Abstract
This study examines the anti-tumor potential of curcumin and C6 ceramide (C6) against osteosarcoma (OS) cell lines when both are encapsulated in the bilayer of liposomal nanoparticles. Three liposomal formulations were prepared – curcumin liposomes, C6 liposomes and C6-curcumin liposomes. Curcumin in combination with C6 showed 1.5 times enhanced cytotoxic effect in the case of MG-63 and KHOS OS cell lines, in comparison with curcumin liposomes alone. Importantly, C6-curcumin liposomes were found to be less toxic on untransformed human cells (human mesenchymal stem cells) in comparison to OS cell lines. In addition, cell cycle assays on a KHOS cell line after treatment revealed that curcumin only liposomes induced G2/M arrest by upregulation of cyclin B1, while C6 only liposomes induced G1 arrest by downregulation of cyclin D1. C6-curcumin liposomes induced G2/M arrest and showed a combined effect in the expression levels of cyclin D1 and cyclin B1. The efficiency of the preparations was tested in vivo using a human osteosarcoma xenograft assays. Using pegylated liposomes to increase the plasma half-life and tagging with folate (FA) for targeted delivery in vivo, a significant reduction in tumor size was observed with C6-curcumin-FA liposomes. The encapsulation of two water insoluble drugs, curcumin and C6, in the lipid bilayer of liposomes enhances the cytotoxic effect and validates the potential of combined drug therapy.
Keywords: Cancer, C6 Ceramide, Curcumin, Liposome, Osteosarcoma
Introduction
Osteosarcoma (OS) is a primary bone cancer that typically occurs in the longer bones of the body, particularly distal femur and proximal tibia. OS tumors, being mesenchymal in origin, are very aggressive and more than 20% of diagnoses are at the metastatic stage (1). It is commonly seen in children and adolescents. Parallel to other solid tumors, OS tumors also contain a highly heterogeneous population of cancer cells in terms of growth rate, karyotype, antigenicity and chemosensitivity (2). Therefore, the intervention of less toxic multi-agent therapy is of utmost importance to treat high grade OS. Recent research has focused on multi-agent therapies in adjuvant as well as neoadjuvant settings as options to treat chemoresistant OS (3–4). Several multi-drug therapies have been explored to treat OS such as; (ifosfamide + methotrexate + cisplatin + doxorubicin) (3), (RAD001 + zoledronic acid) (4), and (biphosphonates + paclitaxel/gemcitabine/doxorubicin) (5). These studies clearly indicate that multi-agent therapy improves OS treatment in wild-type as well as P-glycoprotein overexpressing OS cells (5). It was reported that although these combinations are feasible for the preoperative phase, they are associated with renal and hematological toxicities (3).
Curcumin has shown potent anticancer activity against all stages of cancer by blocking tumor initiation, suppressing tumor progression and by inhibiting invasion/metastasis of cancer cells (6). Tumor inhibitory activity is attributed to its action on NF-κB, TNF-α, VEGF, cyclooxygenase, matrix metalloproteinase and many other signal transduction molecules involved in carcinogenesis (7). Curcumin has become a broad spectrum anticancer drug due to its multi-targeted nature that regulates diverse molecular pathways.
Recently, C6 ceramide (C6) has been shown to potentiate curcumin mediated cell death in melanoma (8). Ceramides are sphingolipids and play an important role in cell differentiation, cell cycle arrest, apoptosis, growth inhibition and senescence (9). Hence, providing exogeneous ceramide along with curcumin could be a better combination to treat cancer cells. Therefore, we have incorporated curcumin and C6 in liposomal nanoparticles to enhance the anticancer effect. However, using C6 and curcumin in free form limits bioavailability because both the drugs are highly water insoluble. To enhance the bioavailability of these drugs, we sought to encapsulate them in the bilayer of lipid vesicles (liposomes). This paper therefore reports on the co-encapsulation of C6 and curcumin in liposome, as a delivery vehicle against the KHOS osteosarcoma cell line.
While focusing on the importance of a combined therapy, we have explored further modified and targeted liposomes (i.e. pegylated and folate (FA) tagged liposomes) to enhance the therapeutic efficacy of the drug in liposomal vesicles. Conventional (nonpegylated) liposomal therapy has two limitations: a short plasma half-life and nonspecific drug delivery. To overcome the rapid clearance effect of the reticuloendothelial system, liposomes were coated with PEG (Polyethylene glycol), thereby enhancing circulation lifetime and accessibility of nanoparticles to tumor. The overexpression of the folate receptor has been reported recently in many osteosarcoma xenograft samples (10). This association of the folate receptor with osteosarcoma has been used for targeted delivery. The FA tagged liposomes would target the OS cells expressing folate receptor and release the curcumin that is incorporated in the liposomes.
Materials and Methods
Materials
Curcumin was purchased from Acros Organics Inc. Morris Plains, New Jersey. C6 Ceramide (N-hexanoyl-D-erythro-sphingosine), DMPG (1,2-Dimyristoyl-sn-glycero-3-(Phospho-rac-(1-glycerol)), DPPC (1,2-dipalmitoyl-sn-glycero-3-Phosphocholine), DSPE-mPEG (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt)), DSPE-PEG2000-Folate (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate(polyethylene glycol)-2000] (ammonium salt)) and Mini-Extruder were from Avanti Polar Lipids Inc. (Alabaster, Alabama). DMEM (Dulbeco’s Modified Eagle’s Medium), α-MEM (Minimum Essential Medium) and FBS (Fetal Bovine Serum) were from Invitrogen, (Carlsbad, CA).
Preparation of C6-curcumin liposomes
Liposomes were prepared by the well-established thin-film evaporation method (11–12). Briefly, the phospholipids DPPC, DMPG and sphingolipid C6 were mixed in the wt% ratio of 40:40:20. Curcumin and lipids were then dissolved in 10 mL of a chloroform + methanol mixture (2:1 v/v). The solution was evaporated using a rotary evaporator for 2.5 hours to form a dry lipid film. The lipid film was then hydrated in PBS for 1 hour at 50°C. The hydrated solution was extruded 11 times through a 400 nm polycarbonate membrane followed by extrusion through a 100 nm membrane at 65°C. Empty liposomes without curcumin and C6 were used as a control. The liposomes containing either C6 or curcumin were synthesized for comparative study. Pegylated liposomes were prepared using 14 wt% (4 mol%) of DSPE-mPEG2000 as reported previously (for folate targeted liposome 3.5 mol% of DSPE-mPEG 2000 and 0.5 mol% DSPE-PEG2000Folate) (13–15). In the preparation of folate targeted liposomes, we used folate conjugated phospholipid (DSPE-PEG 2000-Folate) and curcumin was not conjugated to folate. In this system, the water insoluble curcumin molecule becomes entrapped inside the hydrophobic lipid bilayer and folate will be on the hydrophilic inside and outside layer of liposomes. Hence curcumin’s activity will not be affected by the folate present on the hydrophilic side of the lipid bilayer. Liposomal uptake was visualized by using fluorescent dye DiI (1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindocarbocyanine) from Life Technologies Corp. (Grand Island, New York). Liposomes were made using DPPC: DMPG: DiI in the wt% ratio of 49:49:2 following the same procedure described above.
Cryo-Transmission electron microscopy (Cryo-TEM) and zeta potential
A drop of the liposome suspension was placed on a TEM grid. The drop was blotted to form a thin film using a Whatman filter paper. Thin film was rapidly vitrified in liquid ethane. The vitrified liposome specimens were transferred to a JEOL 2011 microscope equipped with a Gatan cold stage. The specimens were examined under an acceleration voltage of 120 kV in conventional TEM mode. The specimens were maintained at −175°C during the course of imaging. The zeta potential of different liposomal formulations was measured using a Zetasizer Nano ZS, Malvern Instrument, USA. All liposomal formulations were diluted 1:10 with PBS (~2 mg of phospholipid/mL) prior to measurement.
Cell culture
KHOS and MG-63 cells were grown in DMEM supplemented with 10% FBS, 100 units/mL penicillin and 100 µg/mL of streptomycin. Human mesenchymal stem cells (MSCs) were cultured in α-MEM containing 17% FBS, 100 units/mL of penicillin and 100 µg/mL of streptomycin. Cells were maintained at 37°C with 5% CO2 in a humidified incubator. KHOS and MG-63 were obtained from American Type Culture Collection; human MSCs were obtained from Tulane Center for Stem Cell Research and Regenerative Medicine. No further authentication is required because all the experiments were performed within first few passages of the initial isolation/early passages from the source.
Cell viability assay and liposomal uptake study
Cells were seeded in a 96-well plate (5000 cells/well) and allowed to grow for 48 hours. The cells were treated with the different liposomal formulations – empty, C6, curcumin and C6-curcumin. Cells were treated in the range of 4 – 28 µg of curcumin/mL of media. The quantity of C6 liposomes used in the cytotoxicity experiment was equivalent to the quantity of C6-curcumin liposomes (in terms of lipid) for the respective curcumin concentrations. Similarly the empty liposomes were added in proportion to the quantity of curcumin liposomes (in terms of lipid) used in the cell viability experiment. The C6 liposomes and empty liposomes were tested to evaluate the cytotoxicity of C6 and phospholipids, respectively. The concentration of curcumin in C6-curcumin liposome was 25 µg of curcumin/mg of lipid. The percentage of C6 ceramide in both the liposomes (C6 and C6-curcumin) used was 20% by weight. Ceramide being a lipid, participates in the formation of a lipid bilayer structure. Hence all the ceramide used in theliposomal formulation is expected to incorporate into the liposomes (i.e. 100 % loading). Intrinsically, ceramide C6 is insoluble in water, but upon incorporation into liposomes we found no evidence of insoluble precipitates.
Cell viability was estimated after 48 hours of drug treatment using CellTiter 96® Aqueous One Solution cell proliferation assay as per manufacturer’s protocol (Promega Corp. Madison, Wisconsin). Briefly, cells were rinsed with PBS and incubated with 100 µL of DMEM media and 20 µL of CellTiter solution for 2 hours. After 2 hours, the absorbance was measured at 490 nm using a BioTek microplate reader. The mean value and standard deviation for each treatment were determined and then converted values relative to the control. IC50 (50% inhibitory concentration) values were calculated using GraphPad Prism 5.
To visualize the liposomal uptake, KHOS cells were treated with empty liposomes and DiI liposomes in 4 well Nunc Lab-Tek chamber slides (Thermo Scientific, Rochester, New York) for 12 hours. After incubation, cells were rinsed 3 times with cold PBS and fixed with 1.5% glutaraldehyde for 10 minutes. Liposomes are labile to fixatives containing alcohols, and hence such fixatives (formalin or methanol:acetic acid or 100% methanol) are avoided (16). The fixing solution was aspirated and traces of glutaraldehyde were removed by rinsing 3 times with PBS. Cells were then stained with DAPI (4′, 6-diamidino-2-phenylinode) and slides were mounted using a Vectashield mounting medium (H-1000), Vector Laboratories Inc. (Burlingame, California). Slides were observed under a Nikon ECLIPSE 80i fluorescent microscope and images were taken using DAPI/TRITC filter set and merged using IPLab 3.7 software.
Cell cycle assays by flow cytometry and western blot analysis
KHOS cells were grown in a 10 cm dish and treated with five liposomal formulations at 5.5 µg of curcumin/mL (empty, C6, curcumin and C6-curcumin, C6-curcumin-FA) for the period of 12 hours and 24 hours. After drug treatment, the cells were rinsed with PBS and trypsinized. Propidium iodide (PI) staining was performed using a Coulter DNA PREP Reagent kit as per manufacturer’s instructions (Coulter Corporation, Miami, Florida). Briefly, 3 × 105 cells were suspended in 100 µL of PBS and 100 µL of Reagent 1 (RNase and detergent) was added. After 1 minute of vigorous mixing, 1 mL of Reagent 2 (PI) was added, vortexed and incubated in the dark for 1 hour. Samples were run on Beckman Coulter EPICS FC500 flow cytometer using CXP software and modeling software by Variety software house, ModFit version 3.2.
A second set of liposome treated KHOS cells was used for western blot analysis. After drug treatment, the cells were lysed in RIPA buffer (Cell Signaling Technology, Danvers, Massachusetts). After centrifugation, protein in the supernatant was estimated using a Bicinchoninic acid assay kit (Thermo Scientific, Rochester, New York) and subjected to western blot analysis. Proteins were separated through SDS-PAGE using 4–12% NuPAGE from Invitrogen. After electrophoresis, the separated proteins were transferred to a PVDF membrane (GenHunter Corp, Nashville, Tennessee). The PVDF membrane was blocked overnight with 5% nonfat milk (Blotto, SantaCruz Biotech Inc. Santa Cruz, California) and incubated with primary antibody overnight at 4°C. The primary antibodies against cyclin D1and cyclin B1 were tested (Santa Cruz Biotechnology Inc. Santa Cruz, California). The caspase cascade induction after liposome treatment was examined by the expression of Poly (ADP-Ribose) polymerase (PARP), caspase 7, 9 and their respective cleaved forms (Cell Signaling Technology, Danvers, Massachusetts). After overnight incubation in the primary antibody, the membrane was probed with HRP-conjugated rabbit or mouse secondary antibody (Abcam, Cambridge, Massachussets) and the membranes were incubated with ECL western blotting substrate and exposed on CL-Xposure films (Thermo Scientific, Rochester, New York). Films were revealed using a Kodak M35-A X-OMAT processor.
Immunocytochemical analysis of Phospho-cyclin B1
The KHOS cells were plated in 8-chamber slides (Nunc) and allowed to grow up to 80 % confluency. Then cells were treated with empty, C6, curcumin and C6-curcumin liposomes for 24 h (5 µg/ml of curcumin or equivalent of lipids). Slides were fixed in 4% paraformaldehyde and permeabilized with 0.25% Triton X-100 in PBS for 10 min. After 3 washes in PBS + 0.1% BSA, cells were treated overnight with blocking solution (0.05% Tween 20, 1% BSA and 20% goat serum, Sigma). Then cells were incubated overnight at 4°C with phospho-Ser147 Cyclin B1 antibody (Cell SignallingTechnologies, cat# 4131) 1:100 in PBS + 1% BSA. After three washes, cells were incubated 1 hour with goat anti-rabbit AlexaFluor 555 1:250 (Invitrogen, cat# A-21428) and slides mounted in Supermount (Biogenex) + Hoescht 33342 (0.5 µg/mL, Invitrogen) and observed under Nikon Eclipse 80i with NIS-Element software version 3.22.11.
In vivo study using osteosarcoma xenograft model
The in vivo study was approved by the Institutional Animal Care and Use Committee of Tulane University. Mice were regularly monitored over the period of the experiment. GFP-expressing KHOS cells (Refer to supplementary data for generation of GFP-expressing KHOS) were cultured in vitro as described above and prepared for injection in Hank's buffered saline solution. One million cells were injected subcutaneously into immunodeficient nude mice (nu/nu strain, five mice per group) from Charles River Laboratories Inc. (Wilmington, Massachusetts). The tumors were allowed to develop on the posterolateral side of the mice for one week prior to treatment. Mice were randomly assigned to empty liposome treated and C6-curcumin-FA liposome treated group. Mice were treated with empty pegylated liposomes and C6-curcumin-FA liposomes. Liposomes (equivalent to 40 µg of curcumin) were injected intraperitoneally every 48 hours over the period of 2 weeks. Tumor sizes were measured on the day of treatment using a Vernier caliper and tumor volume was calculated using the formula (17) - V = (4/3)πa2b where a = shorter radius in mm and b = longer radius in mm. Mice were euthanized following veterinary advisory protocol at the end of 3 weeks. Harvested tumors were analyzed for histopathology using hematoxylin and eosin staining. Images were taken by Nikon DS-Fi1 microscope using NIS-Elements BR 3.0 software. Tumor inhibition data was analyzed by two tailed unpaired Student’s t-test and P values < 0.05 were considered significant. Error bars represent mean ± SD.
Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay on tumor sections
The TUNEL assay for apoptosis detection was performed using an in situ cell death detection kit following the protocol as per the manufacturer’s instructions (Roche Diagnostics Corp. Indianapolis, Indiana). Paraffin-embedded tumor sections were dewaxed using xylene and hydrated by incubating in decreasing concentrations of ethanol (100%, 95%, 80%, 75% and 50%) for a period of 2 minutes at each concentration. The slides were then rinsed with distilled water and PBS. Cell permeabilization was performed by exposing the slides in a Reveal Decloaker (Biocare Medical, Concord, California) to steam for 10 minutes using a steam heat electronic steamer. The specimens were then blocked for 30 minutes at room temperature in a 3% BSA solution (Bovine Serum Albumin). Following this, the tumor sections were incubated with a labeling mixture (enzyme + labeling solution) for 1 hour at 37°C in a humidified chamber. Endogenous peroxidases were quenched by incubating the slides in a 0.3% hydrogen peroxide solution for 2 minutes. After rinsing with PBS, the specimens were incubated with anti-FITC-horse-radish peroxidase for 30 minutes followed by reaction with substrate DAB (3, 3′-diaminobenzidine). The slides were mounted with Permount mounting media and images obtained using a Nikon DS-Fi1 microscope.
Results
The combined cytotoxic effects of curcumin and C6
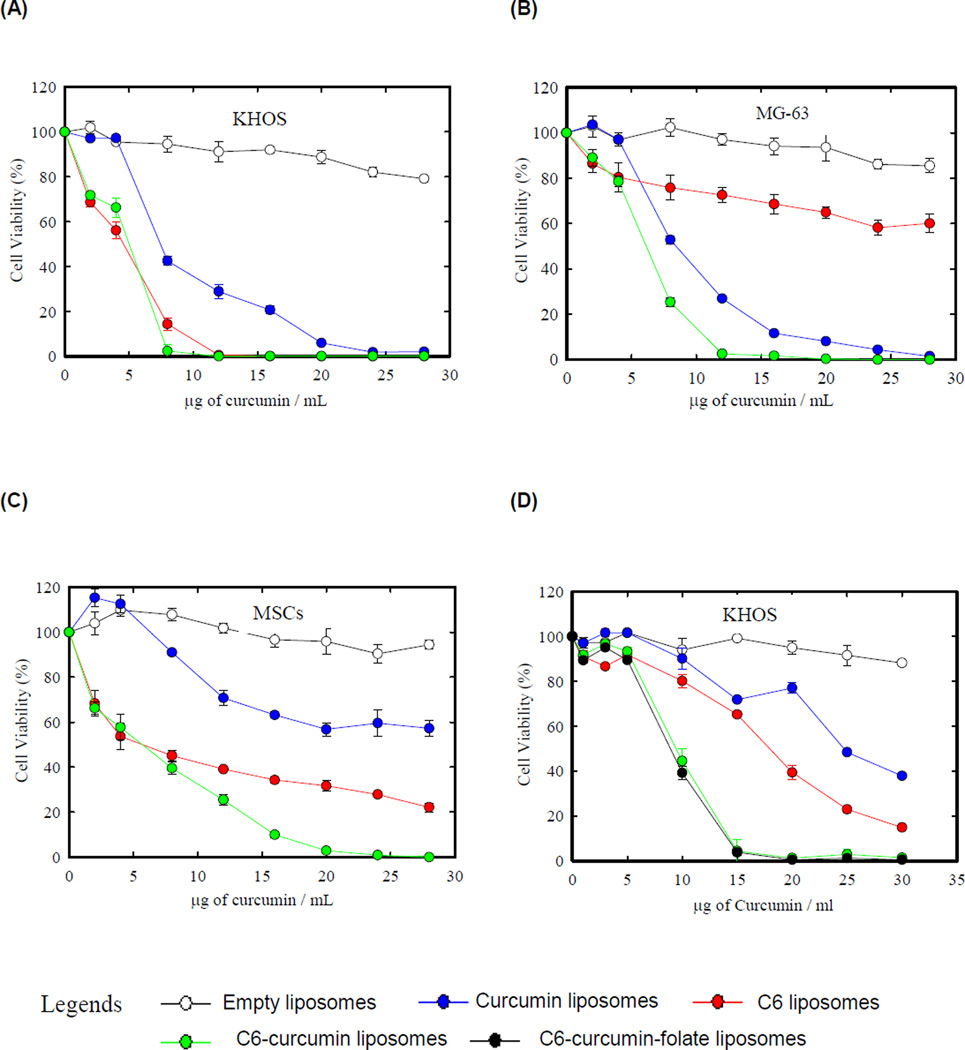
The cytotoxicity study was performed to evaluate the combined effect of both the drugs. Two osteosarcoma cell lines KHOS, MG-63 and untransformed human mesenchymal stem cells (MSCs) were treated with four liposomal formulations – empty, curcumin, C6, and C6-curcumin. Human MSCs were evaluated for toxicity of liposomes on untransformed healthy cells in human body. Figure 1 shows the profile of cell viability against each formulation after 48 hours of treatment. Table 1 shows the IC50 values of curcumin and C6-curcumin formulation against both the osteosarcoma cell lines. The KHOS cell line was similarly sensitive to C6 liposomes and to C6-curcumin liposomes (Fig. 1A). Figure 1B shows that C6-curcumin has a greater cytotoxicity against MG-63 in comparison to C6 or curcumin liposomes alone. All three cells lines show varying sensitivities against the three liposomal formulations. KHOS is 1.5 times more sensitive to C6 and C6-curcumin liposomes than curcumin liposomes alone. MG-63 showed resistance to C6 in used concentration range whereas MSCs were resistant to curcumin. The chemoresistance of MG-63 to C6 Ceramide formulation might be due to the overexpression of ceramide metabolizing enzymes in MG-63 cells as reported earlier in ceramide resistant cell lines (18). The chemoresistance of MSCs to curcumin can be addressed by curcumin’s ability to induce apoptosis in highly proliferating cells. It has been reported that anti-cancer dose of curcumin arrests non-malignant cells in G0 phase reversibly but does not induce apoptosis in them (19). MSCs may be more sensitive to curcumin at higher concentration than used in the present study. The growth rate of the cancer cell line is one of the major parameters that determines the uptake of liposomes (20) and the efficacy of the drug. Additionally, cancer cells undergo multiple mutational bursts and therefore their sensitivity depends on the alteration of the drug target by mutation, deletion or overexpression (21). KHOS and MG-63 have different growth rates and may have mutations in the drug targeting pathway due to which they show differences in sensitivities to curcumin and to C6. All cell lines are sensitive to C6-curcumin liposomes when compared to C6 or curcumin liposomes alone, but interestingly the MSCs show a greater resistance at higher concentration to the C6-curcumin formulation, providing a sufficient therapeutic window for systemic delivery with lowered toxicity (Fig. 1C).
Figure 1.
Curcumin and C6 ceramide shows combined anticancer effect. (A, B & C) Osteosarcoma cell lines (KHOS and MG-63) and human mesenchymal stem cells were treated with four liposomal formulations for 48 hours: curcumin, C6 and C6-curcumin liposomes. Empty liposomes were used as control. The amount of C6 liposomes and empty liposomes used during experiment was equivalent to the quantity of C6-curcumin and curcumin liposomes (in terms of lipid) for the respective curcumin concentration. (D) Effect of pegylated formulations – All the forumulations used were pegylated for this experiement. (E) Liposomal uptake was visualized by fluorescence microscopy. KHOS cells were incubated with empty liposomes and DiI liposomes for 12 hours. Empty liposomes 12 hours (a, b, & c), DiI liposomes 6 hours (d, e & f), DiI liposomes 12 hours (g, h & i). All scale bars represent 100 µm.
Table 1.
IC50 (in µg of curcumin/mL) value for liposomal formulations against KHOS and MG-63.
| Formulation | KHOS | MG-63 |
|---|---|---|
| Curcumin liposomes | 6.36 ± 2.6 | 8.62 ± 0.3 |
| C6-curcumin liposomes | 4.20 ± 1.1 | 5.69 ± 0.3 |
Pegylated formulations were tested both in vitro and in vivo. Figure 1D shows the cytotoxicity profile of pegylated formulations against KHOS. When comparing the effect of nonpegylated formulation (Fig. 1A) to the pegylated formulation, a minor shift in the cytotoxicity profile towards higher drug concentration was observed. This can be explained by the reduced cell interaction of the pegylated formulations as noted in various earlier reports (22). However, this property may reduce nonspecific interactions with human cells in vivo and enhance circulation half-life to allow a sufficient time interval for the formulation to accumulate peritumorally. The data on optimization of C6 wt% during C6-curcumin formulation is shown in the supporting information section (Supplementary data Fig.S1).
Visualization of liposomal uptake
The visualization of liposomes in the KHOS system was performed using a DiI fluorescent marker. Although curcumin is a fluorescent molecule, we have observed that it is susceptible to photobleaching and its fluorescence at the cellular level is very weak for intracellular visualization. DiI is a lipophilic dye with an orange-red fluorescence and liposomal encapsulation properties similar to curcumin. KHOS cells were treated with nonfluorescent empty liposomes and fluorescent DiI liposomes for the period of 12 hours. Panels (a, d, g) and Panels (b, e, h) in Figure 1E illustrate images taken using DAPI and TRITC filters, respectively. To show co-localization with the nucleus, the respective DAPI/TRITC images were merged as shown in Panels (c, f, i). Figure 1E (Panel c) shows empty liposome treated cells for 12 hours where only blue nuclei can be seen. Panel (f) shows a red fluorescence surrounding the nucleus indicating the uptake of DiI liposomes. The fluorescence intensity of DiI was found to increase (Panel (i)) indicating an increased uptake of liposomes at 12 hours in comparison to the uptake in 6 hours. The mixed population of cells in G1 and S phase may be the reason for various sizes of nuclei in all the groups. KHOS is a fast growing osteosarcoma cell line and it can be seen from the cell cycle data (Table S1 & S2) that at a specific time point (12h or 24 h), ~ 42 % of the population is in the G1 phase and ~ 54 % population is in the S phase. Due to DNA synthesis, cells in the S phase are larger than cells in G1 phase.
Characterization of liposomal formulation
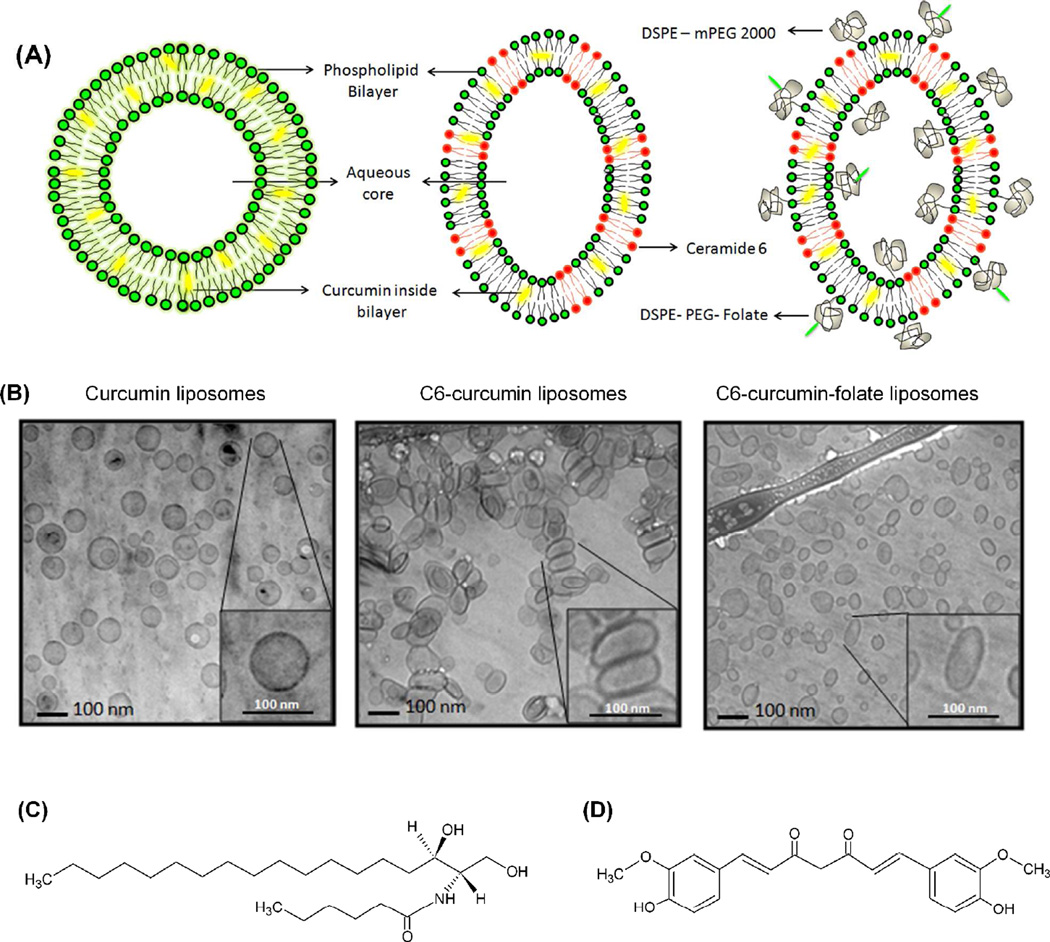
Various ceramide species (along with C6) have been reported to influence the shape of vesicles/cell (23–26). Hence, the morphological characterization of C6 containing vesicles is important. Figure 2A is a schematic representation of liposomal formulations with curcumin in the lipid bilayer and PEG on the surface of liposome. The cryo-TEM images in Figure 2B show spherical morphology of curcumin liposomes. On the other hand, C6-curcumin liposomes were seen to elongate and form ellipsoidal/oblong shapes along with a population of spherical liposomes. The tendency of liposomes to elongate can be attributed to the property of ceramide to form a rigid bilayer of lower curvature (23–24, 27). The chemical structures of C6 and curcumin has been given in Figure 2C&D respectively.
Figure 2.
(A) Schematic representation of curcumin liposomes, C6-curcumin liposomes and C6-curcumin-Folate tagged stealth liposomes (B) Corresponding cryo-TEM images of the three liposomal formulations (a, b & c respectively) (C & D) Chemical structures of C6 ceramide and curcumin respectively.
The zeta potential of liposomes is an indication of its surface charge, colloidal stability and interaction with cells (12, 28). Table 2 lists the zeta potential values for all liposomal formulations used in our studies. Nonpegylated formulations show a higher zeta potential when compared to pegylated formulations. From the data, it is evident that nonpegylated liposomes have a negative charge in the range of −17 mV to −22 mV. This charge is imparted by the negatively charged phospholipid, DMPG. On the other hand, the zeta potential of pegylated formulations decreased to −2 to −3 mV. This decrease in surface charge justifies the charge shielding effect of PEG (29). Studies on pegylated liposomes have reported that 4 mol% of DSPE-PEG2000 forms a mushroom-like surface cover over the liposomes (14). While pegylation appears to decrease electrostatic repulsion between liposomes, the steric barriers created do not allow the liposomes to approach within 2–3 nm of each other (30). This prevents liposome fusion/aggregation and improves colloidal stability.
Table 2.
Zeta potential of nonpegylated and pegylated liposomal formulations
| Formulation | Zeta Potential (mV) |
|---|---|
| Empty liposomes | −20.9 ± 2.3 |
| Curcumin liposomes | −17.6 ± 0.2 |
| C6-curcumin liposomes | −22.8 ± 1.4 |
| C6 liposomes | −17.2 ± 0.8 |
| Pegylated empty liposomes | −2.3 ± 0.7 |
| Pegylated C6-curcumin-FA liposomes | −3.1 ± 0.2 |
The size distribution of the curcumin liposomes (spherical liposomes) has been reported in our previous publication (11). In the case of C6 and C6-curcumin liposomes, a change in shape was observed. As shown in cryo-TEM images C6 containing liposomes show both an elongated population and spherical population. During the formulation study, we attempted to make a homogeneous population of C6 containing liposomes. However, an increase in C6 in lipid bilayer after certain amount destabilizes the liposome bilayer structure. The hydrodynamic radius measured by dynamic light scattering or any other means will only give an approximate idea about the polydispersity index due to difference in the shape of vesicles. Therefore, cryo-TEM is a preferred technique to analyze the shape of vesicles and accurate evaluation of polydispersity in the sample. We have calculated the aspersity (ratio of major axis to minor axis) of C6 containing formulations (1.51 ± 0.5) and spherical liposomes (1.01 ± 0.023) using ImageJ 1.45s. Five different images of liposomal population were measured for each formulation.
Tumor reduction potential of C6-curcumin-FA liposomes in human OS xenograft model
The in vivo study was performed with the most potent formulation (C6-curcumin liposome) screened during the in vitro study. To make the best use of the encapsulated drug in a vesicle, two surface modifications were performed – (i) liposomal formulations were pegylated to enhance the circulation time and hence the accessibility of liposomes to tumor cells (i.e. passive targeting) (ii) liposomes were folate tagged for active targeting of C6-curcumin liposomes to the tumor. Recent reports suggest that the folate receptor α is overexpressed in multiple cancers including osteosarcoma (10, 31). This surface receptor is overexpressed in an adverse nutritional environment as seen in solid tumors to acquire folic acid (Vitamin B9) for DNA synthesis and cell proliferation (32). Recently, the folate receptor α has been explored for delivery of therapeutics and in diagnostics for imaging (33).
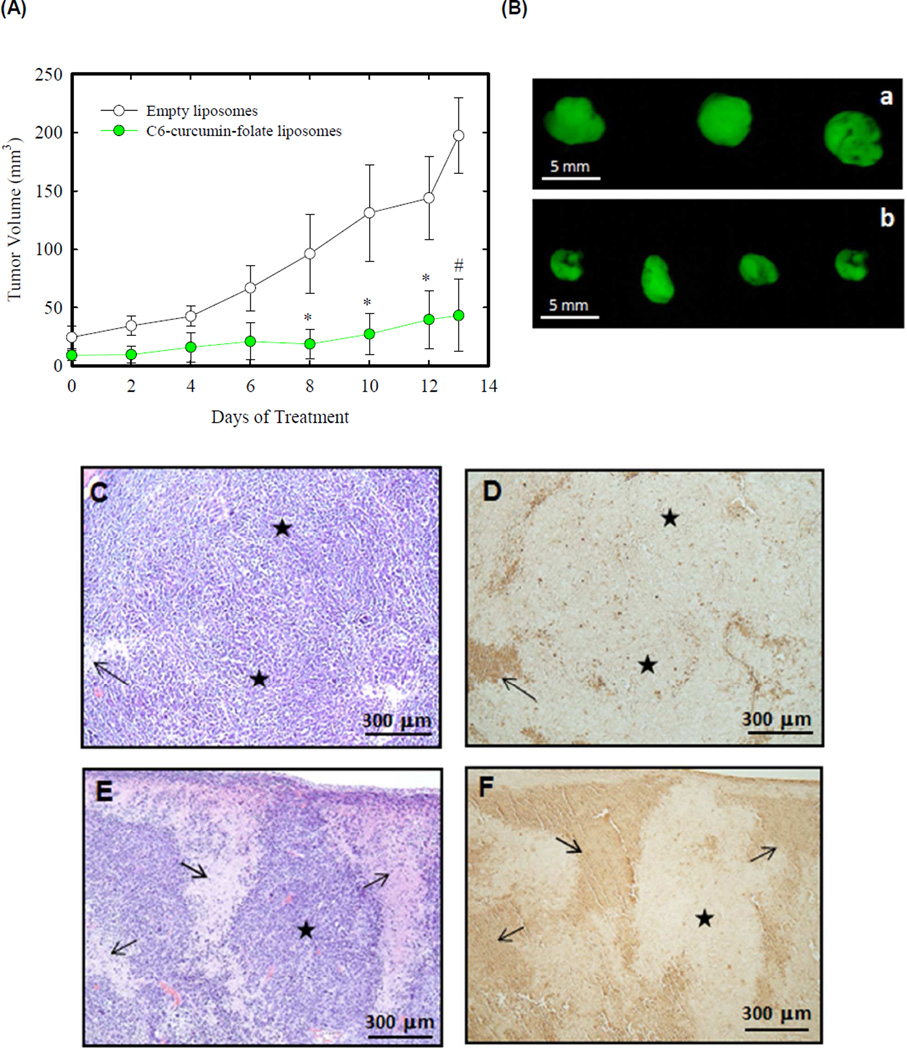
GFP-expressing KHOS cells were cultured and prepared as described in methods section. One million cells were injected subcutaneously into immunodeficient nude mice and tumors were allowed to develop on the posterolateral side of the mice for one week prior to treatment. Mice were grouped into 5 per group and treated intraperitoneally with C6-curcumin-FA liposomes and control tumors were treated with empty liposomes. Treatment was started after 7 days of tumor growth. Figure 3A shows the growth profile of the empty liposome treated tumor and the C6-curcumin-FA liposome treated tumor over a treatment period of 14 days. Mice were sacrificed immediately after a treatment period of 14 days and the tumors were harvested. Figure 3A shows that the tumor growth was inhibited significantly by C6-curcumin-FA liposomes. In this observation, C6-curcumin-FA liposome treated mice were healthier and more energetic as compared to the control mice at the end of 3 weeks. We used fluorescence from GFP to visualize tumor size after necropsy and the tumor reduction potential of C6-curcumin-FA liposomes can be observed from Figure 3B.
Figure 3.
C6-curcumin-Folate liposomes inhibit tumor growth and induce apoptosis in KHOS xenografts. (A) Human osteosarcoma xenograft growth profile: KHOS xenografts were obtained by subcutaneous injection in nude mice (five mice per group). Tumors were allowed to grow for 1 week and mice were treated intraperitoneally with liposomes (equivalent to 40 µg of curcumin) every 48 hours for 2 weeks. * indicates p < 0.05 and # indicates p < 0.001 (B) Representative images of KHOS-GFP tumors in each group - a: Empty liposomes, b: C6-curcumin-FA liposomes. (C&D) Empty liposome treated tumor sections and (E&F) C6-curcumin-FA liposome treated tumor sections. Arrow indicates (↑) area of dead cells for H&E images (C&E) and apoptotic area for TUNEL images (D&F). The star symbol (★) indicates proliferative area in all the images.
In vivo apoptosis detection by TUNEL assay
The TUNEL assay detects DNA fragmentation which is the hallmark of apoptosis. Terminal deoxynucleotidyl transferase adds dUTP specifically to the broken ends of the DNA present in the apoptotic cells (34). Tumor sections were also stained for hematoxylin-eosin staining for histopathological analysis. Figure 3 shows hematoxylin-eosin (H&E) staining and TUNEL assay on treated tumor sections. Empty liposome treated tumors (Fig. 3C) show less necrotic/apoptotic areas whereas areas of dead cells are significant in tumors treated with C6-curcumin-FA liposomes (Fig. 3E). The dark brown areas as shown by the arrow in Figure 3D and F indicate the apoptotic region corresponding to the dead cell area in H&E. The C6-curcumin-FA liposome treated tumor shows more apoptosis as compared to tumors treated with the empty liposomes.
Cytostatic effect and caspase cascade induction by various liposomal formulations
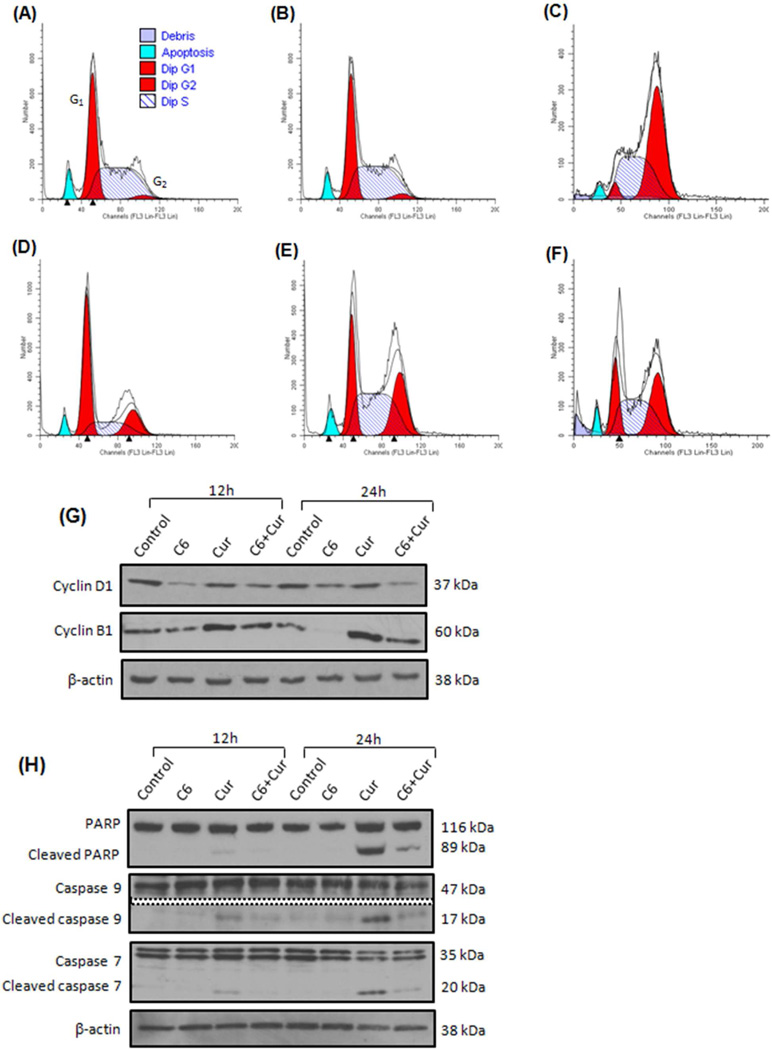
Anticancer drugs have been reported to regulate proteins required for cell cycle progression (35). Different classes of chemotherapeutic drugs have the ability to block specific phases of the cell cycle (36). To study the effects of C6 and curcumin on the KHOS cell cycle, cells were treated with five liposomal formulations (empty, C6, curcumin, C6-curcumin and C6-curcumin-FA) and cell cycle arrest was detected by propidium iodide staining using flow cytometry. Table S1 & S2 in supplementary data lists the population at each stage of cell cycle (G0–G1/S/G2-M). Figure 4 shows the cell cycle profiles of KHOS after liposomal treatment for 12 hours. Both untreated cells and empty liposome treated cells (Fig. 4A and B) follow a similar cell cycle pattern, indicating no effect of phospholipid alone on cell toxicity. Curcumin liposome treated cells show G2/M accumulation (Fig. 4C) whereas C6 liposomes induce G1 arrest in KHOS (Fig. 4D) and the population arrested was found to increase from 12 hours to 24 hours (Supplementary data Table S1 & S2). C6-curcumin liposomes and C6-curcumin-FA liposomes show G2/M arrest (Fig. 4E and F) and the population in the S phase at 24 hours was found to increase in comparison to the corresponding values with cells treated independently with curcumin or C6. The cell cycle data depicts the different molecular targets for curcumin and C6 because of the stage of the cells that are arrested in the G2/M and G1 phase, respectively.
Figure 4.
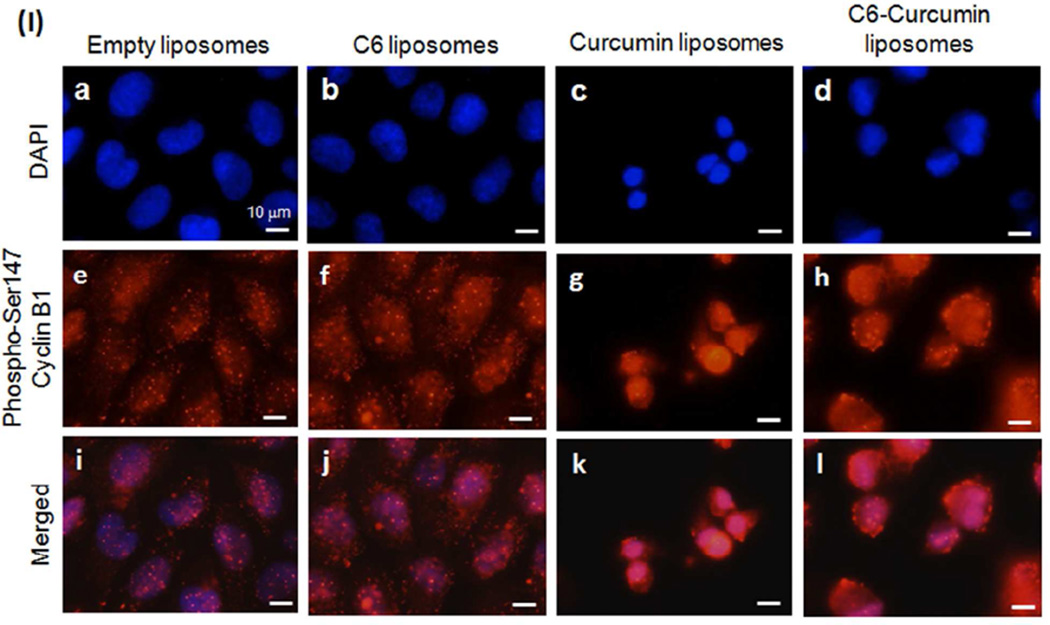
C6-curcumin induces growth arrest in KHOS cells. Cells were treated with five liposomal formulations for 12 hours (A) Untreated (B) Empty liposomes (C) Curcumin liposomes (D) C6 liposomes (E) C6-curcumin liposomes (F) C6-curcumin-Folate liposomes. In cell cycle profile, x-axis represents propidium iodide intensity and y-axis represents number of counts. Numeric data are presented in Supplementary data Table S1 & S2. In vitro treatment of KHOS with C6-curcumin induces both growth arrest and activates apoptotic cascade. Western blot analysis - The KHOS cells were treated with various liposomal formulations for 12 hours and 24 hours. The cell lysate was analyzed for the expression of (G) Cell cycle proteins and (H) Proteins in the caspase cascade pathway. (I) KHOS cells were treated with various liposomal formulations for 24 hours and immunostained to detect expression and localization of Phospho-cyclin B1. All scale bars represent 10 µm.
To determine the cell cycle proteins involved in G1/S and G2/M arrest, expression levels of cyclin D1 and cyclin B1 were analyzed by immunoblot. Cyclin D1 forms a complex with cdk4 and gets transported into the nucleus where it plays an important role in the G1/S transition (37). In the nucleus, it inactivates the retinoblastoma (Rb) protein by phosphorylation and thus facilitates G1/S transition. From Figure 4G, it can be seen that cyclin D1 is downregulated in cells treated with the ceramide containing formulation. This decrease in cyclin D1 levels can be one of the molecular events responsible for G1/S arrest. Curcumin treated cells show no change in the cyclin D1 expression level. This indicates that curcumin treated cells transit normally through the G1/S phase which is in agreement with cell cycle study (Figure 4C). In some reports (38–39), no change in the expression of cyclin D1 was seen after curcumin treatment in cyclin D1 deregulated cells, and cells where cyclin D1 is overexpressed by genetic alterations. A similar mechanism may be the cause in present study where no change in the expression of curcumin treated cells was seen.
Another cell cycle protein, Cyclin B1 is associated with cdk1 to carry out the G2/M transition in the cell cycle. The phosphorylation of cyclin B1 in the cytoplasm drives its translocation into the nucleus along with cdk1 (40). This complex carries out multiple events in mitosis such as chromosome condensation and nuclear breakdown. Western blot assays show an increase in cyclin B1 levels in cells treated with curcumin containing formulation. In the case of C6 liposome treated cells, the cyclin B1 level decreases presumably because cells are arrested in the G1 phase over time. KHOS cells treated with C6-curcumin liposomes show a combined effect of C6 and curcumin in the expression level of cyclin D1 and B1.
The expression of cleaved forms of caspase 7, 9 and PARP indicates caspase dependent cell death (41). It can be seen from Figure 4H that curcumin containing formulations, either curcumin or C6-curcumin liposomes, show expression of these markers in caspase dependent cell death. Caspase cleavage was not observed in C6 liposome treated cells indicating caspase independent cell death.
From cell cycle study and western blot it is evident that cells treated with curcumin liposomes are getting arrested in G2/M phase in spite of increase in cyclin B1 levels. Accordingly, we conducted immunocytochemical assays to confirm the expression of Phospho-cyclin B1 and its cytoplasmic/nuclear localization that has a role in G2/M arrest. Figure 4I, shows cells treated with curcumin & C6-curcumin formulations show nuclear localization of Phospho-cyclin B1. On the contrary, these formulations are cause G2/M arrest and apoptotic cell death simultaneously. This shows curcumin induced mitotic catastrophe (cell death during mitotsis) in KHOS. Previously, Subramaniam et al. (40) reported curcumin induced mitotic catastrophe with increase in expression and nuclear localization of cyclin B1.
Discussion
In the era of multi-agent drug therapy, several different drug combinations have been reported over the last decade. The importance of multi-agent therapy over single-agent therapy is increasingly being considered for the treatment of high grade OS. The single-drug regimen is susceptible to mutational bursts occurring in cancer cells that favor gene mutations in the drug targeting pathway. Indeed, the low survival rate (≤ 20%) of patients with metastatic osteosarcoma has been attributed to chemoresistance (42). Cancer is a complex multifactorial disease with changing nature at every stage, bringing up the need to use a multi-targeted, multi-agent therapy that will be less susceptible to mutational bursts. In the present work, we have reported a combination of curcumin and C6 in liposomal formulation. Although ceramide has been shown to be a potent anticancer drug, it has been found to be associated with drug resistance in cancer cells overexpressing glycosylceramide synthase. Such cells upregulate enzymes involved in ceramide metabolism pathway and thereby escape ceramide-induced apoptosis (18). However, curcumin has shown multidrug resistance (MDR) reversal potential in many MDR cancer cells by decreasing P-glycoprotein function and expression (43). Additionally, curcumin has been reported to induce caspase dependent as well as independent apoptosis displaying its multi-targeted nature (44). Curcumin is increasingly being advised in dietary supplements as a cancer preventive measure and has a long history of medicinal value throughout the Indian subcontinent. Hence coupling C6 and curcumin may represent an ideal combination against a heterogeneous population of cells within the solid OS tumor. Our in vitro and in vivo data also confirms the importance of this combination.
Curcumin and C6 have shown promising anticancer potential individually in various types of cancer, but their hydrophobic nature limits cellular uptake. Although C6 is a cell permeable analogue, the structure of C6 retains two aliphatic chains that appear to limit intercalation into the plasma membrane (45). Hence liposomal encapsulation confers an easy systemic delivery of two water insoluble drugs, and also offers a protective shield against enzymatic degradation of the drug in blood circulation and within the cell. Additionally, based on our earlier results (11), liposomal curcumin is 4–5 time more effective than curcumin as a pure compound against osteosarcoma. The IC50 for DMSO-curcumin is 22.8 µg/ml and the IC50 for liposomal-curcumin is 5.4 µg/ml. Nonpegylated liposomes can reduce the drug metabolism/clearance to some extent but the reticuloendothelial system (RES) still plays a major role in decreasing the circulation half-life of liposomal nanoparticles (46). The pegylation of liposome has been studied extensively over the last 2–3 decades and has now become a standard step in the preparation of liposomes for in vivo use to improve circulation half-life (14). It extends the plasma half-life by sterically inhibiting the adsorption of various blood components/proteins on the surface of liposomes thereby reducing liposomal uptake by RES (47). Due to the leaky vasculature of tumors, long circulating stealth liposomes accumulate near the tumor and show passive targeting (48). Our in vitro study (Fig. 1C) has shown that C6-curcumin liposomes are less toxic on untransformed human cells. For better targeting effect and to enhance the drug efficacy, we have used folate targeting on the liposomes to broaden the therapeutic application of C6-curcumin formulation.
C6 has been evaluated in cutaneous breast cancer patients for Phase II clinical trial (49). Although C6 has not been fully explored in cancers at the clinical level, curcumin has already shown promising anticancer effects in clinical trials against various cancers (50). Our findings about the tumor reduction potential of the C6-curcumin combination pave the way for further evaluation at the clinical level against specific drug resistant cancers.
Supplementary Material
Acknowledgement
We thank Professor Brian Rowan for allowing us to use their cell culture facility and Murali Anabalagan for technical assistance. The authors thank Hope Luebbert for assistance with animal handling during in vivo study.
Funding Sources
This research was supported by the Louisiana Cancer Research Consortium, the Louisiana Gene Therapy Research Consortium and the National Institute of Health (Grant - CA151851) for R. Pochampally, and V. John gratefully acknowledges support from the Department of Defense, Grant: W81XWH-10-1-0377.
Footnotes
Supporting Information Available
This section encompasses detailed information on (i) C6 ceramide wt% optimization (ii) Cell cycle arrest data and (iii) Generation of GFP expressing KHOS. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res. 2010;5:78. doi: 10.1186/1749-799X-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuczek T, Axelrod DE. Tumor cell heterogeneity: divided-colony assay for measuring drug response. Proc Natl Acad Sci. 1987;84:4490–4494. doi: 10.1073/pnas.84.13.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the italian and scandinavian sarcoma groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 4.Moriceau G, Ory B, Mitrofan L, Charrier C, Brion R, Pilet P, et al. 7.P.51 Synergistic inhibition of osteosarcoma cell growth by combination of RAD001 (everolimus) and zoledronic acid. J Bone Joint Surg Br. 2010;92-B:475. [Google Scholar]
- 5.Horie N, Murata H, Kimura S, Takeshita H, Sakabe T, Matsui T, et al. Combined effects of a third-generation bisphosphonate, zoledronic acid with other anticancer agents against murine osteosarcoma. Br J Cancer. 2007;96:255–261. doi: 10.1038/sj.bjc.6603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–E449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52:1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 8.Yu T, Li J, Sun H. C6 ceramide potentiates curcumin-induced cell death and apoptosis in melanoma cell lines in vitro. Cancer Chemother Pharmacol. 2010;66:999–1003. doi: 10.1007/s00280-010-1374-1. [DOI] [PubMed] [Google Scholar]
- 9.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11:3465–3474. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 10.Yang R, Kolb EA, Qin J, Chou A, Sowers R, Hoang B, et al. The folate receptor α is frequently overexpressed in osteosarcoma samples and plays a role in the uptake of the physiologic substrate 5-methyltetrahydrofolate. Clin Cancer Res. 2007;13:2557–2567. doi: 10.1158/1078-0432.CCR-06-1343. [DOI] [PubMed] [Google Scholar]
- 11.Dhule SS, Penfornis P, Frazier T, Walker R, Feldman J, Tan G, et al. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed Nanotech. 2012;8:440–451. doi: 10.1016/j.nano.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhankumar AB, Slagle-Webb B, Wang X, Yang QX, Antonetti DA, Miller PA, et al. Efficacy of interleukin-13 receptor–targeted liposomal doxorubicin in the intracranial brain tumor model. Mol Cancer Ther. 2009;8:648–654. doi: 10.1158/1535-7163.MCT-08-0853. [DOI] [PubMed] [Google Scholar]
- 13.Chiu SJ, Marcucci G, Lee RJ. Efficient delivery of an antisense oligodeoxyribonucleotide formulated in folate receptor-targeted liposomes. Anticancer Res. 2006;26:1049–1056. [PubMed] [Google Scholar]
- 14.Allen C, Dos Santos N, Gallagher R, Chiu GN, Shu Y, Li WM, et al. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol) Biosci Rep. 2002;22:225–250. doi: 10.1023/a:1020186505848. [DOI] [PubMed] [Google Scholar]
- 15.Tagami T, Ernsting MJ, Li SD. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J Control Release. 2011;152:303–309. doi: 10.1016/j.jconrel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Truneh A, Machy P, Horan PK. Antibody-bearing liposomes as multicolor immunofluorescence markers for flow cytometry and imaging. J Immunol Methods. 1987;100:59–71. doi: 10.1016/0022-1759(87)90173-6. [DOI] [PubMed] [Google Scholar]
- 17.Richtig E, Langmann G, Mullner K, Richtig G, Smolle J. Calculated tumour volume as a prognostic parameter for survival in choroidal melanomas. Eye. 2004;18:619–623. doi: 10.1038/sj.eye.6700720. [DOI] [PubMed] [Google Scholar]
- 18.Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, et al. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 2008;22:2541–2551. doi: 10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- 19.Sa G, Das T. Anti cancer effects of curcumin: cycle of life and death. Cell Division. 2008;3:14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Sánchez R, Rodríguez-Enríquez S, Marin-Hernández A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 21.Foo J, Michor F. Evolution of resistance to targeted anti-cancer therapies during continuous and pulsed administration strategies. PLoS Comput Biol. 2009;5(11):e1000557. doi: 10.1371/journal.pcbi.1000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller CR, Bondurant B, McLean SD, McGovern KA, O'Brien DF. Liposome–cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 23.Xu P, Tan G, Zhou J, He J, Lawson LB, McPherson GL, et al. Undulating Tubular Liposomes through Incorporation of a Synthetic Skin Ceramide into Phospholipid Bilayers. Langmuir. 2009;25:10422–10425. doi: 10.1021/la9010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-Montero I, Rodriguez N, Cribier S, Pohl A, Vélez M, Devaux PF. Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem. 2005;280:25811–25819. doi: 10.1074/jbc.M412052200. [DOI] [PubMed] [Google Scholar]
- 25.López-Montero I, Vélez M, Devaux PF. Surface tension induced by sphingomyelin to ceramide conversion in lipid membranes. BBA – Biomembranes. 2007;1768:553–561. doi: 10.1016/j.bbamem.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Glombitza B, Müller-Goymann CC. Influence of different ceramides on the structure of in vitro model lipid systems of the stratum corneum lipid matrix. Chem Phys Lipids. 2002;117:29–44. doi: 10.1016/s0009-3084(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 27.Bollinger CR, Teichgräber V, Gulbins E. Ceramide-enriched membrane domains. BBA-Mol Cell Res. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Holovati JL, Gyongyossy-Issa MIC, Acker JP. Effect of liposome charge and composition on the delivery of trehalose into red blood cells. Cell Preserv Technol. 2008;6:207–218. [Google Scholar]
- 29.Bruno K. Using drug-excipient interactions for siRNA delivery. Adv Drug Deliver Rev. 2011;63:1210–1226. doi: 10.1016/j.addr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland JW, Hui C, Cullis PR, Madden TD. Poly(ethylene glycol)–lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry. 1996;35:2618–2624. doi: 10.1021/bi952000v. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliver Rev. 2002;54:675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 32.Antony AC. Folate Receptors. Annu Rev Nutr. 1996;16:501–521. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- 33.Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliver Rev. 2004;56:1205–1217. doi: 10.1016/j.addr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Jia W, Hegde VL, Singh NP, Sisco D, Grant S, Nagarkatti M, et al. Δ9-Tetrahydrocannabinol-induced apoptosis in jurkat leukemia T cells is regulated by translocation of Bad to mitochondria. Mol Cancer Res. 2006;4:549–562. doi: 10.1158/1541-7786.MCR-05-0193. [DOI] [PubMed] [Google Scholar]
- 35.Cai D, Latham VM, Zhang X, Shapiro GI. Combined Depletion of Cell Cycle and Transcriptional Cyclin-Dependent Kinase Activities Induces Apoptosis in Cancer Cells. Cancer Res. 2006;66:9270–9280. doi: 10.1158/0008-5472.CAN-06-1758. [DOI] [PubMed] [Google Scholar]
- 36.Shah MA, Schwartz GK. Cell cycle-mediated drug resistance. Clin Cancer Res. 2001;7:2168–2181. [PubMed] [Google Scholar]
- 37.Alao J. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 39.Chuang SE, Cheng AL, Lin JK, Kuo ML. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem Toxicol. 2000;38:991–995. doi: 10.1016/s0278-6915(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 40.Subramaniam D, Ramalingam S, Linehan DC, Dieckgraefe BK, Postier RG, Houchen CW, et al. RNA Binding Protein CUGBP2/CELF2 Mediates Curcumin-Induced Mitotic Catastrophe of Pancreatic Cancer Cells. PLoS One. 2011;6:e16958. doi: 10.1371/journal.pone.0016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang H, Salinas RA, Leal BZ, Kosakowska-Cholody T, Michejda CJ, Waters SJ, et al. Caspase-mediated apoptosis and caspase-independent cell death induced by irofulven in prostate cancer cells. Mol Cancer Ther. 2004;3:1385–1396. [PubMed] [Google Scholar]
- 42.Walters DK, Steinmann P, Langsam B, Schmutz S, Born W, Fuchs B. Identification of Potential Chemoresistance Genes in Osteosarcoma. Anticancer Res. 2008;28:673–679. [PubMed] [Google Scholar]
- 43.Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from turmeric powder. Biochem Pharmacol. 2004;68:2043–2052. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol Med. 2008;45:1403–1412. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther. 2003;307:468–475. doi: 10.1124/jpet.103.054056. [DOI] [PubMed] [Google Scholar]
- 46.Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res. 2001;7:223–225. [PubMed] [Google Scholar]
- 47.Vaage J, Mayhew E, Lasic D, Martin F. Therapy of primary and metastatic mouse mammary carcinomas with doxorubicin encapsulated in long circulating liposomes. Int J Cancer. 1992;51:942–948. doi: 10.1002/ijc.2910510618. [DOI] [PubMed] [Google Scholar]
- 48.Torchilin VP. Passive and Active Drug Targeting: Drug Delivery to Tumors as an example. In: Schafer-Korting M, editor. Drug Delivery Handbook of Experimental Pharmacology. Vol. 197. Springer Berlin Heidelberg: 2010. pp. 3–53. [DOI] [PubMed] [Google Scholar]
- 49.Jatoi A, Suman VJ, Schaefer P, Block M, Loprinzi C, Roche P, et al. A Phase II Study of Topical Ceramides for Cutaneous Breast Cancer. Breast Cancer Res Treat. 2003;80:99–104. doi: 10.1023/A:1024409123726. [DOI] [PubMed] [Google Scholar]
- 50.ClinicalTrials.gov. [Accessed 15 June 2013];A service of the U.S. National Institutes of Health. Available: http://clinicaltrials.gov/ct2/results?term=curcumin+cancer&Search=Search.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.