Abstract
The glutamate N-methyl-D-aspartate receptor antagonist ketamine has demonstrated antidepressant effects in individuals with treatment-resistant major depressive disorder (TRD) within 24 h of a single dose. The current study utilized functional magnetic resonance imaging (fMRI) and two separate emotion perception tasks to examine the neural effects of ketamine in patients with TRD. One task used happy and neutral facial expressions; the other used sad and neutral facial expressions. Twenty patients with TRD free of concomitant antidepressant medication underwent fMRI at baseline and 24 h following administration of a single intravenous dose of ketamine (0.5 mg kg−1). Adequate data were available for 18 patients for each task. Twenty age- and sex-matched healthy volunteers were scanned at one time point for baseline comparison. Whole-brain, voxel-wise analyses were conducted controlling for a family-wise error rate (FWE) of P<0.05. Compared with healthy volunteers, TRD patients showed reduced neural responses to positive faces within the right caudate. Following ketamine, neural responses to positive faces were selectively increased within a similar region of right caudate. Connectivity analyses showed that greater connectivity of the right caudate during positive emotion perception was associated with improvement in depression severity following ketamine. No main effect of group was observed for the sad faces task. Our results indicate that ketamine specifically enhances neural responses to positive emotion within the right caudate in depressed individuals in a pattern that appears to reverse baseline deficits and that connectivity of this region may be important for the antidepressant effects of ketamine.
Introduction
Major depressive disorder (MDD) is a leading cause of disability worldwide and current treatments fall short of what is required to meet this large-scale public health problem.1, 2, 3 The discovery that the glutamate N-methyl-D-apartate receptor antagonist ketamine produces rapid and robust antidepressant effects within one day of a single administration4—even in patients with treatment-resistant depression (TRD)5, 6, 7, 8, 9—has spurred research and treatment development focused the glutamate system and the N-methyl-D-aspartate receptor in depression. In this context, human in vivo neuroimaging research provides a unique opportunity to examine the neurocircuit functions regulated by ketamine relevant to its putative antidepressant mechanism of action.10
MDD is characterized by dysfunctional processing of social, emotional and reward-related information, leading to the cardinal clinical symptoms of pervasive depressed mood, anhedonia (i.e., reduced capacity to experience pleasure) and a negative cognitive bias.11, 12, 13, 14 In particular, neuropsychological and neuroimaging investigations have confirmed a negative emotion processing bias as a central feature of MDD.12, 15 Patients with MDD demonstrate increased attention and memory for negative social information (for example, pictures of human facial expressions) and a bias away from positive information.11, 16, 17, 18 For example, depressed patients show a bias away from positive facial expressions16, 19 and require a greater intensity of emotional expression to correctly identify happy (but not sad) emotion.11
Functional neuroimaging studies provide convergent evidence for valence-specific alternations in emotion processing in MDD.13, 20, 21 Increased neural responses to negative stimuli within anterior cingulate cortex, amygdala and paralimbic regions are observed in MDD, coupled with reduced responses to positive stimuli within regions of prefrontal cortex (PFC) and striatum, among other regions.13, 20, 21, 22, 23 Hypo-responsiveness to positive self-referential, social or reward-related information within the striatum and related PFC regions in particular is observed across multiple studies in MDD.24, 25, 26, 27
Studies examining the effects of antidepressant treatment on neural responses to social and emotional stimuli are broadly consistent with the hypothesis that treatment leads to improvement in clinical symptoms by normalizing dysfunctional circuit activation.28, 29 Previous studies have reported attenuated responses to negative stimuli within the amygdala or anterior cingulate cortex following treatment with a selective serotonin reuptake inhibitor,22, 30 as well as increased responses to positive stimuli within hippocampus.31 Despite partial convergence, there exists considerable heterogeneity in the published literature and a robust neuroimaging biomarker of treatment response in MDD remains an elusive goal.10, 32, 33
Ketamine results in an antidepressant response within one day of a single intravenous infusion,4, 5, 6, 8, 9 but few studies to date have investigated changes in neurocircuitry following ketamine administration in patients with depression. A single resting state [18F]-fluorodeoxyglucose positron emission tomography study conducted in MDD found that ketamine was associated with reduced regional glucose metabolism within the habenula 2 h following administration.34 A second [18F]-fluorodeoxyglucose positron emission tomography study conducted in bipolar depression reported no significant changes in metabolism two hours following ketamine compared with placebo, however, improvement in depressive symptoms was associated with increased metabolism within the ventral striatum.35 To date, no study has utilized an emotional activation task and functional magnetic resonance imaging (fMRI) to examine changes in neurocircuit activity associated with ketamine treatment in patients with TRD.
In the current study, we used fMRI and two emotion perception tasks23 to examine changes in neural activity during positive and negative emotion perception following ketamine in antidepressant-free patients with TRD. During each task, patients view either affective or neutral human facial expression and are asked to make a simple explicit judgment to identify the emotion of the face. Similar tasks have been shown previously to engage a robust social-emotional processing network in the brain,36 to distinguish individuals with MDD from healthy volunteers23 and to index changes following treatment with selective serotonin reuptake inhibitors.22, 31 We hypothesized that, compared with healthy volunteers, patients with TRD would show reduced neural responses to positive faces and increased neural responses to negative faces within prefrontal–subcortical circuits and that these abnormalities would be rapidly reversed following treatment with ketamine.
Materials and methods
Study design and participants
Male and female individuals with MDD and a history of nonresponse to at least two previous antidepressant medication trials (for example, TRD) were eligible to participate in the current neuroimaging study if they were enrolled in a concurrent ketamine clinical trial (ClinicalTrials.gov Identifiers: NCT00548964, NCT00768430, NCT01880593) and met the following additional required criteria. Eligible participants were at least 21 years of age, had a primary diagnosis of MDD (recurrent or chronic) as assessed with the Structured Clinical Interview for DSM-IV—Patient Edition,37 were free of concurrent antidepressant medication for at least 1 week before imaging and had current depressive symptoms of at least moderate severity as determined by a score of 32 or greater on the Inventory of Depressive Symptomatology—Clinician Rated.38 Individuals were excluded if they had a lifetime history of a psychotic illness or bipolar disorder, current alcohol or substance abuse, unstable medical illness or had contraindications to MRI. The Program for the Protection of Human Subjects at Icahn School of Medicine at Mount Sinai approved the study. After complete description of the study to potential participants, written informed consent was obtained.
Eligible individuals with TRD underwent MRI at two time points: baseline (Time 1) and 24 h following a single intravenous infusion of ketamine (Time 2). Depression severity was assessed at baseline and 24 h post treatment using a version of the Montgomery–Åsberg Depression Rating Scale (MADRS)39 modified to assess symptoms only in the preceding 24 h. Percent change in MADRS score from Time 1 to Time 2 was the primary clinical variable for analyses of the relationship between neural activity and symptom change, consistent with prior reports.34, 35 For baseline comparison, a group of healthy volunteers of similar age and gender to the TRD group underwent a single fMRI scan session.
Ketamine treatment
Following an overnight fast and admission to a clinical research unit, an indwelling catheter was placed in the antecubital vein of the nondominant arm, and pulse, blood pressure, digital pulse oximetry and ECG monitoring were instituted. Ketamine hydrochloride (0.5 mg kg−1) was administered by an anesthesiologist via an infusion pump over 40 min as previously described.8 Patients remained overnight or were discharged home following a 4-h recovery period and underwent the second fMRI session ~24 h following the treatment.
Facial emotion perception task
All study participants underwent event-related fMRI during two separate emotion perception tasks. During each 8-min experiment, participants were presented with stimuli consisting of high emotion, low emotion or neutral facial expressions drawn from a standardized series of prototypical facial expressions.40 Subsets of the prototypical facial expressions were morphed to depict expressions of 50 or 100% affect intensity along the neutral-emotional expression continuum.41 The final stimuli set for each experimental session consisted of 21 prototypically happy or sad expressions (100% emotion), 21 mildly happy or sad expressions (50% emotion) and 21 neutral facial expressions presented in pseudorandomized order using E-prime software (Psychology Software Tools). Each facial stimulus was displayed for 2 s with an interstimulus interval varying from 3 to 13 s, with an average interval of 4.29 s. Participants were instructed to rate the emotional valence expressed in each stimulus by making a response using a fiber optic button system located under the right hand. Response options ranged from 1 to 5 with 1 indicating a very negative affect, 3 indicating a neutral affect and 5 indicating very positive affect. The order of the two experiments was randomized across participants.
Neuroimaging data acquisition
Participants were scanned with a Philips Achieva 3.0 T X-series MRI using an eight-channel birdcage headcoil for radio frequency transmission and reception. Functional data were acquired using a T2*-weighted gradient echo-planar imaging sequence (repetition time 2000 ms; echo time 26.6 ms; voxel dimensions: 2.2 mm × 2.2 mm × 2.5 mm; field of view 210 mm × 210 mm; flip angle=90°) and 38 contiguous and ascending near-axial planes parallel to the AC–PC plane. For co-registration, high-resolution T1-weighted anatomical images were collected using a three-dimensional turbo field echo sequence (repetition time: 7.5 ms; echo time: 3.5 ms; voxel dimensions: 1 mm × 1 mm × 1 mm; field of view 224 mm × 224 mm; flip angle=8°) and 172 sagittal planes.
Neuroimaging data analysis
Preprocessing
The functional imaging data preprocessing was completed using the Statistical Parametric Mapping software (SPM8) and Matlab (Mathworks, Natick, MA, USA) and included slice scan time correction, voxel-wise linear de-trending, intensity normalization, high-pass filtering, motion correction, co-registration, normalization to the Montreal Neurological Institute template, and three-dimensional smoothing (6 mm full width at half maximum).
fMRI modeling of brain activity
Whole-brain, voxel-wise general linear modeling was conducted separately for the two experiments using Neuroelf version 9c (http://neuroelf.net/). Models included three stimuli-type (100% emotion, 50% emotion, neutral) and six nuisance (motion) regressors convolved with a canonical hemodynamic response function. To identify brain regions specifically engaged by emotion perception, single-subject whole-brain maps reflecting the difference between the blood oxygen level-dependent signals recorded during 100% emotion and neutral stimuli (100% happy or 100% sad > neutral) were computed. Although the 50% morph condition was included in the model, our analyses focused on the 100% emotion vs neutral contrast as this contrast is expected to isolate the largest effect of emotion. To identify differences in neural responses between healthy volunteers and TRD participants at baseline (Time 1), difference maps were utilized in independent sample t-tests. To identify changes in neural responses following ketamine, difference maps for TRD participants were used in paired sample t-tests to identify voxels displaying significant changes in the blood oxygen level-dependent contrast between the pre- and posttreatment scans (Time 1 and Time 2, respectively). To control for multiple comparisons, Alphasim was implemented in Neuroelf to identify cluster size thresholds ensuring a whole-brain family-wise error (FWE) rate of P<0.05.
To identify relationships between neural responses and depressive symptom severity, we followed up on the analyses above using significant cluster(s) as functionally defined regions of interest. Percent signal change for the contrast of interest (for example, happy 100% > neutral) within regions identified in the primary analyses were extracted and subjected to linear correlation analysis using either baseline MADRS score or % change in MADRS score. Finally, we examined potential associations between functional connectivity and symptom severity at baseline and following ketamine (see below).
Functional connectivity analyses
We investigated the functional connectivity of regions before and after ketamine that demonstrated main effects of time in the above analyses and the relationship between connectivity and change in depression symptoms following treatment. Following a psychophysiological interaction procedure, we constructed general linear models consisting of the three stimuli-type and six nuisance motion regressors previously described as well as the time course extracted from the seed, and the product of the seed time course and the 100% emotion stimuli regressor. This last regressor enabled the estimation of the degree of covariation between the seed's time course and any voxel's time course during 100% emotion trials only. We next inserted into the model each subject's MADRS score percent change, and generated a second-level statistical map representing regions that showed significant correlations between MADRS score percent change and seed-functional connectivity values.
Results
Participant characteristics and clinical outcomes
Twenty individuals with TRD underwent fMRI at baseline and 24 h following ketamine, and adequate data were available for 18 individuals for each fMRI task; 20 healthy volunteers underwent a single fMRI session (Table 1). The clinical characteristics and treatment response of a subset of individuals in the current study have been previously reported.8, 9
Table 1. Sample characteristics.
| TRD group (n=18)a | Healthy volunteers (n=20) | |
|---|---|---|
| Age, years | 38.1 (13.8) | 35.0 (8.9) |
| Gender, male | 10 (55.6%) | 11 (55%) |
| Race | W: 13, AA: 2, A: 3, M: 0, O: 0 | W: 7, AA: 8, A: 1, M: 3, O: 1 |
| Education, years | 15.8 (2.0) | 16.6 (2.5) |
| Age at illness onset | 14.3 (5.3) | — |
| Duration of illness, years | 24.2 (15.7) | — |
| Duration of episode, years | 16.3 (18.1) | — |
| Number of episodes | 2.4 (1.7) | — |
| Lifetime ADT failures | 4.8 (2.0) | — |
| Baseline MADRS score | 29.9 (6.8) | — |
| Posttreatment MADRS score | 16.4 (11.1) | — |
Abbreviations: A, Asian; AA, African-American; ADT, antidepressant treatment; M, multiple; MADRS, Montgomery–Asberg Depression Scale; MDD, major depressive disorder; O, other; TRD, treatment-resistant depression; W, white.
Values based on participants completing the positive emotion perception task. Values shown are means (s.d.) or count (%).
See Supplementary Information for behavioral results of the neuroimaging tasks.
Neuroimaging results
Baseline comparison between TRD and healthy groups
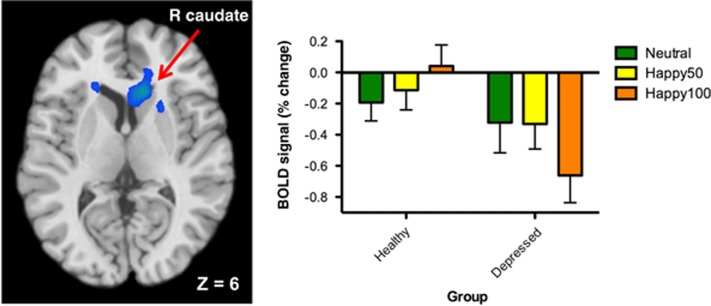
During the positive emotion task, both groups demonstrated main effects of emotion (happy 100% > neutral) and there was a significant group × emotion interaction within the left insula and right caudate (Table 2). In both regions, the TRD group demonstrated hypoactivation for the emotion > neutral contrast compared with the healthy group. In the right caudate, mean blood oxygen level-dependent percent signal for each condition show that responses to positive emotion are reduced in the TRD compared with healthy group, whereas the responses to neutral stimuli are similar (Figure 1). There were no significant results for the happy 50% > neutral contrast.
Table 2. Brain responses during positive emotion task at baseline and following treatment with ketamine.
| Brain region | X (mm) | Y (mm) | Z (mm) | Cluster extent |
|---|---|---|---|---|
| Baseline comparison between TRD and healthy groups | ||||
| L insula | −24 | 24 | 18 | 398 |
| R caudate | 12 | 24 | 6 | 334 |
| Change in TRD group following ketamine | ||||
| R caudate | 12 | 21 | 3 | 951 |
Abbreviations: FWE, family-wise error; L, left; R, right; TRD, treatment-resistant depression.
Clusters indicate regions in which there are significant group (TRD > healthy) × emotion (happy 100% > neutral) or time (Time 2 > Time 1) × emotion (happy 100% > neutral) interactions. Coordinates describe location of cluster peak based on the Montreal Neurological Institute template. For baseline comparison, uncorrected P<0.05, k>170, FWE P<0.05; for treatment effect, uncorrected P<0.05, k>574, FWE P<0.05.
Figure 1.
Differences in brain activation between patients with treatment-resistant depression and healthy volunteers during positive emotion task. Left: analysis yielded activation cluster centered on the right caudate (Peak MNI coordinates: 12,24,6; uncorrected P<0.05, k>170, FWE P<0.05). Right: percent signal change extracted from activation cluster at left depicting neural responses to each condition in depressed individuals and healthy volunteers. BOLD, blood oxygen level-dependent; FWE, family-wise error; MNI, Montreal Neurological Institute.
During the negative emotion task, no main effects of emotion or group × emotion interactions survived correction. See Supplementary Material for additional results.
Effects of ketamine treatment in TRD
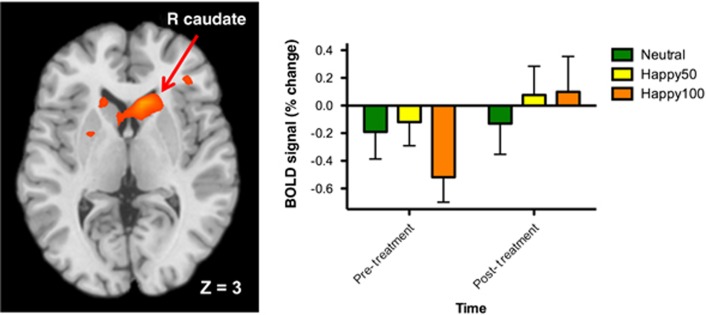
For the positive emotion task, there was a significant interaction between emotion (happy 100% > neutral) and time yielding a large cluster centered in the right caudate (peak coordinates: 12,21,3; k>589, FWE P<0.05; Table 2, Figure 2). Mean blood oxygen level-dependent signal for each condition at Time 1 and Time 2 show that neural responses to positive emotion increased following ketamine whereas responses to the neutral stimuli remained approximately unchanged. There were no significant results for the happy 50% > neutral contrast.
Figure 2.
Regulation of brain responses to positive emotion by ketamine in patients with treatment-resistant depression following ketamine. Left: analysis yielded a single large cluster centered on the right caudate (peak MNI coordinates: 12,21,3; FWE P<0.05). Right: percent signal change extracted from activation cluster at left depicting neural responses to each condition pre- and post-ketamine. BOLD, blood oxygen level-dependent; FWE, family-wise error; MNI, Montreal Neurological Institute.
During the negative emotion task, there was a significant time × emotion interaction within the left middle frontal gyrus. See Supplementary Material for additional results.
Relationships between brain activity and depressive symptoms
Since our primary hypotheses were not supported for the negative emotion task, no further analyses were performed. For the positive emotion task, no correlation was found between brain activation during the main contrast of interest (happy 100% > neutral) and depressive symptoms at baseline or following treatment within the functionally defined regions of interest (for example, insula or caudate) or in a follow-up whole-brain analysis.
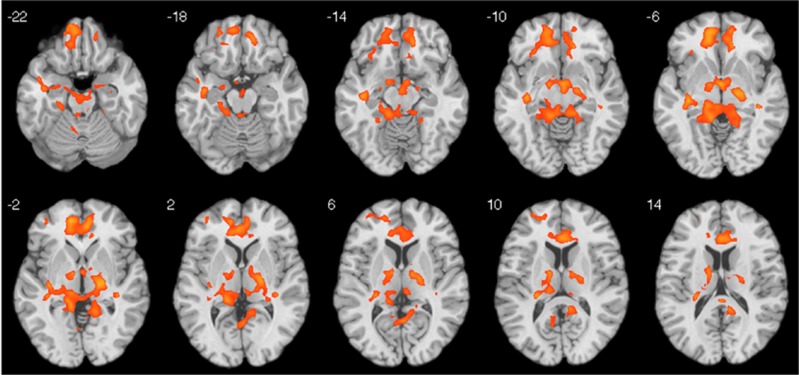
Connectivity analysis revealed a significant correlation between right caudate connectivity following ketamine and clinical improvement using the functionally defined seed region identified in the primary analysis (k>451, FWE P<0.05; Figure 3). The analysis was repeated using an anatomically defined right caudate region and similar whole-brain corrected results were obtained (data not shown). At baseline, there was no association between caudate connectivity and depression symptom severity or subsequent improvement following treatment.
Figure 3.
Correlations between functional connectivity of the right caudate and improvement in depressive symptoms following ketamine. Significant clusters indicate brain regions displaying a positive correlation between connectivity of the right caudate and percent improvement in MADRS score. Results are based on psychophysiological interaction analysis using the functionally defined right caudate as the seed region (peak MNI coordinates: 12,21,3) and are corrected for multiple comparisons (FWE P<0.05). FWE, family-wise error; MADRS, Montgomery–Asberg Depression Scale; MNI, Montreal Neurological Institute.
Discussion
The current study used positive and negative emotion perception tasks and fMRI to investigate the neurocircuitry effects of ketamine and associations with antidepressant response in unmedicated patients with TRD. At baseline, patients with TRD compared with healthy volunteers showed reduced responses to positive emotion within the caudate and insula. Following ketamine, responses to positive emotion were increased within the right caudate on the basis of whole-brain analyses. Functional connectivity of the right caudate during positive emotion was positively correlated with improvement in depression severity following ketamine. We did not find an association between baseline connectivity and change in symptom severity following ketamine. Taken together, our results demonstrate that ketamine regulates neural responses to positive emotion within the right caudate in depressed individuals and that increased caudate connectivity during positive emotion perception is associated with antidepressant effect following ketamine.
Our finding of reduced brain responses within the caudate to positive emotion in TRD is consistent with prior studies showing reduced striatal (caudate or putamen) activation in response to positive social and nonsocial stimuli in MDD13, 20, 23, 42 and in the context of reward processing.25, 26, 27 A recent meta-analysis identified a cluster of hypoactivation in response to positive stimuli within the right caudate and putamen in MDD patients compared with a control group.20 The reason for the laterality of our finding is not completely clear, although we note that our right-sided caudate finding is consistent with the recent meta-analysis by Hamilton et al.20 Prior studies have suggested a general right lateralization of emotional functioning (reviewed in Wager et al.43), although more recent investigations suggest a more complex picture.43, 44 Although our study did not address reward processing per se, it is notable that reduced responses to rewarding stimuli are observed within the caudate and putamen in depressed patients and that smaller caudate volume has been associated with more severe depressive symptoms.26, 27
Our finding of reduced brain responses within the insula to positive emotion in MDD is partially consistent with prior reports,13 although there is considerable variability in the published literature.20, 42 A recent meta-analysis found reduced activation within anterior insula in MDD to negatively valenced stimuli,13 whereas a separate meta-analysis found that insula responses were increased to negative (but not positive) stimuli in MDD.20 The reason for these disparate findings is not completely clear. The insula is known to have a key role in emotion processing, visceral awareness and, in particular, is linked to anxiety and disgust states.45, 46, 47 Future studies using task-based fMRI focused on specific cognitive emotional processes will likely be required to more fully elucidate the role of the insula in depression.
Contrary to our hypotheses, we did not observe robust differences in brain responses to negative emotion between the TRD and healthy control groups. Prior individual studies using emotional faces tasks have found group differences22, 23 and abnormal responses to negative stimuli in MDD more generally are well documented in meta-analyses.13, 20, 42 Although the reason for the absent finding in the current study is not fully known, it is noteworthy that the current study utilized an explicit facial emotion perception task, whereas the study by Surguladze et al. 23 and other studies22 utilized an implicit processing task. We chose to use an explicit emotion perception task to allow us to measure the subjective judgment that subjects made regarding the emotion of the face, although this may have rendered the task less robust in probing limbic activation (for example, amygdala) in response to negative emotion. Another factor that may have contributed to our negative finding is suggested by a recent study showing that heightened amygdala responses to sad faces in depression was confined to a subgroup of patients with histories of childhood trauma.48 Although systematic measurement of childhood trauma was not available in the present study, the influence of trauma history on neurocircuit activation in depression will be an important direction for future research.
We found that ketamine rapidly reversed the blunted response to positive emotion within the caudate in patients with TRD. At the cellular level, subanesthetic doses of ketamine potentiate glutamate signaling in cortical and subcortical circuits49, 50 and facilitate dopaminergic responses within the striatum.51, 52, 53 In vivo human imaging of the D2 receptor suggests that ketamine potentiates amphetamine-induced striatal dopamine release,51 whereas a direct stimulation effect on striatal dopamine is less well supported.53 The striatum has a key role in emotion processing and reward learning.54 The dorsal striatum—corresponding to the caudate head—in particular is involved in linking motivation to action.55, 56 The ventral and dorsal striatum receive dense glutamatergic projections from ventromedial PFC and orbitofrontal cortex conveying stimulus value information.54, 57 Notably, disruption of glutamate signaling between ventromedial PFC and striatum or blockade of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor signaling within striatum results in depressive-like behaviors in animals58, 59 and ketamine is known to enhance postsynaptic AMPA receptor signaling.50 These findings suggest that ketamine may lead to alleviation of depressive symptoms at least in part by reversing impaired glutamate signaling within PFC-striatal pathways.57 Ketamine was recently shown to reverse deficit dopamine signaling in a learned helplessness model of depression and normalized synaptic plasticity within the nucleus accumbens via activation of dopamine D1 receptors.60 Future studies utilizing fMRI tasks specifically designed to assay reward circuitry in humans will be required to more fully understand the impact of ketamine on reward functioning and its association with antidepressant therapeutic effects.
In the current study, ketamine was observed to alter brain responses during negative emotion processing within the left middle frontal gyrus, extending into the orbitofrontal cortex. Specifically, we observed reduced deactivation to sad faces and greater deactivation to neutral faces following ketamine. Since we did not detect baseline differences between the TRD and healthy control groups during sad compared with neutral faces, however, the meaning of the observed findings is not entirely clear. Ventral PFC and orbitofrontal cortex regions are critically involved in value attribution,61, 62 reward processing27 and emotion regulation,63, 64 therefore, it will be important for future studies to more specifically examine the impact of ketamine on these processes.
The current findings do not address the question of the specificity of the observed effect of ketamine on neurocircuit activation since alternative putative rapidly acting antidepressants or conventional antidepressants were not included in the study. Prior studies investigating the neural effects of antidepressant treatment in humans have produced somewhat inconsistent results with regard to the striatum.28, 29, 42 Studies have reported both increases and decreases in response to an affective challenge within the putamen following antidepressant treatment 42 and conventional antidepressants have not been clearly associated with increased striatal responses to positive emotion.29 Our current findings suggest that robust regulation of neural responses to positive emotion within the caudate, in contrast to conventional antidepressants, may either be a relatively unique effect of ketamine or may be characteristic of a rapidly acting antidepressant more generally. Future studies directly comparing ketamine to conventional antidepressants or to other putative rapidly acting agents will be required to further elucidate these issues.
Our study has several limitations. The current study did not include a placebo treatment condition, therefore the potential influence of nonspecific effects related to time or other factors on the observed changes in the TRD group cannot be fully evaluated. The healthy control group did not receive ketamine, therefore, it is not known whether the observed effect of ketamine on caudate activation is specific to depression or if a similar effect would be observed in the absence of depression. Individuals were scanned ~24 h following a single ketamine infusion, thereby limiting interpretations to this timeframe. Image acquisition at the 24-h time point allows us to capture neural changes associated with rapid therapeutic response, while avoiding the confounding effects of acute sedation or dissociation. However, our study cannot address important issues related to durability of antidepressant response. Our healthy control group was scanned at one time point to facilitate the interpretation of baseline brain responses in the TRD group before treatment. However, obtaining repeated scans in the healthy group would have permitted a more thorough evaluation of nonspecific practice effects.
In conclusion, our results show that ketamine rapidly increases brain responses to positive emotion within the caudate in patients with TRD. These changes are consistent with a pattern of normalization of a blunted response to positive emotion in TRD at baseline. Future studies will be required to determine the specificity and duration of this effect and to confirm the specific role of the striatum in the neurobiology of depression and in the antidepressant mechanism of action of ketamine.
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH094707 (Career Development Award to JWM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support for the research reported was provided by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award to JWM), by the Iris & Junming Le Foundation (JWM), by NIMH grant R01MH081870 (SJM) and by grant UL1TR000067 from the NIH National Center for Advancing Translational Sciences. SJM is supported in part by the Marjorie Bintliff Johnson and Raleigh White Johnson, Jr Chair for Research in Psychiatry, and with resources and the use of facilities at the Michael E. Debakey VA Medical Center, Houston, TX, USA.
In the past 3 years, JWM has served on advisory boards for Janssen Research and Development and Genentech, has provided consultation services for ProPhase, LLC and Impel Neuropharma and has received research support from Janssen and Avanir Pharmaceuticals; he is named on a patent pending for neuropeptide Y as a treatment for mood and anxiety disorders. DVI has consulted for Avanir, CNS Response, INSYS Therapeutics, Lundbeck, Otsuka, Servier, Sunovion and he has received grant/research support through Mount Sinai School of Medicine from Alkermes, AstraZeneca, Brainsway, Euthymics Bioscience, Neosync, Roche and Shire. DSC (Dean of Icahn School of Medicine at Mount Sinai) and Icahn School of Medicine at Mount Sinai have been named on a use patent on ketamine for the treatment of depression. The Icahn School of Medicine has entered into a licensing agreement for the use of ketamine as therapy for treatment-resistant depression. DSC and Icahn School of Medicine at Mount Sinai could potentially benefit if ketamine were to gain approval for the treatment of depression. DSC is named on a patent pending for ketamine as a treatment for PTSD and for neuropeptide Y as a treatment for mood and anxiety disorders; he has received funding from the U.S. Department of Defense, NIH, NIH/NIMH, NARSAD, USAMRAA; he has severed on the scientific advisory board for the Institute of Medicine Committee on DHS Workforce Resilience and on the editorial board of CNS Spectrums. SJM has received consulting fees from Bristol-Myers Squibb, Cerecor, Genentech and Naurex, and research support from AstraZeneca, Janssen Research and Development, and Otsuka. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews DC, Furey ML. Human biomarkers of rapid antidepressant effects. Biol Psychiatry. 2013;73:1142–1155. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol. 2006;115:705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem. 2011;96:553–563. doi: 10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Joormann J, Gotlib IH. Judging the intensity of facial expressions of emotion: depression-related biases in the processing of positive affect. J Abnorm Psychol. 2009;118:223–228. doi: 10.1037/a0014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Gotlib IH. Impaired selection of relevant positive information in depression. Depress Anxiety. 2009;26:403–410. doi: 10.1002/da.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Ma Y.Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis Mol Psychiatry 2015. e-pub ahead of print. [DOI] [PubMed]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. 2007;164:599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Simon GE, Perlis RH. Personalized medicine for depression: can we match patients with treatments. Am J Psychiatry. 2010;167:1445–1455. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC, Ibrahim L, Luckenbaugh DA, Brutsche N, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Diazgranados N, Carlson PJ, Ibrahim L, Luckenbaugh DA, Brutsche N, et al. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 2014;16:119–128. doi: 10.1111/bdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders (SCID) Biometrics Research, New York State Psychiatric Institute: New York, NY, USA; 1995. [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press: Palo Alto, CA, USA; 1976. [Google Scholar]
- Young AW, Perret DI, Calder AJ, Sprengelmeyer R, Ekman P. Facial Expressions of Emotion: Stimuli and Tests (FEEST) Thames Valley Test Company: Bury St. Edmunds, UK; 2002. [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, et al. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neurosci Lett. 2009;452:262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012;520:204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R. Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res. 2011;45:886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- Miller DW, Abercrombie ED. Effects of MK-801 on spontaneous and amphetamine-stimulated dopamine release in striatum measured with in vivo microdialysis in awake rats. Brain Res Bull. 1996;40:57–62. doi: 10.1016/0361-9230(95)02144-2. [DOI] [PubMed] [Google Scholar]
- Rabiner EA. Imaging of striatal dopamine release elicited with NMDA antagonists: is there anything there to be seen. J Psychopharmacol. 2007;21:253–258. doi: 10.1177/0269881107077767. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Hikosaka O. Immediate changes in anticipatory activity of caudate neurons associated with reversal of position-reward contingency. J Neurophysiol. 2005;94:1879–1887. doi: 10.1152/jn.00012.2005. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2007;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS One. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry. 2014;76:927–936. doi: 10.1016/j.biopsych.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the h emodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JD. Neural systems supporting the control of affective and cognitive conflicts. J Cogn Neurosci. 2009;21:1842–1855. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.