Abstract
To examine the involvement of opioid receptors in inflammatory pain, we compared Complete Freund’s Adjuvant-induced hyperalgesia in mice lacking mu, delta or kappa receptors under the same experimental conditions. Mechanical allodynia and thermal hyperalgesia were measured using von Frey filaments and the plantar test, respectively. All three receptor-knockout mice, as well as wild-type animals, developed inflammatory hyperalgesia following Complete Freund’s Adjuvant administration. Mu-receptor mutants showed similar hyperalgesia to wild-types in the two tests. Kappa-receptor mutants exhibited enhanced mechanical allodynia compared with wild-type mice but similar thermal hyperalgesia. In contrast, mechanical allodynia and thermal hyperalgesia were both markedly augmented in delta-receptor mutants, indicating a role for an endogenous delta-receptor tone in the control of inflammatory pain. Treatment with the delta-selective agonist SNC80 produced antihyperalgesia, and this effect was abolished in the delta-receptor knockout mice. Altogether, these data demonstrate that delta receptors inhibit inflammatory pain when activated either endogenously or exogenously. We have previously shown enhanced neuropathic pain in delta-receptor knockout mice. The delta receptor definitely represents a promising target for treating chronic pain conditions.
Keywords: allodynia, analgesia, hyperalgesia, kappa, mu
Introduction
Opiates are the most widely used drugs for pain treatment, and the activation of all three opioid receptors (mu, delta and kappa) induces analgesia (Millan, 2002; Fields, 2004). Morphine and other clinically used mu-opioid agonists are most efficient for severe acute pain and cancer pain. However, the use of mu opioids in chronic non-malignant pain has brought controversial results and leads to adverse side-effects (Zollner & Stein, 2007). Kappa-receptor activation results in dysphoria and this strongly limits the use of kappa agonists (DeHaven-Hudkins & Dolle, 2004). The analgesic efficacy of delta opioid agonists has been debated and is complicated by the lack of agonists with high selectivity (Kieffer & Gavériaux-Ruff, 2002; Scherrer et al., 2004) and stability in vivo. Synthesis of the non-peptidic delta agonists BW373U86 and SNC80 has been a major advance in this area (Calderon & Coop, 2004), and the delta receptor is now being explored as a therapeutic target for pain using this novel generation of compounds.
The analysis of knockout animals provides invaluable information on the importance of each opioid receptor in pain control, particularly for delta receptors, for which pharmacological tools are limited. Measures of nociception in mice lacking opioid receptors or peptides have confirmed the existence of antinociceptive tones for mu, delta and kappa receptors (Kieffer & Gavériaux-Ruff, 2002). Recently, a comparative study of single and combinatorial triple opioid receptor-knockout mice has demonstrated that each receptor has a distinct implication in the perception of thermal, mechanical or chemical pain (Martin et al., 2003). Several reports indicate altered responses of mutant animals to chronic pain. Mu-receptor mutant mice show a faster recovery (Qiu et al., 2000) or no change in inflammatory hyperalgesia (Mansikka et al., 2002). Kappa-receptor knockout mice display augmented neuropathic pain whereas dynorphin-null mutants show the opposite phenotype (Xu et al., 2004). We recently reported enhanced neuropathic pain in delta-receptor knockout animals (Nadal et al., 2006).
Previous studies indicate that the endogenous opioid system regulates inflammatory pain. The changes in inflammatory hyperalgesia obtained by preventing enkephalin degradation (Noble & Roques, 2007), or blocking with antibodies to endogenous opioid peptides (Stein et al., 1990; Ossipov et al., 1996) or with opioid antagonists (Binder et al., 2004) suggest that endogenous opioid peptides inhibit inflammatory hyperalgesia. Noticeably, the antinociceptive effect of opioid drugs is generally enhanced in the inflamed state, reflecting changes in the opioid endogenous system (Przewlocki & Przewlocka, 2001; Zollner & Stein, 2007). To our knowledge, however, no study has directly compared the individual opioid-receptor knockout animals in chronic inflammatory pain. The aim of this study therefore was to compare mu-, delta- and kappa-receptor knockout mice in a well-established model of inflammatory pain induced by Complete Freund’s Adjuvant (CFA). All mutants harbour the same genetic background and were observed in parallel experiments under the same conditions. In addition, we have assessed the effectiveness of the prototypic delta opioid agonist SNC80 on inflammatory hyperalgesia. Our data reveal a prominent role of delta receptors in alleviating CFA-evoked pain.
Materials and methods
Animals
Mice lacking mu, delta or kappa opioid receptors were generated by homologous recombination and have been described earlier (Matthes et al., 1996; Simonin et al., 1998; Filliol et al., 2000). Mice were originally obtained on a hybrid 50% 129/SvPas 50% C57BL/6J genetic background and were fully backcrossed onto the C57BL/6J background (wild-type counterparts are referred as to WT). These mice have been characterized for their acute antinociceptive response (Martin et al., 2003). Twelve- to 16-week-old males and females were housed under standard conditions in a 12-h dark–light cycle with free access to water and food. Four days prior to the experiments, mice were handled and habituated to the experimental setting. All experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). Protocols were approved by the local ethical committee (Comite Regional d’Ethique en Matiere d’Experimentation Animale de Strasbourg; CREMEAS).
Inflammatory pain
Under inhalation anaesthesia (Flothane 2%), a volume of 8 μL of CFA (Sigma F-5881, Sigma, Saint Quentin Fallavier, France) was injected subcutaneously into the plantar surface of the left hindpaw using a 50 μL Hamilton syringe with a 27-gauge needle.
Behavioural testing
Baseline thresholds to thermal and mechanical stimuli were determined for each animals prior to CFA injection and then measured daily after CFA. In all experiments, genotype and treatment were unknown to the experimenter until data analysis.
Mechanical allodynia was measured by von Frey filament application to the hindpaw. Mice were placed in clear plexiglass boxes (9 × 9 × 7 cm) on an elevated mesh screen. Calibrated von Frey microfilaments were applied to the plantar surface of each hindpaw in a series of logarithmically ascending forces (ranging between 0.008 and 2.75 g). The responses were recorded as number of paw withdrawals in five applications of one filament. Values with filament force triggering 60% response, i.e. three responses over five applications, are represented.
Thermal hyperalgesia was evaluated using the Hargreaves Plantar test (Ugo Basile, Comerio, Italy). Mice were placed in clear plexiglass boxes (7 × 9 × 7 cm) on a glass surface and testing began when exploration and grooming behaviours ended (~5–20 min). A calibrated, constant radiant heat source was applied to the plantar surface of each hindpaw from an infrared beam underneath the glass surface; this produces a continuous rise in temperature until the pain threshold in the animal is reached. Paw withdrawal latencies were automatically measured when the light beam was interrupted due to movement of the paw. The stimulus intensity was set to produce a paw withdrawal latency between 4 and 7 s in naive mice, with a cutoff of 15 s to prevent damage to the skin.
Drug treatment
SNC80 at a dose of 10 mg/kg (Tocris, Bristol, UK) in saline, or saline control, were administered intraperitoneously. SNC80 was injected 48 h post-CFA, at the time of maximal inflammatory pain, and animals were tested 45 min and 1 h after drug administration for mechanical allodynia and thermal hyperalgesia, respectively. Percentages of antiallodynia and antihyperalgesia were calculated as follows:
Statistical analysis
To investigate the effect of opioid receptor gene deletion on thermal and mechanical nociceptive thresholds, data from 21 animals for WT (10 males and 11 females) and 20 animals for each knockout line (10 males and 10 females for each genotype). We analyzed data obtained from the plantar and von Frey tests on both paws with two-way anova with genotype and gender as factors, followed by a post hoc Bonferroni test.
To investigate the development of inflammatory thermal hyperalgesia and mechanical allodynia, we used the same animals as for baseline analysis, i.e. 21 animals for WT and 20 animals for each knockout line (10 males and 11 or 10 females for each genotype). We analysed data obtained from the plantar and von Frey tests for the CFA effect. Statistical analysis was performed using repeated-measures three-way anova with genotype, gender and paw side as factors. When appropriate, consecutive post hoc comparisons, using the Bonferroni test, were carried out. Results of post hoc analyses are presented in the tables for time and gender effects. Results of post hoc analyses for genotype effects are presented in Figures, with stars representing the level of significance of a difference. One star corresponds to P < 0.05, two stars to P < 0.01 and three stars to P < 0.001.
To investigate the effect of SNC80 on inflammatory pain, statistical analysis was performed using two-way anova with genotype and treatment as factors. When appropriate, consecutive post hoc comparisons were made using Bonferroni tests.
Results
Basal nociception in opioid receptor-knockout mice
The unilateral hindpaw inflammation induced by intraplantar injection of CFA was used as a well-characterized behavioural model of inflammatory pain (Stein et al., 2003). We first measured basal (before CFA) nociceptive thresholds for thermal and mechanical nociception in both males and females of the different opioid receptor-knockout mouse lines and their WT counterparts (Fig. 1).
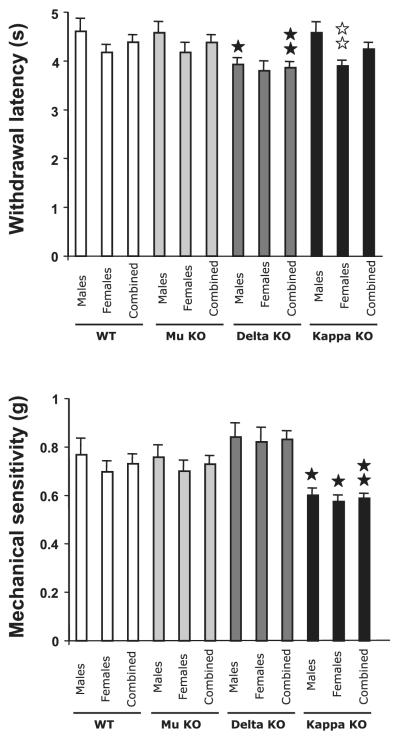
Fig. 1.
Nociceptive thresholds for WT, mu (Mu-KO), delta (Delta-KO) and kappa (Kappa-KO) opioid receptor-knockout mice. Baseline sensitivity to heat was evaluated using the plantar test (top panel) and baseline mechanical nociception was measured using the von Frey filaments (bottom panel). Data are expressed as mean ± SEM of both paws for males, females and combined animals (n = 40/42 for both paws of 20/21 WT mice, 10 males and 11 females; n = 40 for both paws of 20 (10 males and 10 females) mice for each receptor-mutants genotype. Black stars, effect of genotype; white stars, effect of gender within genotype; one star, P < 0.05; two stars, P < 0.01 (Bonferroni post hoc test).
In the plantar test used to measure thermal nociception, baseline paw withdrawal frequencies ranged from 3.8 to 4.6 s depending on the genotype and gender. WT animals and the different opioid receptor knockouts were compared using two-way ANOVA for genotype and gender effects. The comparison of WT and mu-receptor mutants revealed no effect of genotype and a significant effect of gender [F(1,78) = 4.03; P = 0.048], although this gender effect did not reach significance when assessed by a post hoc comparison within each genotype, WT or mu-receptor mutants (Fig.1 top; Tables 1 and 2). The comparison of WT and kappa-receptor mutants also revealed a significant effect of gender [F(1,78) = 8.59; P = 0.0044] and no effect of genotype. Subsequent post hoc comparisons indicated a gender difference in kappa-receptor knockout mice, with females showing lower withdrawal latencies (3.90 ± 0.11 vs. 4.58 ± 0.21 s; P = 0.0075; Fig. 1 top). The comparison of WT and delta-receptor mutants revealed a significant effect of genotype [F(1,78) = 7.92; P = 0.0062]but no gender effect. Post hoc analyses pointed out a significant effect of delta-receptor knockout in males but not in females, and also in combined males and females (Fig. 1 top). Paw withdrawal latencies of delta-knockout males (3.92 ± 0.13 s) were significantly shorter than those of WT males (4.61 ± 0.26 s; P = 0.027). The analysis of females showed shorter paw responses in delta mutants (3.80 ± 0.19 s) than in WTs (4.18 ± 0. 15 s) although it did not reach significance (P = 0.14). When data from males and females were combined, there was a significant genotype difference between WT (4.38 ± 0.15 s) and delta-knockout mice (3.86 ± 0.12 s; P = 0.0072; Fig. 1 top).
Table 1.
Development of inflammatory pain in WT mice
| Time after CFA | Plantar withdrawal responses |
Von Frey mechanical sensitivity (g) (pooled males and females) |
P-value* (time)† |
||||
|---|---|---|---|---|---|---|---|
| Males, latencies (s) |
Males, P-value* (time)† |
Females, latencies (s) |
Females, P-value (time)† |
P-value (gender) |
|||
| Baseline | 4.57 ± 0.41 | 4.27 ± 0.25 | N.S. | 0.731 ± 0.056 | |||
| 6 h | 1.83 ± 0.23 | < 0.0001 | 1.15 ± 0.12 | < 0.0001 | 0.015 | 0.076 ± 0.012 | < 0.0001 |
| 1 day | 1.41 ± 0.13 | < 0.0001 | 1.14 ± 0.15 | < 0.0001 | N.S. | 0.056 ± 0.010 | < 0.0001 |
| 2 days | 1.41 ± 0.13 | < 0.0001 | 1.24 ± 0.09 | < 0.0001 | N.S. | 0.058 ± 0.007 | < 0.0001 |
| 4 days | 2.03 ± 0.27 | < 0.0001 | 1.85 ± 0.24 | < 0.0001 | N.S. | 0.137 ± 0.020 | < 0.0001 |
| 5 days | 3.00 ± 0.12 | < 0.0001 | 2.24 ± 0.18 | < 0.0001 | 0.0029 | 0.268 ± 0.02 | < 0.0001 |
| 7 days | 3.80 ± 0.26 | N.S. | 3.29 ± 0.20 | N.S. | N.S. | 0.389 ± 0.016 | < 0.0001 |
| 10 days | 3.83 ± 0.16 | N.S. | 3.45 ± 0.46 | N.S. | N.S. | 0.481 ± 0.028 | < 0.0001 |
| 14 days | 4.03 ± 0.19 | N.S. | 3.14 ± 0.14 | N.S. | 0.0014 | 0.498 ± 0.013 | < 0.0001 |
| 20 days | 4.37 ± 0.22 | N.S. | 3.69 ± 0.17 | N.S. | 0.023 | 0.580 ± 0.011 | ≤ 0.0001 |
Values are mean ± SEM. Data from ipsilateral paws are represented separately for males and females in thermal noceptive responses, and for pooled genders in mechanical responses. Data represented are from the CFA-injected paws only.
The effect of time post-CFA is shown for both plantar and von Frey assays (Bonferroni post hoc test). The effect of gender is shown for the Plantar test.
P-values, compared with baseline.
Table 2.
Development of inflammatory pain in mu opioid-receptor knockout mice
| Time after CFA | Plantar withdrawal responses |
Von Frey mechanical sensitivity (g) (pooled males and females) |
P-value* (time)† |
||||
|---|---|---|---|---|---|---|---|
| Males, latencies (s) |
Males, P-value* (time)† |
Females, latencies (s) |
Females, P-value (time)† |
P-value* (gender) |
|||
| Baseline | 4.61 ± 0.34 | 4.27 ± 0.24 | N.S. | 0.728 ± 0.049 | |||
| 6 h | 1.91 ± 0.21 | < 0.0001 | 1.14 ± 0.16 | < 0.0001 | 0.0097 | 0.065 ± 0.005 | < 0.0001 |
| 1 day | 1.33 ± 0.21 | < 0.0001 | 0.97 ± 0.11 | < 0.0001 | N.S. | 0.044 ± 0.006 | < 0.0001 |
| 2 day | 1.20 ± 0.13 | < 0.0001 | 1.07 ± 0.12 | < 0.0001 | 0.035 | 0.053 ± 0.005 | < 0.0001 |
| 4 day | 2.35 ± 0.17 | < 0.0001 | 1.81 ± 0.21 | < 0.0001 | N.S. | 0.093 ± 0.007 | < 0.0001 |
| 5 day | 2.73 ± 0.19 | < 0.0001 | 2.27 ± 0.18 | < 0.0001 | N.S. | 0.246 ± 0.020 | < 0.0001 |
| 7 day | 3.30 ± 0.26 | < 0.0001 | 2.57 ± 0.26 | < 0.0001 | N.S. | 0.352 ± 0.017 | < 0.0001 |
| 10 day | 3.81 ± 0.20 | N.S. | 3.03 ± 0.24 | < 0.0001 | 0.023 | 0.404 ± 0.021 | < 0.0001 |
| 14 day | 4.12 ± 0.08 | N.S. | 3.30 ± 0.27 | 0.0009 | 0.0083 | 0.477 ± 0.020 | < 0.0001 |
| 20 day | 4.46 ± 0.15 | N.S. | 3.59 ± 0.13 | N.S. | 0.0004 | 0.559 ± 0.020 | < 0.0001 |
See footnote to Table 1.
The effect of time post-CFA is shown for both plantar and von Frey assays (Bonferroni post hoc test). The effect of gender is shown for the Plantar test.
P-values, compared with baseline.
Assessment of baseline mechanical nociception with the von Frey filament assay revealed withdrawal thresholds ranging from 0.57 and 0.84 g depending on genotype and gender (Fig. 1 bottom). WT animals and the different opioid receptor knockouts were compared using two way anova for genotype and gender effects. The comparison of WT and mu-receptor mutants revealed no effect of genotype or gender. Similarly, WT and delta-receptor mutants comparison revealed no effect of genotype or gender. The comparison of WT and kappa-receptor mutants revealed a significant effect of genotype [F(1,78) = 10.8; P = 0.0015] but no effect of gender. Post hoc analyses showed that kappa-receptor mutant animals were sensitive to a lower force than WT (0.60 g in knockout vs. 0.77 g in WT for males, P = 0.0308; 0.57 g in knockout vs. 0.70 g in WT for females, P = 0.024; and 0.59 g in knockout vs. 0.73 g in WT for combined animals, P = 0.0016; Fig. 1 bottom). This indicated a higher sensitivity to mechanical stimulation in the absence of kappa receptors.
Inflammatory thermal hyperalgesia in opioid receptor-knockout mice
We administered an amount of CFA that allowed the production of significant inflammatory pain in our mouse strains. Hence, CFA administration into the hindpaw induced a rapid and long-lasting decrease in paw withdrawal latency to the thermal stimulus in animals of both genders of all genotypes (Fig. 2). The different opioid receptor knockouts were compared to WT animals for the development of inflammatory heat hyperalgesia over time using three-way repeated-measures anova with genotype, gender and paw side as factors. The comparison of WT and mu-receptor mutants revealed an effect of paw side [F(1,740) = 422; P < 0.0001] and gender [F(1,740) = 38; P < 0.0001], and no effect of genotype. Similarly, the comparison of WT and kappa-receptor mutants revealed an effect of paw side [F(1,740) = 433; P < 0.0001] and gender [F(1,740) = 38; P < 0.0001] and no effect of genotype. The comparison of WT and delta-receptor mutants indicated an effect of paw side [F(1,740) = 414; P < 0.0001], gender [F(1,740) = 26.3; P < 0.0001] and genotype [F(1,740) = 50.5; P < 0.0001]. As anova revealed a gender effect for the three comparisons, data for males and females were analysed separately (see Fig. 2).
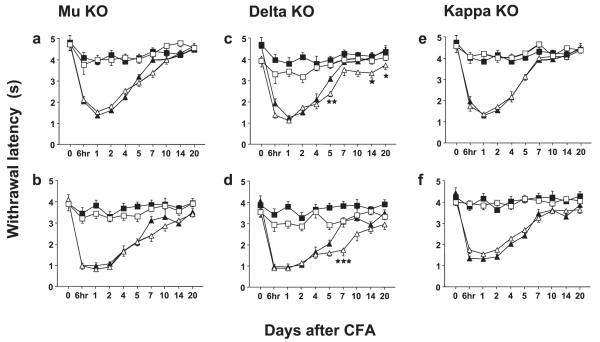
Fig. 2.
Development of thermal hyperalgesia in mu (mu-KO), delta (delta-KO) and kappa (kappa-KO) opioid receptor-knockout mice and their WT counterparts. Thermal hyperalgesia was measured using the plantar test. (a and b) Mu-KO and WT; (c and d) delta-KO and WT; (e and f ) kappa-KO and WT. Upper panels (a, c and e) represent data for males and bottom panels (b, d and f) for females. Data are expressed as mean ± SEM paw withdrawal latencies in s for WT (black symbols) and knockout (white symbols) mice in ipsilateral (triangles) or contralateral (squares) paws; n = 21 for WT mice (10 males and 11 females) and n = 20 for each mutant (10 males and 10 females). Significant genotype effects at ipsilateral paws are represented by one star, P < 0.05; two stars, P < 0.01; and three stars, P < 0.001 (Bonferroni post hoc test).
Each genotype was analysed first for the effect of time after CFA. WT mice displayed a marked decrease in withdrawal latency of the ipsilateral paw after CFA (Fig. 2). Post hoc analysis vs. baseline indicated a significant effect of time after CFA at 6 h to day 5, for both males and females (Table 1). Similarly, mu-, delta- and kappa-receptor knockout mice showed inflammatory thermal hyperalgesia in the ipsilateral paw (Fig. 2 left, middle and right panels, respectively). Mu-receptor knockout animals had a significant hyperalgesia from 6 h to day 7 for males and to day 14 for females (Table 2). Delta-receptor mutants displayed a significant hyperalgesia from 6 h to day 5 for males and to day 10 for females (Table 3). Kappa-receptor mutants had a significant hyperalgesia from 6 h to day 5 for males and to day 7 for females (Table 4). Paw oedema following CFA was similar in the four mouse lines (data not shown).
Table 3.
Development of inflammatory pain in delta opioid-receptor knockout mice
| Time after CFA | Plantar withdrawal responses |
Von Frey mechanical sensitivity (g) (pooled males and females) |
P-value* (time)† |
||||
|---|---|---|---|---|---|---|---|
| Males, latencies (s) |
Males, P-value* (time)† |
Females, latencies (s) |
Females, P-value (time)† |
P-value (gender) |
|||
| Baseline | 3.97 ± 0.19 | 3.84 ± 0.30 | N.S. | 0.832 ± 0.047 | |||
| 6 h | 1.29 ± 0.14 | < 0.0001 | 1.08 ± 0.10 | < 0.0001 | N.S. | 0.070 ± 0.008 | < 0.0001 |
| 1 day | 1.02 ± 0.06 | < 0.0001 | 1.07 ± 0.11 | < 0.0001 | N.S. | 0.039 ± 0.005 | < 0.0001 |
| 2 days | 1.65 ± 0.12 | < 0.0001 | 1.33 ± 0.11 | < 0.0001 | N.S. | 0.054 ± 0.010 | < 0.0001 |
| 4 days | 1.80 ± 0.21 | < 0.0001 | 1.73 ± 0.18 | < 0.0001 | N.S. | 0.070 ± 0.010 | < 0.0001 |
| 5 days | 2.31 ± 0.15 | < 0.0001 | 1.81 ± 0.15 | < 0.0001 | 0.031 | 0.179 ± 0.014 | < 0.0001 |
| 7 days | 3.44 ± 0.28 | N.S. | 1.94 ± 0.27 | < 0.0001 | 0.0011 | 0.341 ± 0.018 | < 0.0001 |
| 10 days | 3.33 ± 0.20 | N.S. | 2.72 ± 0.34 | 0.0004 | N.S. | 0.315 ± 0.017 | < 0.0001 |
| 14 days | 3.28 ± 0.22 | N.S. | 2.96 ± 0.20 | N.S. | N.S. | 0.413 ± 0.026 | < 0.0001 |
| 20 days | 3.66 ± 0.21 | N.S. | 3.16 ± 0.22 | N.S. | N.S. | 0.598 ± 0.034 | < 0.0001 |
See footnote to Table 1.
The effect of time post-CFA is shown for both plantar and von Frey assays (Bonferroni post hoc test). The effect of gender is shown for the Plantar test.
P-values, compared with baseline.
Table 4.
Development of inflammatory pain in kappa opioid-receptor knockout mice
| Time after CFA | Plantar withdrawal responses |
Von Frey mechanical sensitivity (g) (pooled males and females) |
P-value* (time)† |
||||
|---|---|---|---|---|---|---|---|
| Males, latencies (s) |
Males, P-value* (time)† |
Females, latencies (s) |
Females, P-value (time)† |
P-value (gender) |
|||
| Baseline | 4.67 ± 0.34 | 3.97 ± 0.15 | N.S. | 0.585 ± 0.027 | |||
| 6 h | 1.61 ± 0.23 | < 0.0001 | 1.57 ± 0.17 | N.S. | < 0.0001 | 0.071 ± 0.013 | < 0.0001 |
| 1 day | 1.23 ± 0.13 | < 0.0001 | 1.37 ± 0.17 | N.S. | < 0.0001 | 0.041 ± 0.005 | < 0.0001 |
| 2 days | 1.58 ± 0.14 | < 0.0001 | 1.57 ± 0.20 | N.S. | < 0.0001 | 0.050 ± 0.006 | < 0.0001 |
| 4 days | 2.04 ± 0.23 | < 0.0001 | 2.11 ± 0.22 | N.S. | < 0.0001 | 0.103 ± 0.013 | < 0.0001 |
| 5 days | 3.01 ± 0.17 | < 0.0001 | 2.51 ± 0.13 | 0.031 | < 0.0001 | 0.234 ± 0.019 | < 0.0001 |
| 7 days | 3.89 ± 0.21 | N.S. | 3.09 ± 0.21 | 0.0007 | 0.015 | 0.341 ± 0.018 | < 0.0001 |
| 10 days | 3.97 ± 0.20 | N.S. | 3.42 ± 0.13 | N.S. | 0.033 | 0.359 ± 0.015 | < 0.0001 |
| 14 days | 3.94 ± 0.13 | N.S. | 3.43 ± 0.18 | N.S. | 0.034 | 0.478 ± 0.024 | < 0.0001 |
| 20 days | 4.26 ± 0.10 | N.S. | 3.46 ± 0.17 | N.S. | 0.0007 | 0.519 ± 0.022 | N.S. |
See footnote to Table 1.
The effect of time post-CFA is shown for both plantar and von Frey assays (Bonferroni post hoc test). The effect of gender is shown for the Plantar test.
P-values, compared with baseline.
Post hoc analysis for a gender effect on the development of hyperalgesia in ipsilateral paw indicated for WTs a significant difference at 6 h and 5, 14 and 20 days (Table 1), for mu-mutants a difference at 6 h and 2, 10, 14 and 20 days (Table 2), for delta-mutants at days 5 and 7 (Table 3) and for kappa-mutants at days 5–20 (Table 4). At these time points, females showed a greater hyperalgesia than males. Noticeably, the gender effect was greater for the development of thermal hyperalgesia than for that of mechanical allodynia (see below).
Withdrawal latencies of contralateral paws in WT females and WT males were not statistically significantly different from baseline values, as shown by one-way anova for time. There was also no statistically significant effect of time post-CFA on contralateral paw reactions in mu-, delta- or kappa-receptor mutants.
The analysis of the effect of genotype showed that neither mu- nor kappa-knockout animals were different from WTs in the magnitude of inflammatory hyperalgesia, as indicated above by three-way anova (Fig. 2 left and right panels, respectively). Noticeably, delta-receptor knockout mice showed an augmented inflammatory hyperalgesia that statistically differed from control mice on days 5, 14 and 20 for males and on day 7 for females, as shown by post hoc analysis (Fig. 2 middle panels).
Inflammatory mechanical allodynia in opioid-receptor knockout mice
In all the genotypes, CFA administration into the hindpaw strongly decreased the paw withdrawal threshold to von Frey filament application (Fig. 3). The different opioid-receptor knockouts were compared to WT animals for the development of inflammation-induced mechanical allodynia over time using three-way repeated-measures anova with genotype, gender and paw side as factors. The comparison of WT and mu-receptor mutants revealed an effect of paw side [F(1,740) = 249; P < 0.0001] and no effect of genotype or gender. WT and kappa-receptor mutant comparison indicated an effect of paw side [F(1,740) = 310; P < 0.0001] and genotype [F(1,740) = 18; P < 0.0001] but no gender effect. The comparison of WT and delta-receptor mutants indicated an effect of paw side [F(1,740) = 365; P < 0.0001] no effect of gender or genotype, although there was a tendency for a genotype effect [F(1,740) = 2.38; P = 0.126]. As there were no gender effects in any of the genotypes, data from males and females in each genotype were pooled.
Fig. 3.
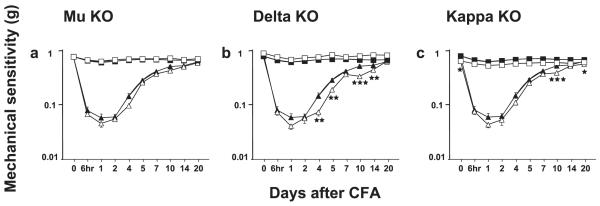
Development of mechanical allodynia in mu (mu-KO), delta (delta-KO) and kappa (kappa-KO)-knockout mice as well as their WT counterparts. Mechanical allodynia was measured using the von Frey test. (a) Mu-KO and WT, (b) delta-KO and WT, (c) kappa-KO and WT. Data are expressed as mean ± SEM paw withdraw thresholds for pooled male and female WT (black symbols) and knockout (white symbols) mice in ipsilateral (triangles) or contralateral (squares) paws; n = 21 for WT mice (10 males and 11 females) and n = 20 for each mutant (10 males and 10 females). Significant genotype effects at ipsilateral paws are represented by one star, P < 0.05; two stars, P < 0.01; and three stars, P < 0.001 (Bonferroni post hoc test).
WT and mu-, kappa- and delta-receptor mutant animals were analysed for the effect of time after CFA by post hoc analysis using pooled males and females. CFA-evoked inflammation led to a marked mechanical allodynia in the ipsilateral paw of WT mice (Fig. 3). Post hoc analysis of the effect of time vs. baseline indicated a significant effect of time from 6 h to 20 days (Table 1). Similarly, mu-, delta- and kappa-receptor knockout mice showed mechanical allodynia in the ipsilateral paw (Fig. 3). Mu- and delta-receptor knockout animals had a significant allodynia from 6 h to 20 days (Tables 2 and 3) and kappa-receptor mutants from 6 h to 14 days (Table 4).
Mechanical sensitivity values of contralateral paws after CFA were similar to values before CFA in WT and delta-receptor mutant mice except on day 1 (P = 0.0012 and P < 0.0001 vs. baseline, respectively). In mu- and kappa-receptor mutants, there was no statistically significant mechanical allodynia in the contralateral paw.
When genotypes were compared, kappa-receptor knockout mice differed from WT mice in inflammation-induced mechanical allodynia at baseline and on days 10 and 20 (post hoc analysis; Fig. 3 right panel). Delta-mutants showed a significant enhancements in mechanical allodynia as compared with WT at days 4, 5, 10 and 14 (post hoc analysis; Fig. 3 middle panel).
Activity of the delta opioid agonist SNC80 on CFA-induced inflammatory pain
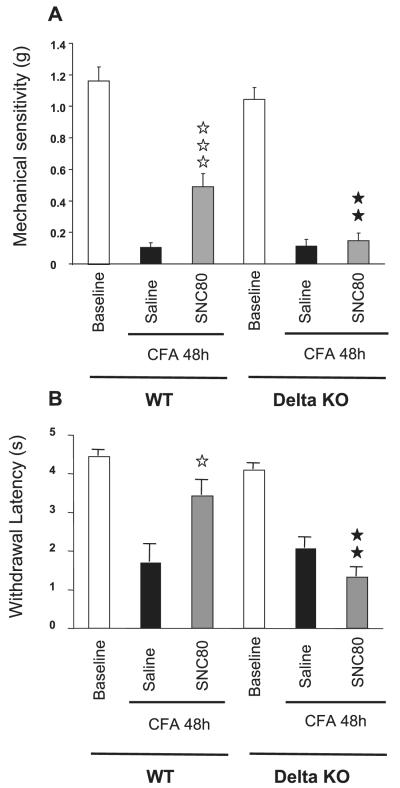
SNC80 was used as the prototypic non-peptidic delta agonist (Bilsky et al., 1995; Calderon & Coop, 2004). SNC80 produced 35.3% antiallodynia when tested with von Frey hairs in WT animals (Fig. 4A; P < 0.0001, SNC80 vs. vehicle). SNC80 also produced 62.8% antihyperalgesia in the plantar assay in WT animals when compared with the vehicle-treated group (Fig. 4B; P < 0.05 SNC80 vs. vehicle). SNC80 showed no significant effect in delta-opioid receptor-knockout mice (Fig. 4A and B). The absence of delta receptors, therefore, prevented the antiallodynic and antihyperalgesic activities of SNC80 on inflammatory pain.
Fig. 4.
Effect of the delta opioid agonist SNC80 on inflammatory pain. (A) von Frey test; (B) Plantar test. WT and delta-receptor knockout mice (delta-KO) were treated with SNC80 (n = 10 for each genotype, five males and five females) or saline (n = 10 for each genotype, five males and five females) as a control 48 h after CFA. Animals were tested 45 min and 1 h after drug administration for mechanical allodynia and thermal hyperalgesia, respectively. Data are expressed as mean ± S.E.M. withdrawal thresholds of ipsilateral paws. White bars represent baseline (before CFA) values, black bars the CFA–control saline groups and grey bars the CFA–SNC80 groups. Significant effects of SNC80 are represented by white stars (one star, P < 0.05; three stars, P < 0.0001; SNC80 vs. saline) and of genotype by black stars (two stars, P < 0.01; delta-KO vs. WT).
Discussion
Inflammation induces the release of cytokines and neuropeptides that produce pain, and also activates the endogenous opioid system which reduces pain (Brack et al., 2004). Here we have investigated the role played by opioid receptors in the control of inflammatory pain using CFA as an inducer of inflammation. This is the first study assessing inflammatory hyperalgesia simultaneously in all three strains of mu-, delta- and kappa-receptor knockout mice. Mu-receptor knockout animals developed inflammatory pain similarly to WTs, while kappa-receptor mutants had an augmented mechanical allodynia vs. WTs when analysed for thermal hyperalgesia and mechanical allodynia. Remarkably, delta-receptor knockout mice showed both enhanced thermal hyperalgesia and mechanical allodynia. This result strongly suggests a major element of endogenous delta-receptor tone limiting the severity of pain under inflammatory conditions.
Basal nociceptive thresholds prior to CFA administration were compared in mutant and WT mice. In the plantar test, we found no difference in baseline paw withdrawal latencies between WT, mu- and kappa-receptor mutant animals, as shown previously (Fuchs et al., 1999; Mansikka et al., 2004; Xu et al., 2004; Nadal et al., 2006). Delta-receptor mutants showed a higher sensitivity to thermal plantar stimulus. This was not found in a previous study using the plantar test (Nadal et al., 2006), possibly because of different experimental conditions. Using von Frey filaments we found no change for baseline mechanical nociception in the mu- and delta-receptor mutants, as described earlier (Mansikka et al., 2004; Nadal et al., 2006). Kappa-receptor mutants displayed higher sensitivity to mechanical stimulation. Previous analysis of kappa-receptor knockout mice showed a stronger response to visceral pain, with no modifications of other nociceptive measures including mechanical stimulation, in the tail pressure test (Simonin et al., 1998; Martin et al., 2003) and the von Frey assay (Xu et al., 2004). In the present study, therefore, the higher mechanical sensitivity of kappa mutants reveals a kappa-receptor-mediated tone regulating mechanical nociception, which is subtle and detectable only under specific experimental conditions.
CFA administration produced inflammatory pain which developed similarly in mu-receptor knockout mice and WT controls. The lack of behavioural phenotype for these mutants is in accordance with studies using the late-phase formalin assay (Martin et al., 2003) or the carrageenan model (Mansikka et al., 2002). Other studies have suggested that mu receptors located at sensory peripheral endings, activated by immune cell-derived opioid peptides, control inflammatory pain (Binder et al., 2004; Puehler et al., 2004). In addition, mu receptors have been shown to be upregulated in superficial layers dorsal horn after CFA (Mousa et al., 2002) or in monoarthritic rats (Besse et al., 1992), contributing to increased mu-receptor tone. These mu-receptor activities may be too local to result in a measurable increase in pain under our experimental conditions. Alternatively, the lack of phenotype for mu-mutants in our experiments might be explained by a different genetic background or by compensatory changes occurring in knockout mice during embryogenesis or while inflammation develops. In the future, the regional- and time-controlled genetic deletion of mu receptors at distinct sites of pain pathways will clarify the contribution of mu receptors in the control of inflammatory pain. Kappa-receptor knockout mice displayed the same thermal hyperalgesia as WTs while they showed enhanced mechanical allodynia on days 10 and 20. Xu et al. (2004) previously demonstrated a strong increase in both thermal hyperalgesia and tactile allodynia in kappa-knockout mice using a model of neuropathic pain. These results, together with our findings, suggest that kappa-receptor tone may be important in the control of neuropathic pain but less so for the control of inflammatory pain.
Delta-receptor knockout mice clearly displayed enhanced inflammatory pain in this study. This finding strongly suggests that delta-receptor-mediated inhibitory mechanisms moderate the severity of pain in WT animals. This delta-receptor activity is demonstrated in both thermal hyperalgesia and mechanical allodynia, two distinct expression modes of inflammatory pain (Ossipov et al., 1999; Lewin et al., 2004). Moreover, both male and female delta-mutants showed more hyperalgesia and allodynia than their WT counterparts, indicating that the delta-receptor tone controls inflammatory pain in both genders. These data also show the relevance of testing both male and female animals, as demonstrated by Mogil & Chanda (2005). Although sex differences in pain perception have been reported in both humans and animals (Wiesenfeld-Hallin, 2005), and although female delta-mutants displayed more thermal hyperalgesia than delta-mutant males at days 5 and 7 in the present study, the effect of delta-receptor deletion was shown in both genders, strengthening the notion of a role for this receptor in chronic pain. Both thermal and mechanical responses were also augmented, to the same extent, in these mutant animals using a model of neuropathic pain (Nadal et al., 2006). Additionally, delta-knockout mice showed increased responses in the late inflammatory phase of the formalin test (Martin et al., 2003). Altogether, data from knockout mice consistently highlight a major role for the delta receptor in several situations of chronic pain. Delta receptors therefore represent an interesting therapeutic target for treating chronic pain that bears complex neurobiological bases.
The important role for endogenous delta-receptor activity in the control of chronic pain control contrasts with its minor implication in acute pain (see Kieffer & Gavériaux-Ruff, 2002). A number of findings have, in fact, reinforced the notion that the delta-opioid system is functionally activated during persistent pain. First, delta agonists are more potent under inflammatory conditions (Fraser et al., 2000; Hurley & Hammond, 2000; Cahill et al., 2003; Petrillo et al., 2003; Pacheco & Duarte, 2005) and this has been postulated to result from additive or synergistic effects of delta drugs with endogenous opioid peptides. Indeed, enkephalins are augmented by inflammation (Machelska & Stein, 2000; Hurley & Hammond, 2001; Spetea et al., 2002; Mousa et al., 2007). Second, inflammation modifies the expression of delta receptors at mRNA and protein (Cahill et al., 2003) and binding-site (Besse et al., 1992) levels. Inflammation also decreases delta-receptor expression in DRGs (Ji et al., 1995), suggesting a differential effect on pre- and post-synaptic receptors. Inflammation indeed leads to an augmentation of axonal opioid receptor transport to sensory nerve terminals (Hassan et al., 1993). Inflammation-induced recruitment of delta receptors to the neuronal surface has also been proposed to account for their increase in functionality (Cahill et al., 2007). Chronic inflammation alters supraspinal pain-controlling sites (Przewlocki & Przewlocka, 2001; Tsuruoka et al., 2003) and activates descending opioidergic pain-controlling pathways (Fraser et al., 2000; Fields, 2004), which in turn impacts on pain perception. This may explain the augmented activity of delta agonists administered in the raphe magnus (Ma et al., 2006). Another explanation is that increased inflammatory pain in the delta-mutants may be due to a generalized production of inflammatory cytokines in blood, cerebrospinal fluid or glial cells (Samad et al., 2001; Watkins et al., 2007). Cytokines in turn upregulate cyclooxygenase-2, increasing the amount of prostaglandinE-2 in cerebrospinal fluid and causing systemic hyperalgesia. Cytokines also activate the bradykinin system, which enhances delta-receptor function (Ferreira et al., 2001; Patwardhan et al., 2005). Alternatively, an alteration of the neuroglial communication induced by inflammation would increase delta-receptor functional competence (Holdridge et al., 2007). Although our analysis of delta-receptor-deficient mice does not allow determination of whether delta-receptor function evolves during inflammation, the clear phenotype of these animals compared to the less pronounced phenotype in mice lacking mu or kappa receptors is fully consistent with the hypothesis that delta-receptor activity is enhanced during inflammation. The analysis of enkephalin-, endorphin- and dynorphin-knockout animals should identify the endogenous opioid peptides that activate delta receptors during inflammation (Kieffer & Gavériaux-Ruff, 2002).
Finally, we have shown that the delta-receptor agonist SNC80 administered systemically effectively reduces inflammatory hyperalgesia. This is consistent with previous studies showing the effects of SNC80 on dynorphin-A allodynia (Kawaraguchi et al., 2004), prostaglandinE-2-hyperalgesia (Pacheco & Duarte, 2005) and CFA-inflammatory pain (Fraser et al., 2000; Gendron et al., 2007) after i.c.v., i.t. or local administration in the paw. It may be of interest to compare the systemic effect of SNC80 to systemic effects of mu- and kappa-opioid receptor-selective agonists under our experimental conditions. Importantly, our study shows the lack of effect of SNC80 in delta-receptor knockout mice. This is the first genetic demonstration that antihyperalgesic activities of the compound are indeed mediated by delta receptors in vivo, and therefore confirms that the delta opioid receptor is a useful therapeutic target for chronic pain treatment. Novel delta agonists have been developed which diminish chronic pain after systemic administration: SB-235863 for inflammatory and neuropathic pain (Petrillo et al., 2003), UK321,130 for inflammatory bowel disease (Middleton et al., 2006) and DVal2-Ala5-Enk for bone cancer pain (Brainin-Mattos et al., 2006). Noticeably, the potential therapeutic utility of delta agonists is now broadened by findings on their beneficial effects on tumour growth (Gomez-Flores et al., 2005), neuroprotection (Narita et al., 2006a) and mood disorders. Delta agonists are under consideration for their use as anxiolytics and antidepressants (Broom et al., 2002; Jutkiewicz, 2006) and could be involved in pain-induced depression (Narita et al., 2006b) or the anxiolytic effects of benzodiazepines (Primeaux et al., 2006).
Altogether, the present findings show that inflammatory pain is augmented in delta-receptor-deficient animals, that the delta-receptor agonist SNC80 produces antihyperalgesia, and that SNC80 activity is abolished in delta-knockout animals. Together, the data strengthen the notion of an important role for delta receptors in inflammatory pain. Delta-receptor activation, by either endogenous opioids or exogenous agonists, probably modulate inflammatory pain at several sites in pain-processing pathways. In the future, the contribution of distinct delta-receptor populations within pain circuits may be addressed by the conditional knockout approach (Gaveriaux-Ruff & Kieffer, 2007).
Acknowledgements
We thank Florent Hartmann for his help. This work was funded by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université Louis Pasteur, the Institut UPSA de la Douleur, and a NIDA grant (NIH-NIDA #DA 05010). L.K. was supported by NIH-NIAAA#U01AA13481.
Abbreviations
- CFA
complete Freund’s adjuvant
- WT
wild-type
References
- Besse D, Weil-Fugazza J, Lombard MC, Butler SH, Besson JM. Monoarthritis induces complex changes in mu-, delta- and kappa-opioid binding sites in the superficial dorsal horn of the rat spinal cord. Eur. J. Pharmacol. 1992;223:123–131. doi: 10.1016/0014-2999(92)94830-o. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J. Pharmacol. Exp. Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- Binder W, Mousa SA, Sitte N, Kaiser M, Stein C, Schafer M. Sympathetic activation triggers endogenous opioid release and analgesia within peripheral inflamed tissue. Eur. J. Neurosci. 2004;20:92–100. doi: 10.1111/j.1460-9568.2004.03459.x. [DOI] [PubMed] [Google Scholar]
- Brack A, Labuz D, Schiltz A, Rittner HL, Machelska H, Schafer M, Reszka R, Stein C. Tissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociception. Anesthesiology. 2004;101:204–211. doi: 10.1097/00000542-200407000-00031. [DOI] [PubMed] [Google Scholar]
- Brainin-Mattos J, Smith ND, Malkmus S, Rew Y, Goodman M, Taulane J, Yaksh TL. Cancer-related bone pain is attenuated by a systemically available delta-opioid receptor agonist. Pain. 2006;122:174–181. doi: 10.1016/j.pain.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Rice KC, Traynor JR, Woods JH. Behavioral effects of delta-opioid receptor agonists: potential antidepressants? Jpn. J. Pharmacol. 2002;90:1–6. doi: 10.1254/jjp.90.1. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, O’Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol. Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Calderon SN, Coop A. SNC 80 and related delta opioid agonists. Curr. Pharm. Des. 2004;10:733–742. doi: 10.2174/1381612043453054. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Dolle RE. Peripherally restricted opioid agonists as novel analgesic agents. Curr. Pharm. Des. 2004;10:743–757. doi: 10.2174/1381612043453036. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Campos MM, Pesquero JB, Araujo RC, Bader M, Calixto JB. Evidence for the participation of kinins in Freund’s adjuvant-induced inflammatory and nociceptive responses in kinin B1 and B2 receptor knockout mice. Neuropharmacology. 2001;41:1006–1012. doi: 10.1016/s0028-3908(01)00142-3. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat. Rev. Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fraser GL, Gaudreau GA, Clarke PB, Menard DP, Perkins MN. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. Br. J. Pharmacol. 2000;129:1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PN, Roza C, Sora I, Uhl G, Raja SN. Characterization of mechanical withdrawal responses and effects of mu-, delta- and kappa-opioid agonists in normal and mu-opioid receptor knockout mice. Brain Res. 1999;821:480–486. doi: 10.1016/s0006-8993(99)01060-4. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Conditional gene targeting in the mouse nervous system: insights into brain function and diseases. Pharmacol. Ther. 2007;113:619–634. doi: 10.1016/j.pharmthera.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007;150:807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Flores R, Caballero-Hernandez D, Tamez-Guerra R, Rodriguez-Padilla C, Tamez-Guerra P, Rice KC, Hicks ME, Weber RJ. Increased survival of tumor-bearing mice by the delta opioid SNC 80. Anticancer Res. 2005;25:4563–4567. [PubMed] [Google Scholar]
- Hassan AH, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993;55:185–195. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- Holdridge SV, Armstrong SA, Taylor AM, Cahill CM. Behavioural and morphological evidence for the involvement of glial cell activation in delta opioid receptor function: implications for the development of opioid tolerance. Mol. Pain. 2007;3:7. doi: 10.1186/1744-8069-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J. Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J. Neurosci. 2001;21:2536–2545. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R-R, Zhang Q, Law P-Y, Low HH, Elde R, Hökfelt T. Expression of μ-, δ-, and κ-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J. Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM. The antidepressant-like effects of delta-opioid receptor agonists. Mol. Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- Kawaraguchi Y, Kawaguchi M, Takahashi M, Horiuchi T, Sakamoto T, Furuya H. Delta-opioid agonist SNC80 can attenuate the development of dynorphin A-induced tactile allodynia in rats. Anesthesiology. 2004;101:546–549. doi: 10.1097/00000542-200408000-00040. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gavériaux-Ruff C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Lu Y, Park TJ. A plethora of painful molecules. Curr. Opin. Neurobiol. 2004;14:443–449. doi: 10.1016/j.conb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol. Pharmacol. 2006;69:1137–1145. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- Machelska H, Stein C. Pain control by immune-derived opioids. Clin. Exp. Pharmacol. Physiol. 2000;27:533–536. doi: 10.1046/j.1440-1681.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- Mansikka H, Zhou L, Donovan DM, Pertovaara A, Raja SN. The role of mu-opioid receptors in inflammatory hyperalgesia and alpha 2-adrenoceptor-mediated antihyperalgesia. Neuroscience. 2002;113:339–349. doi: 10.1016/s0306-4522(02)00189-6. [DOI] [PubMed] [Google Scholar]
- Mansikka H, Zhao C, Sheth RN, Sora I, Uhl G, Raja SN. Nerve injury induces a tonic bilateral mu-opioid receptor-mediated inhibitory effect on mechanical allodynia in mice. Anesthesiology. 2004;100:912–921. doi: 10.1097/00000542-200404000-00022. [DOI] [PubMed] [Google Scholar]
- Martin M, Matifas A, Maldonado R, Kieffer BL. Acute antinociceptive responses in single and combinatorial opioid receptor knockout mice: dictinct mu, delta and kappa tones. Eur. J. Neurosci. 2003;17:1–8. doi: 10.1046/j.1460-9568.2003.02482.x. [DOI] [PubMed] [Google Scholar]
- Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, LeMeur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Middleton DS, Maw GN, Challenger C, Jessiman A, Johnson PS, Million WA, Nichols CL, Price JA, Trevethick M. Highly potent and selective zwitterionic agonists of the delta-opioid receptor. Part 1. Bioorg. Med. Chem. Lett. 2006;16:905–910. doi: 10.1016/j.bmcl.2005.10.102. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Machelska H, Schafer M, Stein C. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J. Neuroimmunol. 2002;126:5–15. doi: 10.1016/s0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- Mousa S, Straub R, Schafer M, Stein C. b-endorphin, Metenkephalin and corresponding opioid receptors within synovium of patients with joint trauma, ostheoarthritis and rheumatoid arthritis. Ann. Rheum. Dis. 2007;66:871–879. doi: 10.1136/ard.2006.067066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal X, Banos JE, Kieffer BL, Maldonado R. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur. J. Neurosci. 2006;23:830–834. doi: 10.1111/j.1460-9568.2006.04569.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Miyatake M, Sato F, Wachi H, Seyama Y, Suzuki T. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J. Neurochem. 2006a;97:1494–1505. doi: 10.1111/j.1471-4159.2006.03849.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Narita M, Kaneko C, Hareyama N, Miyatake M, Shindo K, Miyoshi K, Nakajima M, Nagumo Y, Sato F, Wachi H, Seyama Y, Suzuki T. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J. Neurochem. 2006b;97:1369–1378. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- Noble F, Roques BP. Protection of endogenous enkephalin catabolism as natural approach to novel analgesic and antidepressant drugs. Expert. Opin. Ther. Targets. 2007;11:145–159. doi: 10.1517/14728222.11.2.145. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Kovelowski CJ, Wheeler-Aceto H, Cowan A, Hunter JC, Lai J, Malan TP, Jr, Porreca F. Opioid antagonists and antisera to endogenous opioids increase the nociceptive response to formalin: demonstration of an opioid kappa and delta inhibitory tone. J. Pharmacol. Exp. Ther. 1996;277:784–788. [PubMed] [Google Scholar]
- Ossipov MH, Bian D, Malan TP, Jr, Lai J, Porreca F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain. 1999;79:127–133. doi: 10.1016/s0304-3959(98)00187-0. [DOI] [PubMed] [Google Scholar]
- Pacheco DF, Duarte ID. Delta-opioid receptor agonist SNC80 induces peripheral antinociception via activation of ATP-sensitive K+ channels. Eur. J. Pharmacol. 2005;512:23–28. doi: 10.1016/j.ejphar.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J. Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo P, Angelici O, Bingham S, Ficalora G, Garnier M, Zaratin PF, Petrone G, Pozzi O, Sbacchi M, Stean TO, Upton N, Dondio GM, Scheideler MA. Evidence for a selective role of the delta-opioid agonist [8R-(4bS*,8aalpha,8abeta, 12bbeta)]7,10-dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]iso quinoline hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responses. J. Pharmacol. Exp. Ther. 2003;307:1079–1089. doi: 10.1124/jpet.103.055590. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, McDonald AJ, Mascagni F, Wilson MA. The role of delta opioid receptors in the anxiolytic actions of benzodiazepines. Pharmacol. Biochem. Behav. 2006;85:545–554. doi: 10.1016/j.pbb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur. J. Pharmacol. 2001;429:79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, Stein C. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience. 2004;129:473–479. doi: 10.1016/j.neuroscience.2004.06.086. [DOI] [PubMed] [Google Scholar]
- Qiu C, Sora I, Ren K, Uhl G, Dubner R. Enhanced delta-opioid receptor-mediated antinociception in mu-opioid receptor-deficient mice. Eur. J. Pharmacol. 2000;387:163–169. doi: 10.1016/s0014-2999(99)00813-4. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur. J. Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja S, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the κ-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective κ-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea M, Rydelius G, Nylander I, Ahmed M, Bileviciute-Ljungar I, Lundeberg T, Svensson S, Kreicbergs A. Alteration in endogenous opioid systems due to chronic inflammatory pain conditions. Eur. J. Pharmacol. 2002;435:245–252. doi: 10.1016/s0014-2999(01)01554-0. [DOI] [PubMed] [Google Scholar]
- Stein C, Gramsch C, Herz A. Intrinsic mechanisms of antinociception in inflammation: local opioid receptors and beta-endorphin. J. Neurosci. 1990;10:1292–1298. doi: 10.1523/JNEUROSCI.10-04-01292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat. Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- Tsuruoka M, Arai YC, Nomura H, Matsutani K, Willis WD. Unilateral hindpaw inflammation induces bilateral activation of the locus coeruleus and the nucleus subcoeruleus in the rat. Brain Res. Bull. 2003;61:117–123. doi: 10.1016/s0361-9230(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the ‘bad guys’: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav. Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend. Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Terman GW, Chavkin C. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J. Neurosci. 2004;24:4576–4584. doi: 10.1523/JNEUROSCI.5552-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner C, Stein C. Opioids. Handb. Exp. Pharmacol. 2007;177:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]