Abstract
Pharmacogenetics may allow for a personalized treatment, but a combination with clinical variables may further enhance prediction. In particular, in the present paper, we investigated early partial improvement (EPI) defined as 20% or more improvement by rating scales 2 weeks after treatment, in combination with selected gene variants as a predictor of treatment outcome in patients with major depressive disorder. Two randomized controlled trials with 168 Japanese depressed patients were used. A stepwise multiple linear regression model with HAM-D score change at week 6 as the dependent variable and genotypes, EPI, baseline HAM-D score, age and sex as independent variables was performed in paroxetine, fluvoxamine and milnacipran, respectively, to estimate the prediction of HAM-D change at week 6. In the paroxetine sample, only EPI (P<0.001) was significantly associated with HAM-D change (n=81, R2=0.25, P<0.001). In the fluvoxamine sample, 5-HTTLPR La/Lg, S (P=0.029), FGF2 rs1449683C/T (P=0.013) and EPI (P=0.003) were associated with HAM-D change (n=42, R2=0.43, P<0.001). In the milnacipran sample, HTR-1A-1019C/G (P=0.001), ADRA2A-1297C/G (P=0.028) and EPI (P<0.001) were associated with outcome (n=45, R2=0.71, P<0.001). EPI in combination with genetic variants could be a useful predictor of treatment outcome and could strengthen the practical use of pharmacogenetic data in clinical practice.
Introduction
Major depressive disorder is a debilitating disease that causes a significant burden not only on patients but also on society. At present, major depressive disorder ranked third among all diseases in the world and ranked top in 50 high-income countries in the disability-adjusted life year in 2004 (http://apps.who.int/gho/data/node.main.921?lang=en) despite its relatively low mortality. The introduction and development of antidepressant drugs has largely contributed to the treatment of major depressive disorder, however, the evaluation of antidepressant efficacy in the treatment of major depressive disorder usually takes more than 4 weeks and 60% patients do not show a significant remission after 12 weeks of treatment even with sufficient dose of antidepressant.1, 2 This leads to an increase in the number of medications used, poor response1 and high health-care costs.3 The failure of an initial and subsequent treatments results in patients spending long periods of suffering. Despite results coming from attempts to identify the best antidepressant for all patients, it is clear from clinical practice that each patient differs in the response to each compound.4 An accurate baseline prediction of antidepressant response would help to evaluate the best therapeutic tool for each patient. In this context, a large amount of effort has been directed to the search of genetic predictors of drug efficacy in mood disorders in the last few years and a number of papers have reported positive associations between genetic variants and treatment response to antidepressants. Then in 2007 and 2010, we have reported two comprehensive meta-analyses of antidepressant pharmacogenetic findings to accumulate and translate findings into clinical practice.5, 6 But the variance explained by single-gene variant for antidepressant response is low and this is in accordance with the range of effect of single-gene variants in complex disorders7 and it means that multiple single-nucleotide polymorphisms (SNPs) have a role in individual difference of treatment response. Moreover, genes may interact with the environment in determining the final phenotype8 that in turn interacts with the drug. On the other hand, apart from biological factors, large meta-analysis showed that early partial improvement (EPI) defined as 20% or more improvement by rating scale such as the Hamilton Rating Scale for Depression (HAM-D)9 or the Montgomery–Åsberg Depression Rating Scale10 2 weeks after treatment, is a robust predictor of outcome with high sensitivity and negative predictive value.11 But changing the antidepressant agent in week 2 is not common in clinical practice. Therefore, we hypothesized that the combination of genetic factors and EPI, having insufficient utility as predictor by themselves, could constitute practical predictors for antidepressant response that could be specific across different therapeutic agents as well as different genetic variants.
Materials and methods
Subject and treatment
The analysis was performed using the clinical and genetic data retrieved from our two open-label randomized controlled trials in patients suffering from major depressive disorder (Figure 1).12, 13, 14 The method and design of these two clinical trials were described elsewhere.13, 14 Briefly, a total of 201 Japanese patients consecutively admitted to the Department of Neuropsychiatry at Kansai Medical University Osaka, affected by major recurrent depression, were evaluated at baseline and bi-weekly thereafter until week 6 using the 21-item HAM-D administered by trained senior psychiatrists masked to genetic data. Patients were either drug-free or taking ineffective antidepressants and after 10 days of washout, paroxetine (n=51) or fluvoxamine (n=49; trial 1) or either paroxetine (n=50) or milnacipran (n=51; trial 2) was randomly assigned to reach therapeutic doses from days 8 to 11 until the end of trial (fluvoxamine: 150 mg per day; paroxetine: 40 mg per day, milnacipran 100 mg per day). Concomitant psychotropic drugs were not allowed, except for a low dose of sleep-inducing hypnotic agents at bedtime. Response was evaluated by the percentage HAM-D score change. EPI was defined as at least 20% HAM-D decrease at 2 weeks after antidepressant medication. The study was approved by the ethics committee of Kansai Medical University and Osaka University. Written informed consent was obtained from all the participants before entry into the study. Plasma levels of paroxetine, fluvoxamine and milnacipran were determined by liquid chromatography-tandem mass spectrometry after at least 2 weeks of stable dosage. The patients with plasma levels exceeding the mean value of the sample+1.96 s.d. were excluded from the study to avoid the possibility that extreme differences in the bioavailability of the drug could influence the clinical response.
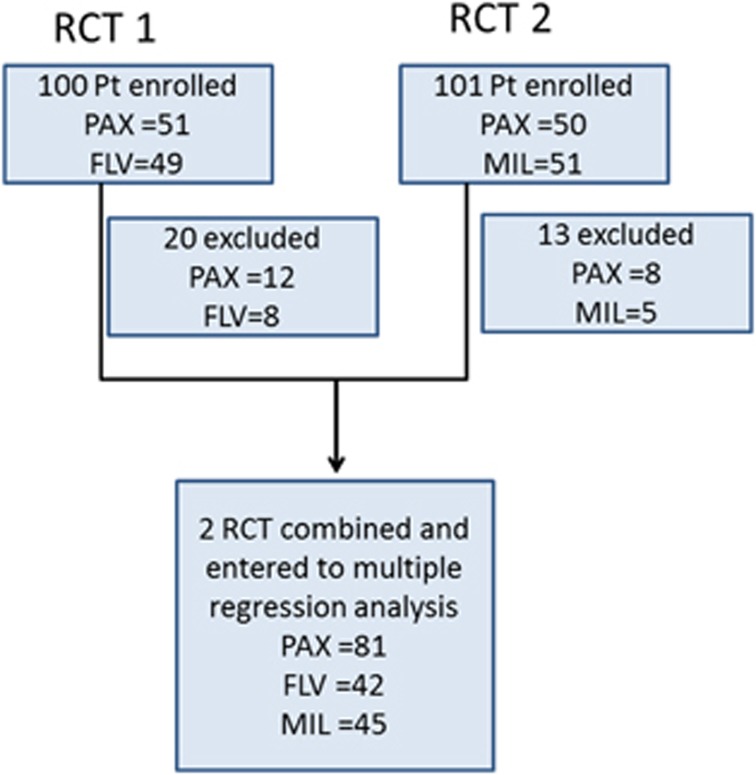
Figure 1.
Flowchart of the subjects included in the study from two randomized controlled studies. FLV, fluvoxamine; MIL, milnacipran; PAX, paroxetine; RCT, randomized controlled trial.
Genotyping
Candidate genetic variants were selected on the basis of our previous meta-analyses and pharmacogenetic studies and entered into the analysis if their contributions to antidepressant response were significant in those studies.5, 6, 14, 15, 16, 17 In detail, serotonin transporter (SLC6A4) gene promoter polymorphism (5-HTTLPR), serotonin-2A receptor gene (HTR2A)-1438A/G (rs6311), tryptophan hydroxylase 1 gene (TPH1) 218A/C (rs1800532) and brain-derived neurotrophic factor (BDNF) gene 66Val/Met (rs6265) were selected on the basis of the results of meta-analyses.5, 6 Serotonin-1A receptor gene (HTR1A)-1019C/G (rs6295),18 alpha 2A-adrenergic receptor (ADRA2A) gene-1297C/G (rs1800544), basic fibroblast growth factor (FGF2) gene rs1449683C/T17 and ATP-binding cassette subfamily B, membrane 1 transporter (ABCB1) gene G2677T/A (rs2032582)15 were selected on the basis of our previous trials. In addition, functional SNP rs25532 within long variant of 5-HTTLPR was also entered to analysis in combination with 5-HTTLPR based on our previous study.16 Genomic DNA was isolated from peripheral leukocytes using a QIAGEN blood Maxi kit (Qiagen, Tokyo, Japan). Each variant was determined by TaqMan(r) SNP Genotyping Assays or real-time polymerase chain reaction method according to a previous study.14, 15, 16, 17 Genotyping of our subjects was done in duplicate together with DNA samples with known genotype as internal control and, in case of disagreement, the analysis was repeated.
Statistical analysis
A stepwise multiple linear regression model with percent changes in HAM-D scores at week 6 as the dependent variable and eight genetic variants, EPI, baseline HAM-D score, age and sex as the independent variables was performed in each antidepressant and a prediction formula was developed. For eight genetic variants, dichotomous variables were used based on their significant contribution observed in previous studies, in detail, 5-HTTLPR+rs25531:LA allele carrier vs S'(LG or S) homozygotes, HTR2A-1438A/G: G/G vs A allele carrier, TPH1 218A/C: C/C vs A allele carrier, BDNF 66Val/Met: Met carrier vs Val homozygotes, HTR1A-1019C/G: G/G vs C-allele carrier, ADRA2A-1297C/G: C-allele carrier vs G/G, FGF2 rs1449683C/T: T allele carrier vs C/C and ABCB1 G2677T/A: T or A homozygotes vs G carrier. The difference of sociodemographic and clinical characteristics among three antidepressants were analyzed using analysis of variance or the Chi-square test. Results were considered significant with an alpha level <0.05. An ‘intention-to-treat' analysis was instead carried out for the patients who underwent a baseline and EPI (week 2) assessments; the last observation was carried forward on the HAM-D. Statistical analyses were carried out using IBM SPSS statistics 19 for Windows (IBM, Chicago, IL, USA). In our sample, considering an alpha value of 0.05, we had sufficient power (0.80) to detect, as an example for BDNF in univariate analyses, a medium−small effect size of d=0.46 which corresponds to a difference at final HAM-D percent change of ~10.6 percent.19 For other variants and multivariate analyses, the power was generally marginally lower.
Result
Out of 201 patients, seventeen were removed for intolerable adverse events, four subjects for lack of compliance as displayed by low plasma levels and thirteen patients for untraceability (that is, lost to follow-up), therefore 168 patients were used for subsequent analyses (Figure 1). Excluded subjects did not differ from the analyzed sample as for sociodemographic variables (data not shown). The genotype frequency of each SNP was the same as NCBI data (http://www.ncbi.nlm.nih.gov/SNP/) that is: 5-HTTLPR in combination with rs25531:LA, 26.8%, HTR2A-1438A/G: G/G 22.0%, TPH1 218A/C: C/C 16.7%, BDNF gene 66Val/Met: Met 66.7%, HTR1A-1019C/G: G/G 4.2%, ADRA2A-1297C/G: C 51.8%, FGF2 rs1449683C/T: T 26.2% and ABCB1 G2677T/A: T or A homozygotes 34.5%. All SNPs were in Hardy–Weinberg equilibrium.
Table 1 reports sociodemographic characteristics of the sample, the mean HAM-D scores at baseline, HAM-D score change at week 6 and rate of EPI, for all subjects and each antidepressant. No significant differences were found for age, sex or rate of EPI, while HAM-D score at baseline and HAM-D change at week 6 were significantly different among three antidepressants. Lower HAM-D baseline was observed in the milnacipran group and higher HAM-D change was observed in the paroxetine group. In the multiple linear regression model for the whole sample, 5-HTTLPR LA/S'(beta=−0.16, P=0.013) and EPI (beta=0.58, P<0.001) were significantly associated with HAM-D score change at week 6 (n=168, R2=0.37, P<0.001). Subsequent analyses were performed in the paroxetine, fluvoxamine and milnacipran samples to estimate the regression equation of HAM-D score change at week 6. In the paroxetine sample, only EPI (beta=0.50, P<0.001) was significantly associated with HAM-D change (n=81, R2=0.25, P<0.001) and the regression equation was 41.6+33.9 (EPI; yes=1, no=0; Table 2). In the fluvoxamine sample, 5-HTTLPR LA/S' (beta=0.29, P=0.029), FGF2 RS1449683C/T (beta=0.33, P=0.013) and EPI (beta=0.42, P=0.003) were significantly associated with HAM-D change (n=42, R2=0.43, P<0.001) and the regression equation was 39.3+16.3 (5-HTTLPR; LA=1, S'/S'=0)+16.4 (FGF2; C/C=0, T=1)+19.8 (EPI; yes=1, no=0). In the milnacipran sample, HTR-1A-1019C/G (beta=0.30, P=0.001), ADRA2A-1297C/G (beta=0.19, P=0.028) and EPI (beta=0.74, P<0.001) were significantly associated (n=45, R2=0.71, P<0.001) and the regression equation was −28.1+52.8 (HTR1A; C=0, G/G=1)+14.2 (ADRA2A; C=1, G/G=0)+54.4 (EPI; yes=1, no=0). For example, patients carrying 5-HTTLPR LA, HTR1A C, ADRA2A C and FGF2 T alleles were expected to reach at least 41.6% improvement by paroxetine, 72.0% by fluvoxamine and −13.9% by milnacipran and when achieving more than 20% improvement at week 2, the HAM-D improvement at week 6 could reach 75.5, 91.8 and 40.5%, respectively. Other polymorphisms, age and sex did not remain in the formula of multiple linear regression analysis.
Table 1. Sociodemographic characteristics, Hamilton Rating Scale for Depression (HAM-D) baseline, HAM-D score change at week 6 and rate of early partial improvement (EPI).
| Total, N=168 | PAX, N=81 | FLV, N=42 | MIL, N=45 | P | |
|---|---|---|---|---|---|
| Age (years) | 46.3±14.8 | 45.4±15.1 | 45.0±14.9 | 49.3±14.3 | 0.297 |
| Sex male % | 53.6 | 55.6 | 52.4 | 51.1 | 0.897 |
| HAM-D baseline | 21.5±6.1 | 21.7±5.2 | 23.9±7.9 | 19.0±4.6 | 0.001 |
| HAM-D % change 6 weeks | 57.7±32.6 | 63.8±32.4 | 60.2±23.9 | 57.7±32.6 | 0.005 |
| EPI % | 58.3 | 65.4 | 57.1 | 46.7 | 0.122 |
Abbreviations: FLV, fluvoxamine; MIL, milnacipran; PAX, paroxetine.
Table 2. Predictive formula of Hamilton Rating Scale for Depression (HAM-D) % change at week 6.
| PAX; (n=81, R2=0.25, P<0.001)=41.6%+33.9% × A |
| FLV; (n=42, R2=0.43, P<0.001)=39.3%+19.8% × A+16.3% × B +16.4% × E |
| MIL; (n=45, R2=0.71, P<0.001)=−28.1%+54.4% × A+52.8% × C +14.2% × D |
| Alphabets in regression equation and frequency of favorable genotype and EPI |
| A; EPI Yes, (Pax, 70% Flv, 50% Mil, 60%) |
| B; 5-HTTLPR LA (26.8%), C; HTR1A-1019G/G (4.2%) |
| D; ADRA2A C (51.8%), E; FGF2 T (26.2%) |
Abbreviations: EPI, early partial improvement; FLV, fluvoxamine; MIL, milnacipran; PAX, paroxetine.
When the patient has the genotype above, corresponding alphabet in the regression equation is 1. When the patient does not have such a genotype, corresponding alphabet in equation is 0.
Discussion
We confirmed that EPI confers a large contribution to the treatment response at week 6 to paroxetine and milnacipran and less to fluvoxamine and genetic variants in ADRA2A and HTRA1A also influenced milnacipran response and 5-HTTLPR and FGF2 influenced fluvoxamine response in the multiple linear regression analysis.
Trajectories of treatment response to antidepressant largely varied among the patients with major depressive disorder.20 A great deal of effort was made to predict such an individual treatment response from pharmacogenetic point of view with the advancement of technology in genetic analysis. Pharmacogenetic studies in genome-wide analyses approach might be one solution to detect the SNP that could predict antidepressant treatment response. However, genome-wide association studies on antidepressant efficacy have yielded only modest results with lack of consistency among studies.21, 22, 23 A possible reason could be that the variance explained by single-gene variant for antidepressant response is low and this is in accordance with the range of effect of single-gene variants in complex disorders. Furthermore, different conditions of intervention, such as specific antidepressants and concomitant medications could bias the efficacy of individual genetic difference. Unfortunately, even if the robust genetic predictor could be found in combined analysis of some studies with different treatment setting, we could not get an answer to the clinical question of how genetic factor can help to select the best antidepressant for each patient in clinical practice. Translational approach is therefore needed to help to decipher the result of pharmacogenetic studies into real-world clinical practice. In the present paper, we hypothesized one possible approach to translate pharmacogenetic result into clinical use and suggested considering genetic variants for non-EPI subjects before changing the antidepressant agent in week 2.
Given the comparatively low R2 of one predictor, EPI, detected in the paroxetine sample, other genetic factors may contribute to treatment response. 5-HTTLPR LA/S', useful predictor in the formula of fluvoxamine, is a well-known functional polymorphism in the transcriptional control region upstream of the serotonin transporter coding sequence in combination with rs25531, another functional SNP within 5-HTTLPR and the L variant with an adenosine at SNP rs25531 (LA) has been reported to have higher activity compared with the long variant with a guanine (LG)1 (Hu et al.24) and LG expression is nearly equal to the s-allele. In our previous study, LG carriers showed a reduced improvement to fluvoxamine but not to paroxetine.16 FGF2 is a neurotrophic molecule that is highly expressed in the adult brain and is protective for a wide range of neurons.25 Reduced FGF2 expression was described in the brain of subjects with major depression26, 27 and the altered FGF2 expression was attenuated by the administration of SSRIs. Rs1449683C/T of FGF2 gene is the functional polymorphism28 and contributed to SSRI response.17 As for prediction formula of milnacipran treatment, -1019C/G variant in the promoter region of HTR1A was associated with an altered expression and the function of HTR1A.29, 30 G-allele is associated with an increase of serotonin-1A autoreceptors and a reduction of serotonergic neurotransmission31 and subjects carrying this allele showed better treatment response to SSRI/SNRI in our previous study18 and meta-analysis. ADRA2A is an auto receptor in the noradrenaline system and its increased expression was observed in the brain of suicidal patients with major depression.32 The C-allele carrier of the ADRA2A-1297C/G polymorphism contributed to a more favorable response than the G/G homozygote to milnacipran, not paroxetine in our previous study.14 Other genetic polymorphisms that have found to be associated with antidepressant treatment response in our previous studies and meta-analysis such as HTR2A-1438A/G, TPH1 218A/C, BDNF 66Val/Met and ABCB1 G2677T/A might be lacking of utility to predict paroxetine, fluvoxamine and milnacipran treatment response in combination with EPI may be due to small effect size compared with EPI, that is to say that these SNPs' contribution might be limited in real-world clinical practice to predict treatment response of these antidepressants.
Several limitations need to be acknowledged. The relatively small sample size lacking of genomic control is liable for stratification bias, however, the Japanese population is considered genetically homogeneous33 and no patient from other regions was included in the study. A further limitation is linked to the lack of control for possible clinical confounders such as personality disorder, number of previous episodes, family history and previous treatments. Candidate gene approach could not assess the SNPs other than hypothetical genes and other genes could have sufficient effect to treatment response that should be included to the prediction formula. Finally, we did not test the model in an independent sample to fully validate it.
In conclusion, we propose a practical method to apply results of pharmacogenetic studies into clinical practice. EPI with specific genetic variants could be a useful predictor of treatment outcome and help personalized treatment of depression. Further prospective studies in which an antidepressant is selected or changed on the basis of these predictive formulae will be needed to confirm the result of this study.
This study was supported by grants from SENSHIN Medical Research Foundation and Grant-in-Aid for Scientific Research (KAKENHI). GlaxoSmithKline, Asahi Kasei Pharma Corporation and Meiji Seika Kaisha Ltd determined plasma level of paroxetine milnacipran and fluvoxamine and provided no further support. The authors declare no conflict of interest.
References
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry. 2007;68:1062–1070. doi: 10.4088/jcp.v68n0713. [DOI] [PubMed] [Google Scholar]
- Russell JM, Hawkins K, Ozminkowski RJ, Orsini L, Crown WH, Kennedy S, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004;65:341–347. doi: 10.4088/jcp.v65n0309. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Kendler KS. ‘A gene for...': the nature of gene action in psychiatric disorders. Am J Psychiatry. 2005;162:1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70:344–353. doi: 10.4088/jcp.07m03780. [DOI] [PubMed] [Google Scholar]
- Kato M, Ikenaga Y, Wakeno M, Okugawa G, Nobuhara K, Fukuda T, et al. Controlled clinical comparison of paroxetine and fluvoxamine considering the serotonin transporter promoter polymorphism. Int Clin Psychopharmacol. 2005;20:151–156. doi: 10.1097/00004850-200505000-00005. [DOI] [PubMed] [Google Scholar]
- Kato M, Fukuda T, Wakeno M, Fukuda K, Okugawa G, Ikenaga Y, et al. Effects of the serotonin type 2A, 3A and 3B receptor and the serotonin transporter genes on paroxetine and fluvoxamine efficacy and adverse drug reactions in depressed Japanese patients. Neuropsychobiology. 2006;53:186–195. doi: 10.1159/000094727. [DOI] [PubMed] [Google Scholar]
- Wakeno M, Kato M, Okugawa G, Fukuda T, Hosoi Y, Takekita Y, et al. The alpha 2A-adrenergic receptor gene polymorphism modifies antidepressant responses to milnacipran. J Clin Psychopharmacol. 2008;28:518–524. doi: 10.1097/JCP.0b013e31818455fc. [DOI] [PubMed] [Google Scholar]
- Kato M, Fukuda T, Serretti A, Wakeno M, Okugawa G, Ikenaga Y, et al. ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:398–404. doi: 10.1016/j.pnpbp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kato M, Nonen S, Azuma J, Serretti A, Tetsuo S, Takekita Y, et al. 5-HTTLPR rs25531A>G differentially influence paroxetine and fluvoxamine antidepressant efficacy: a randomized, controlled trial. J Clin Psychopharmacol. 2013;33:131–132. doi: 10.1097/01.jcp.0000426182.66701.76. [DOI] [PubMed] [Google Scholar]
- Kato M, Okugawa G, Wakeno M, Takekita Y, Nonen S, Tetsuo S, et al. Effect of basic fibroblast growth factor (FGF2) gene polymorphisms on SSRIs treatment response and side effects. Eur Neuropsychopharmacol. 2009;19:718–725. doi: 10.1016/j.euroneuro.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Kato M, Fukuda T, Wakeno M, Okugawa G, Takekita Y, Watanabe S, et al. Effect of 5-HT1A gene polymorphisms on antidepressant response in major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:115–123. doi: 10.1002/ajmg.b.30783. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates: Hillsdale, NJ, USA; 1988. pp. 8–14. [Google Scholar]
- Gueorguieva R, Mallinckrodt C, Krystal JH. Trajectories of depression severity in clinical trials of duloxetine: insights into antidepressant and placebo responses. Arch Gen Psychiatry. 2011;68:1227–1237. doi: 10.1001/archgenpsychiatry.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Perroud N, Ng MY, Hauser J, Henigsberg N, Maier W, et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry. 2010;167:555–564. doi: 10.1176/appi.ajp.2009.09070932. [DOI] [PubMed] [Google Scholar]
- Garriock HA, Kraft JB, Shyn SI, Peters EJ, Yokoyama JS, Jenkins GD, et al. A genomewide association study of citalopram response in major depressive disorder. Biol Psychiatry. 2010;67:133–138. doi: 10.1016/j.biopsych.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Lucae S, Binder EB, Bettecken T, Uhr M, Ripke S, et al. A genomewide association study points to multiple loci that predict antidepressant drug treatment outcome in depression. Arch Gen Psychiatry. 2009;66:966–975. doi: 10.1001/archgenpsychiatry.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dono R. Fibroblast growth factors as regulators of central nervous system development and function. Am J Physiol Regul Integr Comp Physiol. 2003;284:R867–R881. doi: 10.1152/ajpregu.00533.2002. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Schulz S, Kohler K, Schagdarsurengin U, Greiser P, Birkenmeier G, Muller-Werdan U, et al. The human FGF2 level is influenced by genetic predisposition. Int J Cardiol. 2005;101:265–271. doi: 10.1016/j.ijcard.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Stahl S. 5HT1A receptors and pharmacotherapy. Is serotonin receptor down-regulation linked to the mechanism of action of antidepressant drugs. Psychopharmacol Bull. 1994;30:39–43. [PubMed] [Google Scholar]
- Meana JJ, Barturen F, Garcia-Sevilla JA. Alpha 2-adrenoceptors in the brain of suicide victims: increased receptor density associated with major depression. Biol Psychiatry. 1992;31:471–490. doi: 10.1016/0006-3223(92)90259-3. [DOI] [PubMed] [Google Scholar]
- Cavalli Sforza L. The History and Geography of Human Genes. Princeton University Press: Princeton, NJ, USA; 1994. [Google Scholar]