary inactivation of the CeA with the GABAA agonist muscimol reduced DAMGO (D-Ala2-NMe-Phe4-Glyol5-enkephalin)-induced and baseline food intake, whereas intra-BLA muscimol selectively blocked only DAMGO-induced food intake, leaving baseline feeding intact. However, although inactivation of the BLA reduced DAMGO-induced food intake to control levels, this treatment led to exaggerated number and duration of food hopper entries after food intake had ended. A subsequent experiment under conditions of limited access to the diet found the identical pattern of behavior following intra-Acb administration of DAMGO, regardless of whether the BLA was inactivated. Last, BLA inactivation was shown to have no influence on feeding driven by a state of negative-energy balance (24-hr food deprivation), demonstrating a specific influence of the BLA on opioid-driven feeding. These findings suggest that BLA mediates palatability-driven feeding and that this influence is particular to the consummatory act of ingestion.
Keywords: palatability, food reward, muscimol, feeding, food deprivation, high-fat diet
The rate of obesity in the United States has risen dramatically over the past 30 years (Ogden et al., 2006). The abundant availability and increased consumption of energy-dense foods high in sugar and fat, combined with a general decrease in physical activity, is largely responsible (Drewnowski & Levine, 2003; Hill, Wyatt, Reed, & Peters, 2003; Prentice & Jebb, 1995). Although feeding behavior is driven by a variety of factors, including homeostatic mechanisms (Saper, Chou, & Elmquist, 2002) and learned associations (Petrovich, Ross, Gallagher, & Holland, 2007), the process by which the hedonic nature of food drives feeding (Kelley, Baldo, Pratt, & Will, 2005) is critical to understanding the obesity epidemic. In particular, determining the neural substrates that underlie the motivational aspects of craving, seeking, and consuming energy-dense palatable food will greatly advance the efforts toward reversing the current trend of obesity in America and other developed countries.
The endogenous opioid peptides have received particular attention from both animal (Carr, 1984; Cooper, 1983; Giraudo, Grace, Welch, Billington, & Levine, 1993; Johnson, Stellar, & Paul, 1993; Weldon, O’Hare, Cleary, Billington, & Levine, 1996) and human (Drewnowski, Krahn, Demitrack, Nairn, & Gosnell, 1992; Yeomans & Gray, 1996, 2002) studies for their role in mediating food intake driven by palatability or the hedonic nature of food. One of the more extensively characterized animal models of opioid-mediated feeding involves opioid activation of the nucleus accumbens (Acb; Kelley et al., 2005). Indeed, intra-Acb administration of the µ-opioid agonist D-Ala2-NMe-Phe4-Glyol5-enkephalin (DAMGO) markedly increases food intake and preferentially enhances the intake of highly palatable substances such as fat, sucrose, and salt (Zhang & Kelley, 2002; Bakshi & Kelley, 1994). This effect has been shown to be dependent on the activation of a distributed network of cortical, limbic, and brainstem feeding-related structures (Will, Franzblau, & Kelley, 2003).
The amygdala, shown to be important for regulating emotion and motivation, is an integral part of this distributed opioid-driven feeding network (Will, Franzblau, & Kelley, 2004). Both of the major amygdala subregions, the basolateral (BLA) and central nucleus (CeA) of the amygdala, have reciprocal connections to brain regions that have been shown to influence feeding behavior. Indeed, both amygdala subregions receive inputs including prefrontal and gustatory cortex, whereas the CeA receives additional ascending gustatory information through the parabrachial nucleus and nucleus of the solitary tract (Fulwiler & Saper, 1984; Norgren, 1976; Saper & Loewy, 1980). Moreover, both the CeA and the BLA have direct projections to hypothalamic feeding circuitry and motor output pathways involved in eliciting feeding behavior (Alheid, 2003; Swanson, 2000, 2003; Swanson & Petrovich, 1998). However, unlike the BLA, the CeA has no direct cortical or ventral striatal (Acb) projections (Kelley, Domesick, & Nauta, 1982), suggesting that these two amygdala subregions may provide distinct contributions to feeding behavior.
Previous research has demonstrated that the BLA and CeA appear to have differential involvement in mediating intake of a high-fat diet, depending on whether feeding was observed following control treatment or intra-Acb opioid activation in sated animals (Will et al., 2004). Briefly, whereas CeA activity was necessary for both baseline and opioid-driven intake, BLA activity was only required to observe intake driven above baseline levels. Therefore, BLA inactivation had no influence on baseline intake of the high-fat diet but specifically prevented the robust increase observed following intake of intra-Acb opioids (Will et al., 2004). The different connectivity patterns of the BLA and the CeA with other feeding-related regions likely contribute to this observed difference (Alheid, 2003; Swanson, 2003). For instance, the BLA and the CeA have been shown to differentially regulate the dopamine and opioid signaling within the Acb. Indeed, temporary inactivation of the CeA has been shown to inhibit both baseline and feeding-induced dopamine efflux in the Acb, whereas BLA inactivation had no effect on either measure (Ahn & Phillips, 2003). It is interesting to note that, in the current model of opioid-induced high-fat feeding, inhibiting intra-Acb dopamine has no effect on food intake (Will et al., 2006). However, the role of dopamine in regulating locomotor activity and approach behavior is well documented and examining the parallel behaviors of approach and food intake should provide insight into the underlying neurochemistry mediating the influence of the amygdala on intra-Acb opioid-mediated feeding.
One possible interpretation of the previous data indicating differential influences of CeA and BLA on intra-Acb DAMGO-induced food intake (Will et al., 2004) is that the BLA specifically mediates palatability-driven feeding, as opposed to feeding driven by an energy-deficit (see Kelley et al., 2005). For example, it has already been shown that CeA activity is required to observe feeding driven by an energy deficit (24-hr food deprivation) (Baldo, Alsene, Negron, & Kelley, 2005; Minano, Meneres Sancho, Sancibrian, Salinas, & Myers, 1992). Furthermore, inactivation of the CeA, but not the BLA, blocks intra-Acb muscimol-induced feeding (Baldo et al., 2005), a pharmacological model that parallels the motivational state induced by energy deficit (i.e., food restriction; see Kelley et al., 2005, for review). Evidence for this includes the findings that intra-Acb DAMGO, but not intra-Acb muscimol, preferentially increases palatable food intake (Zhang, Gosnell, & Kelley, 1998) and increases progressive ratio responding for sucrose pellets (Zhang, Balmadrid, & Kelley, 2003). The lack of an influence after BLA inactivation on intra-Acb muscimol-induced feeding suggest that the BLA is specifically involved in palatability-driven feeding. This would predict that BLA activity is not required to observe the increased feeding that follows acute food deprivation; however, this has yet to be demonstrated.
In the present set of experiments, the role of the amygdala in mediating palatability-driven feeding behavior was examined within the model of intra-Acb opioid-mediated feeding of a high-fat diet. Whereas similar previous studies were limited to only measuring food intake, the present experiments were able to simultaneously assess multiple feeding behaviors (including general locomotor activity, number and duration of food hopper entries, and food intake) and compare these behaviors across time intervals using automated feeding chambers. In the first set of experiments, rats were given ad libitum access to high-fat diet after bilateral opioid stimulation of the Acb with the µ-opioid agonist DAMGO, and either the BLA or CeA was pharmacologically inactivated with the GABAA agonist muscimol. This method of pharmacological inactivation has been widely used to induce potent temporary neural inactivation (Herry et al., 2008; Krupa, Thompson, & Thompson, 1993; Simmons, Brooks, & Neill, 2007) without affecting fibers of passage. The influence of BLA inactivation on intra-Acb DAMGO-induced feeding was also examined under limited access conditions. Last, we examined energy deficit-driven feeding of the high-fat diet, induced by 24-hr food deprivation, while the BLA was pharmacologically inactivated with the GABAA agonist muscimol.
Method
Rats
Thirty-two adult male Sprague–Dawley rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) weighing 300–400 g, were housed in groups of two in Plexiglas cages in a climate-controlled colony room at a temperature of 22 °C. The rats were maintained on a 12-hr light–dark cycle, and all experiments were conducted during the light phase (0700–1900) between the hours of 1200 and 1500. Unless otherwise noted, rats had free access to laboratory chow and drinking water before and throughout the experiment. Experimental and control groups contained 6–9 rats unless otherwise noted. All experimental procedures were in accord with protocols approved by the University of Missouri Institutional Animal Care and Use Committee.
Surgery
Rats were anesthetized with a mixture of ketamine and xylazine (90 mg/kg and 9 mg/kg, respectively; Sigma, St. Louis, MO), and bilateral guide stainless steel cannulas (23 gauge, 10 mm) were sterotaxically implanted into a region near the border of the Acb core and lateral shell (Experiments 1–3). In addition, each rat was also implanted with bilateral cannulas into either the CeA (Experiment 1) or the BLA (Experiments 2–4). Therefore, each rat was implanted with four cannulas, except for Experiment 4, in which the rats received only two bilateral cannulas aimed at the BLA. Guide cannulas were secured to the skull with stainless steel screws and light curable resin (Dental Supply of New England, Boston) using standard flat-skull techniques. After surgery, wire stylets were placed in the guide cannulas to prevent occlusion. Coordinates for the aimed sites are as follows: Acb: AP, +1.4; ML, ±2.0; DV, −7.8; BLA: AP, −2.8; ML, ±4.7; DV: −8.6; CeA: AP, −2.0; ML, +/−4.0; DV: −8.3.
Apparatus
Behavioral assessment of feeding took place in a room separate from the colony room in eight Plexiglas (30.5 cm × 24.1 cm × 21.0 cm) custom-built feeding chambers (Med Associates, St. Albans, VT). Rats had access to water ad libitum and approximately 35 g of high-fat diet (except for Experiment 3, during which access was limited to 8 g). Feeding chambers were equipped with four infrared locomotor activity beams located 6 cm apart across the length of the chamber and 4.3 cm above the floor. An automated weigh scale for the food hopper continuously monitored the intake of food while automatically correcting for spillage. An additional infrared beam spanning the entrance of the food hopper determined the number and duration of each head entry into the hopper area. The feeding hopper and water bottle were located on the same side (opposite corners) of one chamber wall, and a removable waste tray was located beneath the bar floor. All measurements were automatically summed for every 10-min interval throughout the 2-hr test period. The measurements included locomotor activity (number of horizontal beam breaks), duration of hopper entry (duration of beam break at the entrance of the hopper), hopper entries (number of beam breaks at the entrance to the hopper), and amount consumed (grams of diet consumed). Testing periods consisted of 2 hr of continuous behavioral monitoring in the feeding chambers by a computer running Med-PC software (Med Associates Version IV, St. Albans, VT).
Procedure
Drug Microinjection
D-Ala2, NMe-Phe4, Glyol5-enkephalin (DAMGO; Research Biochemicals, Natick, MA) and muscimol (Sigma, St. Louis, MO) were both dissolved in sterile 0.9% saline. The vehicle control was always sterile 0.9% saline. Infusions were delivered with a microdrive pump (Harvard Apparatus, South Natick, MA), connected by means of polyethylene tubing (PE-10), while rats were gently handheld. Thirty-three-gauge 12.5-mm injectors were used, extending 2.5 mm beyond the end of the guide cannulas. The rate of injection was 0.32 µl/min for the Acb and 0.16 µl/min for the amygdala subregions (BLA and CeA), with the total duration of infusion being 93 s, resulting in 0.5-µl and 0.25-µl volumes, respectively. One additional minute was allowed for diffusion.
Behavioral Assessment of Feeding
Four groups of rats were used, each having either bilateral cannulas aimed at the Acb and CeA (Experiment 1), Acb and BLA (Experiments 2 and 3), or only the BLA (Experiment 4). All behavioral testing began 1 week postsurgery and occurred in the Med-Associates monitors described earlier. Rats were placed in these cages for 2 hr daily until stable food intake across 3 days was obtained, usually occurring within 6 days. To acclimate the rats to the treatment procedure, we gave them 2 days of sham injections over the last 2 days of the baseline period. On the first day of this acclimation procedure, a 10-mm injector was inserted and left in place for 2 min, with no volume injected. The following day, a 12.5-mm injector was inserted, and saline was administered for 93 s. With a within-subject design, all groups of rats received each of four drug treatment combinations on four separate treatment days in a counterbalanced order. On each test day, muscimol (20 ng/0.25 µl/side bilaterally) or saline was infused into the selected amygdala subregion, followed immediately by DAMGO (0.25 µg/0.5 µl/side bilaterally) or saline (Experiments 1–3) into the Acb, thus resulting in four possible treatment combinations. For the final experiment (Experiment 4), muscimol (20 ng/0.25 µl/side bilaterally) or saline was administered into the BLA after either a 24-hr period of home cage chow deprivation or ad libitum access. The 2-hr test session began immediately after the last injection. There was at least 1 day between treatment days.
Design
Experiment 1
Rats (n = 6) were examined for the feeding behaviors in the presence of a full hopper of high-fat diet (approximately 35 g) for 2-hr after intra-Acb administration of DAMGO or saline immediately after the CeA was infused with either muscimol or saline. After administration of one of the four possible drug treatments in a counterbalanced manner, all rats were immediately placed in the feeding chamber for a 2-hr period.
Experiment 2
Rats (n = 9) were examined under the same conditions as in Experiment 1, including DAMGO into the Acb; however, the BLA, rather than the CeA, was targeted for inactivation with muscimol.
Experiment 3
Rats (n = 7) were examined under the same conditions as in Experiment 2, except the amount of high-fat diet available in the hopper was reduced from 35 g to 8 g, as this latter amount is the average food intake typically observed after saline control treatment. The intention was to maintain similar food intake levels across all treatment conditions while restricting consumption below satiation levels for only the intra-DAMGO treatment condition (see Discussion for additional rationale).
Experiment 4
Rats (n = 6) were given 1-week access to high-fat diet as in the previous experiments. Next, in a nonde-prived state, all rats received intra-BLA administration of either saline or muscimol in a counterbalanced order, separated by at least 3 days, and given 2-hr access to the high-fat diet. During the following week, all rats were placed under 24-hr food home cage chow deprivation, followed by intra-BLA administration of either saline or muscimol in a counterbalanced order. Therefore, all rats received each of the four possible treatments, separated by at least 3 days to allow body weight and home cage food intake to return to baseline.
Specialized diet
The specialized sweetened high-fat diet was obtained from Teklad, Inc., Madison, WI. The diet contained 278.3 g/kg vitamin-free casein, 4.2 g/kg DL-methionine, 100.0 g/kg sucrose, 441.2 g/kg shortening, 77.7 g/kg safflower oil, 26.3 g/kg cellulose, 53.3 g/kg mineral mix, 15.2 g/kg vitamin mix, and 3.8 g/kg choline chloride. All components are expressed as weight (g). On the basis of energy, the diet is 6.2 kcal/g.
Histology
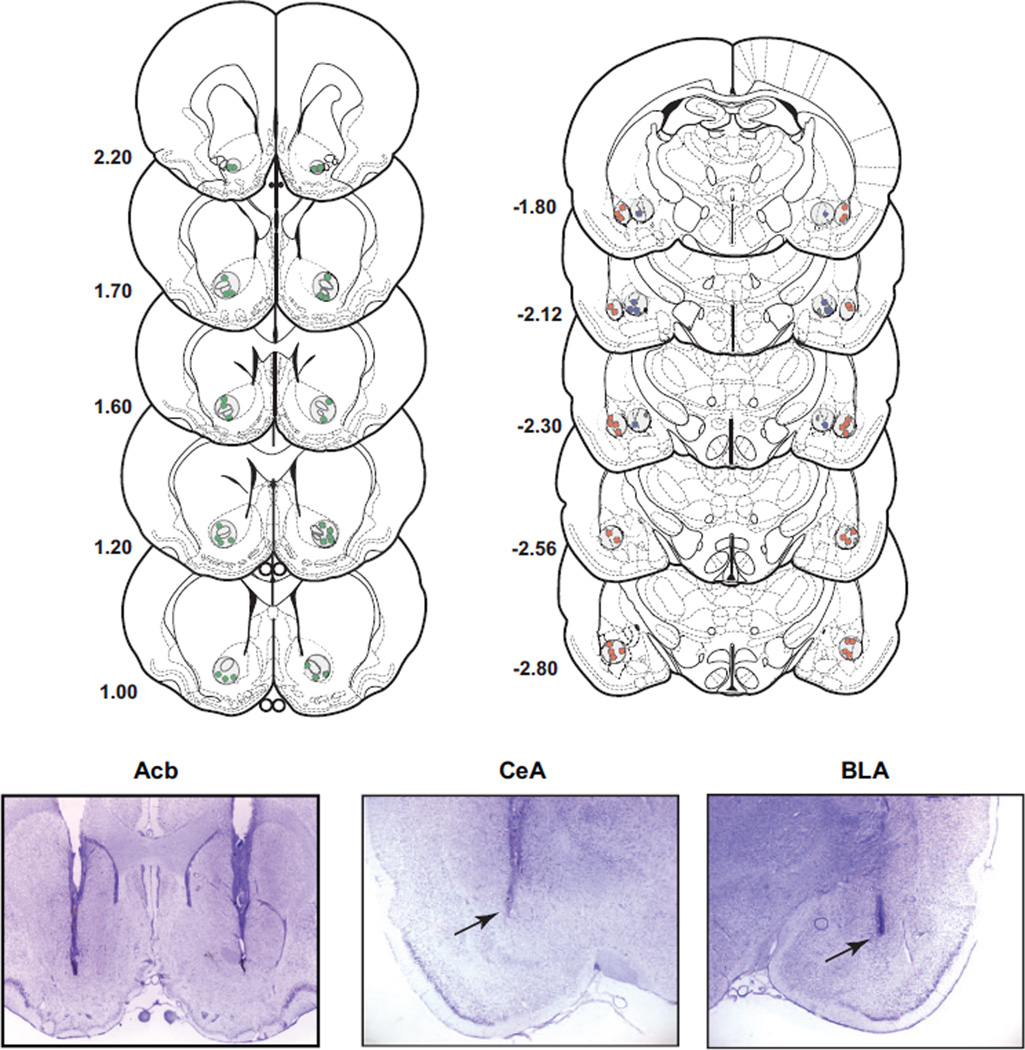
After behavioral testing was completed, rats were overdosed with sodium pentobarbital and perfused transcardially with heparinized saline (200 ml), followed immediately by 500 ml of a 10% buffered formalin solution. The brains were then removed and placed in 10% formalin−20% sucrose for 1 week. Frozen serial sections (50 µm) were collected through the entire extent of the injection site, mounted on gelatinized slides, and counterstained with cresyl violet. Cannula placement profiles were then analyzed for accuracy, and data from rats with misplaced cannulas were not included in the analyses. The placement of all CeA and BLA cannulas and a representative number of Acb cannulas are represented in histological reconstructions (see Figure 5). Also, representative photomicrographs of an injector track are shown for all three targeted regions (see Figure 5).
Figure 5.
Histological analysis and placement for all muscimol injections in the basolateral (BLA) and central nucleus of the amygdala (CeA) and a representative sample of DAMGO (D-Ala2-NMe-Phe4-Glyol5-enkephalin) injections. In the three lower panels are examples of injector tracks for all three targeted regions. Drawings and coordinates are based on the atlas of Paxinos and Watson (1998).
Statistical analysis
For Experiments 1–3, all feeding measures (food intake, locomotor activity, hopper entries, duration of hopper entry) for the total 2-hr session and across the various treatment conditions were analyzed with a two-factor within-subject analysis of variance (ANOVA; Acb Treatment × Amygdala Treatment), with the levels for each factor being either vehicle or drug. For Experiment 4, these same measures were also analyzed using a two-factor within-subject ANOVA (Food Deprivation State × Amygdala Treatment), with the levels for each factor being either nondeprived or deprived and vehicle or muscimol. Preplanned contrasts of means were conducted across treatments, between drug or vehicle and between each brain region (Experiments 1–3) or drug or vehicle and deprivation state (Experiment 4).
Results
Intra-Acb DAMGO significantly enhanced high-fat diet intake to approximately 200–300% above saline-injected control levels in all cannula placement groups (Experiments 1–3). This effect was very robust and consistent, with the majority of the feeding occurring in the first hour of the 2-hr session.
Experiment 1: Influence of CeA Inactivation on Feeding Behaviors Following Intra-Acb DAMGO
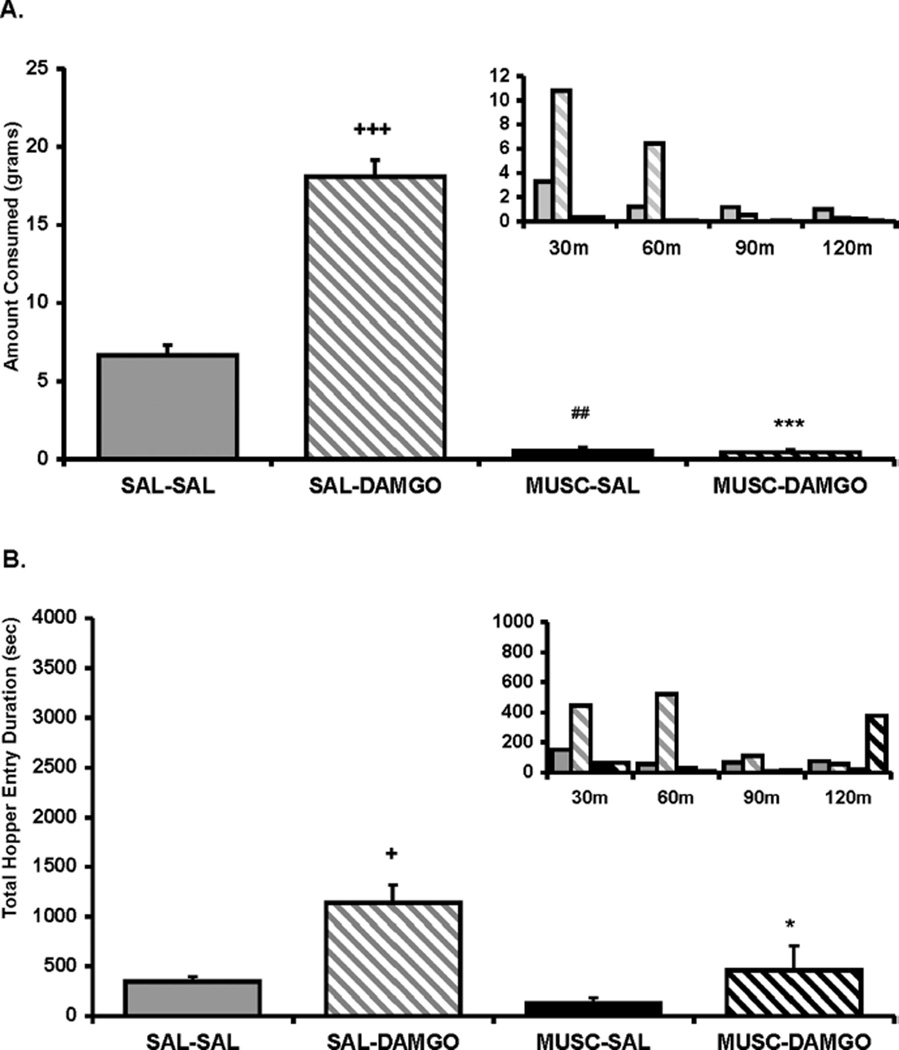
An ANOVA conducted on the food intake data for Experiment 1 revealed a significant main effect of intra-Acb DAMGO treatment, F(1, 5) = 321.06, p < .0001; intra-CeA muscimol treatment, F(1, 5) = 56.324, p < .001; and a significant Acb Treatment × CeA Treatment interaction, F(1, 5) = 79.89, p < .001. As displayed in Figure 1A, contrasts of means revealed that inactivation of the CeA with muscimol significantly reduced both baseline (p < .001) and DAMGO-elicited food intake (p < .0001), in line with previously reported data (Will et al., 2004). An ANOVA conducted on the combined duration of hopper entries across the 2-hr feeding session revealed a significant main effect of intra-Acb DAMGO treatment, F(1, 5) = 31.86, p < .005; but no main effect of intra-CeA muscimol treatment, F(1, 5) = 4.2, ns; or Acb Treatment × CeA Treatment interaction, F(1, 5) = 1.7, ns. As displayed in Figure 1B, contrasts of means revealed that intra-Acb DAMGO significantly increased total duration of hopper entries compared with saline treatment and that CeA inactivation prevented this increase (p < .05). In contrast to the food intake data, CeA inactivation by itself had no influence on hopper entry duration (p = .38). An ANOVA conducted on the measure of duration per hopper entry showed no main effect of intra-Acb DAMGO, F(1, 5) = 3.2, ns; or intra-CeA muscimol, F(1, 5) = 4.8, ns (data not shown). Finally, an ANOVA conducted on locomotor activity, F(1, 5) = 0.98, ns; and number of individual hopper entries (F5,15) = 2.66, ns) revealed no significant effect of treatment (see Tables 1 and 2).
Figure 1.
(A) Amount of food intake and (B) hopper entry duration (duration of beam break at entry of hopper) after intra-accumbens (intra-Acb) DAMGO ([D-Ala2-NMe-Phe4-Glyol5-enkephalin] 0.25 µg/0.5 µl per side) or saline (SAL) administration immediately after muscimol (MUSC; 20 ng/0.25 µl per side) or SAL administration into the central nucleus of the amygdala (CeA). Small inset graphs display the same data across 30-min intervals (y axis represents the same measure as the larger corresponding graph). The x axis labels refer to treatment for the two regions (i.e., Acb treatment/CeA treatment). Values represent group means (plus or minus standard error of the mean). Plus sign represents DAMGO/SAL versus SAL/SAL; pound sign represents MUSC/SAL versus SAL/SAL; asterisk represents MUSC/DAMGO versus SAL/DAMGO. Level of significance is indicated by number of symbols (e.g., *p< .05, **p< .01, and ***p< .001).
Table 1.
Feeding Bouts
| Experiment and feeding time (min) |
SAL-SAL | SAL-DAMGO | MUSC-SAL | MUSC-DAMGO |
|---|---|---|---|---|
| Experiment 1 | ||||
| 0–30 | 43.5 ± 4.6 | 61.8 ± 18.2 | 48.2 ± 11.0 | 50.2 ± 21.1 |
| 30–60 | 16.7 ± 5.6 | 50.0 ± 14.9 | 6.3 ± 4.9 | 10.8 ± 5.1 |
| 60–90 | 22.5 ± 8.2 | 26.7 ± 9.4 | 1.5 ± 1.0 | 3.7 ± 2.9 |
| 90–120 | 18.7 ± 11.4 | 24.7 ± 7.2 | 2.3 ± 2.3 | 15.5 ± 9.8 |
| Total | 101.3 ± 23.2 | 163.2 ± 34.6 | 58.3 ± 16.2 | 80.2 ± 25.9 |
| Experiment 2 | ||||
| 0–30 | 30.4 ± 3.3 | 66.1 ± 17.0 | 40.2 ± 7.6 | 120.7 ± 29.3 |
| 30–60 | 14.0 ± 3.5 | 56.8 ± 17.9 | 14.3 ± 5.3 | 110.3 ± 30.4 |
| 60–90 | 10.6 ± 2.3 | 40.2 ± 13.1 | 4.7 ± 2.2 | 89.7 ± 39.1 |
| 90–120 | 7.0 ± 3.5 | 15.6 ± 3.9 | 3.4 ± 2.6 | 92.8 ± 45.1 |
| Total | 62.0 ± 7.9 | 178.7 ± 47.2 | 62.7 ± 12.4 | 413.4 ± 138.0* |
| Experiment 3 | ||||
| 0–30 | 56.6 ± 21.3 | 63.3 ± 14.6 | 40.3 ± 6.9 | 100.9 ± 18.6 |
| 30–60 | 44.1 ± 14.8 | 67.7 ± 30.2 | 9.6 ±4.1 | 66.1 ± 17.4 |
| 60–90 | 37.9 ± 19.8 | 105.7 ± 31.4 | 5.9 ± 3.0 | 48.9 ± 18.8 |
| 90–120 | 45.0 ± 26.0 | 134.0 ± 37.8 | 1.6 ± 1.4 | 38.3 ± 12.1 |
| Total | 183.6 ± 76.6 | 370.7 ± 108.7+ | 57.3 ± 11.1 | 254.1 ± 56.3 |
| Experiment 4 |
Nonrestricted saline |
Nonrestricted muscimol |
Restricted Saline |
Restricted muscimol |
| 0–30 | 24.8 ± 3.4 | 24.0 ± 5.3 | 59.3 ± 13.3 | 47.2 ± 12.4 |
| 30–60 | 8.7 ± 3.5 | 11.8 ± 5.6 | 12.0 ± 5.2 | 4.2 ± 2.1 |
| 60–90 | 13.0 ± 7.0 | 7.7 ± 3.9 | 7.2 ± 1.9 | 7.5 ± 1.9 |
| 90–120 | 8.0 ± 2.7 | 5.5 ± 3.9 | 9.2 ± 5.8 | 7.3 ± 3.4 |
| Total | 54.5 ± 11.1 | 49.0 ± 10.5 | 87.7 ± 20.6 | 66.2 ±11.3 |
Note. Values represent group means plus or minus the standard error of the mean for a 2-hr measure of food hopper entries (no. of beam breaks at entry of hopper) presented in 30-min trials and a combined 2-hr total after each treatment. For Experiments 1–3, a superscript plus sign represents DAMGO/S AL vs. SAL/SAL and a superscript asterisk represents MUSC/DAMGO vs. SAL/DAMGO (each pairing represents the order of administration). For Experiment 4, there were no significant differences. Level of significance is indicated by the number of symbols (i.e.,
p < .05).
SAL = saline; DAMGO = D-Ala2-NMe-Phe4-Glyol5-enkephalin; MUSC = muscimol.
Table 2.
Locomotor Activity
| Experiment and trial (in minutes) |
SAL-SAL | SAL-DAMGO | MUSC-SAL | MUSC-DAMGO |
|---|---|---|---|---|
| Experiment 1 | ||||
| 0–30 | 499.0 ± 44.9 | 273.5 ± 37.3 | 541.0 ± 80.0 | 514.3 ± 175.8 |
| 30–60 | 225.5 ± 41.5 | 253.2 ± 78.1 | 156.5 ± 36.9 | 479.3 ± 139.4 |
| 60–90 | 141.2 ± 42.8 | 281.8 ± 40.1 | 69.8 ± 12.7 | 225.5 ± 87.1 |
| 90–120 | 132.7 ± 35.5 | 210.8 ± 23.5 | 84.5 ± 21.8 | 164.3 ± 22.4 |
| Total | 998.3 ± 134.1 | 1019.3 ± 130.8 | 851.8 ± 90.6 | 1383.5 ± 414.9 |
| Experiment 2 | ||||
| 0–30 | 485.2 ± 73.3 | 413.8 ± 82.5 | 379.6 ± 62.9 | 707.0 ± 135.9 |
| 30–60 | 191.1 ± 40.8 | 382.0 ± 97.9 | 117.0 ± 33.7 | 425.0 ± 93.1 |
| 60–90 | 146.8 ± 29.6 | 321.5 ± 74.9 | 112.8 ± 25.7 | 300.8 ± 74.2 |
| 90–120 | 105.2 ± 36.6 | 275.2 ± 68.1 | 34.5 ± 10.7 | 217.0 ± 61.5 |
| Total | 928.3 ± 170.1 | 1392.6 ± 273.1+ | 644.0 ± 98.3 | 1649.8 ± 271.7Ψ |
| Experiment 3 | ||||
| 0–30 | 611.3 ± 45.7 | 362.0 ± 72.5 | 400.1 ± 43.8 | 448.9 ± 85.8 |
| 30–60 | 266.7 ± 16.7 | 331.1 ± 76.6 | 142.4 ± 41.8 | 260.0 ± 60.2 |
| 60–90 | 136.9 ± 38.8 | 320.4 ± 73.4 | 62.9 ± 10.9 | 177.6 ± 40.2 |
| 90–120 | 137.0 ± 45.1 | 259.3 ± 38.8 | 36.4 ± 8.7 | 111.4 ± 29.6 |
| Total | 1151.9 ± 104.5 | 1272.9 ± 210.8 | 641.9 ± 88.7# | 997.9 ± 209.2 |
| Experiment 4 |
Nonrestricted SAL |
Nonrestricted MUSC |
Restricted SAL |
Restricted MUSC |
| 284.2 ± 29.6 | 331.3 ± 39.7 | 270.2 ± 29.5 | ||
| 0–30 | 304.8 ± 23.5 | |||
| 30–60 | 71.7 ± 11.2 | 90.2 ± 25.7 | 125.8 ± 15.7 | 39.8 ± 10.4 |
| 60–90 | 82.8 ± 15.2 | 42.8 ± 9.8 | 75.7 ± 17.5 | 77.2 ± 16.9 |
| 90–120 | 112.2 ± 24.7 | 48.7 ± 24.1 | 94.7 ± 32.1 | 67.3 ± 12.6 |
| Total | 571.5 ± 56.4 | 465.8 ± 63.3# | 627.5 ± 77.3 | 454.5 ± 52.7** |
Note. Values represent group means plus or minus the standard error of the mean for a 2-hr measure of locomotor activity (beam breaks) presented in 30-min trials and a combined 2-hr total after each treatment. For Experiments 1–3, a superscript plus sign represents DAMGO/SAL vs. SAL/SAL; a superscript pound sign represents MUSC/SAL vs. SAL/SAL; a superscript asterisk represents MUSC/DAMGO vs. SAL/DAMGO; and a superscript letter psi represents MUSC/DAMGO vs. SAL/SAL. For Experiment 4, a superscript pound sign represents Nonrestricted saline vs. Nonrestricted muscimol, and a superscript asterisk represents restricted muscimol vs. restricted saline. Level of significance is indicated by the number of symbols (i.e.,
p< .05,
p< .01,
p< .001).
SAL = saline; DAMGO = D-Ala2-NMe-Phe4-Glyol5-enkephalin; MUSC = muscimol.
In summary, the results replicate previous findings demonstrating the necessary role of CeA activity in the expression of intra-Acb DAMGO-induced food intake (Will et al., 2004) and further demonstrate the necessary role of CeA activity in other feeding-related behaviors as well. It is interesting that there have been previous reports that intra-CeA muscimol induces “forepaw treading” (Baldo et al., 2005), although this behavior was not measured in the present study. It cannot be ruled out that, if present, this behavior could have influenced the feeding measures reported. However, at lower doses of intra-CeA muscimol, which did not induce forepaw treading, baseline food intake was still significantly reduced (Baldo et al., 2005) and still in contrast to the lack of intra-BLA muscimol influence on baseline feeding observed in the present studies and those previously reported (Will et al., 2004).
Experiment 2: Influence of BLA Inactivation on Feeding Behavior After Intra-Acb DAMGO
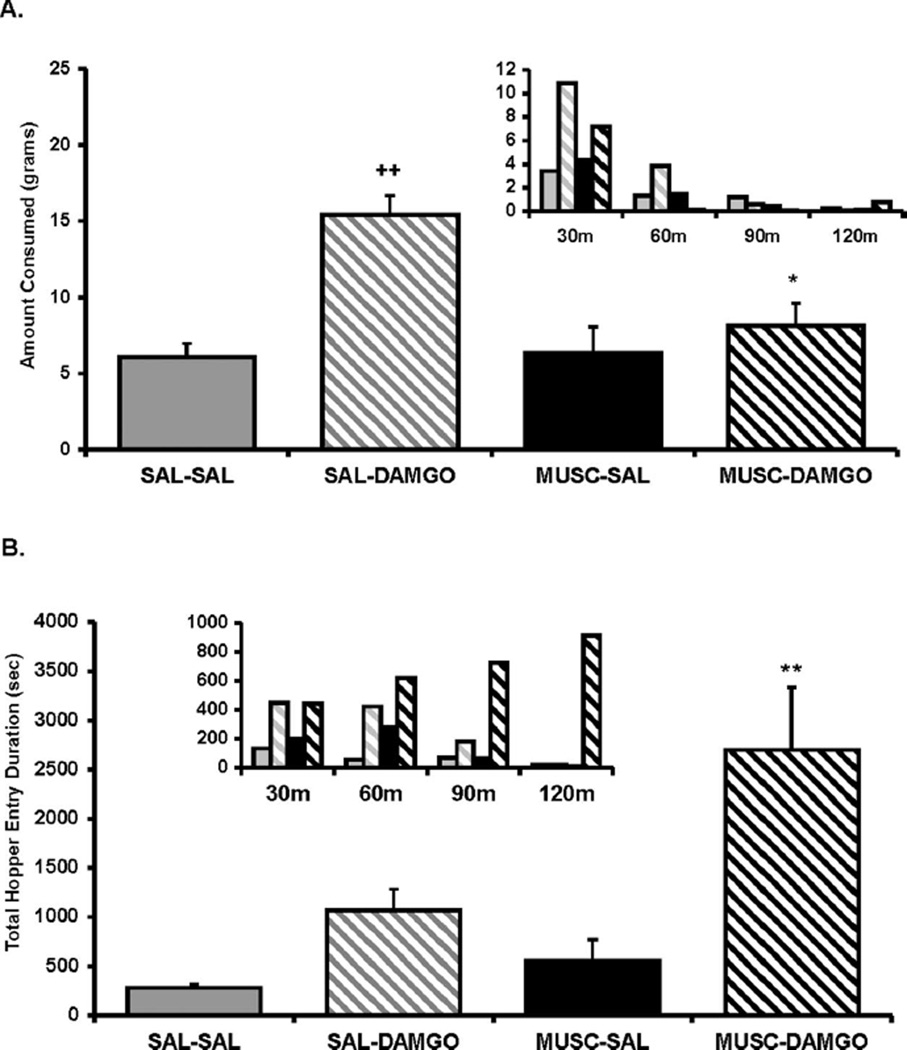
An ANOVA conducted on the food intake data for Experiment 2 revealed a significant main effect of intra-Acb DAMGO treatment, F(1, 8) = 21.34, p < .005; intra-BLA muscimol treatment, F(1, 8) = 11.29, p < .01; and a significant Acb Treatment X BLA Treatment interaction, F(1, 8) = 5.6, p < .05. As displayed in Figure 2A, contrasts of means revealed that, although muscimol administration into the BLA had no effect on baseline intake by itself, this treatment did significantly reduce DAMGO-elicited food intake (p < .05) to baseline levels.
Figure 2.
(A) Amount of food intake and (B) hopper entry duration (duration of beam break at entry of hopper) after intra-accumbens (Acb) DAMGO ([D-Ala2-NMe-Phe4-Glyol5-enkephalin] 0.25 µg/0.5 µl per side) or saline (SAL) administration immediately after muscimol (MUSC; 20 ng/0.25 µl per side) or SAL administration into the basolateral amygdala (BLA). Small inset graphs display the same data across 30-min intervals (y axis represents the same measure as the larger corresponding graph). The x axis labels refer to treatment for the two regions (i.e., Acb treatment/BLA treatment). Values represent group means (plus or minus standard error of the mean). Plus sign represents DAMGO/SAL versus SAL/SAL; asterisk represents MUSC/ DAMGO versus SAL/DAMGO. Level of significance indicated by number of symbols (i.e., *p< .05, **p< .01, and ***p< .001).
An ANOVA conducted on the combined duration of all hopper entries across the 2-hr feeding session revealed a significant main effect of intra-Acb DAMGO treatment, F(1, 8) = 18.8,p < .005; intra-BLA muscimol treatment, F(1, 8) = 6.2, p < .01; and nearly significant Acb Treatment × BLA Treatment interaction, F(1, 8) = 3.8, p = .08. A contrast of means demonstrated that, just as in Experiment 1, intra-Acb DAMGO increased the total duration of hopper entries compared with saline treatment, although not reaching significance (p = .14; see Figure 2B). It is interesting that, whereas BLA inactivation reduced DAMGO-elicited food intake to control levels, post hoc analysis revealed that this treatment actually led to exaggerated DAMGO-elicited hopper entry duration (p < .01) compared with DAMGO treatment alone. As shown in the Figure 2B inset graph, the trend for total combined duration of hopper entries gradually increased over the 2-hr session, in direct contrast to the trend for food intake. Therefore, the results demonstrate that the hopper entry behavior appeared to dissociate from food intake behavior (see Figure 2A and 2B, inset graphs) after muscimol administration to the BLA during concurrent DAMGO activation of the Acb.
An analysis of number of hopper entries and duration per entry data suggest that the changes observed in total duration of hopper entries was not the result of an increase in duration per hopper entry, as there was no significant effect of intra-Acb DAMGO treatment, F(1, 8) = 4.2, ns; intra-BLA muscimol treatment, F(1, 8) = 1.3, ns; or an Acb Treatment × BLA Treatment interaction, F(1, 8) = .03, ns (data not shown). Instead, the significant difference observed for total duration of hopper entries was driven more by an increase in the total number of hopper entries as demonstrated by a significant main effect of intra-Acb DAMGO treatment, F(1, 8) = 13.6, p < .01; and a tendency toward a significant effect of intra-BLA muscimol treatment, F(1, 8) = 2.9, p = .12 and Acb Treatment × BLA Treatment interaction, F(1, 8) = 2.8, p = .12. As displayed in Table 1, contrasts of means revealed that, although intra-BLA muscimol had no effect on baseline levels of hopper entries by itself, concurrent intra-Acb DAMGO and intra-BLA muscimol treatment significantly increased hopper entries compared with intra-Acb DAMGO treatment alone (p < .05).
It is important to note that the increase in hopper entries was not the result of a generalized increase in locomotor activity, as although an ANOVA conducted on the locomotor activity revealed a significant main effect of intra-Acb DAMGO treatment, F(1, 8) = 9.0, p < .02; there was no main effect of intra-BLA muscimol treatment, F(1, 8) = .003, ns; or Acb Treatment × BLA Treatment interaction, F(1, 8) = 2.6, ns. A contrast of means revealed that intra-Acb DAMGO, with or without concurrent intra-BLA muscimol treatment, increased locomotor activity, and these treatments did not significantly differ from each other. (see Table 2).
Experiment 3: Influence of BLA on Feeding Behavior (Limited Access Condition)
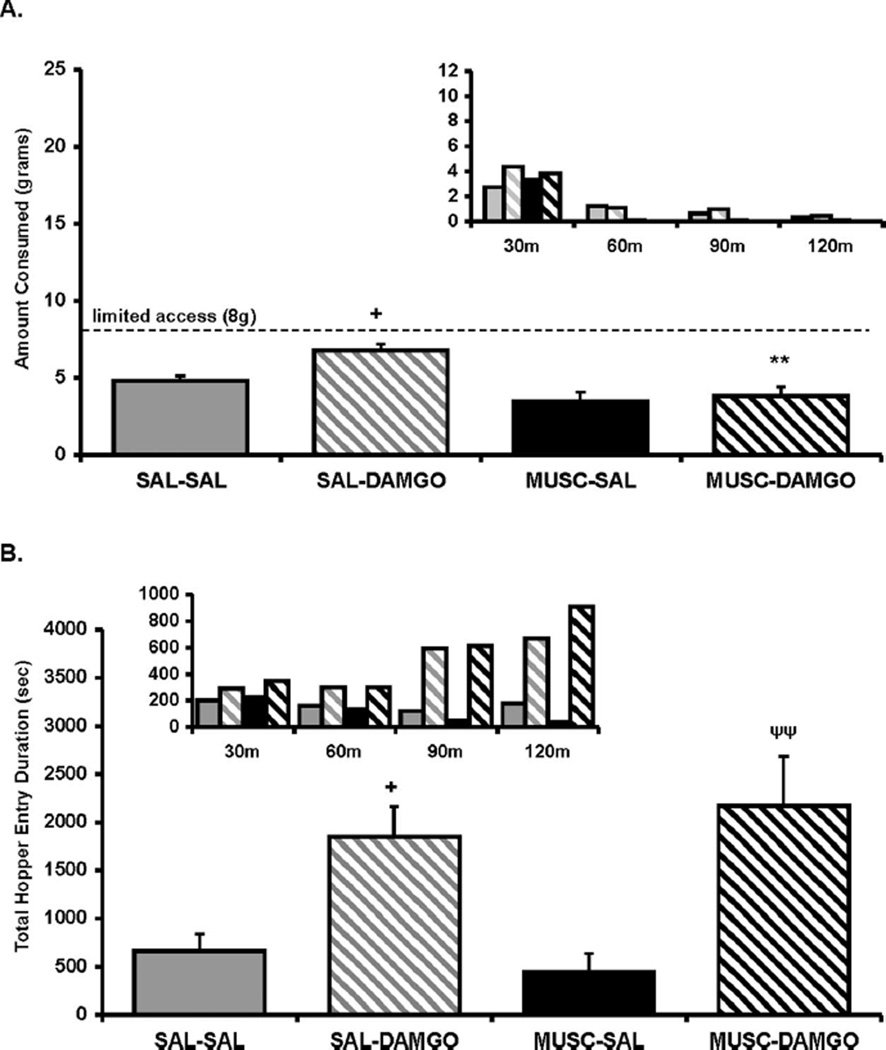
The goal of Experiment 3 was to assess the noningestive feeding behaviors, specifically hopper entry duration, following the same treatments administered in Experiment 2 but under conditions in which food intake across treatments would be held to similar levels, regardless of treatment. Although limiting the available high-fat diet to 8 g did reduce the range of food intake to only 3 g between treatments, significant differences still remained. An ANOVA conducted on the food intake data for Experiment 3 revealed a significant main effect of intra-Acb DAMGO treatment, F(1, 6) = 6.9, p < .05; intra-BLA muscimol treatment, F(1, 6) = 50.0, p < .001; and a tendency toward a significant Acb Treatment × BLA Treatment interaction, F(1, 6) = 2.4, p = .16. As displayed in Figure 3A, contrasts of means revealed that, although muscimol administration into the BLA had no effect on baseline intake by itself, this treatment did significantly reduce DAMGO-elicited food intake (p < .01).
Figure 3.
(A) Amount of food intake and (B) hopper entry duration (duration of beam break at entry of hopper) after intra-accumbens (Acb) DAMGO ([D-Ala2-NMe-Phe4-Glyol5-enkephalin] 0.25 µg/0.5 µl per side) or saline (SAL) administration immediately after muscimol (MUSC; 20 ng/0.25 µl per side) or SAL administration into the basolateral amygdala (BLA). Small inset graphs display the same data across 30-min intervals (y axis represents the same measure as the larger corresponding graph). The x axis labels refer to treatment for the two regions (i.e., Acb treatment/BLA treatment). Values represent group means (plus or minus standard error of the mean). Plus sign represents DAMGO/SAL versus SAL/SAL; asterisk represents MUSC/DAMGO versus SAL/DAMGO; φ represents MUSC/DAMGO versus SAL/SAL. Level of significance indicated by number of symbols (i.e., *p< .05, **p< .01, and ***p< .001).
An ANOVA conducted on the combined duration of all hopper entries across the 2-hr feeding session revealed a significant main effect of intra-Acb DAMGO treatment, F(1, 6) = 35.5, p < .001; but no main effect of intra-BLA muscimol treatment, F(1, 6) = .028, ns; and no Acb Treatment × BLA Treatment interaction, F(1, 6) = 1.14, ns. Contrast of means revealed that, as predicted, DAMGO treatment significantly increased the total duration of hopper entries compared with saline control treatment (p < .001), and the pattern (see Figure 3B, inset graph) was nearly identical to what was observed after concurrent intra-Acb DAMGO and intra-BLA muscimol treatment. Therefore, DAMGO treatment, with or without BLA inactivation, led to the same pattern of exaggerated hopper entry behavior, especially during the second hour (see Figure 3B, inset graph), even when maximum allowed food intake was held to similar levels (see Discussion for further explanation). This exaggerated total hopper entry duration, similar to the results in Experiment 2, was not a result of a longer average duration per hopper entry. An ANOVA conducted on the duration per hopper entry showed no main effect of either intra-Acb DAMGO, F(1, 6) = 4.61, ns; or intra-BLA muscimol, F(1, 6) = 2.97, ns (data not shown). Instead, similar to the Experiment 2 results, the longer total hopper entry durations were driven more by an increase in the number of hopper entries (see Table 1). An ANOVA conducted on the combined number of all hopper entries across the 2-hr feeding session revealed a significant main effect of intra-Acb DAMGO treatment, F(1, 6) = 19.12, p < .005; but as predicted, no main effect of intra-BLA muscimol treatment, F(1, 6) = 3.47, ns; and no Acb Treatment × BLA Treatment interaction, F(1, 6) = .008, ns. Contrasts of means revealed that intra-Acb DAMGO significantly increased the total number of hopper entries compared with saline treatment (p < .05). Although intra-Acb DAMGO and concurrent BLA inactivation treatment also had a tendency to increase hopper entries compared with saline treatment, this did not quite approach significance (p = .15).
Finally, an ANOVA conducted on locomotor activity revealed no main effect of DAMGO treatment, F(1, 6) = 1.66, ns; but a significant effect of intra-BLA muscimol treatment, F(1, 6) = 22.29, p < .005; compared with saline control treatment (see Table 2). Although the conditions of Experiments 2 and 3 provided different levels of available diet (ad libitum access vs. limited access), the reduction of locomotor activity by intra-BLA muscimol in Experiment 3 might suggest further support for hopper entry measures being food directed. Indeed, the observation that both number and duration of hopper entry measures increased despite a significant reduction in general locomotor activity add further support for the validity of hopper entry measures being representative of motivated food-directed behavior.
Experiment 4: Role of BLA in Mediating Feeding Driven by Negative Energy Balance
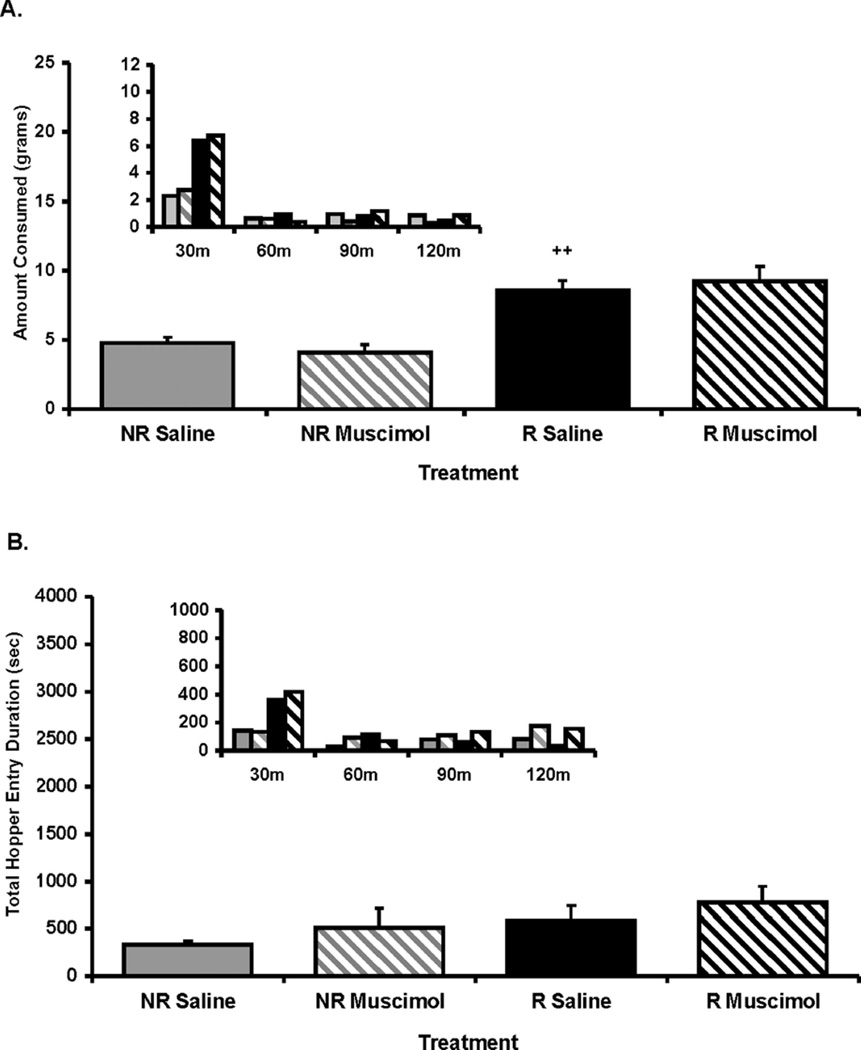
Twenty-four-hour food deprivation led to a significant increase in high-fat intake, as an ANOVA revealed a significant main effect of deprivation state, F(1, 5) = 76.35, p < .0005 (see Figure 4). In contrast to the DAMGO-elicited food intake observed in Experiment 2, BLA inactivation had no influence on this increase, regardless of the deprivation state, F(1, 5) = .00007, ns. An ANOVA conducted on the combined duration of all hopper entries across the 2-hr feeding session revealed no main effect of deprivation state, F(1, 5) = 2.0, ns; and also no influence of intra-BLA muscimol treatment, F(1, 5) = 1.17, ns. There was also no main effect of deprivation state, F(1, 5) = 5.1, ns; or intra-BLA muscimol treatment, F(1, 5) = 1.16, ns; on the number of hopper entries (see Table 2). Finally, an ANOVA conducted on the locomotor activity revealed no main effect of deprivation state, F(1, 5) = 0.6, ns; but a main effect of intra-BLA muscimol treatment, F(1, 5) = 15.2, p < .05. Contrasts of means revealed that, compared with saline treatment, intra-BLA muscimol treatment reduced locomotor activity but only in the deprived condition (p < .05; see Table 2), suggesting locomotor activity under the influence of this motivational state (food deprived) is BLA dependent.
Figure 4.
(A) Amount of food intake and (B) hopper entry duration (duration of beam break at entry of hopper) after 24-hr food deprivation (R) or no deprivation (NR) after either muscimol (MUSC; 20 ng/0.25 µl per side) or saline (SAL) administration into the basolateral amygdala (BLA). Small inset graphs display the same data across 30-min intervals (y axis represents the same measure as the larger corresponding graph). The x axis labels refer to deprivation state and intra-BLA treatment (i.e., deprivation state/BLA treatment). Values represent group means (plus or minus standard error of the mean). Plus sign represents NR/SAL versus R/SAL. Level of significance is indicated by number of symbols (i.e., *p< .05, **p< .01, and ***p< .001).
Discussion
The present findings demonstrate that the CeA and the BLA have unique roles in mediating feeding behavior and that these roles can be dissociated by origin of motivational state (striatal opioids vs. food restriction), as well the specific phase of feeding behavior (appetitive vs. consummatory). Confirming previous reports (Will et al., 2004), inactivation of the BLA completely prevented the opioid-induced enhancement of high-fat diet intake yet left baseline intake unchanged, whereas inactivation of the CeA abolished all food intake under both baseline and opioid-driven conditions. However, although inactivation of the BLA prevented the exaggerated food intake driven by intra-Acb DAMGO, it also led to exaggerated approach responses, as indicated by the number and duration of food hopper entries. It is interesting that these exaggerated approach measures were mostly exhibited after the termination of food intake. In other words, hopper entries occurred in the absence of food intake, despite the majority of food still remaining available. When high-fat diet availability was restricted (limited access condition) to produce similar food intake levels across the various treatments, intra-Acb DAMGO administration led to this same pattern of increased number and duration of hopper entries, regardless of whether the BLA was inactivated. Last, BLA inactivation had no influence on the feeding increase after 24-hr food deprivation, suggesting that the BLA has a specific role in mediating palatability-driven feeding.
The findings from Experiments 1 and 2 further characterized the influence of the CeA and the BLA on intra-Acb opioid-driven high-fat diet intake, including their distinct influences on noningestive feeding behaviors. As the present findings demonstrate, BLA activity appears necessary to observe DAMGO-driven food intake, but not DAMGO-driven food approach behavior, whereas CeA activity is necessary to observe both behaviors. After intra-Acb DAMGO and concurrent BLA inactivation, the two feeding behaviors of food intake and hopper entries were almost entirely dissociated in the last hour of the 2-hr feeding session. As in all the present experiments, the majority of food intake occurred during the first hour of the 2-hr feeding session. However, the results from Experiment 2 demonstrate that concurrent intra-Acb DAMGO and intra-BLA muscimol treatments led to exaggerated hopper entry behavior in the absence of food intake. The majority of total hopper entries occurred during the final hour of the 2-hr feeding session, when very little, if any, food intake occurred. It is important to note that this increased hopper entry behavior cannot be explained by changes in general locomotor activity levels, as intra-Acb DAMGO administration produced similar locomotor activity levels, regardless of whether the BLA was inactivated. Furthermore, the number of hopper entries associated with each treatment did not correlate with their respective influence on general locomotor activity, suggesting that the number of hopper entries reflects a specific food-directed motivated behavior. Therefore, although DAMGO-elicited feeding was blocked by BLA inactivation, the other behavioral indices suggest that this influence was specific to the consummatory act of food intake.
The observation that intra-Acb DAMGO and concurrent BLA inactivation led to continued hopper entries, in the absence of food intake, was a surprising dissociation that required further characterization. Therefore, in Experiment 3, rats received the same drug treatments as in Experiment 2, but under conditions of limited access to high-fat diet. The level of diet available (8 g) was specifically chosen to ensure that all treatments except intra-DAMGO treatment would allow rats to reach satiation. It was predicted that intra-DAMGO treatment would lead to consumption of the entire available diet, followed by behaviors reflective of a motivation to consume more. Comparing the pattern of hopper approach responses across treatments during this latter phase (i.e., the second hour of the session) would help determine whether the results in question from Experiment 2 reflected a similar state of motivation. The results demonstrated that intra-Acb DAMGO, under limited access to high-fat diet, led to exaggerated hopper entry behavior after ingestion of the available food. This behavior was most pronounced during the second hour of the feeding session, when food intake levels after all treatments were similar. It is interesting that hopper entry durations were equally elevated after intra-Acb DAMGO, regardless of whether the BLA was inactivated. BLA inactivation alone produced no change in baseline food intake or hopper entry duration, compared with saline control treatment. Although food intake levels between treatment groups were significant, despite the small range, a subsequent pilot study (n = 6) limited high-fat diet access to 1 g, and the same pattern of exaggerated hopper entry durations was observed after intra-Acb DAMGO, whether the BLA was inactivated (µ = 2143 s) or not (µ = 1831 s).
These findings suggest that the extended hopper entry duration after intra-Acb DAMGO and concurrent BLA inactivation in the absence of food intake, whether under ad libitum access (Experiment 2) or limited-access (Experiment 3) conditions, are related to an unexplained motivation to approach, but not consume, the high-fat diet. Although the exact nature of this motivational state is unknown, the behavioral pattern parallels what was observed after intra-Acb DAMGO treatment by itself, and this motivational state has been well characterized (Pecina & Berridge, 2005; Zhang et al., 1998; Zhang & Kelley, 2002; Zhang et al., 2003). Therefore, the results of Experiment 3 suggest that the behavioral pattern after intra-Acb DAMGO and concurrent BLA inactivation is similar to a sustained motivation to approach and ingest the high-fat diet, yet BLA inactivation has seemingly interfered with the expression of the latter phase of ingestion. In other words, the results support the existence of dissociable substrates mediating intra-Acb opioid-driven approach and consumption behavior, and BLA activity only appears necessary for the latter. Additional studies using more controlled protocols to dissociate approach and consummatory behaviors (i.e., operant tasks) might provide intriguing clues to the nature of these effects.
These findings provide further evidence for the existence of distinct yet overlapping neural substrates that mediate the different motivational phases involved in not only feeding, but other reward-motivated behavior as well (Baldo & Kelley, 2007; Berridge, 2004; Burgdorf & Panksepp, 2006). Historically, the process of feeding has been described as two distinct behaviors, the appetitive and consummatory phase (Craig, 1918). The appetitive phase has since been typically defined as the motivated approach behaviors involved in seeking food-related reward stimuli, whereas the consummatory phase is equated with the actual ingestive behavior. In a recent review, Baldo and Kelley (2007) discussed these motivational phases within the context of feeding behavior, specifically those behaviors that are mediated by the various neurotransmitter systems within the Acb. Briefly, they review evidence demonstrating that goal-seeking behaviors of the approach phase are dopamine mediated (Baldo, Sadeghian, Basso, & Kelley, 2002; Berridge & Robinson, 1998; Blackburn, Phillips, & Fibiger, 1987; Cousins, Wei, & Salamone, 1994; Nowend, Arizzi, Carlson, & Salamone, 2001), whereas the consummatory act of ingestion is more specifically influenced by amino acid and opioid systems (Baldo & Kelley, 2007; Kelley et al., 2005; Zhang et al., 2003). In line with this evidence, intra-Acb DAMGO feeding of high-fat diet is not dependent on dopamine signaling, as intra-Acb administration of dopamine antagonists are without effect (Will, Pratt, & Kelley, 2006). However, although food-seeking behaviors were not assessed in this earlier study, it could be predicted that disruption of dopamine signaling within the Acb would reduce the motor acts of approach without significantly influencing opioid-induced high-fat diet intake. However, such a hypothesis awaits further testing.
The dissociation between the influence that BLA and CeA inactivation on opioid-elicited consummatory and appetitive feeding behaviors is likely an indication of their distinct anatomical connectivity patterns with other brain regions (Alheid, 2003; Swanson, 2003). More specifically, it may be revealing as to the degree to which the BLA and the CeA have different control over dopamine and opioid signaling within the Acb. Indeed, one of the most notable distinctions between their connectivity to other brain regions is the direct projection from the BLA, but not the CeA, to the Acb (Kelley et al., 1982). Considering the evidence for the role of dopamine and opioids mentioned earlier, it may be predicted that, after intra-Acb DAMGO, BLA inactivation is without influence on increases in striatal dopaminergic activity, at least in regard to the role of dopamine driving DAMGO-elicited approach behavior. Although it is already known that intra-Acb opioid-induced food intake is unchanged by previous intra-Acb administration of dopamine antagonists (Will et al., 2006), it has also been shown that dopamine stimulation of the Acb has little or no influence on food intake behavior (Hanlon, Baldo, Sadeghian, & Kelley, 2004; Swanson & Petrovich, 1998). However, dopamine has been shown to be integral in driving food seeking, as demonstrated by increasing breakpoint thresholds in the progressive ratio task (Zhang et al., 2003).
Overall, the evidence observed might predict that intra-Acb DAMGO increases the appetitive behaviors of approach through an increase in dopamine signaling within the Acb and that this is unaffected by BLA inactivation. In support of this theory, a distinction has already been shown between the influences of BLA and CeA inactivation on dopamine efflux within the Acb that also correspond with baseline feeding levels. Indeed, temporary inactivation of the CeA inhibited both baseline and feeding-induced dopamine efflux in the Acb, whereas BLA inactivation had no effect on either measure (Ahn & Phillips, 2003). Although these findings provide insight into the differential effect of BLA and CeA muscimol treatment on baseline feeding, the mechanism by which the BLA appears to be specifically involved in only the DAMGO-induced consummatory phase of ingestion awaits further testing.
The last experiment was conducted to determine whether the BLA would have a similar influence on feeding driven by negative energy balance (i.e., 24-hr period of food deprivation). As has been previously suggested, the BLA may mediate exaggerated feeding in sated rats only under circumstances driven by increased palatability, as is evidenced to occur after intra-Acb opioid administration (Kelley et al., 2002; Pecina & Berridge, 2005; Will et al., 2004). Therefore, the BLA would be predicted to have little or no influence on exaggerated feeding driven by an energy deficit. In support of this theory, results from Experiment 4 demonstrated that 24-hr food deprivation led to a twofold increase in high-fat diet intake, and BLA inactivation had no effect on this increase. The majority of the feeding occurred in the first 30 min in food-deprived rats, and BLA inactivation had no effect on this increase. BLA inactivation also had no effect on food intake in nondeprived rats, compared with saline control treatment. Hopper entry durations after food deprivation, regardless of whether the BLA was inactivated, were paralleled by food intake across the session. Therefore, BLA activity appears to have little or no influence on feeding of a palatable diet driven by energy deficit (food deprivation). This is in line with previous findings that suggest a role for the CeA, but not the BLA, in mediating palatability-driven feeding. Indeed, inactivation of the CeA has been shown to prevent increased feeding of chow driven by acute food deprivation (Baldo et al., 2005). In addition, the hyperphagia driven by intra-Acb muscimol treatment, a pharmacological model that parallels the motivational state induced by energy deficit (see Kelley et al., 2005, for a review), appears to recruit systems related to energydeficit feeding and is blocked by inactivation of the CeA, but not the BLA (Baldo et al., 2005).
In summary, the present experiments provide new evidence demonstrating that the BLA has a very specific and critical role in mediating intra-Acb opioid-driven feeding behavior associated with a palatable food. BLA activity is necessary to observe the specific feeding phase of consumption but not the approach behavior that is driven by intra-Acb opioid administration. Lastly, the lack of influence of BLA inactivation on energy-deficit driven feeding behavior confirms the specificity of the BLA in influencing opioid-driven feeding. This is especially intriguing when considering that one of the major underlying causes of the current obesity epidemic is overconsumption of palatable tasty food in a nondeprived state. Therefore, furthering our understanding of the feeding networks and environmental variables that contribute to this behavior is of considerable importance.
References
- Ahn S, Phillips AG. Independent modulation of basal and feeding-evoked dopamine efflux in the nucleus accumbens and medial prefrontal cortex by the central and basolateral amygdalar nuclei in the rat. Neuroscience. 2003;116:295–305. doi: 10.1016/s0306-4522(02)00551-1. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Annals of the New York Academy of Sciences. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Sensitization and conditioning of feeding following multiple morphine microinjections into the nucleus accumbens. Brain Research. 1994;648:342–346. doi: 10.1016/0006-8993(94)91139-8. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Alsene KM, Negron A, Kelley AE. Hy-perphagia induced by GABAA receptor-mediated inhibition of the nucleus accumbens shell: Dependence on intact neural output from the central amygdaloid region. Behavioral Neuroscience. 2005;119:1195–1206. doi: 10.1037/0735-7044.119.5.1195. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: Insights from nucleus accum-bens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behavioural Brain Research. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiology and Behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Fibiger HC. Dopamine and preparatory behavior: I. Effects of pimozide. Behavioral Neuroscience. 1987;101:352–360. doi: 10.1037//0735-7044.101.3.352. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience and Biobehavioral Reviews. 2006;30:173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Carr KD. The physiology of opiate hedonic effects and the role of opioids in motivated behavior. Advances in Alcohol and Substance Abuse. 1984;3:5–18. doi: 10.1300/J251v03n03_02. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Effects of opiate agonists and antagonists on fluid intake and saccharin choice in the rat. Neuropharmacology. 1983;22:323–328. doi: 10.1016/0028-3908(83)90247-2. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Wei W, Salamone JD. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: Effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology (Berl) 1994;116:529–537. doi: 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biological Bulletin. 1918;34:91–107. [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and preferences for sweet high-fat foods: Evidence for opioid involvement. Physiology and Behavior. 1992;51:371–379. doi: 10.1016/0031-9384(92)90155-u. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Levine AS. Sugar and fat-From genes to culture. Journal of Nutrition. 2003;133:829S–830S. doi: 10.1093/jn/133.3.829S. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Grace MK, Welch CC, Billington CJ, Levine AS. Naloxone’s anorectic effect is dependent upon the relative palatability of food. Pharmacology, Biochemistry and Behavior. 1993;46:917–921. doi: 10.1016/0091-3057(93)90222-f. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAer-gic, opioid, or dopaminergic stimulation of the nucleus accumbens: Is it hunger? Psychopharmacology (Berl) 2004;172:241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: Where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32:1305–1314. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiology and Behavior. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiology and Behavior. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalos-triatal projection in the rat—An anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Minano FJ, Meneres Sancho MS, Sancibrian M, Salinas P, Myers RD. GABAA receptors in the amygdala: Role in feeding in fasted and satiated rats. Brain Research. 1992;586:104–110. doi: 10.1016/0006-8993(92)91377-q. [DOI] [PubMed] [Google Scholar]
- Norgren R. Taste pathways to hypothalamus and amygdala. Journal of Comparative Neurology. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacology, Biochemistry and Behavior. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA: Journal of the American Medical Association. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accum-bens shell: Where do mu-opioids cause increased hedonic impact of sweetness? Journal of Neuroscience. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiology and Behavior. 2007;90:362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM, Jebb SA. Obesity in Britain: Gluttony or sloth? British Medical Journal. 1995;311:437–439. doi: 10.1136/bmj.311.7002.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: Homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the para-brachial nucleus in the rat. Brain Research. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Brooks BM, Neill DB. GABAergic inactivation of basolateral amygdala alters behavioral processes other than primary reward of ventral tegmental self-stimulation. Behavioural Brain Research. 2007;181:110–117. doi: 10.1016/j.bbr.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Research. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The amygdala and its place in the cerebral hemisphere. Annals of the New York Academy of Sciences. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in Neurosciences. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Weldon DT, O’Hare E, Cleary J, Billington CJ, Levine AS. Effect of naloxone on intake of cornstarch, sucrose, and polycose diets in restricted and nonrestricted rats. American Journal of Physiology. 1996;270:R1183–R1188. doi: 10.1152/ajpregu.1996.270.6.R1183. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. Journal of Neuroscience. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–1860. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiology and Behavior. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiology and Behavior. 1996;60:439–446. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neuroscience and Biobehavioral Reviews. 2002;26:713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: Contrasting effects revealed by a progressive ratio study in the rat. Behavioral Neuroscience. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics. 1998;285:908–914. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]