Abstract
The objective of this study was to find the optimum dose of flaxseed that would decrease prostaglandins and alter estrogen pathway endpoints implicated in ovarian cancer. Fifty 1.5-year-old chickens per group were fed different percentages of flaxseed (5%, 10%, 15%) for four months then sacrificed to collect blood and tissues. Levels of flaxseed lignan metabolites, Enterolactone (EL) and Enterodiol (ED), were measured in the serum, liver and ovaries by LC-MS/MS while omega-3 and 6 fatty acid levels were measured by GC. Effect of varying flaxseed doses was assessed by measuring levels of prostaglandin E2, estrogen metabolites (16-hydroxyestrone (16-OHE1), 2-hydroxyestrone (2-OHE1)) and analyzing the expression of E2 metabolizing enzymes (CYP3A4, CYP1B1, CYP1A1) and ERα in the ovaries. The ratio of omega-3 fatty acids/ omega-6 fatty acids increased with increase in flaxseed supplementation of diet corresponding to a dose dependent decrease in COX-2 protein and prostaglandin E2 levels. EL and ED increased in the liver, ovary and serum with increasing concentrations of flaxseed. Flaxseed decreased the expression of ERα in the ovary. The ratio of 2-hydroxyestrone to 16-hydroxyestrone in the serum increased significantly in the 15% flaxseed diet with a corresponding increase in CYP1A1 in the liver and a decrease in CYP3A4 in the ovary, respectively. CYP1B1 mRNA also decreased with flaxseed diet in the ovary. The 15% flaxseed supplemented diet significantly decreased inflammatory prostaglandin E2, ERα, CYP3A4, CYP1B1 and 16-OHE1 while it increased CYP1A1 and 2-OHE1, thus reducing the inflammatory and pro-carcinogenic microenvironment of the ovary.
Keywords: estrogen, prostaglandin, flaxseed, ovarian cancer prevention
1. INTRODUCTION
Flaxseed (Linum usitatissimum) is an excellent source of alpha linoleic acid (ALA), a polyunsaturated omega-3 fatty acid (OM-3 FA), dietary fiber and is the richest source of plant lignan (1). Secoisolaricirescinol diglucoside (SDG) is the predominant lignan present in flaxseed (2). Flaxseed is known to exhibit protective effects against a multitude of chronic ailments like cardiovascular diseases, stroke and cancer. Investigations have shown that flaxseed reduces the risk of breast and colon cancer, atherosclerosis, insulin dependent diabetes mellitus (IDDM) and hyperlipoproteinemia (3–5).
Using the chicken model, we have demonstrated that flaxseed is beneficial in mitigating the severity and reducing the incidence of ovarian cancer (6).The anti-oncogenic properties attributed to flaxseed are mainly due to the OM-3 FA and the lignan component. OM-3 FAs inhibit synthesis of certain arachidonic acid (AA) derived prostaglandins like PGE2, which are known to be elevated in many cancers. PGE2 can initiate tumor growth by binding to its receptors on target tissues and activating signaling pathways, which control processes like cell proliferation, migration, apoptosis, and angiogenesis (7). Cyclooxygenase (COX) enzymes catalyze the synthesis of prostaglandins from arachidonic acid. COX-1 enzyme is expressed constitutively and imparts its homeostatic effects by synthesizing prostaglandins that maintain the integrity of the stomach lining. COX-1 is elevated with ovarian cancer in humans (8) as well as the laying hen (9–11). COX-2 is an inducible enzyme and is mainly stimulated under inflammatory responses, commonly by inflammatory cytokines (12). COX-2 is known to be up regulated in a myriad of cancers including gastric adenocarcinoma (13), colorectal cancer (14), breast cancer (15), prostate carcinoma (16), cervical cancer (17), pancreatic cancer (18) and lung cancer (19).
SDG is a precursor to the phytoestrogens, enterolactone (EL) and enterodiol (ED). The initial steps include conversion of SDG to secoisolaricirescinol (SECO) by hydrolysis. Gut flora demethylate and dehydroxylate SECO to form ED, which can get oxidized further to EL (20). ED and EL are known to have phytoestrogenic and anti-oxidant effects. Recent studies have shown that dietary lignan reduces the risk of post-menopausal breast cancer (21,22). In other case control studies, women consuming lignan rich diet also showed decreased risk of breast cancer (23,24).
There is evidence suggesting that in response to estradiol (E2), estrogen receptor alpha (ERα) stimulates growth and invasion in ovarian cancer cells (25). The anti-estrogenic property of the phytoestrogen lignans, which can be attributed to its weak antagonism to ER(26), might mitigate the estrogen dependent aggressiveness in ovarian cancer cells.
Ovarian cancer is one of the deadliest of gynecological malignancies with a five year survival rate of 44% in the U.S (27).This is due to the late stage of detection when the prognosis is poor and treatment options are limited. While identifying early detection markers is the need of the day, developing a preventative approach is equally essential. Ovarian cancer in the laying hen (Gallus domesticus) develops spontaneously and significantly resembles the human disease with respect to the histopathology as well as gross pathology (28–31). We have previously shown that feeding 10% flax diet decreases the incidence and severity of ovarian cancer in chickens with a decrease in levels of COX-2 and prostaglandin E2 (6) in their ovaries (6,32).
The objective of this study was to determine the effect of different doses of flaxseed-supplemented diet on the levels of prostaglandin E2, ER and the E2 metabolizing enzymes involved in the estrogen pathway. Feeding 10% flaxseed diet reduced the aggressiveness of ovarian cancer which was correlated to a decrease in PGE2 (6). The current study was designed to determine the optimum dose of flaxseed in the diet that will maximize the beneficial effects without toxicity. A maximum dose of 15% was selected for the study as an earlier study had indicated that a 20% dose of flaxseed could be hepatotoxic for the chickens.
2. MATERIALS AND METHODS
2.1 Reagents
The reagents were obtained as follows: ER alpha antibody, sc-73479 (Santa Cruz, CA, USA). Biotinylated anti-rabbit IgG, Vector laboratories. AffiniPure Alexa 488 conjugated Donkey Anti-Mouse IgG, Jackson ImmunoResearch. Streptavidin, Alexa Fluor® 488 conjugate, Life technologies. The iScript cDNA synthesis kit and Ssofast EvaGreen supermix (SYBR green) from Bio-Rad Laboratories Inc. (Hercules, CA, USA); TUNEL Apoptosis Detection Kit from GenScript (Piscatway, USA); Estramet ELISA kits used for 2-hydroxyestrone (2-OHE1) and 16-hydroxyestrone (16-OHE1) analysis from Immuna Care Corp; ED and EL standards from Sigma-Aldrich (NSW, Australia); Genistein-d4 (4-hydroxyphenyl-2, 3’, 5’, 6-d4, 98% atom% D) from C/D/N Isotopes Inc. (Quebec, Canada); β-Glucuronidase enzyme, ≥30,000 units/g solid and secoisolaricirescinol Diglucoside (SDG) standard from Sigma-Aldrich, St Louis, MO. The HPLC analysis was done on Shimadzu LC-20A HPLC system (Shimadzu Co., Kyoto, Japan). The GC analysis was performed on the GC 2010 (Shimadzu Co., Kyoto, Japan). All the other reagents were as used for previous studies.(9)
2.2 Animals
Two hundred Single Comb White Leghorn hens (Gallus domesticus), aged 1.5 years were used for the study. Hens were exposed to a photoperiod of 17 h light: 7 h dark, with lights turned on at 05:00 h and turned off at 22:00 h. Animal management and procedures were reviewed and approved by the Institutional Animal Care and Use Committees at the University of Illinois at Urbana-Champaign and Southern Illinois University at Carbondale.
Chickens were fed different percentages (0%, 5%, 10% and 15%) of flaxseed-supplemented diet for a period of 4 months with 50 birds per group. At the end of 4 months, birds were euthanized and tissues were harvested.
2.3 Diet Composition
The composition of the test diets is shown in Table 1. Hens consumed 110 g of food per day and were provided water ad libitum. The content of SDG and ALA were routinely analyzed to ensure that the chicken diets were consistent.
Table 1.
Composition (%) of diets containing different levels of flaxseed.
| Ingredient | Control | 5% Flaxseed | 10% Flaxseed | 15% Flaxseed |
|---|---|---|---|---|
| Corn | 67.40 | 63.20 | 58.70 | 53.60 |
| Soybean meal | 18.30 | 18.30 | 18.30 | 18.30 |
| Corn gluten meal | 3.00 | 1.80 | 0.60 | 0.00 |
| Flaxseed | 0.00 | 5.00 | 10.00 | 15.00 |
| Calculated analysis: | ||||
| CP4, % | 16.5 | 16.5 | 16.5 | 16.5 |
| TME4, kcal/kg | 2,915 | 2,915 | 2,915 | 2,915 |
| Calcium, % | 3.75 | 3.75 | 3.75 | 3.75 |
| Available phosphorus, % | 0.38 | 0.38 | 0.38 | 0.38 |
| Methionine + cysteine,% | 0.67 | 0.67 | 0.67 | 0.67 |
| Each diet contains Limestone (8.75); Dicalcium phosphate (1.50); Iodized salt (0.30); DL-Methionine (0.10); Vitamin premix1 (0.20); Trace mineral premix2 (0.15); Solka Floc (cellulose) 3(0.30) | ||||
Provided per kilogram of diet: retinyl acetate, 4,400 IU; cholecalciferol, 25 µg; DL-∝-tocopheryl acetate, 11 IU; vitamin B12, 0.01 mg; riboflavin, 4.41 mg; D-Ca-pantothenate, 10 mg; niacin, 22 mg; menadione sodium bisulfite, 2.33 mg.
Provided as milligrams per kilogram of diet: Mn, 75 from MnO; Fe, 75 from FeSO4• 7H2O; Zn, 75 from ZnO; Cu, 5 from CuSO4•5H2O; I, 0.75 from ethylene diamine dihydroiodide; Se, 0.1 from Na2SeO3.
International Fiber Corp., North Tonawanda, NY.
CP = crude protein; TME = true metabolizable energy.
2.4 Tissue collection
All the birds included in the study were euthanized and tissues were collected on necropsy as described previously (6). Histology was performed to confirm that all ovaries were normal (33).
2.5 Histology and Immunofluorescence
Formalin fixed ovarian tissue was embedded in paraffin and 5-micrometer thick sections were cut and mounted on SuperFrost Plus microscope slides. Followed by deparaffinization, slides were rehydrated by running them through xylene and graded ethanol solutions. Hematoxylin and eosin staining was performed on the slides as described by Sheehan and Haarpchak (34). Slides were also used for assessing COX-1, COX-2 and estrogen receptor alpha tissue expression and localization by immunofluorescence. Antigen retrieval was performed by using 0.9% Antigen unmasking solution (Vector Laboratories) and pressure cooked at 20 psi for 5 min. Slides were allowed to cool and sections were blocked with 5% normal goat serum (COX-1 and COX-2) or 5% normal horse serum (ER alpha) in 1× TBS for an hour at room temperature. Sections were incubated with either anti-human COX-1 (1:200) / anti-human COX-2 (1:200) / anti-human ER alpha antibody at 4°C overnight. For COX-1 and COX-2 staining, sections were washed with 1× Tris-buffered saline (TBS) and incubated with biotinylated anti-rabbit secondary antibody (1:200) at room temperature for 30 mins. Secondary antibody was decanted, followed by incubation with Streptavidin Alexa Fluor 488 conjugate for 30m at room temperature. For ER alpha staining, sections were incubated with an AffiniPure Alexa 488 conjugated Donkey Anti-Mouse IgG secondary antibody. Sections were rinsed in 1× TBS with 0.5% Tween 20. Slides were mounted using Dapi Fluoromount G (Southern Biotech). All antibody dilutions were made in blocking solution. Control sections were incubated with non-immune rabbit anti IgG (COX-1 and COX-2) or without any primary antibody (ER alpha).
2.6 RNA extraction and analysis
Total RNA was extracted from ovarian tissue using Trizol reagent as described previously (9,35). Quantification was performed by determination of absorbance at A260 and RNA quality was assessed by Experion RNA StdSens Analysis. RNA samples were then treated with RQ1 RNase-free DNase prior to reverse transcription reaction. cDNA was synthesized using the Bio-rad iScript kit.
2.7 Real time PCR
Target gene mRNA levels were analyzed with Real time quantitative PCR using the CFX384 Real Time System. Gene specific primers were used for target as well as reference gene amplification (Table 2). Three housekeeping genes were used to normalize target gene expression. Amplification conditions: 95°C for 30s followed by 40 cycles of 95°C for 10s and 58°C for 15s with melt curve measured at 65 °C to 95 °C every 0.5 °C gradient for 5s. A non-template control was run for every target gene amplified.
Table 2.
Primers used for real time qPCR
| Gene symbol | Common name | Gene id. | Forward primer | Reverse primer |
|---|---|---|---|---|
| Target | ||||
| PTGS1 | Cyclooxygenase 1 | 427752 | 5’TCAGGTGGTTTCTGGGACATCA3’ | 5’TGTAGCCGTACTGGGAGTT GA3’ |
| PTGS2 | Cyclooxygenase 2 | 396451 | 5’CTGCTCCCTCCCATGTCAGA3’ | 5’CACGTGAAGAATTCCGG TGTT3’ |
| ESR1 | Estrogen Receptor 1 | 396099 | 5’CCACAGCATTCGTGAGAGG3’ | 5’GCATAGTCGTTGCACAC AGC3’ |
| CYP1B1 | Cytochrome P450, family 1, subfamily B, polypeptide 1 | 421466 | 5’CAAGATTCCTGGATGAGAACG3’ | 5’GCTGCACCTTGGA TAATTCC3 |
| CYP1A1 | Cytochrome P450, family 1, subfamily A, polypeptide 1 | 396051 | 5’TGGATACCCTCTGCCTCTCT3’ | 5’GGAACTAAGGGGAAG CGTG3’ |
| CYP3A4 | Cytochrome P450, family 3, subfamily A, polypeptide 4 | 416477 | 5’CCATCTTGGTGAAGGAGTGC3’ | 5’TTCCACTGGTC ATCCATAGC3’ |
| Housekeeping | ||||
| TBP | TATA box binding protein | 395995 | 5’ CGTGAGGGAAATAGGCA 3’ | 5’GACTGGCAGCAAGGAAG 3’ |
| SDHA | Succinate dehydrogenase complex, subunit A | 395758 | 5’ CAGGGATGTAGTGTCTCGT 3’ | 5’GGGAATAGGCTCCTTAGTG 3’ |
| RPL4 | Ribosomal protein L4 | 415551 | 5’ TTATGCCATCTGTTCTGCC 3’ | 5’GCGATTCCTCATCTTACCCT 3’ |
2.8 Cell apoptosis and proliferation staining
Five µm paraffin embedded histological sections of chicken ovarian tissue from the control and all the dosage groups were assessed for apoptotic and proliferating cells using TdT mediated dUTP nick end labeling (TUNEL) and proliferating cell nuclear antigen (PCNA) staining respectively, as previously described (36).
2.9 PGE2 EIA
Six ovary tissue samples from each dietary group were analyzed for levels of PGE2 using a PGE2 specific enzyme immunoassay kit from Cayman Chemicals. Snap frozen ovarian tissues maintained at −80 °C were pulverized on dry ice and re-suspended in homogenizing buffer and transferred to clean tubes, ready for solid phase extraction. Extraction and analysis were carried out as described previously (6).
2.10 2-hydroxyestrone and 16-hydroxyestrone ELISA assay
Blood samples were collected from all the chickens during necropsy. Serum samples from control and each of the dosage groups were analyzed for levels of both the 2-hydroxyestrone (2-OHE1) and the 16-hydroxyestrone (16-OHE1) metabolites, using the 2-hydroxyestrone and 16-hydroxyestrone Estramet double ELISA kit (Immuna Care Corp.). Measurement of the estrone (E1) metabolites is proportional to the concentrations of the E1 and E2 metabolites combined.
2.11 Western Blot Analysis
Snap frozen ovary tissue samples (25 µg) from all groups were analyzed for COX-1/COX-2/ER alpha expression and normalized to β-Actin, as described previously (6).
2.12 Gas Chromatography for fattyacid analysis
The OM-3 FA and OM-6 FA levels in the chicken ovaries were analyzed using Shimadzu GC-2010 (Shimadzu Co. Kyoto, Japan). A solution containing 12.5µg/ml C17:0 standard (Sigma-Aldrich) in methanol was added to the tissue sample (25µg) as a recovery standard. Extraction and analysis was performed as described previously (36).
2.13 High Performance Liquid Chromatography for SDG analysis
2.13.1 SDG Extraction
One g flaxseed was hydrolyzed with 50 mL of 0.5 M NaOH at 135 W; microwaving intermittently (30 s on/off) for 3 min. Acidified hydrolysate (pH=3, 5 N H2SO4) was added to100 mL of methanol to precipitate the carbohydrates and proteins. After a 10 min centrifugation at 3,000 rpm, supernatants were filtered through a 0.22-µm filter and analyzed by HPLC.
2.13.2 HPLC analysis
All extracts were analyzed by a Shimadzu LC-20A HPLC system (Shimadzu Co., Kyoto, Japan) by measuring A280 nm using a variable wavelength detector. SDG was separated on a Kinetex 2.6u C18 column (2.6 µm, 50×3.0 mm, Phenomenex) using a slightly modified version of the gradient elution method (37). Column thermostat was set to 40°C and injection volume was set to 10 µL. The mobile phase consisted of 1% acetic acid (solvent A) and methanol (solvent B) mixed A/B (v/v): 0 min (95: 5), 12 min (40: 60), 18 min (60: 40), 18.2 min (95: 5), and used at a flow rate of 0.4 mL/min. The SDG peaks were identified and quantified by comparing them to the SDG standard.
2.14 Liquid Chromatography Tandem Mass Spectrometry for ED and EL analysis
2.14.1 Extraction
Tissues from all the different diet groups were finely homogenized in citrate buffer (25 mM, pH 5.0) while serum samples were centrifuged before extraction. Four times the volume of methanol was added to the samples, followed by treatment with 90 units of β-Glucuronidase enzyme. Majority of lipid was removed using n-hexane. A 1:4 solution of the EL and ED standards were prepared in methanol. Internal standard genistein-d4 was added to all samples and standards. The supernatant was applied to a preconditioned SPE cartridge and bound compounds were then eluted in methanol. The eluted sample was concentrated and the residue was then reconstituted with the loading solvent and acetonitrile/water (1:3).
2.14.2 LC MS/MS Analysis
All extracts were analyzed by a Shimadzu Prominence UFLC-8080 system (Shimadzu Co., Kyoto, Japan). The ED and EL were separated from other compounds in the extract by chromatography on a Water XTerra MS C18 column (3.0 µm, 2.1×50 mm). The mobile phase consisted of 0.2 % formic acid in water (solvent A) and acetonitrile (solvent B) mixed A/B (v/v): 0.3 min (80: 20), 1.8 min (10: 90), 1.81 min (80: 20), 2.50 min (end) and used at a flow rate of 0.6 mL/min and an injection volume of 5 µL. The compound peaks were identified and quantified by comparison with those of standard ED, EL and genistein-d4. The molecules with the m/z ratio of 301, 296.9 and 273.1 corresponded to ED, EL and genistein-d4 respectively.
2.14 Statistics
All the experiments were performed in duplicates and target values were normalized to control. Statistical calculations were done using GraphPad Instat software by employing one-way ANOVA analysis. A P < 0.05 was considered significant while a P<0.01 was considered highly significant.
3. RESULTS
3.1 ELISA analysis for Prostaglandin E2 in ovary
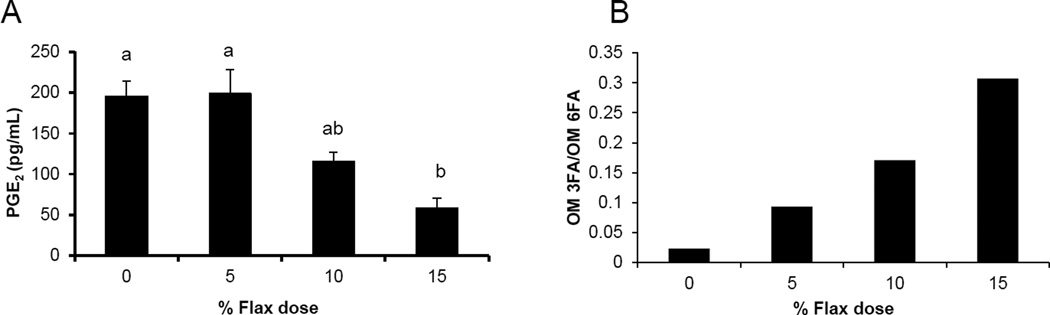
PGE2 levels in the ovarian tissue decreased with increase in percentage of flaxseed in the diet. PGE2 levels were significantly lower in the 15% diet group compared to control and 5% group (Figure 1A).
Figure 1.
Flaxseed dose dependent alteration in the levels of omega-3 fatty acids and prostaglandins. A, PGE2 levels were assessed in the ovary tissue of control and flaxseed diet - fed hens using an ELISA assay kit, n=6, (Control (0%) vs. 15%, P<0.01), (5% vs. 15%, P<0.01). B, Ovary tissue levels of OM 3FAs and OM 6FAs were measured using GC.
3.2 H and E staining on ovary sections
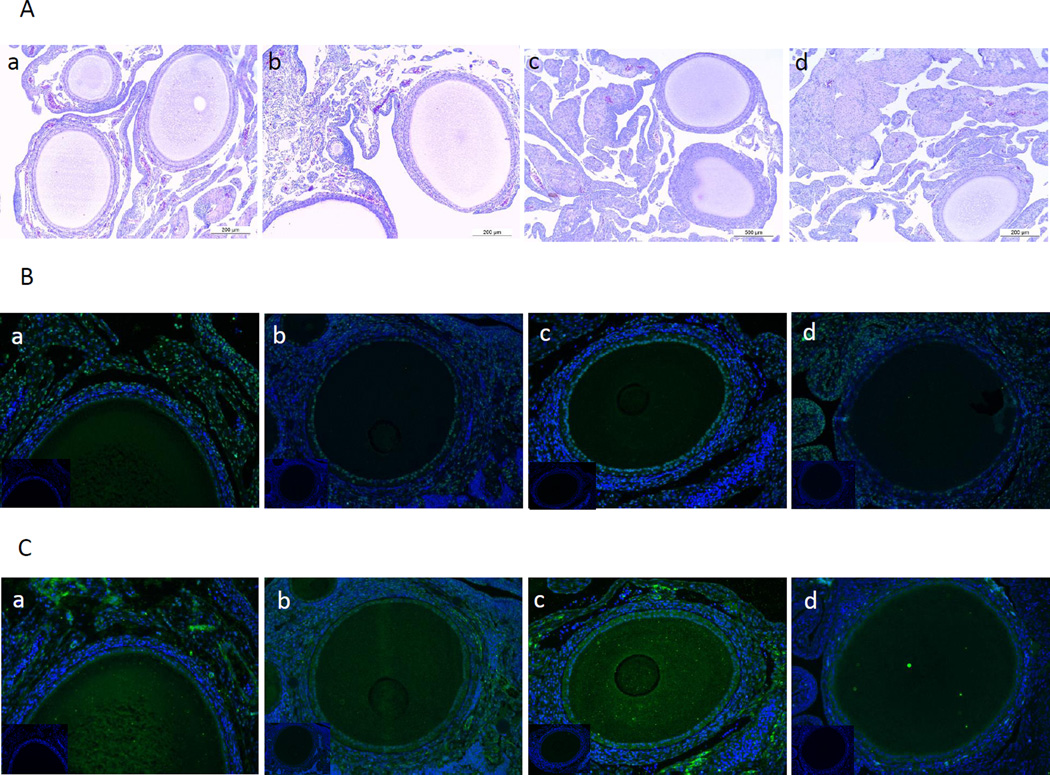
Ovary tissues from all the diet groups were assessed using hematoxylin and eosin staining. Ovaries from all the diet groups appeared normal histologically (Figure 2A).
Figure 2.
H and E staining on ovarian section and immunofluorescence staining for COX-1 and COX-2 proteins. A, Ovary tissue sections from control (a), 5% (b), 10% (c) and 15% (d) flaxseed diet groups were stained using hematoxylin and eosin and assessed histologically under 100× magnification, n=3. B and C, Ovary sections from control (a), 5% (b), 10% (c) and 15% (d) were stained for COX-1 and COX-2 proteins, respectively and observed at 200× magnification. (Insert: non-immune IgG, 200×), n=3.
3.3 COX-1 and COX-2 enzyme expression
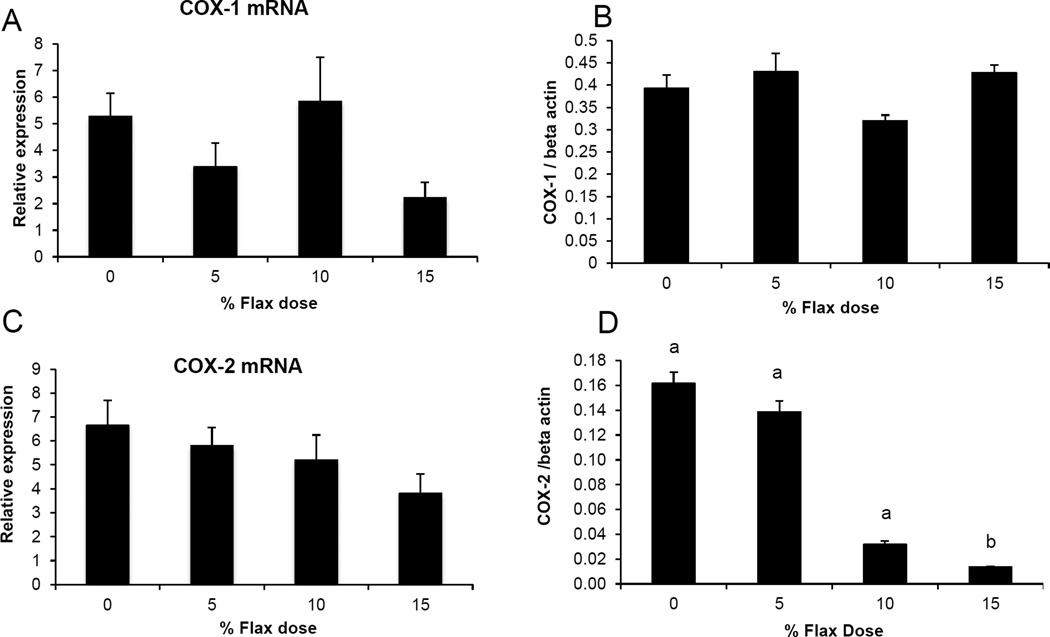
COX-1 expression was observed in the granulosa cells and also in some stromal cells while COX-2 was predominantly expressed in the granulosa cells and the ovarian surface epithelium (Figure 2B and 2C). COX-1 protein was expressed consistently across all the groups (Figure 2B). COX-2 protein was deceased in the 15% flaxseed diet group (Figure 2Cd). COX-1 mRNA and protein levels in the ovaries were not affected by flaxseed diet (Figure 3A and 3B). COX-2 mRNA levels appeared to be consistent through all the different diet groups (Figure 3C) while COX-2 protein expression was significantly reduced in the ovaries of 15% flaxseed diet fed chicken (Figure 3D).
Figure 3.
COX-1 and COX-2 protein and mRNA expression in the ovary. A and B, COX-1 enzyme mRNA expression was measured using real time PCR while protein levels were analyzed using western blotting in control and flaxseed diet-fed chickens, respectively, (n=6). C and D, COX-2 enzyme mRNA expression was measured using real time PCR while protein levels were analyzed using western blotting in control and flaxseed diet-fed chickens, respectively, (n=6). (0% vs. 15% (P<0.01), 5% vs.15% (P<0.05) and 10% vs. 15% (P<0.05)).
3.4 Omega 3 fatty acid and omega 6 fatty acid levels in ovary
Omega 3 fatty acid and omega 6 fatty analysis revealed that higher the dose of flaxseed diet higher was the incorporation of OM-3 FA in the chicken ovaries. The OM-3 FA / OM-6 FA ratio increased with higher doses of flaxseed diet (Figure 1B).
3.5 Cell proliferation and apoptosis assay
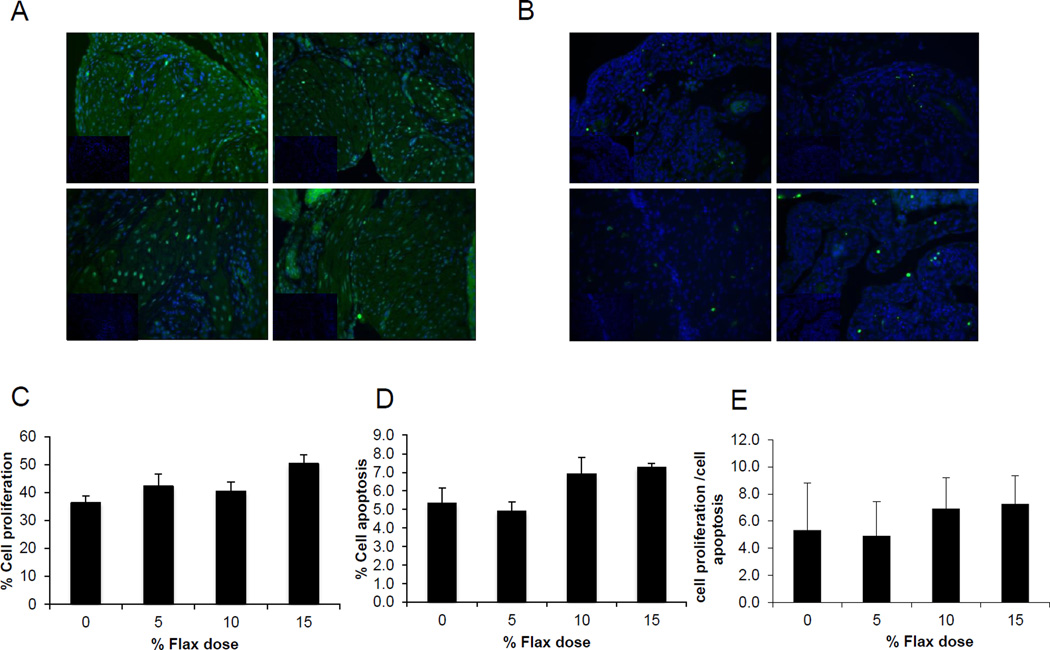
TUNEL staining for detection of apoptotic cells indicated that there was no significant difference with respect to the number of apoptotic cells in the ovarian tissue, among the birds in different dietary groups (Figures 4B and 4D). Similarly, proliferation marker, PCNA expression was not affected by the flax diet (Figures 4A and 4C). As a result, the ratio of proliferating to apoptotic cells in the ovary was comparable across all the diet groups (Figure 4E).
Figure 4.
Effect of 5%, 10%, and 15% flaxseed-supplemented diet on cell proliferation and apoptosis in the ovary. A, Green fluorescent staining indicated PCNA positive proliferating cells for ovary sections from control (a), 5% (b), 10% (c) and 15% (d) diets. B. Green fluorescent staining indicated TUNEL positive apoptotic cells for ovary sections from control (a), 5% (b), 10% (c) and 15% (d) diets, observed at 200× magnification (Insert: no primary control, 200×).
C, Graph indicating percent cell proliferation. D, Graph indicating % cells apoptosis. E, Graph indicating ratio of cell proliferation to cell apoptosis in normal ovary tissue from chickens, n=3.
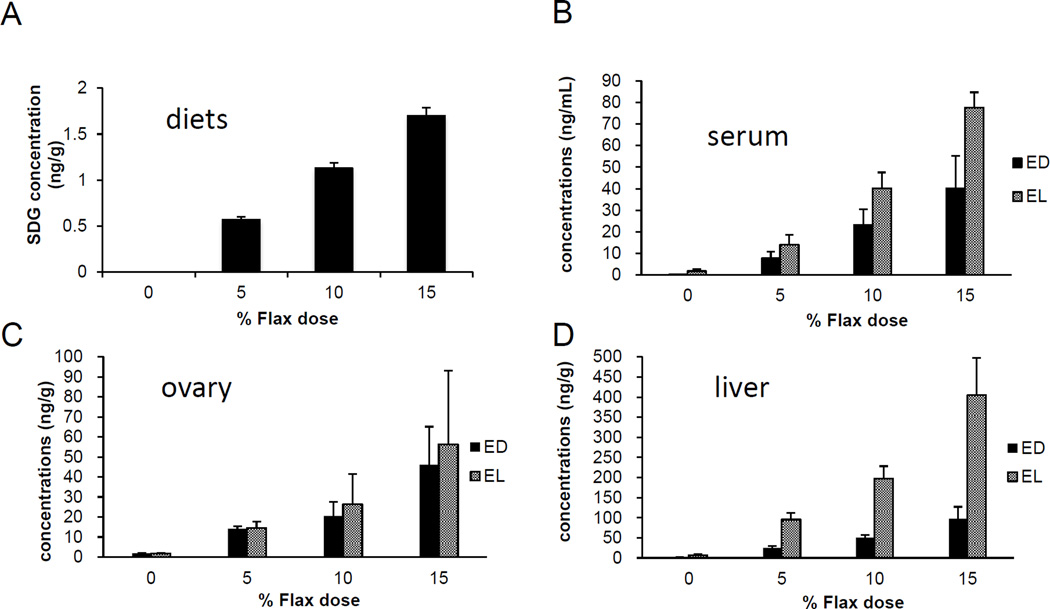
3.6 SDG concentration in different diets
The level of SDG was measured in the 0%, 5%, 10% and 15% flaxseed diet groups using liquid Chromatography. As expected, the level of SDG increased proportionally with the percentage of flaxseed (Figure 5A). The SDG concentrations were 0.56mg/g, 1.12mg/g and 1.65mg/g, in the 5%, 10% and 15% flaxseed diets, respectively.
Figure 5.
Levels of SDG and ED, EL in the diets and tissues, respectively. SDG is hydrolyzed and ultimately metabolized by the gut flora to Enterolactone and Enterodiol. A, Levels of SDG were measured in the varying percentage of flaxseed-supplemented diets, using LC. B, C and D, Levels of ED and EL were analyzed in the different flaxseed-supplemented diets groups using LC/MS/MS analysis, in the serum, ovary and liver tissues, respectively, n=6.
3.7 EL and ED levels in the tissue were proportional to the flaxseed content of the diet
The concentrations of phytoestrogen lignans, EL and ED were measured in the ovary, liver and serum samples from birds that were fed different doses of flaxseed in their diets. With increase in supplementation of flax in the diet, the levels of EL and ED in serum (Figure 5B), ovary (Figure 5C) and liver (Figure 5D) tissues increased proportionally to the flaxseed dose. The EL levels were considerably higher than that of ED in the liver tissue for all the dosage groups, while in the serum, EL levels were lower than ED for all the doses. ED and EL levels were comparable in the ovarian tissues.
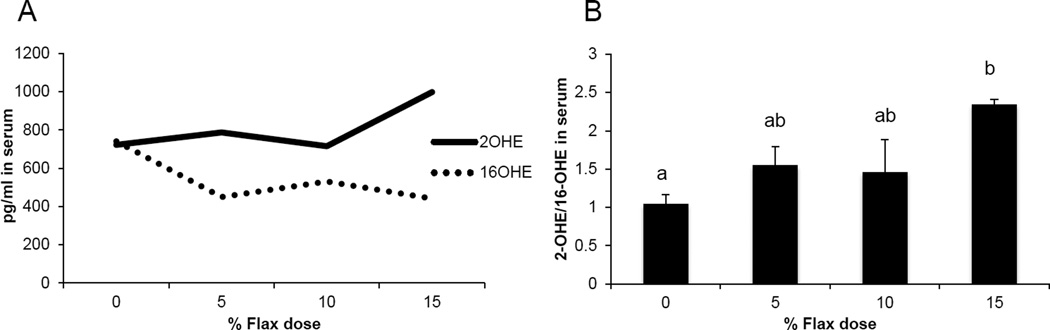
3.8 Flax diet influences levels of Estrogen metabolites
Serum samples from the different diet groups were analyzed for the levels of certain estrogen metabolites. The level of 16-OHE1 was considerably reduced in the flax fed birds in comparison to control, whereas, the level of 2-OHE1 increased substantially in the 15% flax diet group (Figure 6A). The clinically relevant 2-OHE1/16-OHE1 ratio was seen to increase significantly in the serum of 15% dosage group birds (Figure 6B).
Figure 6.
Serum levels of 2-hydroxyestrone and 16-hydroxyestrone. A, 2-OHE1 and 16-OHE1 were analyzed in the serum samples from control and flaxseed-supplemented diet groups, using the 2-hydroxyestrone and 16-hydroxyestrone Estramet double ELISA kit, n=3 for 5%, 10% and 15% diet groups, n=9 for 0% group (0% vs. 15%, P<0.05). B, Ratio of 2-OHE1/16-OHE1 in the serum samples of chickens fed varying doses of flaxseed supplemented diets.
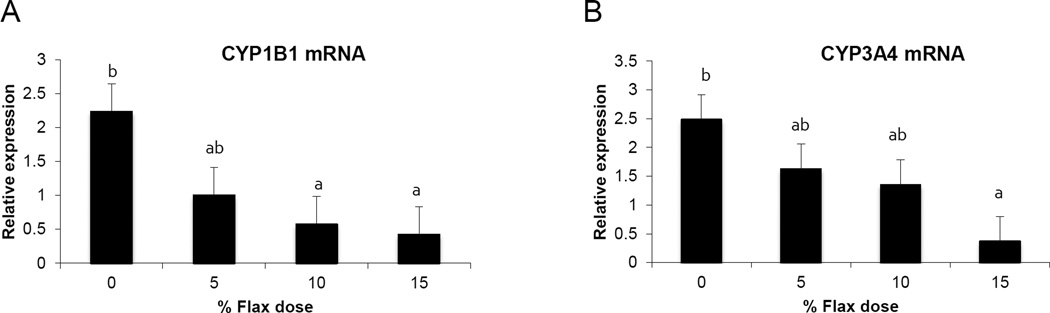
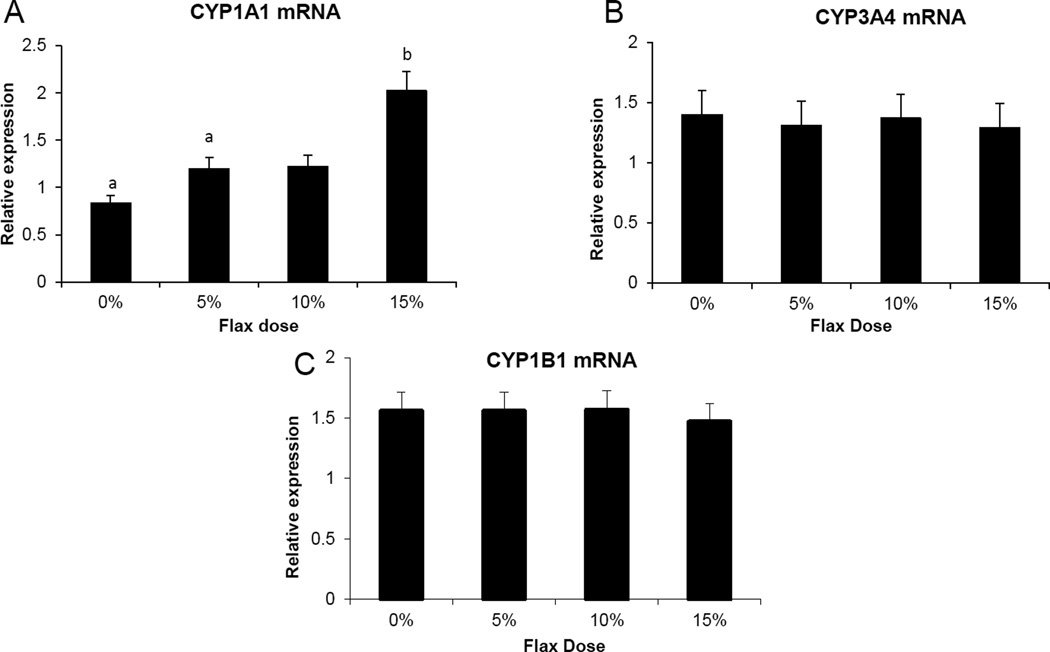
3.9 Cytochrome p450 enzyme expression
CYP1B1 (Cytochrome P450 family 1 subfamily B polypeptide 1), CYP1A1 (Cytochrome P450, family 1, subfamily A, polypeptide 1) and CYP3A4 (Cytochrome P450, family 3, subfamily A, polypeptide 4) enzymes metabolize E2 to 4-hydroxyestradiol(38), 2-hydroxyestradiol and 16-hydroxyestradiol (Estriol), respectively (39). Ovarian tissue expression of CYP1B1 and CYP3A4 mRNA decreased proportionally with the dose of flaxseed diet (Figure 7A and 7B). CYP1A1 mRNA expression in the liver increased with increase in flaxseed content of the diets (Figure 8A), while its expression was negligible in the ovary. The mRNA expression of CYP3A4 and CYP1B1 in the liver remained unchanged between groups (Figure 8B and 8C respectively).
Figure 7.
CYP1B1 and CYP3A4 enzyme mRNA expression in the ovary tissue. A, Cyp1B1 mRNA expression was measured using real time qPCR, n=6, (0% vs. 10% P<0.05, 0% vs.15% P<0.05) B, Cyp3A4 mRNA expression was measured using real time qPCR, n=6, (0% vs.15% P<0.01).
Figure 8.
Cytochrome P450 enzyme CYP1A1, CYP1B1 and CYP3A4 mRNA expression, in the liver. A, Cyp1A1 mRNA levels were quantified using real time qPCR, n=6, (0% vs. 15%, 0% vs. 10%, P <0.05) B and C, CYP1B1 and CYP3A4 mRNA levels were analyzed using real time PCR, n=6.
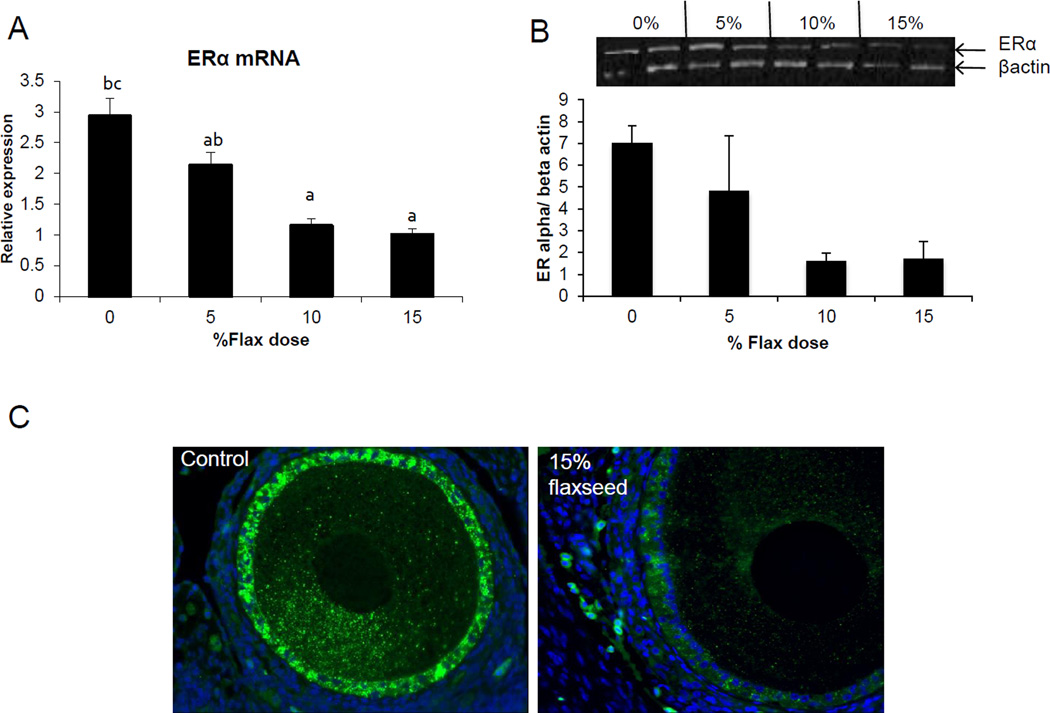
3.10 Estrogen receptor expression decreases with flax diet
An increase in dosage of flax in the diet was observed to parallel the decrease in ER alpha mRNA expression (Figure 9A). mRNA levels were significantly decreased in the 10% and 15% diet groups in comparison to control. Correspondingly, ER alpha protein decreased in the 10% and 15% flax diet groups, as shown by western blot analysis (Figure 9B). ERα protein was localized in the granulosa cells of the follicles and its expression was decreased in the 15% flaxseed diet group (Figure 9C).
Figure 9.
ERα expression in the ovary. A, ERα mRNA levels were analyzed using real time qPCR in the different diet groups. n=6, (0% vs. 10% P<0.05, 0% vs. 15% P<0.05,) B, ERα protein levels were analyzed using western blotting, n=2. C, ERα protein tissue localization was observed using immunofluorescence staining, n=3, 200× magnification.
4. DISCUSSION
From our previous studies we demonstrated that feeding 2.5-year-old chickens a 10% flaxseed diet for one year reduced the severity but not the incidence of ovarian cancer (32). When younger (22 weeks old) chickens in their first lay were fed flaxseed diet for four years, the severity as well as incidence of the ovarian cancer was found to be reduced (6). These findings suggest that flaxseed diet is most effective in combating ovulation-induced inflammation, when consumed at the start of ovulation and continued throughout their reproductive life. The objective of this study was to determine the optimum dose of flaxseed in the diet by analyzing levels of surrogate end points that are representative of its effect on ovarian cancer initiation and progression.
We have previously established that COX-2 enzyme expression increases with age while consumption of flaxseed decreases COX-2 expression (35). COX enzymes act on omega 6 fatty acids to generate series-2 pro-inflammatory prostaglandins like PGE2, while their action on omega 3 fatty acids generate series-3 anti-inflammatory prostaglandins (40). Flaxseeds are a rich source of OM3 FA, which competes as a substrate with OM6 FA for COX enzymes, in turn leading to a possible decrease in the synthesis of pro-inflammatory prostaglandins that are known to stimulate the release of inflammatory cytokines (41), associated with cancer. In this study we observed that an increase in the percentage of flaxseed in the diet led to an increase in the OM3 FA to OM6 FA ratio with a concurrent statistically significant reduction in the ovarian PGE2 levels, in 15% flax-fed diet group hens (Figure 1B). COX-1 mRNA and protein expression was not altered significantly with flax diet, which was consistent with our previous finding (6) that suggested flax diet did not have any effect on COX-1 expression in young normal chickens (Figure 2B, 3A and 3B). Also consistent with our previous results, we observed that COX-2 protein expression decreased significantly with 10% and 15% flax diet (Figure 3D). With the present study, we confirm our previous results (6,35) and take a step further by demonstrating that not only does flax diet decrease the levels of inflammatory PGE2 (35), but it does so in a dose - dependent fashion. This further suggests that the anti-apoptotic and pro-angiogenic (42) COX-2 enzyme can be targeted by flaxseed diet.
Phase I of estrogen metabolism begins with hydroxylation; initiated by the CYPs (Cytochrome P450 enzymes) CYP1B1, CYP1A1 and CYP3A4 that act at different positions on E2 to form 4-OHE2, 2-OHE2 and 16-OHE2 respectively. Phase II of estrogen metabolism includes conjugation by glucuronidation, sulfation or O-methylation by COMT (Catechol-O-methyl transferase) (43). 4-OHE2, 2-OHE2 and 16-OHE2 metabolites are known to have varying degrees of estrogenicity themselves (39). The corticle follicles and small follicles in the chicken ovary produce E2 (44), thus providing a substrate for these metabolizing enzymes. The 4-OHE2 is further oxidized to estrogen-3, 4-quinones, which can form depurinating DNA adducts that can cause mutations resulting in the initiation of cancer (45). 16-OHE2 is known to be procarcinogenic (46), with evidence suggesting that 16-OHE2 increases risk of breast cancer by increasing the rate of cell proliferation through binding covalently to the ER(47). The 2-OHE2 has a relatively low binding affinity for ER and has no carcinogenicity unlike some other E2 catechols, as seen by Fernandez et. al., who demonstrated that 2-OHE2 could not induce kidney tumor in hamsters, whereas 4-OHE2 could (48). 2-OHE2 is also known to be protective against hormone-dependent cancers by interfering with the binding of sex hormones to sex steroid binding protein (49). It is also converted to 2-methoxyestradiol (2-MEOH E2) at a much faster rate by COMT, compared to the other catechol estrogens. The 2-MEOH E2 has anti-proliferative and anti-angiogenic properties(50).
The serum analysis suggested that 2-OHE1 increased considerably in the 15% flax diet while the 16-OHE1 decreased in all the flax diet samples and significantly in the 15% group. A decrease in the ratio of 2-OHE1 to 16-OHE1 can be correlated to an increased risk for a number of cancers including breast, endometrium and cervix (51–54). In our study we observed that the ratio of 2-OHE1/16-OHE1 increased significantly in the 15% flax diet in comparison to control. Consistent with the 16-OHE1 levels, we observed that feeding flax seed diet to chickens led to a down regulation of CYP3A4 mRNA expression in the ovary (Figure 7B). CYP1A1 expression could not be detected in the ovaries of control or flax-fed birds, but its expression in the liver increased with flax diet (Figure 8A). The mechanism through which flaxseed regulates the expression of these CYP enzymes is currently being investigated.
E2 induces CYP1B1 expression in the female reproductive tract as well as in ER positive breast cancer cells and ER over-expressing endometrial cancer cells (38). We have previously shown that CYP1B1 expression is up regulated in cancerous chicken ovaries and is the highest in the POF 3 (post ovulatory follicle 3), which is usually buried inside the ovary where the E2 concentration is high (45). E2 levels in the ovary are about 100-fold higher than circulating levels (55). The high estrogen levels in the ovary could not only facilitate increased CYP1B1 expression but also provide a large substrate pool for CYP1B1, resulting in an increase in genotoxic 4-OHE2 catechol levels. Different doses of flaxseed in the diet did not alter E2 concentrations in the chicken sera (data not shown); however, concentrations in the ovary were not measured. In this study, CYP1B1 mRNA expression was seen to decrease in the ovary with flax diet in a dose dependent manner (Figure 7A). A decrease in CYP1B1 expression in the ovary with flax diet demonstrates the anti-estrogenic and possibly anti-oncogenic properties of flaxseed.
Estrogen stimulates cell proliferation and can promote lymph node metastasis in ER positive ovarian cancer tumors but not in ER negative cancers (56), demonstrating the significance of ER in promoting tumor growth and mobilization in ovarian cancer. CYP1B1 is primarily regulated by aryl hydrocarbon receptor (AHR) and its dimerising partner, aryl hydrocarbon receptor nuclear translocator (ARNT) (57). Tsuchiya et. al. have shown that E2 can up regulate CYP1B1 through ER, independent of AHR (38), further suggesting the role of ER in inducing estrogen mediated toxicity. We observed that feeding hens a flaxseed-supplemented diet led to a dose - dependent decrease in the expression of the ERα mRNA and protein (Figure 9A and 9B). Decrease in ERα expression does not alter the egg laying frequency of these birds, indicating that flaxseed does not impair the normal functioning of the ovary. We have previously published that hens that were fed a diet of 10% flaxseed had an average egg laying frequency of about 71% per week (58). From one of our recent studies, we know that hens that were fed 15% flaxseed diet produced eggs at 69.4% frequency per week while the control group produced at 72.23 % per week. These data clearly indicate that the different doses of flaxseed diet do not affect the egg laying frequency of the birds. Effect of flaxseed diet on ERα could be due to the flaxseed lignan, SDG, which is ultimately converted to the weakly estrogenic/anti-estrogenic EL and ED. The partial agonist/antagonist property of these compounds could be responsible for decreasing ER activity and eventually its expression, since ER transactivates its own expression (10).
In our previous long-term study we showed that feeding 10% flaxseed supplemented diet decreased the levels of inflammatory prostaglandins E2, which also correlated with reduced incidence and severity of ovarian cancer (6). We observed that levels of PGE2 were also higher in older chickens regardless of pathology, suggesting that there are other factors contributing to ovarian cancer initiation and dissemination. Our present findings indicate that 15% flaxseed dose is most effective in decreasing the levels of the highly potent inflammatory prostaglandins, PGE2, carcinogenic estrogen metabolites and ER in the ovary of young, normal chickens without any toxic effects. This suggests that a dose of 15% flaxseed diet can beneficially affect a number of clinically relevant surrogate endpoints implicated in cancer. It is especially significant after it was recently demonstrated that ER positive ovarian cancer is more chemo-resistant than ER negative ovarian cancer, making it extremely challenging to treat (59). The ER positive status of these tumors possibly plays an important role in progression of the disease.
Taken together, chemo-preventative properties of flaxseed alleviate the expression of inflammatory prostaglandin E2, ER, toxic estrogen metabolites and enzymes, which might be contributing in the development of ovarian cancer later on. As a result, our data strongly supports early dietary intervention using flaxseed, as a preventative approach for ovarian cancer.
Table 3.
Table of abbreviations
| Abbreviation | Definition |
|---|---|
| E2 | Estradiol |
| COX-1 | Cyclooxygenase-1 |
| COX--2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandin E2 |
| ER | Estrogen receptor |
| AHR | Aryl hydrocarbon receptor |
| AHNT | Aryl hydrocarbon receptor nuclear translocator |
| CYP1A1 | Cytochrome p450 family 1, subfamily A, polypeptide 1 |
| CYP1B1 | Cytochrome p450 family 1, subfamily B, polypeptide 1 |
| CYP3A4 | Cytochrome p450 family 3, subfamily A, polypeptide 4 |
| CYPs | Cytochrome p450 enzymes |
| TBP | TATA binding protein |
| RPL4 | Ribosomal protein L4 |
| SDHA | Succinate dehydrogenase |
| COMT | Catechol –O- methyl transferase |
| Nrf2 | Nuclear-like factor 2 |
| HO-1 | Hemeoxygenase 1 |
| GST | Glutathione- S- transferase |
| 2-OHE1 | 2-hydroxyestrone |
| 16-OHE1 | 16-hydroxyestrone |
| 2-OHE2 | 2-hydroxyestradiol |
| 16-OHE2 | 16-hydroxyestradiol |
| 4-OHE2 | 4-hydroxyestradiol |
| PCNA | Proliferating cellular nuclear antigen |
| TUNEL | TdT mediated dUTP nick end labeling |
| ALA | Alpha linoleic acid |
| SDG | Secoisolaricirescinol diglucoside |
| SECO | Secoisolaricirescinol |
| OM3 FA | Omega-3fattyacid |
| OM6 FA | Omega-6fattyacid |
| ED | Enterodiol |
| EL | Enterolactone |
ACKNOWLEDGMENTS
This work was funded by the NIH RO1 AT00408 grant. We are extremely grateful to the poultry farm management that includes Chet Utterback, Pam Uterback, Doug Hilgendorf, and Carl Parsons for helping us with the diet formulations. Dr. Richard van Breeman and his mass spectrometry lab members helped us extensively with the enterolactone and enterodiol analysis at the University of Illinois, Chicago. We also thank Dr. Janice Bahr for her continued guidance and Dr. Karen Hales for her valuable suggestions.
ABBREVIATIONS
- E2
Estradiol
- COX-1
Cyclooxygenase-1
- COX-2
Cyclooxygenase-2
- PGE2
Prostaglandin E2
- ER
Estrogen receptor
- AHR
Aryl hrdrocarbon receptor
- AHNT
Aryl hydrocarbon receptor nuclear translocator
- CYP1A1
Cytochrome p450 family 1, subfamily A, polypeptide 1
- CYP1B1
Cytochrome p450 family 1, subfamily B, polypeptide 1
- CYP3A4
Cytochrome p450 family 3, subfamily A, polypeptide 4
- CYPs
Cytochrome p450 enzymes
- TBP
TATA binding protein
- RPL4
Ribosomal protein L4
- SDHA
Succinate dehydrogenase
- COMT
Catechol-O-methyl transferase
- 2-OHE1
2-hydroxyestrone
- 16-OHE1
16-hydroxyestrone
- 2-OHE2
2-hydroxyestradiol
- 4-OHE2
4-hydroxyestradiol
- 16-OHE2
16-hydroxyestradiol
- PCNA
proliferating cellular nuclear antigen
- TUNEL
TdT mediated dUTP nick end labeling
- ALA
Alpha linoleic acid
- SDG
Secoisolaricirescinol diglucoside
- SECO
Secoisolariciresinol
- ED
Enterodiol
- EL
Enterolactone
- OM3 FA
Omega-3 fatty acid
- OM6 FA
Omega-6 fatty acid
Footnotes
Disclosure statement: the authors have nothing to disclose.
REFERENCES
- 1.Thompson L, Boucher B, Liu Z, et al. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54(2):184–201. doi: 10.1207/s15327914nc5402_5. [DOI] [PubMed] [Google Scholar]
- 2.Axelson M, Sjovall J, Gustafsson BE, et al. Origin of lignans in mammals and identification of a precursor from plants. Nature. 1982;298(5875):659–660. doi: 10.1038/298659a0. [DOI] [PubMed] [Google Scholar]
- 3.Prasad K. Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: Effect of secoisolariciresinol diglucoside (SDG) Molecular and Cellular Biochemistry. 2000;209(1–2):89–96. doi: 10.1023/a:1007079802459. [DOI] [PubMed] [Google Scholar]
- 4.Daun JK, Barthet VJ, Chornick T, et al. Structure, composition, and variety development of flaxseed. In: Thompson L, Cunnane SC, editors. Flaxseed in human nutrition. 2 ed. Urbana: AOCS Publishing; 2003. pp. 1–40. [Google Scholar]
- 5.Thompson L, Cunnane S. Flaxseed, lignans, and cancer. In: Thompson L, Cunnane SC, editors. Flaxseed in human nutrition. 2 ed. Urbana: AOCS Publishing; 2003. pp. 194–222. [Google Scholar]
- 6.Eilati E, Bahr JM, Hales DB. Long Term Consumption of Flaxseed Enriched Diet Decreased Ovarian Cancer Incidence and Prostaglandin E2 in Hens. Gynecologic Oncology. 2013;3(130):620–628. doi: 10.1016/j.ygyno.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambros V, Chen X. The regulation of genes and genomes by small RNAs. pp. 1635–1641. Vol 1342007. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RA, Tejada LV, Tong BJ, et al. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003;63(5):906–911. [PubMed] [Google Scholar]
- 9.Eilati E, Pan L, Bahr JM, et al. Age dependent increase in prostaglandin pathway coincides with onset of ovarian cancer in laying hens. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2012;87(6):177–184. doi: 10.1016/j.plefa.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hales DB, Zhuge Y, Lagman JAJ, et al. Cyclooxygenases expression and distribution in the normal ovary and their role in ovarian cancer in the domestic hen (Gallus domesticus) Endocrine. 2008;33(3):235–244. doi: 10.1007/s12020-008-9080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giles JR, Olson LM, Johnson PA. Characterization of ovarian surface epithelial cells from the hen: a unique model for ovarian cancer. Exp Biol Med. 2006;231(11):1718–1725. doi: 10.1177/153537020623101108. [DOI] [PubMed] [Google Scholar]
- 12.Vane JR, Bakhle YS, Botting RM. Cyclooxgenase 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Ristimäki A, Honkanen N, Jänkälä H, et al. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Research. 1997;57(7):1276–1280. [PubMed] [Google Scholar]
- 14.Eberhart C, Coffey R, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 15.Parrett M, Harris R, Joarder F, et al. Cyclooxygenase-2 gene expression in human breast cancer. International journal of oncology. 1997;10(3):503–507. doi: 10.3892/ijo.10.3.503. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Srivastava M, Ahmad N, et al. Over-expression of cyclooxygenase - 2 in human prostate adenocarcinoma. The Prostate. 2000;42(1):73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni S, Rader JS, Zhang F, et al. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clinical Cancer Research. 2001;7(2):429–434. [PubMed] [Google Scholar]
- 18.Molina MA, Sitja-Arnau M, Lemoine MG, et al. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Research. 1999;59(17):4356–4362. [PubMed] [Google Scholar]
- 19.Hida T, Kozaki K-i, Muramatsu H, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clinical Cancer Research. 2000;6(5):2006–2011. [PubMed] [Google Scholar]
- 20.Wang L-Q, Meselhy MR, Li Y, et al. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chemical and Pharmaceutical Bulletin-Tokyo. 2000;48(11):1606–1610. doi: 10.1248/cpb.48.1606. [DOI] [PubMed] [Google Scholar]
- 21.Buck K, Zaineddin AK, Vrieling A, et al. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. The American journal of clinical nutrition. 2010;92(1):141–153. doi: 10.3945/ajcn.2009.28573. [DOI] [PubMed] [Google Scholar]
- 22.Velentzis L, Cantwell M, Cardwell C, et al. Lignans and breast cancer risk in pre-and post-menopausal women: meta-analyses of observational studies. British Journal Of Cancer. 2009;100(9):1492–1498. doi: 10.1038/sj.bjc.6605003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCann SE, Moysich KB, Freudenheim JL, et al. The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. The Journal of nutrition. 2002;132(10):3036–3041. doi: 10.1093/jn/131.10.3036. [DOI] [PubMed] [Google Scholar]
- 24.McCann SE, Muti P, Vito D, et al. Dietary lignan intakes and risk of pre- and postmenopausal breast cancer. Int. J. Cancer. 2004;111(3):440–443. doi: 10.1002/ijc.20262. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell AJ, Macleod KG, Burns DJ, et al. Estrogen receptor-alpha mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr Relat Cancer. 2005;12(4):851–866. doi: 10.1677/erc.1.01039. [DOI] [PubMed] [Google Scholar]
- 26.Mueller SO, Simon S, Chae K, et al. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor α (ERα) and ERβ in human cells. Toxicological Sciences. 2004;80(1):14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda A, Katanoda K. Five-year Relative Survival Rate of Ovarian Cancer in the USA, Europe and Japan. Japanese journal of clinical oncology. 2014;44(2):196. doi: 10.1093/jjco/hyu007. [DOI] [PubMed] [Google Scholar]
- 28.Barua A, Bitterman P, Abramowicz JS, et al. Histopathology of ovarian tumors in laying hens: a preclinical model of human ovarian cancer. Int J Gynecol Cancer. 2009;19(4):531–539. doi: 10.1111/IGC.0b013e3181a41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lengyel E, Burdette J, Kenny H, et al. Epithelial ovarian cancer experimental models. Oncogene. 2013 doi: 10.1038/onc.2013.321. Epub doi: 10.1038/onc.2013.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson KA. The standard of perfection: thoughts about the laying hen model of ovarian cancer. Cancer prevention research (Philadelphia, Pa. 2009;2(2):97–99. doi: 10.1158/1940-6207.CAPR-08-0244. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PA, Giles JR. Use of genetic strains of chickens in studies of ovarian cancer. Poult Sci. 2006;85(2):246–250. doi: 10.1093/ps/85.2.246. [DOI] [PubMed] [Google Scholar]
- 32.Ansenberger K, Richards C, Zhuge Y, et al. Decreased severity of ovarian cancer and increased survival in hens fed a flaxseed-enriched diet for 1 year. Gynecologic Oncology. 2010;117(2):341–347. doi: 10.1016/j.ygyno.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch MJRS, Mellor LD, Spare PD, Inwood MJ. Medical Laboratory Technology and Clinical Pathology. 2nd edition. Philadelphia London Toronto: WB Saunders Co.; 1969. [Google Scholar]
- 34.Sheehan D, Hrapchak B. Theory and practice of histology. St. Louis: CV Mosby Co.; 1980. [Google Scholar]
- 35.Eilati E, Hales K, Zhuge Y, et al. Flaxseed enriched diet-mediated reduction in ovarian cancer severity is correlated to the reduction of prostaglandin E2 in laying hen ovaries. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2013;89(4):179–187. doi: 10.1016/j.plefa.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eilati E, Small CC, McGee SR, et al. Anti-inflammatory effects of fish oil in ovaries of laying hens target prostaglandin pathways. Lipids in health and disease. 2013;12(1):152. doi: 10.1186/1476-511X-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muir AD, Westcott ND. Quantitation of the lignan secoisolariciresinol diglucoside in baked goods containing flax seed or flax meal. Journal of agricultural and food chemistry. 2000;48(9):4048–4052. doi: 10.1021/jf990922p. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya Y, Nakajima M, Kyo S, et al. Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Research. 2004;64(9):3119–3125. doi: 10.1158/0008-5472.can-04-0166. [DOI] [PubMed] [Google Scholar]
- 39.Yager J, Leihr J. Molecular mechanisms of estrogen carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1996;36(1):203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 40.Larsson SC, Kumlin M, Ingelman-Sundberg M, et al. Dietary long-chain n− 3 fatty acids for the prevention of cancer: a review of potential mechanisms. The American journal of clinical nutrition. 2004;79(6):935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 41.Sheibanie AF, Yen J-H, Khayrullina T, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23→ IL-17 axis. The. Journal of Immunology. 2007;178(12):8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 42.Arico S, Pattingre S, Bauvy C, et al. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J. Biol. Chem. 2002;277(31):27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer letters. 2005;227(2):115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Lee K, Bahr J. Utilization of substrates for testosterone and estradiol-17β production by small follicles of the chicken ovary. Domestic Animal Endocrinology. 1994;11(3):307–314. doi: 10.1016/0739-7240(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 45.Zhuge Y, Lagman JA, Ansenberger K, et al. CYP1B1 expression in ovarian cancer in the laying hen Gallus domesticus. Gynecol Oncol. 2009;112(1):171–178. doi: 10.1016/j.ygyno.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Telang NT, Suto A, Wong GY, et al. Induction by Estrogen Metabolite 16α;-Hydroxyestrone of Genotoxic Damage and Aberrant Proliferation in Mouse Mammary Epithelial Cells. Journal of the National Cancer Institute. 1992;84(8):634–638. doi: 10.1093/jnci/84.8.634. [DOI] [PubMed] [Google Scholar]
- 47.Davis DL, Bradlow HL, Wolff M, et al. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environmental Health Perspectives. 1993;101(5):372. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liehr JG, Wan-Fen F, Sirbasku DA, et al. Carcinogenicity of catechol estrogens in Syrian hamsters. J. Steroid Biochem. 1986;24(1):353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 49.Martin ME, Haourigui M, Pelissero C, et al. Interactions between phytoestrogens and human sex steroid binding protein. Life Sciences. 1995;58(5):429–436. doi: 10.1016/0024-3205(95)02308-9. [DOI] [PubMed] [Google Scholar]
- 50.LaVallee TM, Zhan XH, Herbstritt CJ, et al. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors α and β. Cancer Research. 2002;62(13):3691–3697. [PubMed] [Google Scholar]
- 51.Bradlow HL, Hershcopf RJ, Martucci CP, et al. Estradiol 16 alpha-hydroxylation in the mouse correlates with mammary tumor incidence and presence of murine mammary tumor virus: a possible model for the hormonal etiology of breast cancer in humans. Proceedings of the National Academy of Sciences. 1985;82(18):6295–6299. doi: 10.1073/pnas.82.18.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradlow H, Hershcopf R, Martucci C, et al. 16α - Hydroxylation of Estradiol: A Possible Risk Marker for Breast Cancer. Annals of the New York Academy of Sciences. 1986;464(1):138–151. doi: 10.1111/j.1749-6632.1986.tb16001.x. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi M, Shimomoto T, Miyajima K, et al. Effects of estrogens and metabolites on endometrial carcinogenesis in young adult mice initiated with N-ethyl-N'-nitro-N-nitrosoguanidine. Cancer Lett. 2004;211(1):1–9. doi: 10.1016/j.canlet.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 54.Bell MC, Crowley-Nowick P, Bradlow HL, et al. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecologic Oncology. 2000;78(2):123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 55.Ho S-M. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1(1):73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spillman MA, Manning NG, Dye WW, et al. Tissue-specific pathways for estrogen regulation of ovarian cancer growth and metastasis. Cancer Research. 2010;70(21):8927–8936. doi: 10.1158/0008-5472.CAN-10-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Savas Ü, Alexander DL, et al. Characterization of the mouse CYP1B1 gene Identification of an enhancer region that directs aryl hydrocarbon receptor-mediated constitutive and induced expression. J. Biol. Chem. 1998;273(9):5174–5183. doi: 10.1074/jbc.273.9.5174. [DOI] [PubMed] [Google Scholar]
- 58.Eilati E, Hales K, Zhuge Y, et al. Flaxseed enriched diet-mediated reduction in ovarian cancer severity is correlated to the reduction of prostaglandin E< sub> 2</sub> in laying hen ovaries. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2013;89(4):179–187. doi: 10.1016/j.plefa.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brasseur K, Leblanc V, Fabi F, et al. ERα-Targeted Therapy in Ovarian Cancer Cells by a Novel Estradiol-Platinum (II) Hybrid. Endocrinology. 2013;154(7):2281–2295. doi: 10.1210/en.2013-1083. [DOI] [PubMed] [Google Scholar]