Abstract
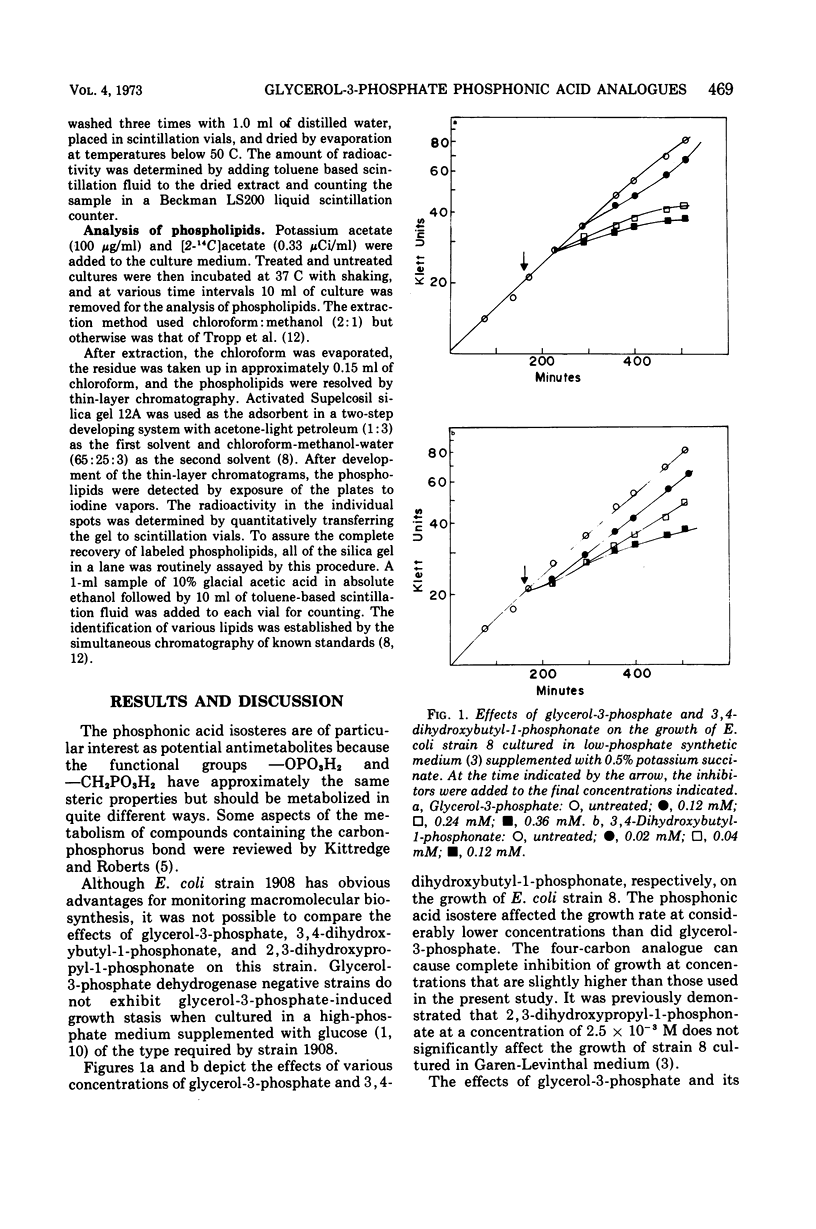
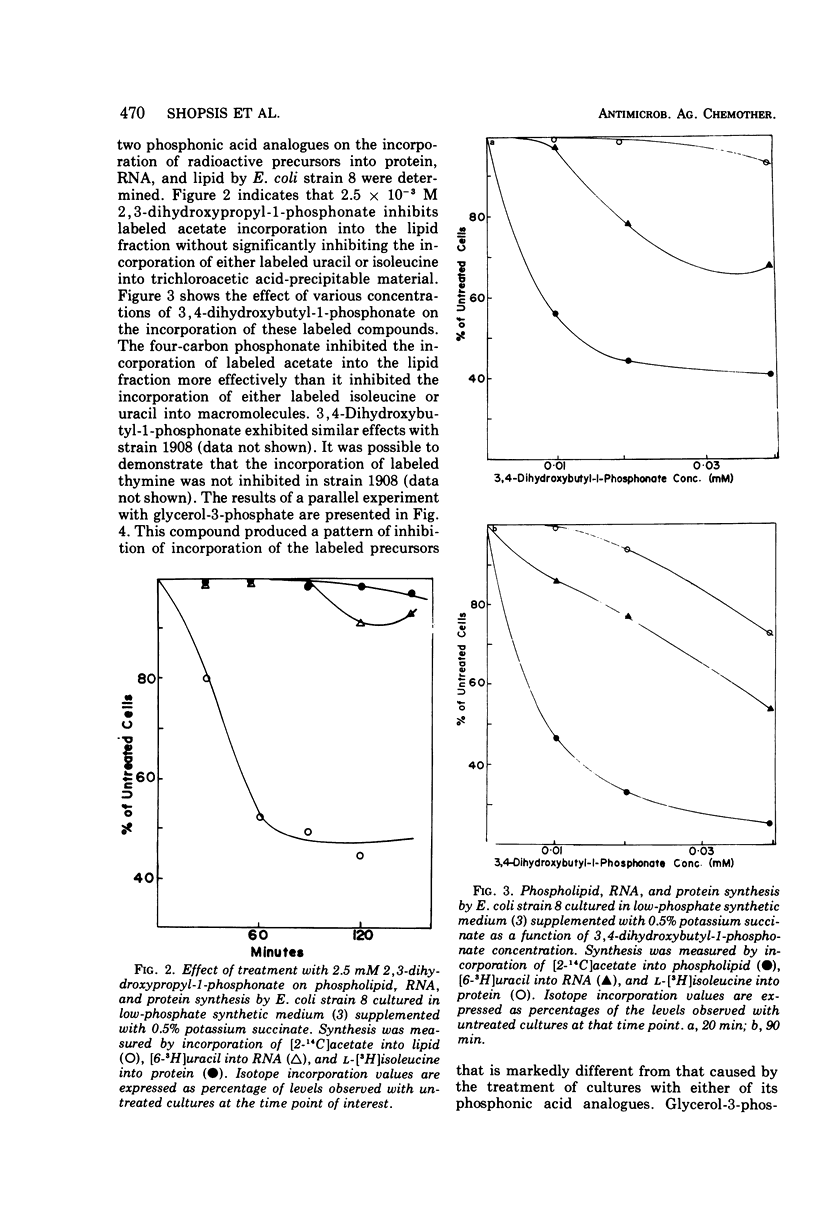
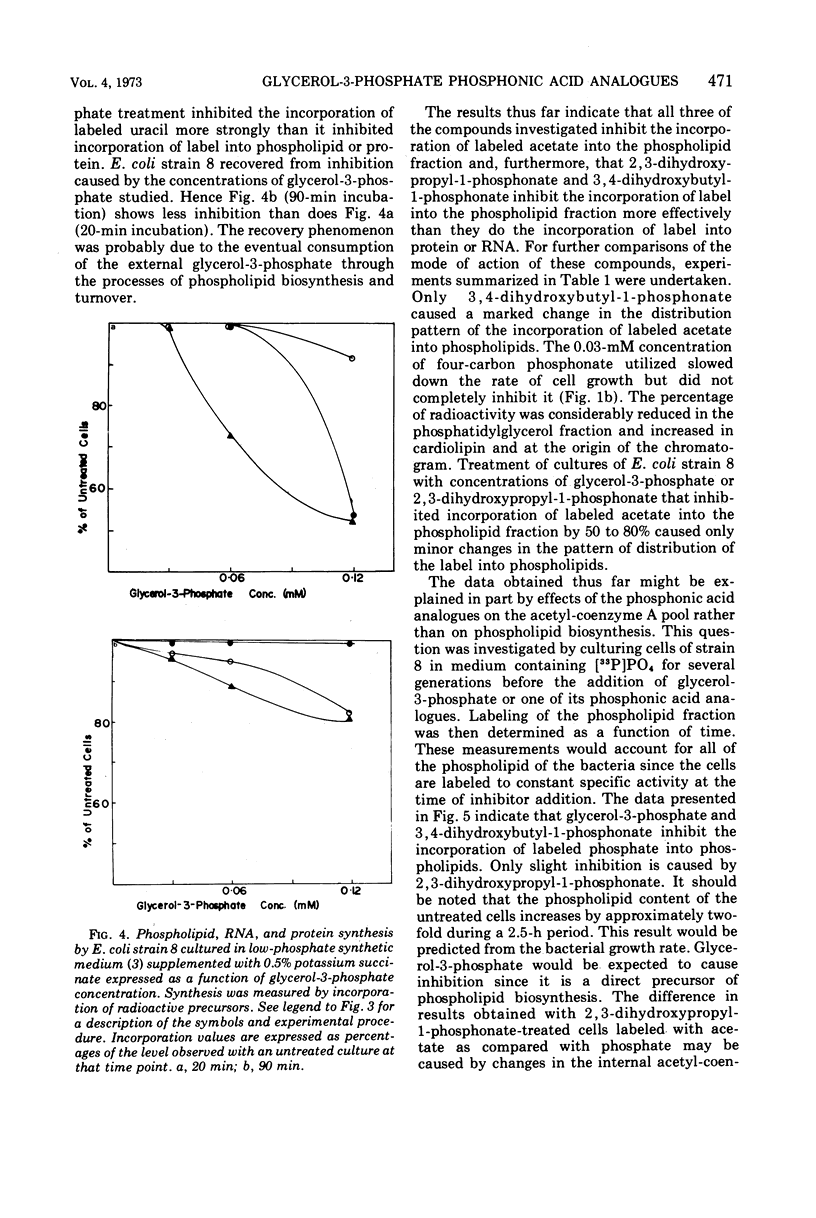
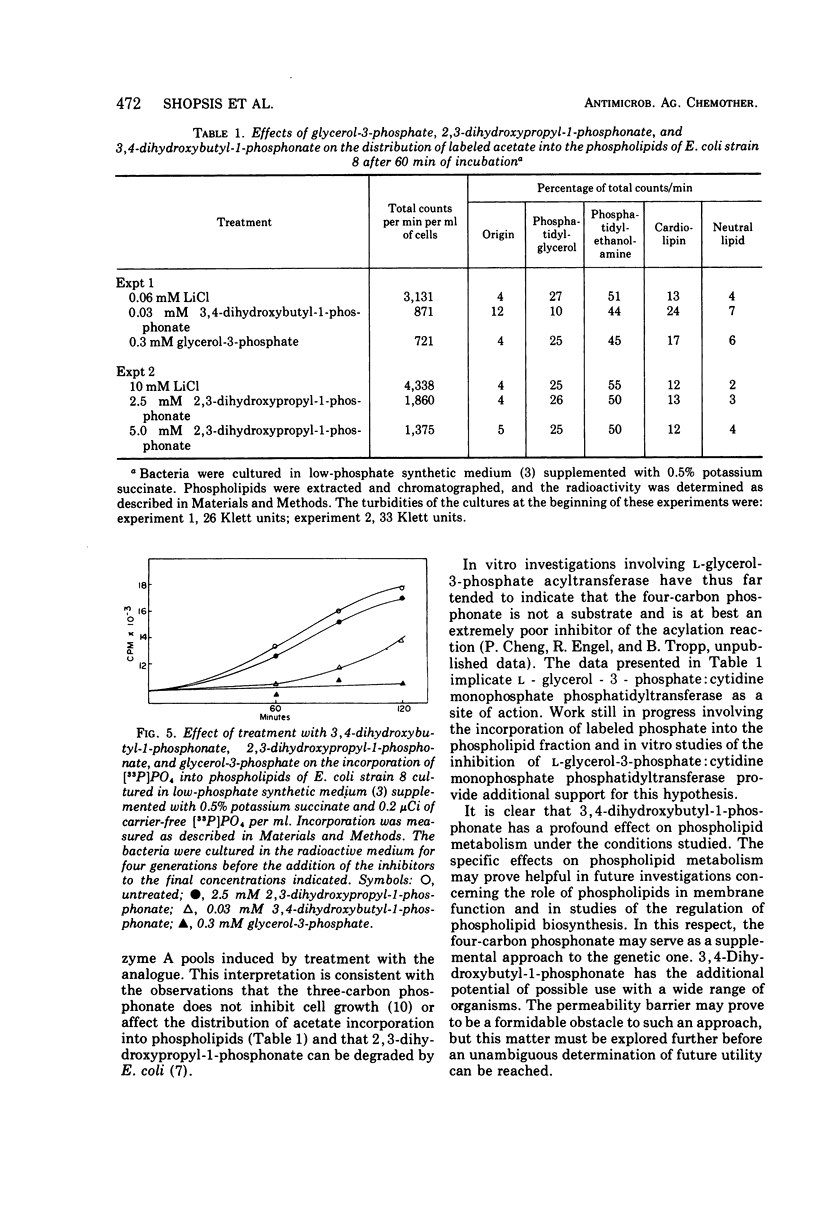
The effects of glycerol-3-phosphate, 3,4-dihydroxybutyl-1-phosphonate, and 2,3-dihydroxypropyl-1-phosphonate on the metabolism of Escherichia coli strains 8 and 1908 were determined. These strains lack the membrane-bound glycerol-3-phosphate dehydrogenase and are constitutive for the glycerol-3-phosphate transport system. Such cells were more sensitive to growth inhibition by the four-carbon phosphonate than by glycerol-3-phosphate. The three-carbon phosphonate did not appear to inhibit cell growth. The incorporation of labeled precursors of lipid, protein, ribonucleic acid, or deoxyribonucleic acid into bacterial cells was measured in the presence of either glycerol-3-phosphate or one of its phosphonic acid analogues. The phosphonic acid analogues inhibited the uptake of labeled acetate into the lipid fraction to the greatest extent. The incorporation of [33P]PO4 into phospholipids was strongly inhibited by 3,4-dihydroxybutyl-1-phosphonate but was only slightly affected by 2,3-dihydroxypropyl-1-phosphonate. Glycerol-3-phosphate inhibited the incorporation of labeled uracil to the greatest extent during the first 20 min; however, this effect was largely reversed after 90 min. Only 3,4-dihydroxybutyl-1-phosphonate altered the distribution of labeled acetate into the phospholipids of strain 8, decreasing the percentage of counts in the phosphatidylglycerol fraction. The three-carbon phosphonate probably alters acetate incorporation by affecting the acetyl-coenzyme A pool, whereas the 3,4-dihydroxybutyl-1-phosphonate has a definite effect upon phospholipid metabolism. It is suggested that l-glycerol-3-phosphate:cytidine monophosphate phosphatidyltransferase is the probable site of action.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- Kabak J., DeFilippe L., Engel R., Tropp B. Synthesis of the phosphonic acid isostere of glycerol 3-phosphate. J Med Chem. 1972 Oct;15(10):1074–1075. doi: 10.1021/jm00280a022. [DOI] [PubMed] [Google Scholar]
- Kittredge J. S., Roberts E. A carbon-phosphorus bond in nature. Science. 1969 Apr 4;164(3875):37–42. doi: 10.1126/science.164.3875.37. [DOI] [PubMed] [Google Scholar]
- MASTALERZ P., WIECZOREK Z., KOCHMAN M. UTILIZATION OF CARBON-BOUND PHOSPHORUS BY MICROORGANISMS. Acta Biochim Pol. 1965;12:151–156. [PubMed] [Google Scholar]
- Nunn W. D., Tropp B. E. Effects of phenethyl alcohol on phospholipid metabolism in Escherichia coli. J Bacteriol. 1972 Jan;109(1):162–168. doi: 10.1128/jb.109.1.162-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsis C. S., Engel R., Tropp B. E. Effects of phosphonic acid analogues of glycerol-3-phosphate on the growth of Escherichia coli. J Bacteriol. 1972 Oct;112(1):408–412. doi: 10.1128/jb.112.1.408-412.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropp B. E., Meade L. C., Thomas P. J. Consequences of expression of the "relaxed" genotype of the RC gene. Lipid synthesis. J Biol Chem. 1970 Feb 25;245(4):855–858. [PubMed] [Google Scholar]
- Weiner J. H., Heppel L. A. Purification of the membrane-bound and pyridine nucleotide-independent L-glycerol 3-phosphate dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1360–1365. doi: 10.1016/0006-291x(72)90222-7. [DOI] [PubMed] [Google Scholar]


