Abstract
Atrial fibrillation (AF) is a very common cardiac arrhythmia, and its prevalence is increasing along with aging in the developed world. This review discusses racial differences in the epidemiology and treatment of AF between African-American and Caucasian patients. Additionally, the effect of race on warfarin and novel oral anticoagulant use is discussed, as well as the role that physicians and patients play in achieving optimal treatment outcomes. Despite having a lower prevalence of AF compared with Caucasians, African-Americans suffer disproportionately from stroke and its sequelae. The possible reasons for this paradox include poorer access to health care, lower health literacy, and a higher prevalence of other stroke-risk factors among African-Americans. Consequently, it is important for providers to evaluate the effects of race, health literacy, access to health care, and cultural barriers on the use of anticoagulation in the management of AF. Warfarin-dose requirements vary across racial groups, with African-American patients requiring a higher dose than Caucasians to maintain a therapeutic international normalized ratio; the novel oral anticoagulants (dabigatran, rivaroxaban, and apixaban) seem to differ in this regard, although data are currently limited. Minority racial groups are not proportionally represented in either real-world studies or clinical trials, but as more information becomes available and other social issues are addressed, the treatment disparities between African-American and Caucasian patients should decrease.
Keywords: antithrombotic, atrial fibrillation, stroke, warfarin, race
Introduction
Atrial fibrillation (AF) is highly prevalent in the US, and with the incidence of AF continuing to grow, the costs of treatment are also increasing.1 Stroke is a serious but preventable complication of AF, but therapeutic anticoagulation for the prevention of stroke can lead to an increased risk of bleeding;2,3 therefore, clinicians must be aware of the optimum benefit:risk ratio for the treatment of these patients. Despite African-Americans having a lower incidence of AF than Caucasians, the burden remains high, with one in nine African-Americans receiving a diagnosis before 80 years of age.4
The objectives of this review are: 1) to provide clinicians with an overview of AF and the importance of stroke prevention; 2) to discuss stroke prevention in underrepresented patient populations, particularly African-American patients; 3) to highlight knowledge gaps related to stroke prevention in underrepresented patient populations; 4) to discuss racial differences in the prevalence of AF and response to anticoagulants; and 5) to discuss the role clinicians may play in the care of patients with AF.
Description, prevalence, and consequences of atrial fibrillation and stroke
AF, defined as an abnormal rate or rhythm of the heart, is the most common type of arrhythmia. In patients with AF, multiple disorganized electrical impulses lead to rapid and chaotic contractions that prevent the atria from contracting properly, and as a result blood does not travel normally through the heart and vasculature.1 Although many patients with AF do not experience any symptoms, some patients can experience heart palpitations, lack of energy, dizziness, or shortness of breath. Additionally, AF can lead to an increase in the risk of stroke by allowing clots to form within the heart, which may travel to the brain. Nonvalvular AF (NVAF), defined as AF in the absence of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair, is the most common type of AF.3,5
The prevalence of AF is increasing along with aging in the developed world, and AF is associated with a fivefold-increased risk of stroke, with a fifth of all strokes associated with AF.6,7 Strokes are classified by the type of brain injury (eg, ischemic, hemorrhagic) and the type and location of the vascular lesion.8 Ischemic strokes are by far the more common type, causing over 80% of all strokes, and 20% of all ischemic strokes result directly from AF.9,10 The absolute risk of stroke in patients with AF can vary 20-fold, and this risk is dependent on the number of additional risk factors that a patient may have, such as heart failure, hypertension, older age, diabetes, prior stroke, vascular disease, and female sex.2,11,12 Strokes related to AF tend to be associated with greater morbidity and mortality compared with non-AF-related strokes;13,14 however, the risk of stroke can be reduced with anticoagulant treatment.2,3,6,15
The total health care cost of all strokes in the US was $53.9 billion in 2010, and of that figure, the cost of AF-related strokes was estimated to range from $3.2 billion to nearly $13 billion. By 2050, the economic burden of strokes related to AF in the US is estimated to approach $30 billion.16
Are there racial/ethnic differences in the epidemiology of AF?
The incidence of AF is lower in African-Americans than in Caucasians; the reasons for this remain unclear.17–21 It has been suggested that the incidence of AF in African-Americans may be underreported, and that the number of African-Americans with AF might be higher if more sensitive methods for detection were used.18,20,22 A study investigating racial differences in the incidence of and risk factors for AF in older adults found that for all associations examined, risk factors were similar in African-Americans and Caucasians, except for left ventricular posterior-wall thickness, which was more strongly associated with AF in African-Americans.23
Conversely, the relative rate of stroke is higher in African-Americans than in Caucasians,21 which appears to contradict the strong association between stroke and AF. One possible reason for the higher incidence and mortality rate of stroke in African-Americans may be the higher prevalence of other stroke-risk factors, including hypertension, obesity, and diabetes, in this population.17,19
In general, cryptogenic stroke (defined as ischemic stroke without a well-defined etiology) accounts for 34%–54% of ischemic strokes in most modern stroke registries and databases.24–29 Additionally, a population-based study showed that the rate of cryptogenic stroke in African-Americans was approximately double the rate observed in Caucasians (risk ratio 1.9, 95% confidence interval 1.5–2.3).28 Use of sophisticated heart-monitoring techniques like implanted loop recorders might detect unrecognized paroxysmal AF as the cause of some cryptogenic strokes in African-Americans.30 This suggestion is further supported by results from the REGARDS (REasons for Geographic And Racial Differences in Stroke) study, a national, population-based, longitudinal study of African-American and Caucasian adults aged at least 45 years in the US, which found that the odds of an association of stroke with race (African-American versus Caucasian) and AF progressively decreased with increasing test sensitivity.22
Treatment of AF: reducing the risk of stroke with oral anticoagulants
There are many treatment options for patients with AF that aim to control irregular heart rate or prevent stroke.31 Options include cardioversion, ablation, and pharmacological agents, which may be used for rate or rhythm control.32 Additionally, antithrombotic therapy can be used to reduce the risk of stroke in patients with AF, and that is the focus of this article.
Antithrombotic therapy to prevent thromboembolism is recommended for all patients with AF, except those with lone AF or contraindications, and the selection of an antithrombotic agent should be based on absolute risks of stroke and bleeding and the relative risk and benefit for a given patient.31
Guidelines for oral anticoagulant use in patients with AF in both the US and Europe are summarized in Table 1;2,3,6,33,34 however, it should be noted that some of these were published prior to the approval of apixaban and edoxaban.
Table 1.
Guideline recommendations on the use of warfarin and novel oral anticoagulants for the prevention of stroke in patients with nonvalvular atrial fibrillation
| Guideline | Guidance by risk | Guidance by agent | Guidance by renal function |
|---|---|---|---|
| AHA/ACC/HRS33 | • CHA2DS2-VASc score ○ ≥2 or prior stroke or TIA: use warfarin, dabigatran, rivaroxaban, or apixaban ○ 1: no antithrombotic therapy, OAC, or aspirin ○ 0: no antithrombotic therapy |
• Warfarin-treated: determine INR weekly, then monthly when stable • If unable to maintain INR, use dabigatran, rivaroxaban, or apixaban |
• Evaluate prior to NOAC initiation and annually thereafter (or when clinically indicated) • Use warfarin in patients with NVAF with CHA2DS2-VASc ≥2 and end-stage CKD (CrCl <15 mL/min) or undergoing hemodialysis • Consider reduced NOAC dose in patients with NVAF with moderate-to-severe CKD and CHA2DS2-VASc ≥2 (safety and efficacy not established) • Dabigatran and rivaroxaban not recommended in AF patients with end-stage CKD undergoing hemodialysis |
| AHA/ASA2,a | • Warfarin (INR 2.0–3.0) in all patients at high risk and many at moderate risk of stroke • Aspirin in patients at low risk and some moderate risk, based on patient preference, bleeding risk, and access to monitoring |
• Warfarin, dabigatran, apixaban and rivaroxaban indicated for first and recurrent stroke prevention in NVAF patients • Dabigatran useful alternative to warfarin in patients with AF and risk factors for stroke or SE who do not have prosthetic heart valve or hemodynamically significant valve disease, severe renal failure (CrCl <15 mL/min), or advanced liver disease • Safety of combining NOACs with antiplatelet agents not established • Apixaban 5 mg BID is an alternative to warfarin (or aspirin in patients unable to take a VKA), in patients with NVAF who have ≥1 additional risk factor and ≥1 of the following characteristics: age ≥80 years, weight #60 kg, serum creatinine ≥1.5 mg/dL • Apixaban 2.5 mg BID is an alternative to warfarin (or aspirin in patients unable to take a VKA) in patients with NVAF who have ≥1 additional risk factor and ≥2 of the following criteria: age ≥80 years, weight #60 kg, or serum creatinine ≥1.5 mg/dL • Rivaroxaban (20 mg/day) is reasonable in NVAF patients at moderate-to-high risk of stroke (prior history of TIA, stroke, or SE, or ≥2 additional risk factors) |
• For patients with renal impairment and NVAF at moderate-to-high risk of stroke (prior history of TIA, stroke, or SE, or ≥2 additional risk factors), with a CrCl of 15–50 mL/min, consider rivaroxaban 15 mg/day (efficacy and safety not established) • Can use dabigatran 150 mg BID instead of warfarin for the prevention of first and recurrent stroke in NVAF patients with ≥1 additional risk factor who have CrCl .30 mL/min • On the basis of PK data, the use of dabigatran 75 mg BID in patients with NVAF and ≥1 additional risk factor who have a low CrCl (15–30 mL/min) (based on PK data only; safety and efficacy not established) |
| ESC6,a | • CHA2DS2-VASc score ○ ≥2: use VKA (INR 2.0–3.0), dabigatran, rivaroxaban, or apixaban, unless contraindicated ○ 1: consider VKA (INR 2.0–3.0), dabigatran, rivaroxaban, or apixaban, based on bleeding risk and patient preference |
• If VKA cannot be used in patients with AF when recommended, use dabigatran, rivaroxaban, or apixaban • Where OAC recommended, use an NOAC rather than VKA for most NVAF patients, based on net clinical benefit • Use dabigatran 150 mg BID, or lower 110 mg BID dose (in Europe) in patients aged ≥80 years, using concomitant interacting drugs (eg, verapamil), with a high bleeding risk (HAS-BLED ≥3) or moderate renal impairment (CrCl 30–49 mL/min) • Use rivaroxaban 20 mg QD, or lower dose (15 mg QD) in patients with a high bleeding risk (HAS-BLED ≥3) or moderate renal impairment (CrCl 30–49 mL/min) |
• NOACs not recommended in patients with severe renal impairment (CrCl <30 mL/min) |
| ACCP3,a | • CHADS2 score ○ 0: no therapy rather than antithrombotic therapy; for those who choose therapy, use aspirin (75–325 mg QD) ○ 1: OAC rather than no therapy, or aspirin; in patients unable to take OACs, use combination therapy with aspirin and clopidogrel ○ ≥2: OAC rather than no therapy, or combination therapy; for patients unsuitable for OACs, use combination therapy rather than aspirin |
• When OACs recommended, dabigatran 150 mg BID suggested rather than VKA (INR 2.0–3.0) | • No recommendation provided by renal function |
| ASA/AHA34 | • Patients with valvular AF with CHA2DS2-VASc ≥2 and acceptably low risk of hemorrhage: use long-term VKA (INR 2.0–3.0) • NVAF: CHA2DS2-VASc score ○ ≥2 OACs recommended, including warfarin, dabigatran, apixaban, and rivaroxaban ○ 1: no antithrombotic therapy, anticoagulant therapy, or aspirin may be considered ○ 0: no antithrombotic therapy |
•Selection of OAC should be based on individual risk factors, cost, tolerability, patient preference, potential drug interactions, and other clinical characteristics | • No recommendation provided by renal function |
Note:
Guidelines reflect the fact that apixaban had not been approved at the time of publication.
Abbreviations: AF, atrial fibrillation; ACC, American College of Cardiology; ACCP, American College of Chest Physicians; AHA, American Heart Association; ASA, American Stroke Association; BID, bis in die (twice daily); CKD, chronic kidney disease; CrCl, creatinine clearance; ESC, European Society of Cardiology; HRS, Heart Rhythm Society; INR, international normalized ratio; NVAF, nonvalvular atrial fibrillation; OAC, oral anticoagulant; PK, pharmacokinetic; QD, quaque die (once daily); SE, systemic embolism; TIA, transient ischemic attack; NOAC, novel oral anticoagulant; VKA, vitamin K antagonist.
Differences in treatment by race/ethnicity
A treatment disparity exists between African-American and Caucasian patients; however, none of the current guidelines for the prevention of stroke in patients with AF states whether any amendments to treatment should be made based on racial differences.2,3,5,6,15,35
African-Americans are undertreated, and thus are at an increased risk of stroke.18,36,37 Differences in access to health care may account in part for lower rates of AF detection in African-Americans than in Caucasians. In the REGARDS study, race and income were both found to be independent predictors of patients being aware that they had AF, with African-Americans being less than a third as likely as Caucasians to be aware.18 Additionally, this study found that race was an independent predictor of warfarin treatment, with the odds of African-American patients being treated with warfarin only a quarter as great as the odds for Caucasians.18 A study using a multiethnic stroke-free cohort of hospitalized patients with nonrheumatic AF found that although the percentage of time on warfarin did not differ by race/ethnicity, the median percentage of time that patients spent within the therapeutic range (international normalized ratio [INR] 2.0–3.0) was lower in African-American than Caucasian patients (47.8% versus 55.2%).38 Additionally, an analysis of the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) study population found that ethnic minority status was an independent predictor of poor INR control, and race was subsequently included in a scoring system used to predict poor INR control in patients with AF treated with warfarin (SAMe-TT2R2: female sex, age <60 years, medical history [more than two comorbidities], treatment [with interacting drugs], tobacco use [doubled], and race [doubled]).39
Results from the Cardiovascular Health Study, a prospective, US-based cohort study of risk factors for cardiovascular disease in community-dwelling adults aged over 65 years, found that African-Americans have poorer access to health care than Caucasians, and were more likely to have no supplemental insurance coverage.40 A study investigating the differences in the type of hospital where minorities and low-income patients received care found that African-American patients and those living in low-income areas more frequently received care in teaching and safety-net hospitals and hospitals with higher bed counts, more intensive care unit beds, and emergency department volume, compared with Caucasians and those living in high-income areas.41 Crude inpatient-mortality rates for patients hospitalized with acute ischemic stroke were significantly lower in African-American than Caucasian patients across all three socioeconomic status cohorts (low, medium, and high income); however, African-American patients were younger, and fewer had any form of AF.41 When adjusted for risk, inpatient mortality was similar in African-American and Caucasian patients, but was significantly higher in low-income-area patients than in high-income-area patients.41 Caucasian patients were more likely than African-American patients to arrive by emergency medical services, to be evaluated by a stroke team, and to have a documented National Institutes of Health Scale score.42 Additionally, African-Americans were less likely to have visited a physician 1 year after their stroke than Caucasians.43 Together, these results highlight the socioeconomic differences between African-American and Caucasian patients, and suggest that there is a need for better access to health care and improvements to education programs for African-American patients.
Warfarin
For over 50 years, warfarin has been the standard of care for the prevention of stroke in patients with AF, and remains the most commonly used anticoagulant in this population. A meta-analysis of six randomized controlled trials showed that warfarin reduced the risk of all strokes (ischemic and hemorrhagic) by 64% versus placebo.44 Although highly efficacious for the prevention of stroke in patients with AF, warfarin has many limitations, including an increased risk of bleeding, multiple drug and food interactions, and the need for regular monitoring and dose adjustment to maintain treatment within the narrow therapeutic range (INR 2.0–3.0).45 These limitations can result in the underuse of warfarin.46 It has been shown that warfarin-dose requirements vary across racial/ethnic groups, with African-American patients requiring a higher dose than Caucasian patients to maintain an INR between 2.0 and 3.0.47 A chart review comparing older African-American and Caucasian patients found that although the dose of warfarin required to maintain a therapeutic INR decreased with age, African-Americans required a higher maintenance dose than Caucasians.48 Therefore, strategies for initiating warfarin therapy based on studies of patients of European ancestry could result in insufficient anticoagulation of older African-American patients, thereby potentially increasing their risk of thromboembolism.48
Several studies have investigated the treatment disparity between African-American and Caucasian patients.49–51 A cohort study of Medicare beneficiaries with AF found that the use, monitoring, and effectiveness of warfarin therapy were suboptimal, especially in African-American patients.49 One possible effect of this is that African-American patients with AF are at greater risk of warfarin-related intracranial hemorrhage.52 Additionally, African-American patients were significantly less likely than Caucasians to fill a warfarin prescription for newly incident NVAF or to receive a warfarin prescription at hospital discharge.50,51
Several genetic factors have recently been identified as possible reasons why African-American patients require a higher dose of warfarin.53,54 Dose variability is affected by single-nucleotide polymorphisms (SNPs) in genes encoding cytochrome P450 (CYP)-2C9, which metabolizes the S-enantiomer of warfarin, and vitamin K epoxide reductase complex 1, which is the target enzyme for warfarin.55,56 These genotypes explain up to 30% of total variability in warfarin-dose requirements in people of European or Asian origin; however, they explain substantially less genetic variability in African-Americans (10%).56 A genome-wide association study identified a novel association between an SNP in CYP2C9 (rs12777823) and warfarin-dose variability in African-American patients.54 Patients carrying this SNP require a significantly lower stable dose of warfarin than those without the variant.54
Novel oral anticoagulants
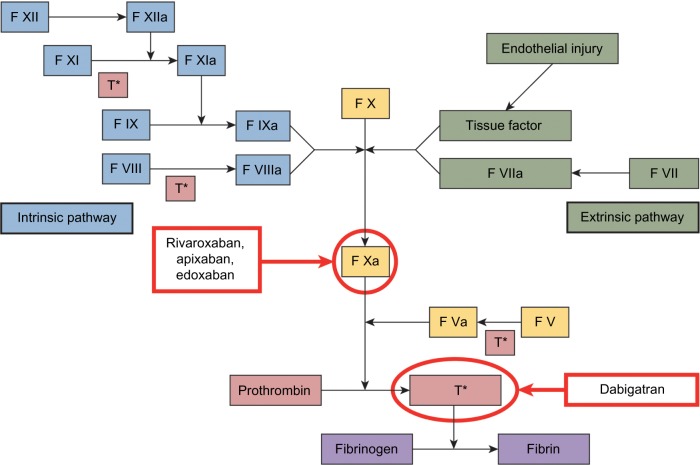
Four novel oral anticoagulants (NOACs) have recently been approved by the US Food and Drug Administration (FDA): a direct thrombin inhibitor (dabigatran), and three factor-Xa inhibitors (apixaban, edoxaban, and rivaroxaban) (Figure 1). Unlike warfarin and other vitamin K antagonists, NOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) do not require dose adjustments to maintain a therapeutic dose and have fewer risks of food and drug interactions.57–60 Lower doses of NOACs may be required based on renal function, weight, and/or age. Their predictable pharmacokinetic (PK) and pharmacodynamic profiles mean that routine monitoring is not necessary with these agents (Table 2);45,57–61 however, no specific antidotes are currently available for the reversal of the anticoagulant effects of NOACs in the event of overdose or hemorrhagic complications.57–60 Several options for the reversal of the anticoagulant effects of these agents are under investigation.62–68
Figure 1.
Sites of NOAC action in the coagulation cascade.
Abbreviations: F, factor; T*, thrombin.
Table 2.
Comparison of pharmacokinetic/pharmacodynamic interactions and dosing of warfarin and novel oral anticoagulants
| Warfarin45 | Dabigatran58 | Rivaroxaban60 | Apixaban59 | Edoxaban57 | |
|---|---|---|---|---|---|
| Mode of action | Inhibitor of factors II, VII, IX, and X, and the anticoagulant proteins C and S | Direct thrombin inhibitor | Factor Xa inhibitor | Factor Xa inhibitor | Factor Xa inhibitor |
| Route of administration | Oral or IV | Oral (tablet) | Oral (tablet or nasal gastric tube) taken with food | Oral (tablet or nasal gastric tube)61 | Oral (solution or tablet) |
| Approved dose (US) | Target INR 2.0–3.0 | 150 mg BID (75 mg BID for patients with CrCl 15–30 mg/mL) | 20 mg QD | 5 mg BID | 60 mg QD (30 mg QD for patients with CrCl 15 to 50 mL/min) Not approved in patients with CrCl .95 mL/min |
| Half-life | 40 hours | 12–17 hours | 5–9 hours | ∼12 hours | 10–14 hours |
| Monitoring | Daily INR determination upon initiation until stable INR 2.0–3.0 achieved; INR every 1–4 weeks thereafter | Prolongs aPTT, ECT, and TT; insensitive to INR measurements | Cannot be monitored using standard laboratory tests; PT, aPTT, and Heptest® (Heptest Laboratories, St Louis, MO, USA) are prolonged dose-dependently; anti-FXa activity is also influenced by rivaroxaban | Prolongs PT, INR, and aPTT, but changes at therapeutic dose are small, subject to a high degree of variability; Rotachrom® heparin (Diagnostica Stago, Parsippany, NJ, USA) chromogenic assay produced a concentration-dependent increase in anti-FXa activity, but is not recommended to assess the anticoagulant effect of apixaban | Prolongs PT and aPTT but changes in PT, INR and aPTT at the expected therapeutic dose are small and subject to a high degree of variability |
| Reversal | • Vitamin K | • A specific reversal agent for dabigatran is not available • Hemodialysis can remove dabigatran; however, clinical experience is limited • aPPCs, rFVIIa, or concentrates of coagulation factors II, IX, or X may be considered, but have not been evaluated in clinical trials • Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of dabigatran • Consider administration of platelet concentrates in cases where thrombocytopenia is present or long-acting antiplatelet drugs have been used |
• A specific antidote for rivaroxaban is not available • Because of high plasma protein binding, rivaroxaban is not expected to be dialyzable • Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban • Partial reversal of PT prolongation has been seen after administration of PCCs in healthy volunteers • The use of other procoagulant reversal agents like aPPC or rFVIIa has not been evaluated |
• A specific antidote for apixaban is not available • Hemodialysis does not appear to have a substantial impact on apixaban exposure • Protamine sulfate and vitamin K would not be expected to affect the anticoagulant effect of apixaban • There is no experience with antifibrinolytic agents in patients receiving apixaban • There is neither scientific rationale for reversal nor experience with systemic hemostatics in patients receiving apixaban • PCCs, aPCC, or rFVIIa may be considered, but havenot been evaluated in clinical trials • Activated charcoal reduces absorption of apixaban, thereby lowering apixaban plasma concentration |
• A specific antidote for edoxaban is not available • Hemodialysis does not significantly contribute to edoxaban clearance • Protamine sulfate, vitamin K, and tranexamic acid are not expected to reverse the anticoagulant activity of edoxaban |
| Reasons for dose reductions | Dose and administration individualized to each patient | Patients with CrCl 15–30 mL/min should take reduced dose (75 mg BID) | Patients with CrCl 15–50 mL/min should take reduced dose (15 mg QD) | ≥2 of the following: age ≥80 years, weight <60 kg, or serum creatinine ≥1.5 mg/dL | Patients with CrCl 15 to 50 mL/min |
| Food interactions | Foods high in vitamin K (eg, kale, spinach, turnip greens, scallions, brussels sprouts, raw broccoli, grapefruit juice, prunes, asparagus, avocado, beef liver) | None | Should be taken with the evening meal; bioavailability decreases if not taken with food | None | None |
| Drug interactions | Monitor INR closely if used concurrently with inhibitors or inducers of: CYP3A4, CYP2C9, or CYP1A2 Closely monitor concurrent use with drugs that can increase bleeding risk |
Avoid concomitant use with P-gp inducers Decrease dose when used concomitantly with P-gp inhibitors, dronedarone, or systemic ketoconazole in patients with moderate renal impairment (CrCl 30–50 mL/min) Bleeding risk increases if used concomitantly with drugs that affect bleeding risk |
Avoid concomitant use with combined P-gp and strong CYP3A4 inhibitors Avoid concomitant use with combined P-gp and strong CYP3A4 inducers Increase monitoring if used concomitantly with NSAIDs or aspirin Avoid concurrent use with other platelet-aggregation inhibitors or antithrombotic agents |
Decrease dose when used concomitantly with strong dual inhibitors of P-gp and CYP3A4 Avoid concomitant use with strong dual inducers of P-gp and CYP3A4 Bleeding risk increases if used concomitantly with drugs that affect hemostasis |
Avoid concomitant use of edoxaban with P-gp inducers (rifampin) Bleeding risk increases if used concomitantly with drugs that affect hemostasis |
| Effect of race on treatment | Asian patients may require lower initiation and maintenance doses of warfarin SNPs in the CYP2C9 and VKORC1 genes have been associated with variable warfarin-dose requirements | No information available | Healthy Japanese subjects were found to have on average 20%–40% higher exposure compared with other ethnicities, including Chinese | No dose adjustment is required based on race/ethnicity | In a population PK analysis, edoxaban exposures in Asian and non-Asian patients were similar |
Abbreviations: aPCC, activated prothrombin complex concentrate; anti-FXa, anti-factor Xa; aPTT, activated partial thromboplastin time; BID, bis in die (twice daily); CrCl, creatinine clearance; ECT, ecarin clotting time; INR, international normalized ratio; IV, intravenous; NSAID, nonsteroidal anti-inflammatory drug; rFVIIa, recombinant factor VIIa; PCC, prothrombin complex concentrate; P-gp, p-glycoprotein; PK, pharmacokinetic; PT, prothrombin time; QD, quaque die (once daily); SNP, single-nucleotide polymorphism; TT, thrombin time.
In their respective Phase III trials, all of the NOACs were found to be noninferior to warfarin for reducing the risk of stroke or systemic embolism (SE) in patients with NVAF; however, only the dabigatran 150 mg twice daily (bis in die [BID]) (P<0.001) and apixaban 5 mg BID (P=0.01) dosages showed superiority (Table 3).69–73 Additionally, dabigatran (110 mg BID), apixaban, and edoxaban (both dosages) significantly reduced the risk of major bleeding compared with warfarin. A summary of other key end points is provided in Table 3.
Table 3.
Phase III clinical trial results for the novel oral anticoagulants
| RE-LY (dabigatran 110 mg)69,70 | RE-LY (dabigatran 150 mg)69,70 | ROCKET AF (rivaroxaban 20 mg)73 | ARISTOTLE (apixaban, 5 mg)72 | ENGAGE AF-TIMI 48 (edoxaban 60 mg)71 | ENGAGE AF-TIMI 48 (edoxaban, 30 mg)71 | |
|---|---|---|---|---|---|---|
| Stroke or systemic embolism | RR 0.90 (95% CI 0.74–1.10) | RR 0.65 (95% CI 0.52–0.81) | HR 0.88 (95% CI 0.75–1.03) | HR 0.79 (95% CI 0.66–0.95) | HR 0.79 (97.5% CI 0.63–0.99)b | HR 1.07 (97.5% CI 0.87–1.31)b |
| P<0.001 (noninferiority) | P<0.001 (noninferiority) | P<0.001 (noninferiority) | P<0.001 (noninferiority) | P<0.001 (noninferiority) | P=0.005 (noninferiority) | |
| P=0.30 | P<0.001 | P=0.12a | P=0.01 | P=0.08 | P=0.10 | |
| Hemorrhagic stroke | RR 0.31 (95% CI 0.17–0.56) | RR 0.26 (95% CI 0.14–0.49) | HR 0.59 (95% CI 0.37–0.93)c | HR 0.51 (95% CI 0.35–0.75) | HR 0.54 (95% CI 0.38–0.77) | HR 0.33 (95% CI 0.22–0.50) |
| P<0.001 | P<0.001 | P=0.024 | P<0.001 | P<0.001 | P<0.001 | |
| Intracranial hemorrhage | RR 0.30 (95% CI 0.19–0.45) | RR 0.41 (95% CI 0.28–0.60) | HR 0.67 (95% CI 0.47–0.93)c | HR 0.42 (95% CI 0.30–0.58)c | HR 0.47 (95% CI 0.34–0.63)c | HR 0.30 (95% CI 0.21–0.43)c |
| P<0.001 | P<0.001 | P=0.02 | P<0.001 | P<0.001 | P<0.001 | |
| Myocardial infarction | RR 1.29 (95% CI 0.96–1.75) | RR 1.27 (95% CI 0.94–1.71) | HR 0.81 (95% CI 0.63–1.06)c | HR 0.88 (95% CI 0.66–1.17) | HR 0.94 (95% CI 0.74–1.19) | HR 1.19 (95% CI 0.95–1.49) |
| P=0.09 | P=0.12 | P=0.121 | P=0.37 | P=0.60 | P=0.13 | |
| All-causemortality | RR 0.91 (95% CI 0.80–1.03) | RR 0.88 (95% CI 0.77–1.00) | HR 0.92 (95% CI 0.82–1.03) | HR 0.89 (95% CI 0.80–0.998) | HR 0.92 (95% CI 0.83–1.01) | HR 0.87 (95% CI 0.79–0.96) |
| P=0.13 | P=0.051 | P=0.15 | P=0.047 | P=0.08 | P=0.006 | |
| Major bleeding | RR 0.80 (95% CI 0.70–0.93) | RR 0.93 (95% CI 0.81–1.07) | HR 1.04 (95% CI 0.90–1.20)c | HR 0.69 (95% CI 0.60–0.80)c | HR 0.80 (95% CI 0.71–0.91)c | HR 0.47 (95% CI 0.41–0.55)c |
| P=0.003 | P=0.32 | P=0.58 | P<0.001 | P<0.001 | P<0.001 |
Notes:
The primary end point for ROCKET-AF used the per-protocol population. In the per-protocol analysis, the HR for stroke or SE with rivaroxaban versus warfarin was 0.79 (95% CI 0.66–0.96, P<0.001 for noninferiority);
modified intention-to-treat population in the treatment period;
safety on-treatment population. All values are for the intention-to-treat populations unless otherwise stated. All P-values are for superiority unless otherwise stated.
Abbreviations: CI, confidence interval; HR, hazard ratio; RR, relative risk; RE-LY, Randomized Evaluation of Long-term anticoagulant therapY; ARISTOTLE, Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation; ROCKET AF, Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation; ENGAGE AF-TIMI 48, Effective aNticoaGulation with factor XA next GEneration in Atrial Fibrillation – Thrombolysis In Myocardial Infarction 48 study.
There is limited information about the effect of race on NOAC treatment, and the number of patients by race was not provided in all of the NOAC Phase III trial publications.69–74 In an analysis of key subgroups in the ROCKET AF (Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) trial, the effects of rivaroxaban versus warfarin were found to be similar across racial groups (Caucasian, African-American, Asian, other) for the outcomes of stroke or SE and major or clinically relevant nonmajor bleeding (P-values for interaction 0.486 and 0.591, respectively).73 Similarly, an analysis of key subgroups in the ENGAGE AF-TIMI (Effective aNticoaGulation with factor XA next GEneration in Atrial Fibrillation – Thrombolysis In Myocardial Infarction) 48 study found that the treatment effects of both doses of edoxaban compared with warfarin were similar across racial groups (Caucasian versus non-Caucasian) for the outcomes of stroke or SE (P-values for interaction 0.28 and 0.49 for edoxaban 60 mg and 30 mg BID, respectively) and for major bleeding (P-values for interaction 0.16 and 0.34 for edoxaban 60 mg and 30 mg BID, respectively).71 No subgroup analyses by race have been published for the RE-LY (Randomized Evaluation of Long-term anticoagulant therapY) or ARISTOTLE (Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation) trials,72,74 and no real-world data are currently available comparing the treatment effects of NOACs by race.
The prescribing information for dabigatran and rivaroxa-ban does not state whether any dose adjustments are needed for race;58,60 however, based on the results of a PK study in healthy subjects that showed no difference in apixaban PK among Caucasians, Asians, and African-Americans, the apixaban prescribing information states that no dose adjustment is required based on race/ethnicity.59
Role of physicians and patients in stroke prevention in patients with AF
There are disparities in stroke awareness between minority groups compared with Caucasians, including lack of awareness of stroke symptoms and signs and lack of knowledge about the need for urgent treatment and the causal role of risk factors.75 The Centers for Disease Control and Prevention analyzed data from an optional module of the 2005 Behavioral Risk Factor Surveillance System that had used data from 13 US states and the District of Columbia to assess public awareness of stroke warning symptoms and the importance of seeking emergency care. The results showed that the proportion of respondents who were able to identify all five stroke warning signs and recognized the need to call emergency services was lower in African-Americans than in Caucasians (29.5% versus 41.3%).76 This and other similar studies empha-size the need for educational efforts to improve knowledge about stroke symptoms among African-Americans.76–81
There are differences in attitudes, beliefs, and compliance among minority groups, including denial of disease, concern for potential or experienced side effects of medications, absence of symptoms, hierarchy of need, burden of filling prescriptions, attending doctor visits, and lower health literacy, all of which influence compliance with treatment.78 It is also important for physicians to evaluate the effects of health literacy, access to health care, and cultural barriers to anticoagulant use.82
Studies have shown that literacy decreases with age, and that this is more pronounced in African-American than Caucasian patients.82 Older patients receiving oral anticoagulants must become active participants in their own care, in that they and their families must read and comprehend written information regarding their treatment.82 It is also important to ensure that the reading materials supplied to patients with NVAF are sufficiently comprehensible to older adults.82 This may in part be easier with NOAC than with warfarin use, due to fewer food and drug interactions associated with NOACs.
Conclusion
AF is less common in African-Americans than in Caucasians; however, African-Americans have a higher risk of stroke. This could be due to African-Americans having a higher prevalence of other stroke-risk factors compared with Caucasians, although this may be an oversimplified view, as there are a number of other reasons why this disparity may occur. These include a need for more sensitive methods of detecting AF and a need for better access to health care and patient education for African-Americans. Warfarin-dose requirements vary across racial/ethnic groups, with African-American patients requiring a higher dose than Caucasians to maintain a therapeutic INR: the NOACs seem to differ in this regard, although data are currently limited. Minority racial groups are not proportionally represented in either real-world studies or clinical trials, and as more information becomes available and other social issues are addressed, the treatment disparities between African-American and Caucasian patients should decrease.
Acknowledgments
Professional medical writing and editorial assistance was provided by Claire Hall, PhD, and Nicole Draghi, PhD, at Caudex Medical, and was funded by Bristol-Myers Squibb and Pfizer Inc.
Footnotes
Disclosure
The author reports grant support from Bristol-Myers Squibb and Pfizer. The author reports no other conflicts of interest in this work.
References
- 1.American Heart Association What is atrial fibrillation? 2013. [Accessed October 30, 2013]. Available from: http://www.heart.org/idc/groups/heart-public/@wcm/@hcm/documents/downloadable/ucm_300294.pdf.
- 2.Furie KL, Goldstein LB, Albers GW, et al. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(12):3442–3453. doi: 10.1161/STR.0b013e318266722a. [DOI] [PubMed] [Google Scholar]
- 3.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123(10):e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 6.Camm AJ, Lip GY, De Caterina R, et al. 2012 Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 9.Marini C, De SF, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115–1119. doi: 10.1161/01.STR.0000166053.83476.4a. [DOI] [PubMed] [Google Scholar]
- 10.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28(3):491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 14.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27(10):1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 15.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 16.Deitelzweig S, Amin A, Jing Y, et al. Medical cost reductions associated with the usage of novel oral anticoagulants vs warfarin among atrial fibrillation patients, based on the RE-LY, ROCKET-AF and ARISTOTLE trials. J Med Econ. 2012;15(4):776–785. doi: 10.3111/13696998.2012.680555. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 18.Meschia JF, Merrill P, Soliman EZ, et al. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41(4):581–587. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SC, Jr, Clark LT, Cooper RS, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation. 2005;111(10):e134–e139. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- 20.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40(4):1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111(10):1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 22.Prineas RJ, Soliman EZ, Howard G, et al. The sensitivity of the method used to detect atrial fibrillation in population studies affects group-specific prevalence estimates: ethnic and regional distribution of atrial fibrillation in the REGARDS study. J Epidemiol. 2009;19(4):177–181. doi: 10.2188/jea.JE20081032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2013;61(2):276–280. doi: 10.1111/jgs.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32(12):2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 25.Lee BI, Nam HS, Heo JH, Kim DI. Yonsei Stroke Registry. Analysis of 1,000 patients with acute cerebral infarctions. Cerebrovasc Dis. 2001;12(3):145–151. doi: 10.1159/000047697. [DOI] [PubMed] [Google Scholar]
- 26.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30(12):2513–2516. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 27.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25(4):382–390. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 28.Schneider AT, Kissela B, Woo D, et al. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke. 2004;35(7):1552–1556. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 29.Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: importance of population-based studies. Stroke. 2003;34(8):2050–2059. doi: 10.1161/01.STR.0000079818.08343.8C. [DOI] [PubMed] [Google Scholar]
- 30.Christensen LM, Krieger DW, Højberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol. 2014;21(6):884–889. doi: 10.1111/ene.12400. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JL, Halperin JL, Albert NM, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(18):1935–1944. doi: 10.1016/j.jacc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson C, Inglis SC, Newton PJ, Middleton S, Macdonald PS, Davidson PM. Atrial fibrillation: stroke prevention in focus. Aust Crit Care. 2014;27(2):92–98. doi: 10.1016/j.aucc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 33.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246–2280. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim SA, Kwoh CK, Harper DL, Baker DW. Racial differences in the utilization of oral anticoagulant therapy in heart failure: a study of elderly hospitalized patients. J Gen Intern Med. 2000;15(2):134–137. doi: 10.1046/j.1525-1497.2000.05199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwamm LH, Reeves MJ, Pan W, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121(13):1492–1501. doi: 10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]
- 38.Shen AY, Yao JF, Brar SS, Jorgensen MB, Wang X, Chen W. Racial/ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke. 2008;39(10):2736–2743. doi: 10.1161/STROKEAHA.107.508580. [DOI] [PubMed] [Google Scholar]
- 39.Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT(2)R(2) score. Chest. 2013;144(5):1555–1563. doi: 10.1378/chest.13-0054. [DOI] [PubMed] [Google Scholar]
- 40.Henry J Kaiser Family Foundation Examining sources of supplemental insurance and prescription coverage among Medicare beneficiaries; findings from the Medicare Current Beneficiary Survey. 2007. [Accessed December 9, 2014]. Available from: http://kff.org/medicare/report/examining-sources-of-supplemental-insurance-and-prescription.
- 41.Hanchate AD, Schwamm LH, Huang W, Hylek EM. Comparison of is chemic stroke outcomes and patient and hospital characteristics by race/ethnicity and socioeconomic status. Stroke. 2013;44(2):469–476. doi: 10.1161/STROKEAHA.112.669341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharya P, Mada F, Salowich-Palm L, et al. Are racial disparities in stroke care still prevalent in certified stroke centers? J Stroke Cerebrovasc Dis. 2013;22(4):383–388. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995;45(4):659–663. doi: 10.1212/wnl.45.4.659. [DOI] [PubMed] [Google Scholar]
- 44.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 45.Bristol-Myers Squibb Coumadin [prescribing information] 2011. [Accessed October 11, 2013]. Available from: http://packageinserts.bms.com/pi/pi_coumadin.pdf.
- 46.Rosenman MB, Simon TA, Teal E, McGuire P, Nisi D, Jackson JD. Perceived or actual barriers to warfarin use in atrial fibrillation based on electronic medical records. Am J Ther. 2012;19(5):330–337. doi: 10.1097/MJT.0b013e3182546840. [DOI] [PubMed] [Google Scholar]
- 47.Dang MT, Hambleton J, Kayser SR. The influence of ethnicity on warfarin dosage requirement. Ann Pharmacother. 2005;39(6):1008–1012. doi: 10.1345/aph.1E566. [DOI] [PubMed] [Google Scholar]
- 48.Garwood CL, Clemente JL, Ibe GN, Kandula VA, Curtis KD, Whittaker P. Warfarin maintenance dose in older patients: higher average dose and wider dose frequency distribution in patients of African ancestry than those of European ancestry. Blood Cells Mol Dis. 2010;45(1):93–97. doi: 10.1016/j.bcmd.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37(4):1070–1074. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 50.Schauer DP, Johnston JA, Moomaw CJ, Wess M, Eckman MH. Racial disparities in the filling of warfarin prescriptions for nonvalvular atrial fibrillation. Am J Med Sci. 2007;333(2):67–73. doi: 10.1097/00000441-200702000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Thomas KL, Piccini JP, Liang L, et al. Racial differences in the prevalence and outcomes of atrial fibrillation among patients hospitalized with heart failure. J Am Heart Assoc. 2013;2(5):e000200. doi: 10.1161/JAHA.113.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50(4):309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 53.Cavallari LH, Aston JL, Momary KM, Shapiro NL, Patel SR, Nutescu EA. Predictors of unstable anticoagulation in African Americans. J Thromb Thrombolysis. 2009;27(4):430–437. doi: 10.1007/s11239-008-0236-8. [DOI] [PubMed] [Google Scholar]
- 54.Perera MA, Cavallari LH, Limdi NA, et al. Genetic variants associated with warfarin dose in African-American individuals: a genome-wide association study. Lancet. 2013;382(9894):790–796. doi: 10.1016/S0140-6736(13)60681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limdi NA, Beasley TM, Crowley MR, et al. VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African-Americans and European-Americans. Pharmacogenomics. 2008;9(10):1445–1458. doi: 10.2217/14622416.9.10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Limdi NA, Arnett DK, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 on warfarin dose, anticoagulation attainment and maintenance among European-Americans and African-Americans. Pharmacogenomics. 2008;9(5):511–526. doi: 10.2217/14622416.9.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daiichi Sankyo Co. Ltd Savaysa™ (edoxaban) prescribing information. 2015. [Accessed January 22, 2015]. Available from http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf.
- 58.Boehringer Ingelheim Pradaxa dabigatran etexilate capsules [prescribing information] [Accessed September 18, 2013]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022512s017lbl.pdf.
- 59.Bristol-Myers Squibb Eliquis® (apixaban tablets) [prescribing information] 2014. [Accessed March 20, 2014]. Available from: http://packageinserts.bms.com/pi/pi_eliquis.pdf.
- 60.Janssen Pharmaceuticals Xarelto® (rivaroxaban tablets) [prescribing information] 2014. [Accessed March 8, 2014]. Available from: http://www.xareltohcp.com/sitesdefault/files/pdf/xarelto_0.pdf#zoom=100.
- 61.Song Y, Wang X, Perlstein I, et al. Bioavailability of apixaban solution formulation and crushed tablet via nasogastric tube. Clin Pharmacol Ther. 2013;93(Suppl 1):S120–S121. doi: 10.1016/j.clinthera.2015.05.497. [DOI] [PubMed] [Google Scholar]
- 62.Boehringer Ingelheim Study to evaluate the safety, pharmacokinetics and pharmacodynamics of BI 655075 administered alone or with dabigatran etexilate. [Accessed June 6, 2013]. Available from: http://clinicaltrials.gov/show/NCT01688830. NLM identifier: NCT01688830.
- 63.Crowther M, Kitt M, Lorenz T, et al. A phase 2 randomized, double-blind, placebo-controlled trial of PRT064445, a novel, universal antidote for direct and indirect factor Xa inhibitors [Abstract] J Thromb Haemost. 2013;11(Suppl 2):30. [Google Scholar]
- 64.Crowther M, Mathur V, Kitt M, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of rivaroxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), an antidote for FXa inhibitors; Poster presented at: 55th ASH Annual Meeting and Exposition; December 7–10, 2013; New Orleans, LA. [Google Scholar]
- 65.Hollenbach SJ, Lu G, Tan S, et al. PRT064445 but not recombinant FVIIa reverses rivaroxaban induced anticoagulation as measured by reduction in blood loss in a rabbit liver laceration model; Poster presented at: 54th ASH Annual Meeting and Exposition; December 8–11, 2012; Atlanta, GA. [Google Scholar]
- 66.Laulicht B, Bakhru S, Lee C, et al. Small molecule antidote for anticoagulants. Circulation. 2012;126:A11395. [Google Scholar]
- 67.Perosphere PER977: an anticoagulant reversal agent. 2013. [Accessed June 6, 2013]. Available from: http://perosphere.com/content/research/per977.htm.
- 68.van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol. 2011;57(14 Suppl 1):E1130. [Google Scholar]
- 69.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus war-farin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 70.Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–1876. doi: 10.1056/NEJMc1007378. [DOI] [PubMed] [Google Scholar]
- 71.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfa-rin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 72.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus war-farin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 73.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfa-rin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 74.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 75.Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(7):2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 76.Centers for Disease Control and Prevention Awareness of stroke warning symptoms – 13 states and the District of Columbia, 2005. MMWR Morb Mortal Wkly Rep. 2008;57(18):481–485. [PubMed] [Google Scholar]
- 77.Greenlund KJ, Neff LJ, Zheng ZJ, et al. Low public recognition of major stroke symptoms. Am J Prev Med. 2003;25(4):315–319. doi: 10.1016/s0749-3797(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 78.Lutfiyya MN, Lipsky MS, Bales RW, Cha I, McGrath C. Disparities in knowledge of heart attack and stroke symptoms among adult men: an analysis of behavioral risk factor surveillance survey data. J Natl Med Assoc. 2008;100(10):1116–1124. doi: 10.1016/s0027-9684(15)31483-8. [DOI] [PubMed] [Google Scholar]
- 79.Lutfiyya MN, Cumba MT, McCullough JE, Barlow EL, Lipsky MS. Disparities in adult African American women’s knowledge of heart attack and stroke symptomatology: an analysis of 2003–2005 Behavioral Risk Factor Surveillance Survey data. J Womens Health (Larchmt) 2008;17(5):805–813. doi: 10.1089/jwh.2007.0599. [DOI] [PubMed] [Google Scholar]
- 80.Lutfiyya MN, Ng L, Asner N, Lipsky MS. Disparities in stroke sympto-mology knowledge among US midlife women: an analysis of population survey data. J Stroke Cerebrovasc Dis. 2009;18(2):150–157. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 81.Zerwic J, Hwang SY, Tucco L. Interpretation of symptoms and delay in seeking treatment by patients who have had a stroke: exploratory study. Heart Lung. 2007;36(1):25–34. doi: 10.1016/j.hrtlng.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Wilson FL, Racine E, Tekieli V, Williams B. Literacy, readability and cultural barriers: critical factors to consider when educating older African Americans about anticoagulation therapy. J Clin Nurs. 2003;12(2):275–282. doi: 10.1046/j.1365-2702.2003.00711.x. [DOI] [PubMed] [Google Scholar]