Abstract
Activity-dependent neuroprotective protein (ADNP) is a most frequent autism spectrum disorder (ASD)-associated gene and the only protein significantly decreasing in the serum of Alzheimer's disease (AD) patients. Is ADNP associated with ASD being more prevalent in boys and AD more prevalent in women? Our results revealed sex-related learning/memory differences in mice, reflecting hippocampal expression changes in ADNP and ADNP-controlled AD/ASD risk genes. Hippocampal ADNP transcript content was doubled in male vs female mice, with females showing equal expression to ADNP haploinsufficient (ADNP+/−) males and no significant genotype-associated reduction. Increased male ADNP expression was replicated in human postmortem hippocampal samples. The hippocampal transcript for apolipoprotein E (the major risk gene for AD) was doubled in female mice compared with males, and further doubled in the ADNP+/− females, contrasting a decrease in ADNP+/− males. Previously, overexpression of the eukaryotic translation initiation factor 4E (eIF4E) led to ASD-like phenotype in mice. Here, we identified binding sites on ADNP for eIF4E and co-immunoprecipitation. Furthermore, hippocampal eIF4E expression was specifically increased in young ADNP+/− male mice. Behaviorally, ADNP+/− male mice exhibited deficiencies in object recognition and social memory compared with ADNP+/+ mice, while ADNP+/− females were partially spared. Contrasting males, which preferred novel over familiar mice, ADNP+/+ females showed no preference to novel mice and ADNP+/− females did not prefer mice over object. ADNP expression, positioned as a master regulator of key ASD and AD risk genes, introduces a novel concept of hippocampal gene-regulated sexual dimorphism and an ADNP+/− animal model for translational psychiatry.
Introduction
Autism spectrum disorder (ASD) affects ~1.5% children with a prevalence of three boys/one girl and with ~50% deficient or borderline intellectual function, in the US.1 The high comorbidity of ASD with intellectual disability suggests common genes and pathways.2, 3, 4 Focusing on sexual dimorphism and cognitive functions and contrasting with ASD, there is a greater prevalence of Alzheimer's disease (AD) in women compared with men, whereas men may have a higher risk of mild cognitive impairment, an intermediate stage between normal aging and dementia.5
Activity-dependent neuroprotective protein (ADNP), recently estimated to be de novo mutated in at least 0.17% of ASD cases,6, 7, 8 was discovered in our laboratory and identified as vital for brain formation.9, 10, 11, 12, 13 Partial deficiency in ADNP resulted in hyperphosphorylation of the MT-associated protein tau (leading to AD/frontotemporal dementia—like tau pathology) paralleled by cognitive deficits. NAP (NAPVSIPQ), a snippet from ADNP,12 enhancing tau-MT interaction14, 15 and inhibiting, in part, tau aggregation,16, 17, 18 reversed ADNP deficiencies in vivo,12 while increasing ADNP-MT end binding protein interaction through the shared SIP domain.19 Tau deposition was also associated with autism.20 GSK3beta-deficient mice (the tau kinase overactivated in ADNP+/− mice12), showed improved social behavior vs control mice,21 suggesting that overactivation of GSK3beta and the resulting tau hyperphosphorylation impairs social behaviors, as seen in autism. In parallel, the MT-associated protein 2 (MAP2) expression was depleted in autistic patients.22 In this respect, ADNP silencing resulted in MAP2 depletion23 and NAP treatment increased MAP2 expression.24
We recently showed that ADNP+/− mice exhibited reduced hippocampal beclin1 (a key factor in the regulation of autophagy, which was also reduced in schizophrenia postmortem hippocampus compared with controls). At the protein level, ADNP co-immuoprecipitated with the MT-associated protein 1 light chain 3, another major regulator of the autophagy process, which was augmented by NAP,25 protecting the autophagic flux.26 Autophagy has been associated with autism,27 for example, in mice haploinsufficient for ambra1 (a positive regulator of beclin1), showing autism-like behavior restricted to the female gender.28 In addition, other converging mechanisms shared by schizophrenia and autism have been shown, including mutations affecting synaptic strength and cytoskeleton activity.29
Importantly, ADNP expression, correlated with related proteins is deregulated in schizophrenia postmortem brains.30
Complete gene expression microarray analysis identified altered transcript content in the ADNP+/− mice12 compared with ADNP+/+, including a solute carrier transcript, involved in intracellular signaling cascade, transforming growth factor, involved in angiogenesis and Pax6,11 involved in neuronal migration and axogenesis, which are either mutated or altered in their expression in individuals with autism.11, 12, 20, 31, 32 In addition, ADNP haploinsufficiency was also associated with deficits in social memory.12, 33
From a diagnostic point of view, assessment of ADNP expression lymphocytes showed increased expression in patients suffering from schizophrenia compared with matched controls, which was reduced with disease progression only in female patients.25 This increased expression in schizophrenia is contrasted by decreased expression in peripheral blood mononuclear cells34 from multiple sclerosis patients, and, at the protein level, complete serum proteomics identified ADNP as the only protein decreasing in serum from AD patients compared with controls.35
Given the male/female differences in ASD and AD, it is of interest to note that ADNP expression in the arcuate nucleus of the rodent hypothalamus, a brain area associated with appetite and sexual behavior, exhibited fluctuations during the estrous cycle, proestrous sections being the most ADNP-immunoreactive and estrous sections the least, whereas male arcuate nucleus ADNP-like immunoreactivity was significantly lower than that of the female estrous.36
Here, we asked if ADNP is differentially expressed in the hippocampus of males and females and if changes in ADNP expression regulate key ASD and AD risk genes, leading to sex-specific cognitive and social differences. Better understanding of ADNP, which regulates >400 genes during the development13 will pave the path to better targeted treatments.
Materials and methods
Animals
All procedures involving animals have been approved by the Animal Care and Use Committee of Tel Aviv University and the Israeli Ministry of Health. ADNP heterozygous mice on a mixed C57BL and 129/Sv background, a model for cognitive impairments,12 were housed in a 12-h light/12-h dark cycle facility with free access to rodent chow and water.
The procedure to generate ADNP+/− animals was described previously.11, 12 Due to severe inbred mating problems, mating with ICR, an outbred mouse line, was implemented allowing for continuous breeding and excellent progeny. Genotyping was performed by Transnetyx (Memphis, TN, USA). ADNP+/− and littermates ADNP+/+ mice were compared. Six-month-old male or female mice were exposed twice daily (5 μl per nostril) to intranasal administration, of a vehicle solution,37 in which each ml included 7.5 mg of NaCl, 1.7 mg of citric acid monohydrate, 3 mg of disodium phosphate dihydrate, and 0.2 mg of benzalkonium chloride solution (50%).
The mice were subjected to behavioral exams (n=16–18 for each of the male groups; n=11 for each of the female groups) as outlined below. A subset of these mice (males only, n=5 per group) were killed and hippocampal RNA was subjected to quantitative real-time RT-PCR. An independent set of groups with hippocampal RNA analyzed by quantitative real-time RT-PCR included naive 5- to 6-month-old mice (n=6–8 per group).
Object recognition
Subject ADNP+/− and ADNP+/+ mice were 7 months of age at the time of testing. The test includes two consecutive days of habituation (5 min per day) and the experimental day which consists of the three phases. In phase 1 (habituation), the open field apparatus (50 × 50 cm) contained two identical objects (plastic or metal, 4 × 5 cm2) and a mouse was placed in the apparatus facing the wall and allowed to freely explore the objects (5 min). After 3 h in the home cage, the mouse was placed back into the apparatus for 3 min for phase 2 (short retention choice), during which one of the familiar objects was replaced with a novel object. Approximately 24 h after the completion of phase 2, the mouse was placed into the apparatus for 3 min for phase 3 (long retention choice), during which one of the familiar objects was replaced with a novel object. The mouse was kept in its home cage between phases 2 and 3. The time spent sniffing/touching each object was measured. Data were analyzed using the discrimination capacity formula: D2=(b−a)/(a+b), where ‘a' designated the time of exploration of the familiar object and 'b' designated the time of exploration of the novel object.38
Social approach task
Subject ADNP+/− and ADNP+/+ mice were 7–8 months of age at the time of testing. Mice used as novel (target mice) to be explored by the subject mice were from the 129/SvJ strain (in our colony), known for their docile nature (7- to 8-month old). The social approach task was previously reported.39 A plexiglas box was divided into three adjacent chambers, each 20 cm (length) × 40.5 cm (width) × 22 cm (height), separated by two removable doors. Steel wire pencil cups (10.16 cm (diameter), 10.8 cm (height)), www.kitchen-plus.com, were used as both containment for the target mice and as inanimate objects (weights prevented the mice from overturning the cups). Experiments were conducted in a dimly lit area during the light phase of the mouse. The brightness of the right and left chambers was measured with a light meter (MRC lab, Holon, Israel) and kept at ~6+0.5 lux before experiments were initiated, to avoid bias. Three sides of the box were covered to prevent the mice from using spatial cues. The long side facing the experimenter was left open for experimenter view.
Target mice (males for males and females for females) were placed inside the wire cup in one of the side chambers for three 10-min sessions on the day before the test for habituation. The next day, each subject mouse was tested in an experiment with three phases, each 10-min long (measured with a simple timer): I and II, the habituation phases (ensuring no bias), and III, the experimental phase, recorded with a video camera. No significant differences were noted between time periods spent in the different chambers in the habituation phase.
In phase III, an empty wire cup (novel object) was placed in the center of the right or left chamber and the cup containing the target mouse was placed in the center of the other chamber. Location of the empty wire cup (novel object) and the novel mice were counterbalanced to avoid confounding side preference. The doors were then removed and the timer for 10-min started. During this phase, the experimenter timed how long the subject mouse explored the empty wire cup and the wire cup with the novel mouse with two silent stopwatches (first novel mouse exposure). The three-chamber apparatus was cleaned between mice.
The social approach task was used as habituation for the social memory task, 3 h after the first phase (3-min exposure), the mouse was placed back into the apparatus for another 3 min (second phase), during which one cup contained the familiar mouse and the other contained a novel mouse. The positions of the familiar and novel mouse during phases 1 and 2 were counterbalanced within and between groups to exclude the possibility of positional effects, but were kept the same for a given animal. The discrimination capacity (social memory) was analyzed using the formula: D2=(b−a)/(b+a), as for the object recognition test.
Odor habituation–dishabituation
This test was performed as described.40
Biochemical and immunochemical procedures
Male ADNP+/− and ADNP+/+ littermates (wild type) mice (5- to 6-months old) were decapitated, the hippocampus was dissected, frozen in liquid nitrogen and maintained frozen (−80 °C) until further processing. RNA and protein were extracted using Macherey-Nagel NucleoSpin RNA/Protein kit (Bethlehem, PA, USA). RNA purity and concentration were determined with a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The protein amount was estimated by the BCA-200 protein kit (Pierce, Rockford, IL, USA). An additional RNA quantification experiment on a limited amount of transcripts was carried out in older mice (9-month-old male mice). Before hippocampal RNA extraction, the 9-month-old mice (n=5 per group) were treated with vehicle (defined above) and were subjected to behavioral tests as outlined in Figures 1 and 2.
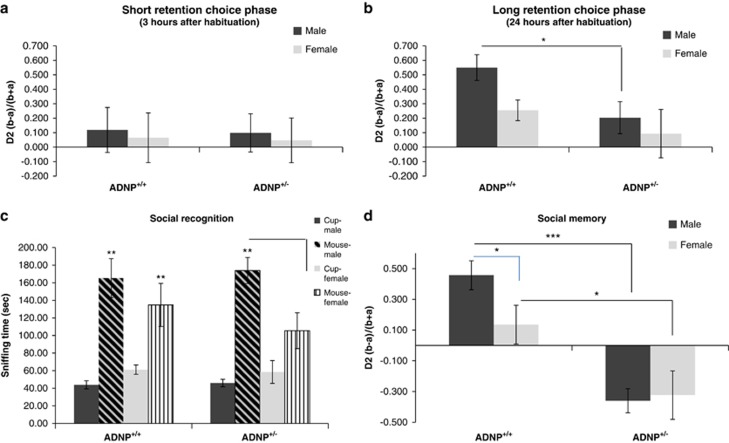
Figure 1.
ADNP+/− differ from ADNP+/+ male mice: object recognition, social interactions and sexual dichotomy. Animal performance in the object recognition test is shown (n=16–18 for each of the male groups; n=11 for each of the female groups). (a, b) ADNP+/− male mice are deficient in object recognition. Two identical objects were first presented, and one of the identical objects was replaced by a novel object 3 h after sniffing the familiar object (short retention choice) (a) or on the following day (~24 h later, long retention choice) (b). Data are expressed as mean (±s.e.m.) by a relative discrimination index (D2=(b−a)/(b+a); b=time (s) sniffing a novel object, a=time (s) sniffing a familiar object). Two-way analysis of variance (ANOVA) revealed no significant differences in the short retention choice (a). In the long retention choice (b), two-way ANOVA showed a significant effect of genotype only in the male group (F(1,49)=5.022, P=0.030). ADNP-deficient male mice spent significantly less time (>2-fold) in exploring the new object as compared with control mice (ADNP+/+). Fisher's LSD post hoc test revealed a significant difference between ADNP+/− male mice compared with ADNP+/+ mice (*P<0.05). (c) Sniffing time of empty cup and novel mouse—social recognition test. Data are expressed as mean (±s.e.m.) total time (s) spent exploring mice or objects. A three-chamber cage was used. Two-way repeated measure ANOVA with group as a fixed factor and sniffed item (that is, mouse vs cup) as repeated factor revealed no main effect for group (F(3,48)=1.051, P=0.379) on the sniffing time periods of a novel mouse and a cup. However, a main effect was found for the sniffed item (F(1,48)=75.761, P<0.001), indicating a strong preference for the novel mouse over the cup. In addition, an interaction effect between group × sniffed item was found (F(3,48)=3.296, P=0.028). Fisher's LSD post hoc test revealed significant differences between sniffing time period of the cup and mouse in all the groups (*P<0.05, ***P<0.001 vs cup in the same group—male or female). Bonferroni post hoc test revealed significant differences between sniffing time period of the cup and mouse in all the groups (**P<0.01) except for ADNP+/− females (P=0.18). The sniffing time of mouse in the latter group was significantly lower than in the ADNP+/− males (#P<0.05), with no change in the sniffing time of the cup (P>0.99 after adjustment for multiple comparisons). (d) ADNP+/− mice displayed a significant decrease in social memory. Animal performance in the social memory test is shown (3 h after the original 3-min exposure). Data are expressed as mean (±s.e.m.) total time (s) spent exploring another mouse as designated by a relative discrimination index (b=time sniffing a novel mouse, a=time sniffing a familiar mouse. The total time allowed for sniffing in the second exposure was 3 min). In the social memory test, the ADNP-deficient male and female mice spent significantly less time exploring the novel mouse as compared with control mice (ADNP+/+). Two-way ANOVA showed a significant genotype effect (F(1,47)=31.357, P<0.001) in the social memory test (ADNP+/+ vs ADNP+/− mice), but no general sex effect (males vs females), (F(1,47)=1.563, P=0.217). Fisher's LSD post hoc test revealed a significant genotype difference between the ADNP+/+ and ADNP+/− in the male group (***P<0.001) as well as in the female group (*P<0.05); there was also a significant sex effect in the ADNP+/+ group (*P=0.05 male vs female). ADNP, activity-dependent neuroprotective protein; LSD, least significant difference.
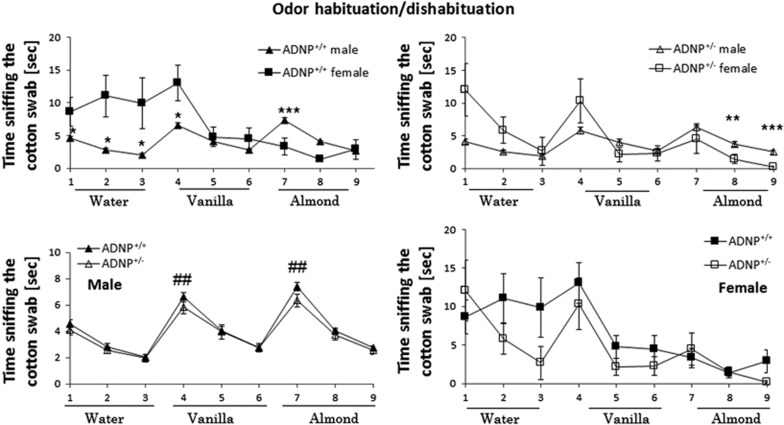
Figure 2.
Odor habituation/dishabituation is intact in the ADNP+/− ICR model exhibiting sexual dichotomy. Each mouse was tested during three consecutive 2-min periods for each odor with 2-min intervals between presentations. The time that the mouse smelled the swab was recorded (beginning when the animal oriented its nostrils toward the odor-scented cotton swab, within 2 cm or less). Male ADNP+/+ mice performed better in the odor habituation/dishabituation test compared with female ADNP+/+ mice. ***P<0.001, *P<0.05 vs female at the same trial, t-test. ##P<0.01 vs previous sniffing (novel vs familiar odor). ADNP, activity-dependent neuroprotective protein.
Immunoprecipitation
Proteins (400–500 μg) were extracted from the hippocampus and diluted in lysis buffer for further immunoprecipitation using the CoIP kit (Pierce) protocol. Twenty microliters of A/G PLUS-Agarose beads were loaded to a column washed with coupling buffer at 90 g for 1 min. Ten micrograms of rabbit mouse ADNP antibody (Bethyl Laboratories, Montgomery, TX, USA) were added to the beads and incubated for 1 h at 24 °C. Cross-linking using 2.5 mM disuccinimidyl suberate was performed by further 1 h incubation. Then, cleared 500 μg of brain lysate were added to the column and incubated (16 h, 4 °C). To detect the eluted antigen, proteins were separated by electrophoresis on 15% acrylamide gel containing 0.1% SDS,10 transferred to nitrocellulose filter (Millipore, Bedford, MA, USA) and immunostained with rabbit polyclonal antibody against mouse eIF4E (1:500), (a kind gift from Professor Orna Elroy-Stein, Tel Aviv University).41 Proteins were visualized using enhanced chemiluminescence reagents and exposure to hyperfilm (Kodak, Petach Tiqwa, Israel). Protein bands on hyperfilm were quantified using photochromatography analysis.
Quantitative real-time RT-PCR
Equal amounts of total RNA (1 μg RNA/sample, obtained from 6-month-old mice) were subjected to reverse transcription (RT) using qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD, USA). Real-time PCR was performed using Powered SYBR Green PCR master mix (Kappa Technologies, Woburn, MA, USA) and ABI PRISM 7900 Sequence Detection System instrument and software (Applied Biosystems, Foster City, CA, USA). RNA expression levels were determined using specific mouse primers: eIF4E sense 5′-TCTGGCTAGAGACACTGCTG-3′, anti-sense 5′-AGTCCATATTGCTATCTTATCACC-3′, ApoE primers sense 5′-ACCGCTTCTGGGATTACCT-3′, anti-sense 5′-ATCAGTGCCGTCAGTTCTT-3′, ADNP sense 5′-ACGAAAAATCAGGACTATCGG-3′, anti-sense 5′-GGACATTCCGGAAATGACTTT-3′, ADNP2 sense 5′-GGAAAGAAGCGAGATACCG-3′, anti-sense 5′-TCCTGGTCAGCCTCATCTTC-3′. Neuroligin (1–4) primers were designed and used as described before.42 Hypoxanthine-guanine phosphoribosyltransferase was chosen as a reference gene with appropriate primers for mouse sense 5′-GGATTTGAATCACGTTTGTGTC-3′, anti-sense 5′-AACTTGCGCTCATCTTAGGC-3′. Results are shown as 2−ΔCT (http://de-de.invitrogen.com/etc/medialib/en/filelibrary/Nucleic-Acid-Amplification-Expression-Profiling/PDFs.Par.83765.File.dat/relative-quant-ct.pdf).43
Bioinformatics
ADNP Sequence analysis was used to identify the consensus KclYcnyLp and cekYkpgVLL—elF4E binding sites as per published literature.44
Statistical analysis
Two-way repeated measures analysis of variance, followed by Fisher's least significant difference test, with group as a fixed factor (group) and sniffed item (that is, cup vs a novel mouse) as a repeated factor, was used to analyze the data in the social recognition task. In all other measurements, two-way analysis of variance followed by Fisher's least significant difference was used. One-way analysis of variance or Student's t-test were used when required. All the analyses were conducted with SigmaPlot software (Chicago, IL, USA) for Windows.
Results
ADNP expression modulated behavior in a sexual-dependent manner: ADNP+/− male, but not female mice display deficits in object recognition
ADNP haploinsufficient mice did not differ in their open field behavior38 (data not shown). In contrast, while there was no difference in the short-term retention choice (3 h, Figure 1a), in the long-term retention choice, ADNP+/+ male mice spent greater than doubled time with the novel object, compared with ADNP+/− male littermates. Thus, a potential deficit in the memory or rather repetitive behavior and preference of the familiar condition was observed with ADNP deficiency (Figure 1b). In contrast to the males, there was no genotype difference in females representing a significant sex difference (Figure 1b).
ADNP+/− mice exhibit sex differences in social recognition
When comparing the preference of an inanimate object to a mouse (male, in the case of males; and female, in the case of females), all males preferred animals over the empty cup (Figure 1c), exhibiting longer sniffing periods with the animal. A significant sex difference was discovered, with ADNP+/− females, showing only a trend of being more interested in the animal over the empty cup (contrasting ADNP+/+ females) and being less socially interested (2-fold less interaction time with the other female mouse compared with males). Comparing males with females, it could be that the ADNP+/− males are deficient in learning to recognize the other animal and thus continue to persist increasing their interaction time, whereas the females may recognize quickly and thus exhibit less interested behavior.
ADNP+/− male mice display a significant preference to a familiar mouse rather than a novel one (decreased social memory)
In the social memory test, there was a significant sex difference in the control mice, with males preferring the novel mouse and females showing no preference (Figure 1d). This was not observed in the ADNP+/− mice, that is, ADNP+/− male mice behave like ADNP+/− females but unlike ADNP+/+ males. Thus, as seen in the object recognition test, ADNP+/− male mice showed a highly significant preference to the familiar over the novel mouse, opposite to the ADNP+/+ mice. Similarly, ADNP+/− female mice also preferred the familiar female. In previous experiments, inbred ADNP+/− male mice (not mated with the ICR mice12, 33) also showed social memory deficits. However, these experiments could not be repeated in the same way (open arena), as the ICR background used here resulted in very aggressive mice, and the only possibility to perform mouse interaction tests was with mice enclosed in an isolated compartment.
ADNP+/− mice show intact odor habituation–dishabituation
The odor habituation–dishabituation measured two parameters including (1) intact olfactory function and (2) olfactory memory. Here, sex comparisons reveals significant differences between males and females in the intact and deficient ADNP groups, with only males showing intact odor habituation–dishabituation and complete identity between the male ADNP+/− and ADNP−/− groups, that is, no genotype differences (Figure 2). Thus, the differences observed above could be attributed to emotional/cognitive disturbance dissociated from olfactory memory.
Mechanism associated with autism: eukaryotic translation initiation factor 4E (eIF4E)
A recent publication addressed central mechanisms of ASDs and showed that overexpression of the eukaryotic translation initiation factor 4E (eIF4E) led to ASD-like phenotype.42
Bioinformatics identified two eIF4E putative binding motif sequences on the ADNP protein sequence KclYcnyLp and cekYkpgVLL—elF4E binding sites44 (Figure 3a). These sequences implicate ADNP as a potential regulator of protein translation in neuronal and glial cytoplasm23, 44 through elF4E interaction. Analysis of elF4E expression by western blotting (Figure 3b) revealed increased protein expression in the hippocampus of ADNP+/− male mice compared with ADNP+/+ male mice (Figure 3b, lanes 1 compared with 2 and 5 compared with 7, two independent experiments). Co-immunoprecipitation with ADNP antibodies followed by western analysis with eIF4E antibody showed specific elF4E precipitation only in the presence of ADNP antibodies (lanes 3,4,6 and 8), with increased precipitation in the ADNP+/− mice as expected from the whole extract results. These findings were corroborated at the RNA level by quantitative real-time RT-PCR. The amounts of hippocampal elF4E mRNA (expressed relative to the amount of hypoxanthine–guanine phosphoribosyltransferase) were significantly increased by about 25% in the ADNP+/− mice compared with the ADNP+/+ mice (Figure 4a).
Figure 3.
Identification and verification of ADNP and eIF4E interacting-sites. (a) Diagram of the predicted eIF4e protein binding motifs on human ADNP.10, 44 (b) ADNP and eIF4E interaction. Co-immunoprecipitation assays were used to analyze ADNP and eIF4E binding. Lanes 1,5: 20 μg of brain lysate from ADNP+/+ male mice and lanes 2,7: 20 μg of brain lysate from ADNP+/− male mice immunoblotted with anti-eIF4E antibody; lane 3: 500 μg of brain lysate of ADNP+/+ male mice and lane 4: 500 μg of brain lysate of ADNP+/− male mice were immunoprecipitated with anti-ADNP antibody and immunoblotted with anti-eIF4E antibody; lane 6: 500 μg of brain lysate of ADNP+/+ and lane 8: 500 μg of brain lysate of ADNP+/− mice were immunoprecipitated without an antibody and immunoblotted with anti-eIF4E antibody. ADNP, activity-dependent neuroprotective protein.
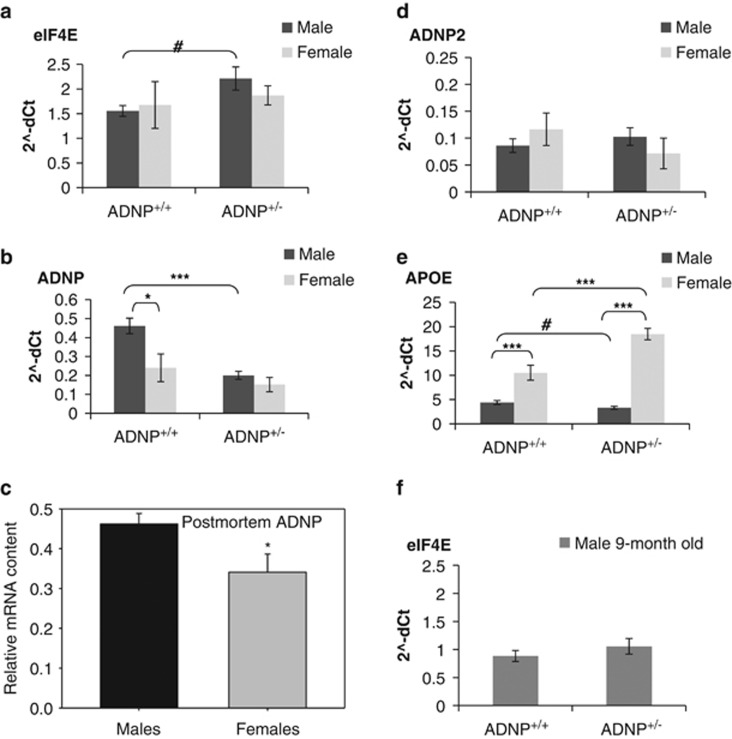
Figure 4.
Autism-specific gene modulation in the hippocampus of ADNP+/− mice: sexual dichotomy. Hippocampal RNA from 5- to 6-month-old mice was analyzed by quantitative real-time PCR (n=6–8 mice per group). (a) eIF4E: Two-way analysis of variance (ANOVA) showed no significant effect of sex (P=0.692) and genotype (P=0.137). However, as there was a marginal genotype trend, one-way ANOVA was also performed showing a significant increase in eIF4E transcripts in the ADNP+/− male mice compared with ADNP+/+ male mice (#P<0.05). (b) ADNP: two-way ANOVA showed a significant effect of sex (F(1,15)=8.760, P=0.010) and a significant effect of genotype (F(1,15)=14.660, P=0.002). Fisher's LSD post hoc test revealed a significant 2-fold decrease in ADNP+/− male mice compared with control mice (***P<0.001). In addition, Fisher's LSD post hoc test revealed a significant 2-fold increase in ADNP+/+ male mice compared with ADNP+/+ female mice (*P<0.05). (c) Human ADNP: Hippocampal human ADNP used before30 was analyzed comparing males and females. As there was no difference at the ADNP transcript levels between normal and schizophrenia subjects, the cohorts were pooled to obtain 22 men and six women. Results showed a statistically significant increase in men (Student's t-test, *P<0.05). (d) ADNP2: two-way ANOVA showed no significant effect of sex (P=0.977) and no significant effect of genotype (P=0.498). (e) ApoE: two-way ANOVA showed a significant effect of sex (F(1,23)=128.734, P<0.001) and a significant effect of genotype (F(1,23)=13.430, P=0.001). Fisher's LSD post hoc test revealed a significant 2-fold increase in ADNP+/− female mice compared with ADNP+/+ female mice (***P<0.001). In addition, Fisher's LSD post hoc test revealed a highly significant decrease in male mice compared with female mice for both ADNP+/+ and ADNP+/− (***P<0.001). One-way ANOVA showed a significant decrease in ADNP+/− male mice compared with ADNP+/+ mice (#P<0.05). (f) Nine-month-old male mice, eIF4E: no specific genotype effect, unlike (a) 5- to 6-month old. ADNP, activity-dependent neuroprotective protein; LSD, least significant difference.
ADNP hippocampal expression exhibits a striking sexual dimorphism
Given the sex and genotype differences in ADNP+/− mice, we were interested to see if ADNP expression is sex-dependent in the hippocampus, a brain area directly associated with learning and memory. We revealed, for the first time, a significant 2-fold decreased ADNP expression in the female hippocampus, similar to the genotype-associated decrease in ADNP+/− males, with the genotype decrease (ADNP haploinsufficiency) in females being insignificant (Figure 4b). To reflect this sex difference to men, we have also reevaluated our previous data in postmortem human hippocampal tissue25, 30 discovering the same sex difference, with males expressing ~25% more ADNP transcript than females (Figure 4c). In contrast to ADNP, no significant differences were found in the related ADNP2 (Figure 4d), agreeing with our previous results showing no effect of the ADNP-deficient genotype of ADNP2 expression.45 ApoE, the major risk gene for AD, which has been shown to be suppressed by ADNP during embryonic development,13 was twice decreased in males compared with females (Figure 4e), contrasting the increased hippocampal ADNP in males (Figure 4b). However, ADNP deficiency did not result in ApoE increase in males, rather, a small decrease was observed, suggesting compensatory mechanisms. Compared with males, ADNP+/+ females, revealed doubled ApoE-transcript content which was further doubled in ADNP+/− females (Figure 4e).
All the above-mentioned experiments were carried out in 5- to 6-month-old-mice. A follow-up experiment utilized 9-month-old-male-mice showing no genotype effect for eIF4E in the older mice (Figure 4f) coupled to a significant decrease in eIF4E transcript levels between ADNP+/+ ‘young' male mice and ADNP+/+ ‘old' male mice (P=0.001, paired t-test, comparing Figure 4f to Figure 4a), a similar age-dependent decrease was also observed in ADNP+/− mice (P=0.001, paired t-test).
Finally, abnormalities in neuroligins, (NLGNs), which are downstream to elF4E, have been suggested as causal for autism42 with sex-specific association of common variants of neuroligin genes (NLGN3 and NLGN4X) with ASD.46 We therefore investigated the 4 neuroligin transcript expression in the ADNP+/− compared with ADNP+/+ male and female mice, respectively, at 5–6 months of age (Figures 5a–d). While results did not show genotype-associated changes in either males or females, there were significant sex differences with significant increases in NLGN1 and 3 (Figures 5a–c) and a decrease in NLGN4 (Figure 5d) in the ADNP+/− females compared to males. NLGN2 did not show differences (Figure 5b), while NLGN1 also showed increased expression in control (ADNP+/+) males, compared with control females (Figure 5a).
Figure 5.
Neuroligins show sex-dependent expression. Hippocampal RNA from 5-to 6-month-old mice was analyzed by quantitative real-time PCR (n=6–8 mice per group). The 4 NGLN genes were compared. (a) NGLN1: two-way analysis of variance (ANOVA) showed a significant effect of sex (F(1,26)=16.754, P<0.001), but no significant effect of genotype (P=0.561). Fisher's LSD post hoc test revealed a significant difference between male mice compared with female mice for both ADNP+/+ and ADNP+/− (*P<0.05). (b) NGLN2: two-way ANOVA showed no significant effect of sex (P=0.777) and no significant effect of genotype (P=0.450). (c) NGLN3: two-way ANOVA showed a significant effect of sex (F(1,24)=7.800, P=0.010), but no significant effect of genotype (P=0.771). Fisher's LSD post hoc test revealed a significant difference between ADNP+/− male mice compared with ADNP+/− female mice (*P<0.05). (d) NGLN4: two-way ANOVA showed a significant effect of sex (F(1,25)=12.258, P=0.002), but no significant effect of genotype (P=0.482). Fisher's LSD post hoc test revealed a significant difference between ADNP+/− male mice compared with ADNP+/− female mice (*P<0.05). (e) Nine-month-old male mice, NGLN1: no specific genotype effect, like (a) 5- to 6-month old. (f) Nine-month-old male mice, NGLN2. (g) Nine-month-old male mice, NGLN3. (h) Nine-month-old male mice, NGLN4. ADNP, activity-dependent neuroprotective protein; LSD, least significant difference.
A follow-up experiment on 9-month-old male mice indicated no genotype-associated changes, as found for the young mice (Figures 5a–h). Analyzing for age-dependent difference, revealed no change in NLGN3 and NLGN4 However, a significant age-dependent reduction was found for the NLGN1 transcript comparing ADNP+/+ ‘young' vs ‘old' male mice (P=0.023, paired t-test, Figures 5a and e) Furthermore, a significant age-dependent reduction was observed for NLGN2 transcripts in ‘old' ADNP+/− male mice compared with ‘young' ADNP+/− male mice (P=0.049, paired t-test, Figures 5b and f).
Discussion
A major surprising finding of the current work was the doubled hippocampal expression of ADNP in the male vs the female mouse which was found to mimic the postmortem human brain, followed by a two-fold decrease in the ADNP haploinsufficient male, and no significant decrease in the ADNP+/− female. The increase in ADNP expression paralleled enhanced behavioral performance in the ADNP intact males compared with females in the social memory task, presenting intact olfactory discrimination. In contrast, ADNP haploinsufficiency resulted in a severe cognitive impairment in the males with somewhat spared females in the object recognition test, mimicking the findings in children with ADNP mutations with the four female patients showing mild intellectual disability, while most of the males (five out of six) showing severe intellectual disability.6
It is also important to bear in mind that our colony, outbred with ICR mice, is still showing dramatic behavioral effects in the ADNP+/− males, which implicates a strong genotype effect, as predicted from human studies. These findings are also reproduced in the females, with ADNP+/− mice only insignificantly trending to prefer mice over objects in the social recognition test, unlike the ADNP+/+ females and males. The sex difference in hippocampal ADNP expression is even more striking when comparing it with previous findings in the arcuate nucleus of the hypothalamus showing an opposite finding, with ADNP decreased expression in males and regulation by the estrous cycle involving estrogen effects.36 In this respect, ADNP is part of the SWI/SNF chromatin remodeling complex47 including BAF57 that specifically regulates estrogen receptorα (ERα)-mediated transcription48 and ADNP was shown to regulate its own expression.13, 49 Furthermore, the ADNP-interacting SWI/SNF member, BRG1, also interacts with endogenous androgen receptor-responsive promoters50 and both BRG1 and BAF57 are involved in the enhancement of androgen receptor activity.51 Our studies suggest direct interaction of ADNP in these processes, explaining in part the sexual divergence associated with ADNP-deficient genotype.
The human study on autistic children showing de novo mutations in ADNP referred to ADNP as an SWI/SNF member6 with predominant nuclear localization, as indicated by our original studies.47 We are now showing an interaction of ADNP with the cytoplasmic eIF4E and increased eIF4E expression in the hippocampus of the 5- to 6-month-old ADNP+/− male mouse, which has been suggested as causal for ASD.42 Interestingly, this increased eIF4E expression did not persist at 9 months of age, which was coupled to an overall decreased eIF4E expression in the older mice. These findings may be related to the fact that overall protein synthesis decreases with brain aging52, 53 coupled to the fact that autism is an early onset disorder associated with deregulation during development.
Besides elF4E, ADNP interacts with several other proteins, including PSF (a tau splicing factor)54 and both PSF and elF4E are phosphorylated by Mnk kinase.55 Thus, ADNP binding to either protein may affect the phosphorylation and activation state of eIF4E, which is associated with the regulation of translation.
Sexual dimorphism was also found at the level of neuroligin expression, shown to be regulated by eIF4E.42 Further, dramatic sexual dimorphism was identified in the expression of ApoE suggesting a broader effect of ADNP on autism and AD-related genes. With ApoE being the major risk gene for AD, a connection is made for a higher AD risk in autistic individuals.
The highly significant increase of ApoE expression in the female hippocampus coupled to further increase in the ADNP+/− female may be correlated with increased prevalence of AD in women compared with men.5 Furthermore, neuroligin1 (doubled in the female mouse hippocampus compared with males) interacts with amyloid beta peptide to increase the formation of amyloid beta oligomers,56 a major pathology in AD. Interestingly, in older mice (9 vs 5–6 months of age), a significant reduction in the NLGN1 transcript was found suggesting that increases in neuroligin1 may be related to the initial stages of AD.
The second major pathology in AD, tau hyperphosphorylation and accumulation of tau neurofibrillary tangles has been shown to be a part of the pathology of ADNP-deficient mice.12 In addition, an initial increase in ADNP expression, followed by an aging-associated dramatic decrease, predict tau pathology in mice with ADNP interacting with tau mRNA splicing.54, 57 As indicated above, comprehensive proteomics identified ADNP as the only protein decreased in the serum of AD patients compared with controls.35
The current study puts ADNP replacement therapy as a major drug target. The ADNP-derived, eight amino acid neuroprotective peptide NAP (davunetide), protected against ADNP-deficiency outcomes in the inbred ADNP+/− mouse, that is, tau pathology and cognitive impairments12 and has shown positive indications of increasing cognitive scores in aging mild cognitive impairment patients.58, 59, 60 Although NAP (davunetide) showed activity in mild cognitive impairment, its clinical results in progressive supranuclear palsy patients were disappointing.61 In contrast, in schizophrenia patients, NAP (davunetide) treatment indicated protection of activities of daily living62 and brain cell function (magnetic resonance spectroscopy measurements).63 The current findings set the stage with a paradigm of ADNP deficiency that provides a new model for (1) better understanding of the pivotal role of ADNP in neurodevelopment and neuroprotection in males and females and paves the path to (2) optimizing and improving NAP (davunetide) and related compounds18, 57 for potential future clinical development in ASD and prevention of later onset of AD, while paying attention to the dramatic sex differences toward precise medical intervention/rational translational psychiatry.
Acknowledgments
Support was provided by the AMN Foundation, Montreal Circle of Friend, Joe and Grace Alter, and the Adams family. Professor Illana Gozes is the incumbent of the Lily and Avraham Gildor Chair for the Investigation of Growth Factors, and the Director of the Dr. Diana and Zelman Elton (Elbaum) Laboratory for Molecular Neuroendocrinology at Tel Aviv University. Professor Gozes served as the Director of the Adams Super Center for Brain Studies for the last 9 years. This work is in partial fulfillment of the requirements for graduate degrees for Anna Malishkevich, Noy Amram and Gal Hacohen-Kleiman. Gal Hacohen-Kleiman is supported in part by a Scholarship from Professor Anat Bardea of the Open University, Israel. Dr Iddo Magen was a VATAT post-doctoral fellow. We thank Professors Galila Agam, Brian Dean and Elisabeth Scarr and Dr Efrat Dresner for the ability to use (reanalyze) previously published results. Professor Gozes is currently a Humboldt Award Recipient and a fellow at the Hanse-Wissenschftenkolleg, Germany. The ADNP+/− model is under patent protection, Ramot@Tel Aviv University.
The authors declare no conflict of interest.
References
- Maenner MJ, Rice CE, Arneson CL, Cunniff C, Schieve LA, Carpenter LA, et al. Potential impact of DSM-5 criteria on autism spectrum disorder prevalence estimates. JAMA Psychiatry. 2014;71:292–300. doi: 10.1001/jamapsychiatry.2013.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C, Billstedt E. Autism and Asperger syndrome: coexistence with other clinical disorders. Acta Psychiatr Scand. 2000;102:321–330. doi: 10.1034/j.1600-0447.2000.102005321.x. [DOI] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkowski ME, Rosenfeld JA, Blumenthal I, Pillalamarri V, Chiang C, Heilbut A, et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012;149:525–537. doi: 10.1016/j.cell.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsmoortel C, Vulto-van Silfhout AT, Coe BP, Vandeweyer G, Rooms L, van den Ende J, et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat Genet. 2014;46:380–384. doi: 10.1038/ng.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. 2001;276:708–714. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther. 2007;323:438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- Mandel S, Rechavi G, Gozes I. Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol. 2007;303:814–824. doi: 10.1016/j.ydbio.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Oz S, Ivashko-Pachima Y, Gozes I. The ADNP derived peptide, NAP modulates the tubulin pool: implication for neurotrophic and neuroprotective activities. PLoS One. 2012;7:e51458. doi: 10.1371/journal.pone.0051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishe S, Cowan CM, Mudher A. NAP (davunetide) rescues neuronal dysfunction in a Drosophila model of tauopathy. Mol Psychiatry. 2013;18:834–842. doi: 10.1038/mp.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev N, Jouroukhin Y, Giladi E, Polyzoidou E, Grigoriadis NC, Rosenmann H, et al. NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis. 2009;34:381–388. doi: 10.1016/j.nbd.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Gozes I, Iram T, Maryanovsky E, Arviv C, Rozenberg L, Schirer Y, et al. Novel tubulin and tau neuroprotective fragments sharing structural similarities with the drug candidate NAP (Davuentide) J Alzheimers Dis. 2014;40:S23–S36. doi: 10.3233/JAD-131664. [DOI] [PubMed] [Google Scholar]
- Gozes I, Schirer Y, Idan-Feldman A, David M, Furman-Assaf S. NAP alpha-aminoisobutyric acid (IsoNAP) J Mol Neurosci. 2014;52:1–9. doi: 10.1007/s12031-013-0103-8. [DOI] [PubMed] [Google Scholar]
- Oz S, Kapitansky O, Ivashco-Pachima Y, Malishkevich A, Giladi E, Skalka N, et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatry. 2014;19:1115–1124. doi: 10.1038/mp.2014.97. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Neumann M, Trojanowski JQ, Lee VM, Feldman G, Norris JW, et al. A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition. Brain. 2010;133:1391–1402. doi: 10.1093/brain/awq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latapy C, Rioux V, Guitton MJ, Beaulieu JM. Selective deletion of forebrain glycogen synthase kinase 3beta reveals a central role in serotonin-sensitive anxiety and social behaviour. Philos Trans R Soc Lond B Biol Sci. 2012;367:2460–2474. doi: 10.1098/rstb.2012.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaetova-Ladinska EB, Arnold H, Jaros E, Perry R, Perry E. Depletion of MAP2 expression and laminar cytoarchitectonic changes in dorsolateral prefrontal cortex in adult autistic individuals. Neuropathol Appl Neurobiol. 2004;30:615–623. doi: 10.1111/j.1365-2990.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- Mandel S, Spivak-Pohis I, Gozes I. ADNP differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J Mol Neurosci. 2008;35:127–141. doi: 10.1007/s12031-007-9013-y. [DOI] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Gozes I, Brenneman DE, D'Andrea MR, Plata-Salaman CR. Activity-dependent neurotrophic factor-9 and NAP promote neurite outgrowth in rat hippocampal and cortical cultures. J Mol Neurosci. 2005;25:225–238. doi: 10.1385/JMN:25:3:225. [DOI] [PubMed] [Google Scholar]
- Merenlender-Wagner A, Malishkevich A, Shemer Z, Udawela M, Gibbons A, Scarr E, et al. Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry. 2013. [DOI] [PMC free article] [PubMed]
- Esteves AR, Gozes I, Cardoso SM. The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson's disease. Biochim Biophys Acta. 2014;1842:7–21. doi: 10.1016/j.bbadis.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Wertz MH, Kwiatkowski E, Tsai PT, Leech JD, Greene-Colozzi E, et al. Neuronal Tsc1/2 complex controls autophagy through AMPK dependent regulation of ULK1. Hum Mol Genet. 2014;23:3865–3874. doi: 10.1093/hmg/ddu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Dahm L, Lu D, Hammerschmidt K, Ju A, Tantra M, et al. Heterozygous ambra1 deficiency in mice: a genetic trait with autism-like behavior restricted to the female gender. Front Behav Neurosci. 2014;8:181. doi: 10.3389/fnbeh.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresner E, Agam G, Gozes I. Activity-dependent neuroprotective protein (ADNP) expression level is correlated with the expression of the sister protein ADNP2: deregulation in schizophrenia. Eur Neuropsychopharmacol. 2011;21:355–361. doi: 10.1016/j.euroneuro.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, et al. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Iwayama Y, Nakamura K, Sato M, Toyota T, Ohnishi T, et al. A novel missense mutation (Leu46Val) of PAX6 found in an autistic patient. Neurosci Lett. 2009;462:267–271. doi: 10.1016/j.neulet.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Shiryaev N, Pikman R, Giladi E, Gozes I. Protection against tauopathy by the drug candidates NAP (davunetide) and D-SAL: biochemical, cellular and behavioral aspects. Curr Pharm Des. 2011;17:2603–2612. doi: 10.2174/138161211797416093. [DOI] [PubMed] [Google Scholar]
- Braitch M, Kawabe K, Nyirenda M, Gilles LJ, Robins RA, Gran B, et al. Expression of activity-dependent neuroprotective protein in the immune system: possible functions and relevance to multiple sclerosis. Neuroimmunomodulation. 2009;17:120–125. doi: 10.1159/000258695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Yang YH, Lu CY, Jong SB, Chen LJ, Lin YF, et al. Activity-dependent neuroprotector homeobox protein: a candidate protein identified in serum as diagnostic biomarker for Alzheimer's disease. J Proteomics. 2012;75:3617–3629. doi: 10.1016/j.jprot.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Furman S, Hill JM, Vulih I, Zaltzman R, Hauser JM, Brenneman DE, et al. Sexual dimorphism of activity-dependent neuroprotective protein in the mouse arcuate nucleus. Neurosci Lett. 2005;373:73–78. doi: 10.1016/j.neulet.2004.09.077. [DOI] [PubMed] [Google Scholar]
- Alcalay RN, Giladi E, Pick CG, Gozes I. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci Lett. 2004;361:128–131. doi: 10.1016/j.neulet.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Merenlender-Wagner A, Pikman R, Giladi E, Andrieux A, Gozes I. NAP (davunetide) enhances cognitive behavior in the STOP heterozygous mouse—a microtubule-deficient model of schizophrenia. Peptides. 2010;31:1368–1373. doi: 10.1016/j.peptides.2010.04.011. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN, et al. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF, et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2008;325:146–153. doi: 10.1124/jpet.107.130526. [DOI] [PubMed] [Google Scholar]
- Bernstein J, Shefler I, Elroy-Stein O. The translational repression mediated by the platelet-derived growth factor 2/c-sis mRNA leader is relieved during megakaryocytic differentiation. J Biol Chem. 1995;270:10559–10565. doi: 10.1074/jbc.270.18.10559. [DOI] [PubMed] [Google Scholar]
- Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–377. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Gosselin P, Martineau Y, Morales J, Czjzek M, Glippa V, Gauffeny I, et al. Tracking a refined eIF4E-binding motif reveals Angel1 as a new partner of eIF4E. Nucleic Acids Res. 2013;41:7783–7792. doi: 10.1093/nar/gkt569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresner E, Malishkevich A, Arviv C, Leibman BS, Alon S, Ofir R, et al. Novel evolutionary-conserved role for the activity-dependent neuroprotective protein (ADNP) family that is important for erythropoiesis. J Biol Chem. 2012;287:40173–40185. doi: 10.1074/jbc.M112.387027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, He X, Yao D, Li Z, Li H, Zhao Z, et al. A sex-specific association of common variants of neuroligin genes (NLGN3 and NLGN4X) with autism spectrum disorders in a Chinese Han cohort. Behav Brain Funct. 2011;7:13. doi: 10.1186/1744-9081-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Gozes I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J Biol Chem. 2007;282:34448–34456. doi: 10.1074/jbc.M704756200. [DOI] [PubMed] [Google Scholar]
- Garcia-Pedrero JM, Kiskinis E, Parker MG, Belandia B. The SWI/SNF chromatin remodeling subunit BAF57 is a critical regulator of estrogen receptor function in breast cancer cells. J Biol Chem. 2006;281:22656–22664. doi: 10.1074/jbc.M602561200. [DOI] [PubMed] [Google Scholar]
- Aboonq MS, Vasiliou SA, Haddley K, Quinn JP, Bubb VJ. Activity-dependent neuroprotective protein modulates its own gene expression. J Mol Neurosci. 2012;46:33–39. doi: 10.1007/s12031-011-9562-y. [DOI] [PubMed] [Google Scholar]
- Dai Y, Ngo D, Jacob J, Forman LW, Faller DV. Prohibitin and the SWI/SNF ATPase subunit BRG1 are required for effective androgen antagonist-mediated transcriptional repression of androgen receptor-regulated genes. Carcinogenesis. 2008;29:1725–1733. doi: 10.1093/carcin/bgn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu C, Tu WH, Yang N, Qin H, Sun Z, et al. ZMIZ1 preferably enhances the transcriptional activity of androgen receptor with short polyglutamine tract. PLoS One. 2011;6:e25040. doi: 10.1371/journal.pone.0025040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Cronin BL, Moskowitz MA. Protein synthesis in rat brain microvessels decreases with aging. J Neurochem. 1981;36:1311–1315. doi: 10.1111/j.1471-4159.1981.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Tavernarakis N. Ageing and the regulation of protein synthesis: a balancing act. Trends Cell Biol. 2008;18:228–235. doi: 10.1016/j.tcb.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Schirer Y, Malishkevich A, Ophir Y, Lewis J, Giladi E, Gozes I, et al. Novel marker for the onset of frontotemporal dementia: early increase in activity-dependent neuroprotective protein (ADNP) in the face of Tau mutation. PLoS One. 2014;9:e87383. doi: 10.1371/journal.pone.0087383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Platanias LC. Mnk kinase pathway: cellular functions and biological outcomes. World J Biol Chem. 2014;5:321–333. doi: 10.4331/wjbc.v5.i3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinamarca MC, Weinstein D, Monasterio O, Inestrosa NC. The synaptic protein neuroligin-1 interacts with the amyloid beta-peptide. Is there a role in Alzheimer's disease. Biochemistry. 2011;50:8127–8137. doi: 10.1021/bi201246t. [DOI] [PubMed] [Google Scholar]
- Gozes I, Iram T, Maryanovsky E, Arviv C, Rozenberg L, Schirer Y, et al. Novel tubulin and tau neuroprotective fragments sharing structural similarities with the drug candidate NAP (Davuentide) J Alzheimers Dis. 2014;40:S23–S36. doi: 10.3233/JAD-131664. [DOI] [PubMed] [Google Scholar]
- Gozes I, Schirer Y, Oz S. Activity-dependent neuroprotective protein (ADNP) is regulated in the brains of mouse models of human tauopathies: toward davunetide replacement therapy. Alzheimers Dement. 2010;6:S578–S579. [Google Scholar]
- Gozes I.Davunetide (NAP) pharmacology: neuroprotection and tauIn: Martinez A (ed). Emerging Drugs and Targets for Alzheimer's Disease Royal Society of Chemistry: Cambridge, UK; 2010108–128. [Google Scholar]
- Gozes I, Stewart A, Morimoto B, Fox A, Sutherland K, Schmeche D, et al. Addressing Alzheimer's disease tangles: from NAP to AL-108. Curr Alzheimer Res. 2009;6:455–460. doi: 10.2174/156720509789207895. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014;13:676–685. doi: 10.1016/S1474-4422(14)70088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF, et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res. 2012;136:25–31. doi: 10.1016/j.schres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Dong Z, Kangarlu A, Colibazzi T, Girgis RR, Kegeles LS, et al. Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology. 2013;38:1245–1252. doi: 10.1038/npp.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]