Abstract
Axon guidance molecules, slit glycoprotein (Slit) and Roundabout receptor (Robo), have implications in the regulation of physiological processes. Recent studies indicate that Slit and Robo also have important roles in tumorigenesis, cancer progression and metastasis. The Slit/Robo pathway can be considered a master regulator for multiple oncogenic signaling pathways. Herein, we provide a comprehensive review on the role of these molecules and their associated signaling pathways in cancer progression and metastasis. Overall, the current available data suggest that the Slit/Robo pathway could be a promising target for development of anticancer drugs.
Keywords: Slit/Robo, cancer progression, cancer metastasis, hormonal-dependent and - independent cancers, drug target
Introduction
The development of the nervous system involves several progressive and regressive events that are mainly driven by axon guidance molecules [1], such as Slit and Roundabout (Robo) [2]. Slit/Robo signaling was first established as an extracellular signature to guide axon path finding, promote axon branching and control neuronal migration. The interaction of Slit and Robo proteins is critically involved in the developmental processes of various vital organs such as breast, lung, liver, kidney, eye and reproductive systems. Slit proteins are highly conserved, secreted glycoproteins that mediate their functions by binding to the transmembrane receptors known as Robo recptors [1]. Slits and Robos are large proteins involved in several cell signaling pathways including axon guidance, cell proliferation, cell motility and angiogenesis [2–4]. Slit and Robo proteins were first discovered as secreted proteins in Drosophila [5–7]. Thereafter, homologs of Slit and Robo proteins have been discovered in rat, mice and humans [8]. Many reports have suggested that, in addition to axon guidance, the Slit/Robo pathway is also involved in the developmental processes and in the regulation of several physiological processes. An aberrant Slit/Robo expression in cells can lead to cancer development, progression and metastasis. Herein, we have reviewed recent advances regarding the roles of the Slit/Robo pathway and proteins in different types of cancer, molecular crosstalk and the modulation of oncogenic signaling pathways.
Structure of Slit and Robo proteins
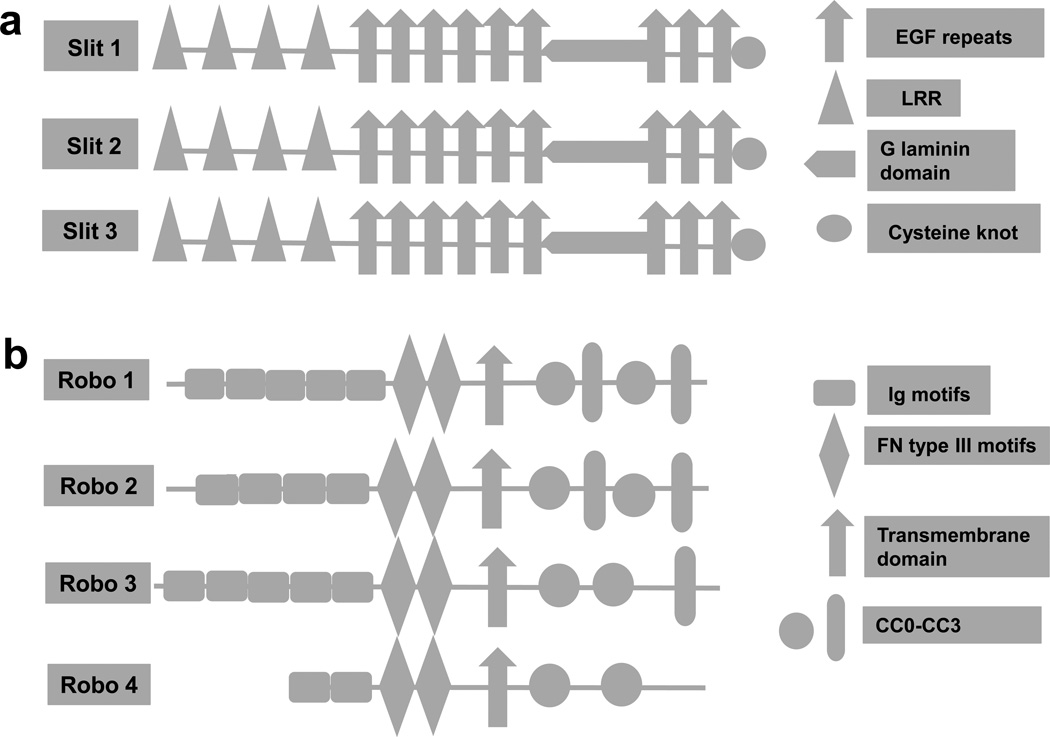
In humans, Slits are composed of a single peptide of about 1500 amino acids, and there are three members: Slit1, Slit2 and Slit3 [9,10]. The primary structure of Slit contains four domains at the N terminus (D1–D4) with leucine-rich repeats (LRR), six EGF-like sequences (EGF), a laminin- G domain and a C terminus with a cysteine-rich knot (Figure 1) [9,11,12]. All vertebrates have similar Slit family protein structures. The D2 region domain of LRR of Slits is highly conserved and plays an important part in binding to Robo proteins [9].
Figure 1.
Structure of the Slit/Robo protein family. (a) Structure of human Slit protein. It is a large molecular weight glycoprotein, comprising (from N to C terminal): four leucine-rich repeats (LRR), seven to nine EGF[s4] repeats, laminin G domain and a cysteine-rich knot. (b) Structure of human Robo proteins. Robos are the receptors of Slit proteins, which contain immunoglobulin (Ig) motifs, three fibronectin (Fn III[s5]) motifs, a transmembrane domain and a conserved cytoplasmic domain (CC0–CC3). Figure modified, with permission, [s6]from [10].
Robos are considerably large molecules and are composed of 1000 to 1600 amino acids, and a transmembrane receptor protein with a conserved cytoplasmic domain [10,13]. In humans, four Robos have been identified thus far. All Robo proteins are composed of five immunoglobulin (Ig) and three fibronectin (Fn III) motifs in the extracellular domain with the exception of Robo4, which has only two Ig domains as well as the Fn III motifs (Figure 1). The Ig proteins are highly conserved, and the different expressions of the conserved cytoplasmic domains expressed determine the interaction of Slit protein with downstream signaling pathways. The conserved domain of Slits (D2 LRR), IG[s1]1 and IG2 are crucial for binding of Slit with Robo. Robo4 has only two Ig, and Fn III is not considered a true receptor. Robo4 is expressed in endothelial cells and is involved in angiogenesis. The interaction of Robo4 with Slits is controversial, and could have its function in a Slit-dependent or -independent manner.
Slit/Robo pathway in cancer progression
The first link between Slit/Robo signaling and cancer was reported by Sundaresan et al. [14]. Subsequent studies indicated that the exon 2 of Robo1 was deleted in lung and breast tumor cell lines [14,15]. Subsequently, various studies have shown that Slit1–3 and Robo1–4 promoters are hypermethylated (epigenetic inactivation) in several different types of cancers [40–44]. [s2]The activation or suppression of the Slit/Robo pathway modulates several oncogenic signaling pathways that are associated with the development and progression of cancer [16,17]. In most of the tumors, expression of Slit and Robo proteins is either suppressed or undetectable, which is primarily caused by promoter hypermethylation. Very limited studies have suggested that the downregulation or suppression of Slit/Robo is not directly associated with the inhibition of cell death or apoptosis in cancer cells but that it occurs through indirect interaction with another axon guidance receptor: deleted in colorectal cancer (DCC). In colorectal cancer, Slit/Robo signaling can induce apoptosis by Slit2–Robo4 interaction. Activated Robo4 physically interacts and suppresses the expression of netrin-1, another axon guidance protein [18]. Netrin-1 (a tumor suppressive protein) binds DCC and prevents DCC-mediated apoptosis in cancer cells [19,20]. Thus, binding of Robo4 with netrin-1 leads to disassociation of netrin-1 from its receptor DCC. Alternatively, Slit2 can also bind to netrin-1 and prevents inhibition of DCC by netrin-1. Free DCC activates caspase-3- and caspase-9-dependent apoptosis and cell cycle arrest in cancer cells [21,22].
Most of the current reports suggest that the Slit/Robo pathway displays its effects in the late stages of cancer [21,23]. It was reported that the Slit/Robo pathway inhibits cell invasion by interacting with E-cadherin and β-catenin in breast cancer and colorectal cancer [24–26]; whereas in liver cancer Slit2/Robo1 specifically inhibited hepatocyte growth factor (HGF)- mediated cell migration [25]. HGF is a tyrosine kinase receptor that interacts with its ligand, Met, and activation of Met correlates with a metastatic phenotype and poor prognosis in several carcinomas [27,28]. Most of the reports suggested that Slit2 inhibits invasion and migration in cancer cells. However, Denk et al. recently demonstrated that Slit3 also inhibited cell migration in melanoma cells through modulation of activator protein-1 (AP-1) [29]. In most of the cancers, Slit/Robo acts as a tumor suppressor by inhibiting cell invasion and migration [2,24,30–34], except in prostate cancer and colorectal cancer [23,35]. The current data indicate that Slit/Robo pathways differentially modulate invasion and migration (Figure 2), which varies according to signaling and type of cancer. It is still not known why the discrepancy occurs in specific types of cancers. One possible reason could be that, out of the three Slits, Slit2 binds more specifically than Slit1 or Slit3 to the Robo1 receptor. Overall, these findings indicate that the Slit/Robo pathway mainly suppresses tumor progression by regulating processes such as invasion, migration and apoptosis.
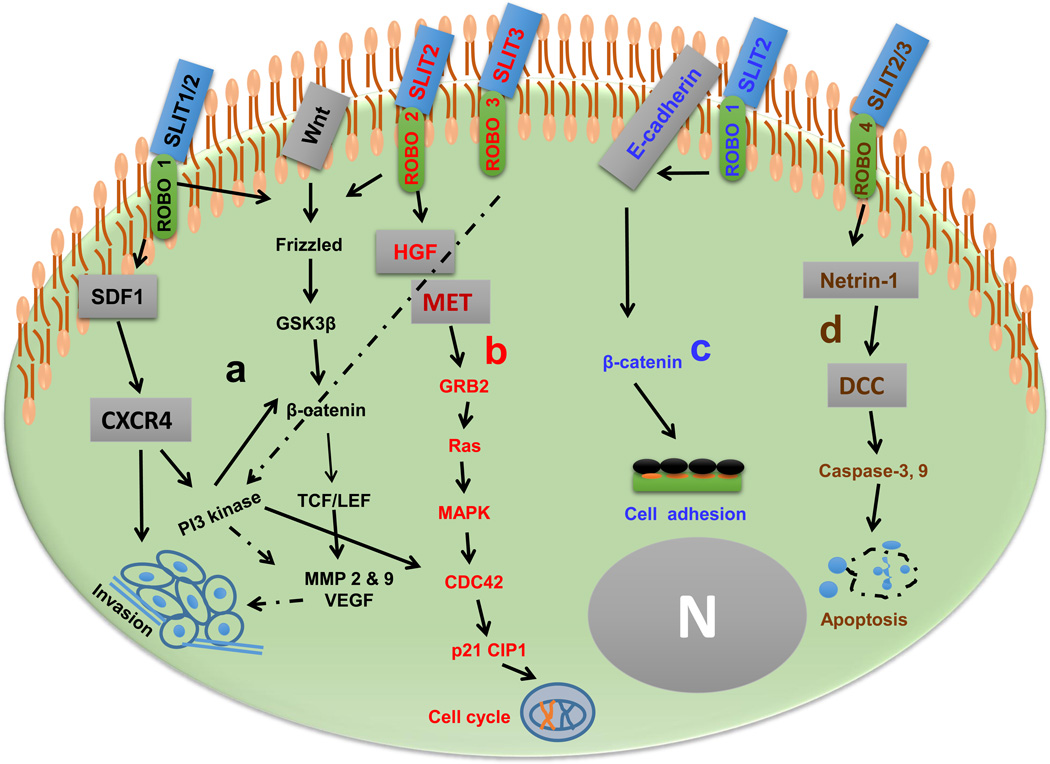
Figure 2.
Slit/Robo interactions and their roles in carcinogenesis. (a) Slit1,2/Robo1 can inhibit cell invasion by inhibiting the stromal-derived factor (SDF)-1/CXCR4 axis and can attenuate cell cycle progression by destruction of β-catenin and cell division control protein (CDC)42 expression. (b) Alternatively, Slit/Robo interactions suppress hepatocyte growth factor (HGF) expression leading to Met[s7][s8]-dependent inhibition of cell cycle arrest. (c) Slit2/Robo1 axis antagonizes E-cadherin/β-catenin-mediated enhanced cell adhesion. (d) Binding of Slit2,3 with Robo4 releases netrin-1, activating DCC[s9]-regulated activation of caspase-dependent apoptotic cell death. Solid lines represent reported studies and dotted lines represent putative functions. Figure modified [s10]with permission, from [2]. Abbreviations: GRB, growth factor receptor bound protein; GSK, glycogen synthase kinase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; N, nucleus; VEGF, vascular endothelial growth factor.
Regulation of tumor microenvironment
At present, there are no convincing reports that suggest whether the Slit/Robo axis has any role in cancer initiation [24,33,36]. Slit/Robo expression is known to inhibit cancer progression (invasion and migration) by modulating chemokines and growth factors [24,29,37,38]. The Slit/Robo pathway plays an important part in the modulation of tumor microenvironment by inhibiting the migration of leukocyte chemotaxis and inducing a chemorepellent effect in cancer cells [24]. Several reports indicate that the Slit/Robo pathway has an important role in the regulation of immunity in various types of cancers [1,2,39]. The activation of Slit2/Robo1 inhibits chemotaxis and chemoinvasion in breast cancer by inhibiting chemokine receptor CXCR4 expression in breast and lung cancer cells [24,40]. The Slit/Robo axis modulates tumor microenvironment by regulating growth factors such as hepatocyte growth factor (HGF), which supports the motility of tumor cells. In another study, it was suggested that Slit2/Robo1 inhibited HGF-dependent cell migration in breast, ovarian and endometrial cancer cells. Slit2 suppression enhanced HGF-dependent metastatic morphogenesis and cell motility in lung, colorectal and breast cancer cell lines [32], suggesting that the Slit/Robo pathway negatively regulates HGF in cancer cells. The mechanism behind regulation of growth-factor-mediated oncogenic signaling pathways by the Slit/Robo pathway is still unclear [25]. Alternatively, activation of Slit2 inhibits cell motility in in vitro studies. Also, addition of recombinant Slit2 decreases colony formation in ovarian cancer, gliomas [41] and breast and lung carcinoma cells [32]. Colonization of tissuespecific cancer cells occurs in the early stage of metastasis, which supports cancer cells for their settlement, adaptation and further progression to distant organs [42,43].
Cancer metastasis
Tumor microenvironment and tumor metabolism are key regulators of metastasis in cancers [44]. Tumor microenvironment can be either tissue-resident or derived from peripheral reservoirs such as the bone marrow and spleen. The macrophages that are in close proximity or within tumors are called tumor-associated macrophages (TAMs). TAMs could be derived either from circulating monocytes or resident-tissue macrophages [45,46]. The interactions between TAMs and growth factors, cytokines and chemokines are the key determinants for progression of tumor invasion and metastasis [47–49]. In many tumors such as breast, ovarian, glioma and lymphoma, the level of TAM infiltration is associated with poor prognosis [50]. Like neuronal cells, migration of leukocytes also requires the recognition of guidance cues, polarization of the cell and mobilization of the actin cytoskeleton [51]. The secretory protein Slit2 and its receptors Robo1 and Robo4 are considered to regulate mobility and permeability of endothelial cells and other cell types. Slit2 was found to inhibit leukocyte migration in the gradient of monocyte chemotactic protein-1 (MCP-1) in an in vitro pancreatic cancer model [52]. Current reports suggest that Slit2 and its receptor Robo1 inhibit metastasis in breast cancer [38], fibrosarcoma and squamous cell carcinoma [53]. In most of the cancers, the Slit–Robo interaction is not directly involved in the progression of metastasis [38,54], but is involved in modulating chemokines, such as stromal-derived factor-1 (SDF-[s3]1) and MCP-1 [38,55,56]. It is known that CXCL12, a ligand of SDF-1, is constitutively expressed and is an inducible chemokine that modulates several biological and physiological processes, including embryonic development and organ homeostasis [57]. Aberrant expression of SDF-1 has been found in various solid tumor tissues such as ovarian cancer [58], breast cancer [59] and other cancers [60–63]. SDF-1 has been shown to promote growth, invasion and metastasis in breast cancer [59], lung cancer [64], pancreatic cancer [65] and other cancers [22,66]. At present, it is not known if Slit/Robo pathways have any role in tumor metabolism; however, it can definitely be considered that the Slit/Robo pathway inhibits metastasis by abrogating multiple oncogenic signaling pathways in tumors [37,67,68].
Role of Slit/Robo pathway in different cancers
The tumor suppressor or antitumor activity of the Slit/Robo pathway is not unanimous, and how cancer-specific expression of Slit and Robo proteins governs the different type of cancers is not clearly comprehended. This section summarizes the recent updates on the regulation of oncogenesis by the Slit/Robo pathway in different cancer types.
Hormone-dependent cancers
Hormone-dependent cancers are primarily driven by sex hormones. Slit and Robo proteins have a crucial role in regulating physiological functions in the reproductive organs of humans. The differential expression and modulation of Slit and Robo proteins determine cell death and proliferation in an autocrine and paracrine manner. Estrogen and selective estrogen receptor modulators (SERMs) have been shown to regulate the expression of Slit1 in bones of ovariectomized rats [2,69]. Therefore, the effect of sex hormones on Slit and Robo proteins in hormone-dependent cancers such as breast, prostate, ovarian, endometrial and kidney cancers is still a major area of research to investigate. Herein, we summarize the current updates on the modulation of metastasis by the Slit/Robo pathway in some hormone-dependent cancers.
Breast cancer
The Slit/Robo pathway has an important role in breast development and morphology by regulating outgrowth of mammary branches, and by inhibiting translocation of cytosolic nuclear β-catenin to the nucleus of the basal myoepithelial cells [70]. In breast cancer, Slit/Robo has antitumor activity. This is evidenced by the fact that most breast tumors have low expression of Slit/Robo and its higher expression is correlated with increased survival rate in cancer patients, whereas low Slit2 expression is associated with poor survival and increased metastasis [22,38,71]. Moreover, downregulation of Slit/Robo is associated with amplification of c-Myc and cyclin D1 gene expression in many breast cancer tumors [72,73]. Recently, Chang et al. demonstrated that activation of Slit2/Robo1 inhibits activation of β-catenin by inhibiting Akt, thereby preventing translocation of cytosolic β-catenin to the nucleus of the fibroblast cells [40]. Upregulation of Robo1 and Slit2 expression inhibited translocation of nuclear β-catenin in breast cancer cells. β-catenin is one of the major oncogenic pathways responsible for the motility, invasiveness and triple-negative phenotype of breast cancer cells [74–76]. Most of the breast cancers initially respond to antiestrogen therapy but, during the course of the treatment, resistance to antiestrogen therapy is very common. Antiestrogen therapy can fail as a result of one or more of the possible mechanisms such as activation of phosphoinositide 3-kinase (PI3K)/Akt and chemokines (CXCL12/CXCR4 or CCL2) [67,77–79]. Prasad et al. reported for the first time that Slit/Robo also regulates chemokine CXCL12. Addition of recombinant Slit2 suppresses CXCL12-induced breast cancer cell chemotaxis, chemoinvasion and adhesion in MDA-MB-231 breast cancer cells [24]. There was no remarkable effect of Slit2 on the CXCL12- induced internalization process of CXCR4, but it inhibits the CXCL12-mediated RAFTK/Pyk2 signaling pathway [38]. Slit2/Robo1 also inhibits CXCL12-induced PI3K/Akt, p44/42 mitogen-activated protein (MAP) kinase and matrix metalloproteinase (MMP)-2 and -9 activities in breast cancer cells [24,38]. Slit2 expression did not inhibit c-Jun N-terminal kinase (JNK) or p38 MAP kinase activities in breast cancer cells [24]. The Slit/Robo pathway regulates chemokine-mediated cellular responses in breast cancer cells and thus inhibits cellular migration by activation of ubiquitin/proteasome degradation pathways [80]. Combined, these findings suggest that the Slit/Robo pathway might be a key regulator of breast cancer metastasis.
Ovarian cancer
Slit–Robo interaction involves autocrine and paracrine mechanisms, which seem to play an important part in the development of fetal ovaries during organogenesis [81]. In humans, normal ovarian tissues and ovarian cancer tissues widely express three components of Slit and Robo proteins (Slit2/3 and Robo1), suggesting that Slit/Robo has an important role in normal and malignant ovarian tissues [21]. However, expression of Robo4 was much higher in ovarian tumor tissues, and Robo1 was also detected in ovarian tumor tissues. Robo4 is known to induce angiogenesis by inhibiting Slit2, however blockage of Robo leads to inhibition of angiogenesis in ovarian cancer in vitro. It is not clear whether overexpression of Robo4 has antior pro-angiogenic effect in ovarian cancer cells [21]. This variation could be the result of the small sample size and the heterogeneous nature of ovarian cancer cells used in this study [21,82]. In vitro cell line models suggested that the ovarian cancer cell lines OVCAR-3 and SKOV-3 express Slit2/3 and Robo1/4. However, addition of recombinant human Slit2 did not significantly affect cell proliferation or induce ERK1/2 and Akt1 phosphorylation in these cells [21]. In another study, it was reported that the basal mRNA expression levels of Slit2,3 and Robo1,2,4 was low in primary cultures of ovarian cancer epithelial cells compared with normal ovarian surface epithelial (OSE) cells [83]. The expression of Slit/Robo also depends on the level of differentiation of ovarian cancer cells [21,83]. In all cases, high-grade malignant cells had lower expression of Slits and Robos. When exogenous cortisol was added, there was a reduced expression of Slit2,3 and Robo1,2,4 in normal OSE and PEO-14 cells. Furthermore, blocking Slit/Robo activity reduced apoptosis in well-differentiated PEO-14 and SKOV-3 tumor cells. When glucocorticoid receptor was knocked-down using siRNA, there was an upregulation of Slit/Robo. One of the possible reasons that can account for this effect is that Slits and Robos are regulated at the transcriptional level by glucocorticoid receptors in ovarian cancer cells [83].
Prostate cancer
Limited studies suggest that all Slits (Slit1–3) are overexpressed in prostate tumors as compared with the normal prostate tissues. However, no significant association was observed in the expression of Slit2 and Slit3 in prostate tumors as compared with the normal prostate tissues. The expression of Slit1 was more frequent in clinically localized tumors. Slit1–3 were overexpressed in hormone refractory tumors as compared with benign tumors [23]. In contrast to Slit1, Robo1 was downregulated in prostate tumors when compared with the normal prostate tissues. Robo2 was highly overexpressed in hormone refractory tumors as compared with malignant tumors [23]. The possible reason for the upregulation of the Slit/Robo pathway could be caused by suppression of the DCC receptor, by another axon guidance cue netrin-1, in prostate tumors and prostate cancer cells [18,20]. Netrin-1 is also known as a survival cue because it controls apoptosis in many cancers. The binding of netrin-1 to DCC or Slits might be a determinant for the antitumor activity of Slit and Robo proteins in prostate cancers [19,23].
Nonhormonal cancers
Although the Slit/Robo family members are primarily expressed in the developing nervous system and are involved in organogenesis, they are also widely expressed outside the nervous system and reproductive organs in adult tissues, suggesting possible roles outside the developing embryo [84]. Several studies have suggested that, in most of the cancers, specific Slit and Robo proteins are downregulated, such as in lung cancer [31,32,85], brain cancer [31,35], cervical cancer [86], liver cancer [32,35], colon cancer [85] and others. Table 1 summarizes the recent updates on the expression of Slit/Robo in cancer cells.
Table 1.
Expression of Slit/Robo
| Cancer types | Cell lines | Proteins | Expression status |
Refs |
|---|---|---|---|---|
| Liver cancer | HuH-7, HepG2 | Slit1, Robo1,2 | ↑ | [35] |
| Slit3, Robo4 | ↓ | |||
| Lung cancer | A549 | Slit1,2,3 Robo1,3 |
↓ | [9,23[s13],31,32,85–88] |
| Colon cancer | RKO | Slit2 | ↑ | [85] |
| Robo1 | ↓ | |||
| Brain cancer and glioma | GAMG | Slit1,2,3 Robo1 |
↓ | [31,35] |
| Prostate cancer | LNCaP, PC-3, DU-145 | Slit1,2,3 Robo1,3 |
↑ | [9,23,88] |
| Cervical cancer | CaSki, HT-3, SiHa, SW756, ME-180, HeLa |
Slit1,2,3 Robo1,3 |
↓ | [86] |
| Breast cancer | MCF-7 | Slit1,2,3 Robo1,3 |
↓ | [9,32,87,88] |
| Kidney cancer | ccRCC | Robo1,3 | ↓ | [88] |
| Acute lymphocytic leukemia |
THP-1, Jurkat | Slit2 Robo1 |
↓ | [54] |
Slit and Robo proteins are expressed differently depending upon the type of cancer and cancer cell line. In lung, brain, cervical, kidney and acute lymphocytic leukemia these proteins are downregulated; whereas in prostate, liver and colon cancers usually upregulation of Slit1,2/Robo1,2 is found. Prostate cancer cells have shown upregulation of all Slit and Robo proteins.
Lung cancer
In lung cancer, the expression of Slit2 is suppressed [9,31,32,36,85] with a high expression of β-catenin in 60% of formalin-fixed, paraffin-embedded lung tumors as compared with normal tissue. Suppression of Slit2 was associated with an upward trend of pathological stage and poor survival rate of lung cancer patients. However, downregulation of Slit2 and βTrCP, and upregulation of β-catenin, can be predicted for postoperative disease relapse [87]. βTrCP, one of the components of the β-catenin–ubiquitin-ligase complex, is a negative regulator of the Wnt signaling pathway. In vitro studies suggested that the suppression of Slit2 is due to activation of Akt/GSK3β/βTrCP in non-small-cell lung carcinoma cells. Activation of the PI3K/Akt pathway toward the GSK3β/β-catenin signaling cascade stimulates cancer cells to begin bone metastasis [88]. Slit2 has an inverse association with lung cancer cells with respect to motility in in vitro systems.
Cervical cancer
In cervical cancer, primary tumors and cells (Caski, HeLa, SiHa, C33A, HT-3 and ME-180) have Slit proteins (Slit1–3) and Robo1,3 that become downregulated or completely suppressed [86]. The suppression of Slit and Robo proteins in cervical cancer is due to promoter hypermethylation, which is an early event in tumor progression [86]. Re-expression of Slit/Robo by a demethylating drug (5-Aza-2'deoxycytidine and trichostatin A) in cervical cancer cells is only partially restored and is found in very few cervical cancer cells. It can be assumed that only demethylation of promoters of Slit/Robo pathway genes does not effectively reactivate gene expression in cervical cancer. The only known mechanism that accounts for this effect is promoter hypermethylation of Slit/Robo.
Glioblastoma
It is well established that, in the central nervous system, Slit–Robo interactions play an important part in controlling the migration of neurons and neural crest cells by Slit-regulated Rho-GTPase-activating proteins and by the inhibition of CDC42, a small Rho GTPase [30,89]. The expression of the Slit2 protein gets suppressed in glioblastoma, as observed in several other cancers. The Slit/Robo pathway has antitumorigenic properties as evidenced by the suppressed expression of Slit2 in primary glioma tumors and invasive glioma cells as compared with normal human brain cells and in control cell type astrocytes. The Slit/Robo pathway inhibits cell invasion and motility in glioblastoma in in vitro and in vivo systems [90]. In vitro studies suggested that addition of recombinant Slit2, or ectopic expression of Slit2, inhibited glioma cell invasion and motility by inhibiting CDC42 activity in glioma cells. CDC42 is one of the members of the Rho GTPase family and it controls cell polarity during migration of cancer cells [41,90]. It has been shown that the activity of CDC42 is upregulated in several cancers, including glioblastoma, and is responsible for high invasive metastasis in cancers [91]. In vivo studies also suggested that the re-expression of Slit2 in SNB19 glioma cells inhibited infiltration of glioma cells into the brain of mice [90]. Activated Slit2 inhibited cell motility without affecting the expression of N-cadherin and β-catenin in glioma cells [90].
Future prospects for Slit/Robo pathway as a drug target
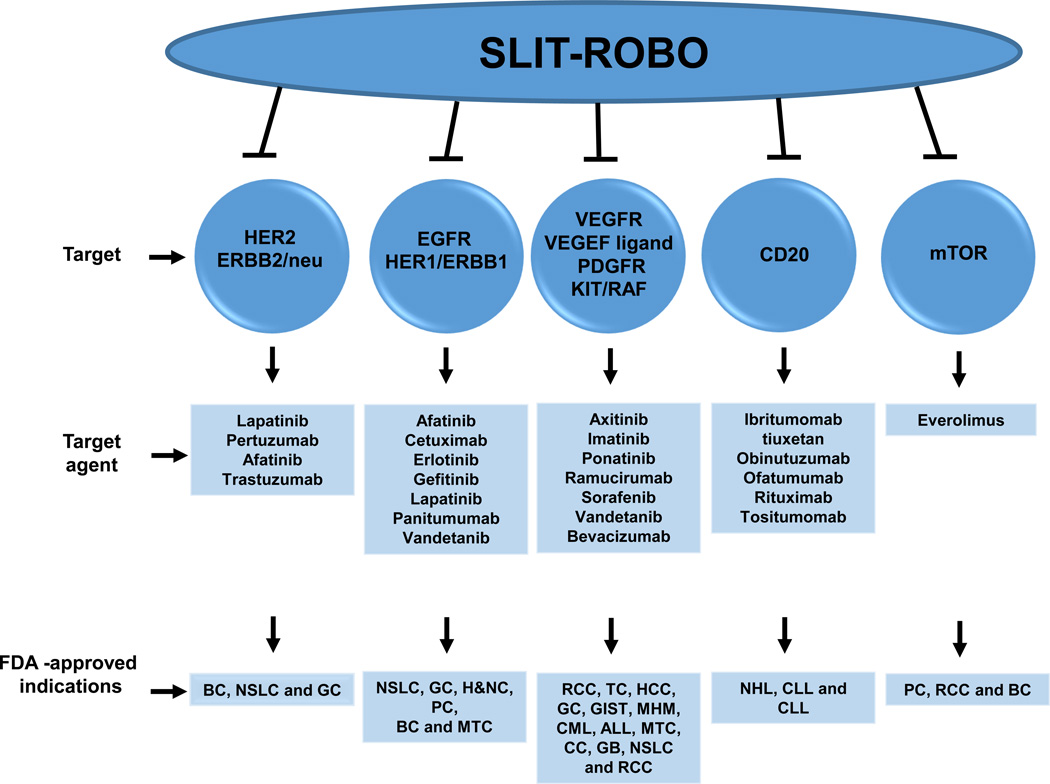
Besides functioning as axon guidance molecules, the Slit/Robo pathway proteins also regulate functions such as morphogenesis of tissue as well as performing many non-neuronal roles such as cell growth, migration and cell survival [92]. Slit/Robo is often downregulated in the advanced stage of most tumors [31,85,86]. In vitro studies suggested that expression of Slit can be regulated by stress and optimum function can be achieved by modulating the endoplasmic reticulum (ER), Ca2+ homeostasis and actin in β cells [92]. For example, cytokines, thapsigargin and palmitate suppress Slit2 expression and serum deprivation downregulates Slit3 [92]. Cancer cells have the tendency to become aggressive and metastatic under extreme stress (low glucose, hypoxic condition) as compared with normoxic conditions [93]. However, robust induction of ER stress upregulates Slit3 and Slit1 expression [92]. It is not known whether a high grade of tumor, besides promoter hypermethylation, deletion of Slit/Robo gene and other factors such as ER, Ca2+ and high glucose, has any role in the modulation of Slit/Robo pathways in cancer cells. Many targeted therapeutic agents inhibit the growth of cancer cells by controlling a specific pathway: HER-2/EGFR/VEGFR/mTOR/CD-20. Overall, the literature suggests that the Slit/Robo pathway is a direct or indirect key regulator of these other pathways (Figure 3). Therefore, we propose that targeting the Slit/Robo pathway using an inhibitor is highly useful and can lead to regulation of a wide variety of oncogenic signaling. Such a strategy could emerge as a new therapeutic modality for cancer. In our opinion, it could be achieved by several methods as outlined below.
Developing specific mimetics that can interfere with Slit/Robo expression. Recently such molecules have been designed and used for semaphorin, an axon guidance protein [94]. This type of mimetic was not developed to target the Slit/Robo pathway so it has not been tested in any cancers.
The Slit/Robo pathway has antitumor effects and, in most of the tumors, its expression is downregulated or undetectable. Like many antioncogenic genes, promoter hypermethylation is one mechanism by which loss of gene function occurs [31,95]. Slit/Robo expression can be restored by histone deacetylase (HDAC) inhibitors. However, in vitro results suggested that very few cell types respond better; rather, their response is poor or they do not respond at all. Even demethylation of promoters of Slit/Robo pathway genes does not effectively reactivate gene expression; thus, the upregulation of Slit/Robo by HDAC inhibitors is not an ideal mechanism [31,86].
Another approach might be the epigenetic modulation of Slit/Robo along with the modulation of intracytoplasmic concentration of intracellular Ca2+ and glucose, or induction of strong ER stress by thapsigargin, or other synthetic or natural compounds. However, these approaches need to be tested in in vitro and in vivo models for their feasibility and applicability for targeted therapies.
Figure 3.
[s11]. Slit/Robo pathway targets various key signaling pathways in cancer. The list of targeted agents that improve clinical therapeutic outcome of breast cancer (BC), non-small-cell lung cancer (NSLC), gastric cancer (GC), renal cell carcinoma (RCC), head and neck cancer (H&NC), pancreatic cancer (PC), medullary thyroid cancer (MTC), thyroid carcinoma (TC), GI stromal tumor (GIST), multiple hematologic malignancies (MHM), chronic mylogenous leukemia (CML), acute lymphoblastic leukemia (ALL), colorectal cancer (CC), glioblastoma (GB), chronic lymphocytic leukemia (CLL), among others. Inhibitors or agents that regulate the Slit/Robo pathway could be useful therapeutic molecules. Abbreviations: EGFR, [s12]epidermal growth factor receptor; HER2, human epidermal growth factor; HCC, hepatocellular carcinoma; mTOR, mammalian target of rapamycin; NHL, non-Hodgkin lymphoma; PDGFR, plateletderived growth factor receptor; VEGFR, vascular endothelial growth factor receptor.
Concluding remarks
The overall data suggest that the axon guidance molecule Slit/Robo has important roles in cancer metastasis, tumorigenesis in many tumors and that several possible tumor-specific expressions need to be explored in future studies. However, additional research is necessary before we can provide a robust, comprehensive mechanism to target the Slit/Robo pathway in the specific cancers. Tumor-specific discrepancy of Slit/Robo by previous authors might simply tempt one to apply neurobiological models to cancer. Also, because tumorigenesis is a complex process and involves a huge array of signaling pathways, it is extremely difficult to determine how Slit/Robo participates in this complex system. Most studies reported are primarily based on basic molecular biology techniques that are totally different to the biological conditions in tumors. Further, local concentration of ligand receptors and intracellular concentration of various molecules might be possible reasons why variations exist in recent studies. Multiple crosstalk signaling needs to be reconsidered before making any final conclusions. There are some major questions in this field concerning how different Slits are able to mediate their disparate actions. Slits have been shown to bind with similar affinity to all of the Robo receptors using evolutionary conserved binding residues. However, in different systems, Slits mediate diverse effects. For instance, in most of the tumors, Slit2 has an antitumor effect, as compared with Slit1 or Slit3. Therefore, it will be important to identify the molecules, such as co-receptors, that mediate such ligand specificities. Another remaining paradox is how increasing or decreasing levels of Robo receptors and/or different combinations of Robo receptors mediate varying effects within a given cell or between neighboring cells. There is no drug molecule that can efficiently target the Slit/Robo pathway. Therefore, vast possibilities exist for carrying out additional basic research in this direction in order to develop efficient targeted anticancer therapies.
Highlights.
Slit and Robo are highly involved in the regulation of physiological processes
The Slit/Robo pathway has a significant role in tumorigenesis and cancer progression
The Slit/Robo pathway is a key regulator of many oncogenic pathways
Molecules targeting the Slit/Robo pathway could be promising anticancer drugs
Acknowledgments
The authors thank Cathy Christopherson (Sanford Research) for editorial assistance. This work was partially supported by grants from: the National Institutes of Health (R01 CA142736 to S.C.C. and U01 CA162106A to S.C.C. and M.J.); Department of Defense (PC073887 to S.C.C. and PC073643 to M.J.); and the College of Pharmacy (2013 Dean’s Seed Grant of the University of Tennessee Health Science Center to M.J. and M.M.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Teaser: Recent studies indicate that Slit and Robo play an important part in tumorigenesis, cancer progression and metastasis. This review suggests that the Slit/Robo pathway could be a promising target for the development of anticancer drugs.
Conflicts of interest
The authors have no conflict of interest to declare regarding this work.
References
- 1.Andrews W, et al. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction. 2010;139:697–704. doi: 10.1530/REP-10-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Refai O, et al. Extension of the Caenorhabditis elegans pharyngeal M1 neuron axon is regulated by multiple mechanisms. G3 (Bethesda) 2013;3:2015–2029. doi: 10.1534/g3.113.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brose K, et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 5.Rothberg JM, et al. Slit: an EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell. 1988;55:1047–1059. doi: 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- 6.Kidd T, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 7.Seeger M, et al. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen-Ba-Charvet KT, Chedotal A. Role of Slit proteins in the vertebrate brain. J. Physiol. 2002;96:91–98. doi: 10.1016/s0928-4257(01)00084-5. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson RE, et al. Epigenetic inactivation of SLIT3 and SLIT1 genes in human cancers. Br. J. Cancer. 2004;91:2071–2078. doi: 10.1038/sj.bjc.6602222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara M, et al. Potential role of the Slit/Robo signal pathway in angiogenesis. Vasc. Med. 2006;11:115–121. doi: 10.1191/1358863x06vm658ra. [DOI] [PubMed] [Google Scholar]
- 11.Rothberg JM, Artavanis-Tsakonas S. Modularity of the slit protein. Characterization of a conserved carboxy-terminal sequence in secreted proteins and a motif implicated in extracellular protein interactions. J. Mol. Biol. 1992;227:367–370. doi: 10.1016/0022-2836(92)90891-m. [DOI] [PubMed] [Google Scholar]
- 12.Rothberg JM, et al. Slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- 13.Domyan ET, et al. Roundabout receptors are critical for foregut separation from the body wall. Dev. Cell. 2013;24:52–63. doi: 10.1016/j.devcel.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundaresan V, et al. Somatic genetic changes in lung cancer and precancerous lesions. Ann. Oncol. 1995;6(Suppl. 1):27–31. doi: 10.1093/annonc/6.suppl_1.s27. [DOI] [PubMed] [Google Scholar]
- 15.Zabarovsky ER, et al. Tumor suppressor genes on chromosome 3p involved in the pathogenesis of lung and other cancers. Oncogene. 2002;21:6915–6935. doi: 10.1038/sj.onc.1205835. [DOI] [PubMed] [Google Scholar]
- 16.Tang H, et al. Axonal guidance signaling pathway interacting with smoking in modifying the risk of pancreatic cancer: a gene- and pathway-based interaction analysis of GWAS data. Carcinogenesis. 2014;35:1039–1045. doi: 10.1093/carcin/bgu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biankin AV, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 19.Paradisi A, et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17146–17151. doi: 10.1073/pnas.0901767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castets M, et al. DCC constrains tumour progression via its dependence receptor activity. Nature. 2012;482:534–537. doi: 10.1038/nature10708. [DOI] [PubMed] [Google Scholar]
- 21.Dai CF, et al. Expression and roles of Slit/Robo in human ovarian cancer. Histochem. Cell Biol. 2011;135:475–485. doi: 10.1007/s00418-011-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid BC, et al. The neuronal guidance cue Slit2 induces targeted migration and may play a role in brain metastasis of breast cancer cells. Breast Cancer Res. Treat. 2007;106:333–342. doi: 10.1007/s10549-007-9504-0. [DOI] [PubMed] [Google Scholar]
- 23.Latil A, et al. Quantification of expression of netrins, slits and their receptors in human prostate tumors. Int. J. Cancer. 2003;103:306–315. doi: 10.1002/ijc.10821. [DOI] [PubMed] [Google Scholar]
- 24.Prasad A, et al. Slit protein-mediated inhibition of CXCR4-induced chemotactic and chemoinvasive signaling pathways in breast cancer cells. J. Biol. Chem. 2004;279:9115–9124. doi: 10.1074/jbc.M308083200. [DOI] [PubMed] [Google Scholar]
- 25.Stella MC, et al. The Slit/Robo system suppresses hepatocyte growth factordependent invasion and morphogenesis. Mol. Biol. Cell. 2009;20:642–657. doi: 10.1091/mbc.E08-03-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wick MJ, et al. Lung Kruppel-like factor (LKLF) is a transcriptional activator of the cytosolic phospholipase A2 alpha promoter. Biochem. J. 2005;387:239–246. doi: 10.1042/BJ20041458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueoka Y, et al. Hepatocyte growth factor modulates motility and invasiveness of ovarian carcinomas via Ras-mediated pathway. Br. J. Cancer. 2000;82:891–899. doi: 10.1054/bjoc.1999.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson KW, Sandler AB. The role of MET receptor tyrosine kinase in non-small cell lung cancer and clinical development of targeted anti-MET agents. Oncologist. 2013;18:115–122. doi: 10.1634/theoncologist.2012-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denk AE, et al. Slit3 inhibits activator protein 1-mediated migration of malignant melanoma cells. Int. J. Mol. Med. 2011;28:721–726. doi: 10.3892/ijmm.2011.742. [DOI] [PubMed] [Google Scholar]
- 30.Lahoz A, Hall A. A tumor suppressor role for srGAP3 in mammary epithelial cells. Oncogene. 2013;32:4854–4860. doi: 10.1038/onc.2012.489. [DOI] [PubMed] [Google Scholar]
- 31.Dallol A, et al. Frequent epigenetic inactivation of the SLIT2 gene in gliomas. Oncogene. 2003;22:4611–4616. doi: 10.1038/sj.onc.1206687. [DOI] [PubMed] [Google Scholar]
- 32.Dallol A, et al. SLIT2, a human homologue of the Drosophila Slit2 gene, has tumor suppressor activity and is frequently inactivated in lung and breast cancers. Cancer Res. 2002;62:5874–5880. [PubMed] [Google Scholar]
- 33.Legg JA, et al. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11:13–21. doi: 10.1007/s10456-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 34.Wang LJ, et al. Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Sci. 2008;99:510–517. doi: 10.1111/j.1349-7006.2007.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehlen P, et al. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat. Rev. Cancer. 2011;11:188–197. doi: 10.1038/nrc3005. [DOI] [PubMed] [Google Scholar]
- 36.Nasarre P, et al. Guidance molecules in lung cancer. Cell Adh. Migr. 2010;4:130–145. doi: 10.4161/cam.4.1.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer K, et al. Slit-2 facilitates interaction of P-cadherin with Robo-3 and inhibits cell migration in an oral squamous cell carcinoma cell line. Carcinogenesis. 2011;32:935–943. doi: 10.1093/carcin/bgr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marlow R, et al. SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4 within breast epithelium. Cancer Res. 2008;68:7819–7827. doi: 10.1158/0008-5472.CAN-08-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JY, et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang PH, et al. Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway. Cancer Res. 2012;72:4652–4661. doi: 10.1158/0008-5472.CAN-12-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mertsch S, et al. Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. J. Neurooncol. 2008;87:1–7. doi: 10.1007/s11060-007-9484-2. [DOI] [PubMed] [Google Scholar]
- 42.Shibue T, Weinberg RA. Metastatic colonization: settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Semin. Cancer Biol. 2011;21:99–106. doi: 10.1016/j.semcancer.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Chang J, Erler J. Hypoxia-mediated metastasis. Adv. Exp. Med. Biol. 2014;772:55–81. doi: 10.1007/978-1-4614-5915-6_3. [DOI] [PubMed] [Google Scholar]
- 44.Bailey KM, et al. Targeting the metabolic microenvironment of tumors. Adv. Pharmacol. 2012;65:63–107. doi: 10.1016/B978-0-12-397927-8.00004-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyler L, et al. Brain metastasis in renal cancer patients: metastatic pattern, tumour-associated macrophages and chemokine/chemoreceptor expression. Br. J. Cancer. 2014;110:686–694. doi: 10.1038/bjc.2013.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang W, et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. 2014;35:1780–1787. doi: 10.1093/carcin/bgu060. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed MM, et al. Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties. Int. J. Biochem. Cell Biol. 2014;46:138–147. doi: 10.1016/j.biocel.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xuan QJ, et al. Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol. Oncol. Res. 2014;20:619–624. doi: 10.1007/s12253-013-9740-z. [DOI] [PubMed] [Google Scholar]
- 49.Yang Q, et al. Antitumour activity of the recombination polypeptide GST-NT21MP is mediated by inhibition of CXCR4 pathway in breast cancer. Br. J. Cancer. 2014;110:1288–1297. doi: 10.1038/bjc.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leek RD, et al. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br. J. Cancer. 1999;79:991–995. doi: 10.1038/sj.bjc.6690158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirakaj V, et al. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6555–6560. doi: 10.1073/pnas.1015605108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gohrig A, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74:1529–1540. doi: 10.1158/0008-5472.CAN-13-1012. [DOI] [PubMed] [Google Scholar]
- 53.Kim HK, et al. Slit2 inhibits growth and metastasis of fibrosarcoma and squamous cell carcinoma. Neoplasia. 2008;10:1411–1420. doi: 10.1593/neo.08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunwell TL, et al. Frequent epigenetic inactivation of the SLIT2 gene in chronic and acute lymphocytic leukemia. Epigenetics. 2009;4:265–269. doi: 10.4161/epi.9137. [DOI] [PubMed] [Google Scholar]
- 55.Kanellis J, et al. Modulation of inflammation by slit protein in vivo in experimental crescentic glomerulonephritis. Am. J. Pathol. 2004;165:341–352. doi: 10.1016/S0002-9440(10)63301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeon ES, et al. Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells. Exp. Mol. Med. 2010;42:280–293. doi: 10.3858/emm.2010.42.4.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ratajczak MZ, et al. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 58.Xue B, et al. Stromal cell-derived factor-1 (SDF-1) enhances cells invasion by alphavbeta6 integrin-mediated signaling in ovarian cancer. Mol. Cell. Biochem. 2013;380:177–184. doi: 10.1007/s11010-013-1671-1. [DOI] [PubMed] [Google Scholar]
- 59.Sobolik T, et al. CXCR4 drives the metastatic phenotype in breast cancer through induction of CXCR2 and activation of MEK and PI3K pathways. Mol. Biol. Cell. 2014;25:566–582. doi: 10.1091/mbc.E13-07-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoellenriegel J, et al. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood. 2014;123:1032–1039. doi: 10.1182/blood-2013-03-493924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang B, et al. SDF-1/CXCR4 axis promotes directional migration of colorectal cancer cells through upregulation of integrin alphavbeta6. Carcinogenesis. 2014;35:282–291. doi: 10.1093/carcin/bgt331. [DOI] [PubMed] [Google Scholar]
- 62.Choi YH, et al. CXCR4, but not CXCR7, discriminates metastatic behavior in nonsmall cell lung cancer cells. Mol. Cancer Res. 2014;12:38–47. doi: 10.1158/1541-7786.MCR-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma J, et al. CXCL12 induces lung cancer cell migration by polarized mtDNA redistribution. Hum. Cell. 2014;27:22–28. doi: 10.1007/s13577-013-0077-4. [DOI] [PubMed] [Google Scholar]
- 64.Mirisola V, et al. CXCL12/SDF1 expression by breast cancers is an independent prognostic marker of disease-free and overall survival. Eur. J. Cancer. 2009;45:2579–2587. doi: 10.1016/j.ejca.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Roy I, et al. CXCL12 Chemokine expression suppresses human pancreatic cancer growth and metastasis. PLoS One. 2014;9:e90400. doi: 10.1371/journal.pone.0090400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao YX, et al. The role of the CXCL12-CXCR4/CXCR7 axis in the progression and metastasis of bone sarcomas. Int. J. Mol. Med. 2013;32:1239–1246. doi: 10.3892/ijmm.2013.1521. [DOI] [PubMed] [Google Scholar]
- 67.Beelen K, et al. PI3K/AKT/mTOR pathway activation in primary and corresponding metastatic breast tumors after adjuvant endocrine therapy. Int. J. Cancer. 2014;135:1257–1263. doi: 10.1002/ijc.28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campbell DS, Okamoto H. Local caspase activation interacts with Slit-Robo signaling to restrict axonal arborization. J. Cell Biol. 2013;203:657–672. doi: 10.1083/jcb.201303072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helvering LM, et al. Expression profiling of rat femur revealed suppression of bone formation genes by treatment with alendronate and estrogen but not raloxifene. Mol. Pharmacol. 2005;68:1225–1238. doi: 10.1124/mol.105.011478. [DOI] [PubMed] [Google Scholar]
- 70.Macias H, et al. SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by limiting basal cell number. Dev. Cell. 2011;20:827–840. doi: 10.1016/j.devcel.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarubin T, et al. Identification of eight genes that are potentially involved in tamoxifen sensitivity in breast cancer cells. Cell Res. 2005;15:439–446. doi: 10.1038/sj.cr.7290312. [DOI] [PubMed] [Google Scholar]
- 72.Barbareschi M, et al. Cyclin-D1-gene amplification and expression in breast carcinoma: relation with clinicopathologic characteristics and with retinoblastoma gene product, p53 and p21WAF1 immunohistochemical expression. Int. J. Cancer. 1997;74:171–174. doi: 10.1002/(sici)1097-0215(19970422)74:2<171::aid-ijc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 73.Kononen J, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 74.Bilir B, et al. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J. Transl. Med. 2013;11:280. doi: 10.1186/1479-5876-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang L, et al. Wnt modulates MCL1 to control cell survival in triple negative breast cancer. BMC Cancer. 2014;14:124. doi: 10.1186/1471-2407-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foldynova-Trantirkova S, et al. Breast cancer-specific mutations in CK1epsilon inhibit Wnt/beta-catenin and activate the Wnt/Rac1/JNK and NFAT pathways to decrease cell adhesion and promote cell migration. Breast Cancer Res. 2010;12:R30. doi: 10.1186/bcr2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang Z, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem. Biophys. Res. Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jordan NJ, et al. Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro. Breast Cancer Res. 2014;16:R12. doi: 10.1186/bcr3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah KN, et al. AKT-induced tamoxifen resistance is overturned by RRM2 inhibition. Mol. Cancer Res. 2014;12:394–407. doi: 10.1158/1541-7786.MCR-13-0219. [DOI] [PubMed] [Google Scholar]
- 80.Yuasa-Kawada J, et al. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14530–14535. doi: 10.1073/pnas.0801262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dickinson RE, et al. Novel regulated expression of the SLIT/ROBO pathway in the ovary: possible role during luteolysis in women. Endocrinology. 2008;149:5024–5034. doi: 10.1210/en.2008-0204. [DOI] [PubMed] [Google Scholar]
- 82.Bast RC, Jr, et al. The biology of ovarian cancer: new opportunities for translation. Nat. Rev. Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dickinson RE, et al. Glucocorticoid regulation of SLIT/ROBO tumour suppressor genes in the ovarian surface epithelium and ovarian cancer cells. PLoS One. 2011;6:e27792. doi: 10.1371/journal.pone.0027792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whitford KL, et al. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 85.Dallol A, et al. SLIT2 axon guidance molecule is frequently inactivated in colorectal cancer and suppresses growth of colorectal carcinoma cells. Cancer Res. 2003;63:1054–1058. [PubMed] [Google Scholar]
- 86.Narayan G, et al. Promoter hypermethylation-mediated inactivation of multiple Slit- Robo pathway genes in cervical cancer progression. Mol. Cancer. 2006;5:16. doi: 10.1186/1476-4598-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tseng RC, et al. SLIT2 attenuation during lung cancer progression deregulates beta-catenin and E-cadherin and associates with poor prognosis. Cancer Res. 2010;70:543–551. doi: 10.1158/0008-5472.CAN-09-2084. [DOI] [PubMed] [Google Scholar]
- 88.Papachristou DJ, et al. Bone metastases: molecular mechanisms and novel therapeutic interventions. Med. Res. Rev. 2012;32:611–636. doi: 10.1002/med.20224. [DOI] [PubMed] [Google Scholar]
- 89.Wong K, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- 90.Yiin JJ, et al. Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro Oncol. 2009;11:779–789. doi: 10.1215/15228517-2008-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao L, et al. Activation of Rho GTPase Cdc42 promotes adhesion and invasion in colorectal cancer cells. Med. Sci. Monit. Basic Res. 2013;19:201–207. doi: 10.12659/MSMBR.883983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang YH, et al. Intraislet SLIT-ROBO signaling is required for beta-cell survival and potentiates insulin secretion. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16480–16485. doi: 10.1073/pnas.1214312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caino MC, et al. Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J. Clin. Invest. 2013;123:2907–2920. doi: 10.1172/JCI67841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shirvan A, et al. Anti-semaphorin 3A antibodies rescue retinal ganglion cells from cell death following optic nerve axotomy. J. Biol. Chem. 2002;277:49799–49807. doi: 10.1074/jbc.M204793200. [DOI] [PubMed] [Google Scholar]
- 95.Dong SM, et al. Concurrent hypermethylation of multiple genes is associated with grade of oligodendroglial tumors. J. Neuropathol. Exp. Neurol. 2001;60:808–816. doi: 10.1093/jnen/60.8.808. [DOI] [PubMed] [Google Scholar]