Abstract
A nutritionally vulnerable older adult has a reduced physical reserve that limits the ability to mount a vigorous recovery in the face of an acute health threat or stressor. Often this vulnerability contributes to more medical complications, longer hospital stays, and increased likelihood of nursing home admission. We have characterized in this review the etiology of nutritional vulnerability across the continuum of the community, hospital, and long term care settings. Frail older adults may become less vulnerable with strong, consistent, and individualized nutritional care. Interventions for the vulnerable older adult must take their nutritional needs into account to optimize resiliency in the face of the acute and/or chronic health challenges they will surely face in their life course.
Keywords: older adults, nutritionally vulnerable, malnutrition, community dwelling, hospital malnutrition, long term care
Introduction
What does it mean to be vulnerable? In the health context, a background of predisposing factors determines the ability of an individual to respond to stressors or precipitants. A vulnerable older person has a greater accumulation of detrimental predisposing factors (e.g., multiple medical conditions, obesity, and limited social support) relative to protective factors (e.g., ample stores of muscle mass). When an acute stress arises, perhaps a bout of influenza or a need for surgery, the individual is unable to mount a vigorous recovery and may never fully return to their pre-stress baseline. The term “nutritionally vulnerable” evokes the classic image of a home-bound elder with limited resources and/or medical disabilities that preclude the consumption of a fully adequate diet. Surely, these individuals remain on the radar of concern in geriatric nutrition. However, a very broad array of dynamic challenges contributes to nutritional vulnerability in today’s older adults. Here we address and explore these varied concerns that span the continuum from older adults living in the community to those in health care institutions, as well as those making transitions among these settings. Well-established topics will be briefly addressed, so that we can more fully explore the newer and understudied topics.
Nutritional Vulnerability in the Community
Many factors impact the quality and quantity of dietary intake in community dwelling older adults. Older adults are predisposed to nutrient deficiency due to a decline in total and resting energy requirements (physical inactivity, loss of lean muscle mass and increased adiposity) that gradually reduces food intake while vitamin and mineral needs remain unchanged or increased [1]. Furthermore, biomedical, psychosocial, and environmental and economic factors also seriously impact nutritional status in older adults. The breadth of potential causes of reduced dietary intake contributing to nutritional vulnerability in older adults is illustrated by examples from the three domains of Physiologic/Biomedical, Psycho/Social, and Environmental/Economic in Table 1.
Table 1.
Factors Associated with Nutritional Vulnerability in Community-Dwelling Older Adults
| Physiologic/Biomedical | Psycho/Social | Environmental/Economic |
|---|---|---|
| Frailty | Mental health | Financial constraints |
| Obesity | Cognition | Access to food |
| Chronic health conditions | Loneliness | Food preparation |
| Anorexia of aging | Social Isolation | Living alone |
| Oral heath | Lack of social support | Transportation |
| Presbyosmia | Alcoholism | Neighborhood walkability |
| Hypochlorhydria | Bereavement | Safety |
| Medication use | Eating alone | |
| Gastritis | ||
| Malabsorption |
Biomedical and Physiologic Determinants
In the physiologic sense, vulnerability equates closely with a more familiar term used to describe age-related decline, namely “frailty”. Frailty is a clinical syndrome characterized by increased vulnerability to adverse health outcomes including acute illness, decline in physiological reserve, and increased risk for adverse outcomes including acute illness, disability, falls, hospitalization, need for long-term care, and death [4]. The definition of frailty varies across disciplines, which makes it difficult to identify the prevalence and impact on community-dwelling older adults. However, commonly agreed upon etiologies include acute or chronic illness, inflammation, malnutrition, and inactivity [5]. In a recent systematic review of the literature, Shamliyan et al. found the pooled prevalence of frailty among community dwelling older adults to be approximately 14% using the phenotype frailty definition (three or more of the following: weight loss, fatigue, exhaustion, weakness, low physical activity and slowness, and mobility impairment), with the greatest prevalence among those who were 80 and older, African American, and female [6]. Sarcopenia, the age-related loss of lean muscle mass; dynapenia, the age-related loss of muscle strength; rapid weight loss, and excessive adiposity are all contributing factors to the development of frailty and all are greatly impacted by dietary adequacy.
A more recent and rapidly growing phenomenon is undernourishment concomitant with obesity in older adults. Individuals in this condition typically are malnourished in micronutrients as a result of chronic overeating, under consumption of nutrient rich foods, and decline in physical activity. As already noted, decreased calorie requirements with age makes the consumption of all the essential nutrients while not overeating a difficult task. This is especially evident in older adults who are participating in the Older Americans Nutrition Act Program (OAANP). Recent research has highlighted not only the increase prevalence of obesity [7] and obesity-related health problems [8], but also mental health conditions [9] poor nutritional status [10, 11], inappropriate eating behaviors [12], and food insecurity [13]. Excess adiposity is associated with many chronic health conditions, including disability, decreased quality of life, and frailty, thus placing this cohort at a great risk for future malnutrition and loss of independence [14, 15].
Life expectancy continues to increase and, as a result, so does the number of older adults with multiple chronic diseases. Sadly, these extra years of life are often filled with physical and psychological distress rather than quality time [16]. Increasingly, the focus for health promotion is on the extension of healthspan as opposed to simply increasing lifespan; healthspan has been said to encompass “optimal longevity”, not just living longer but living well.[17, 18] The challenge is that chronic health conditions are a predominant concern for this population; approximately 46% of older adults have 2 to 3 chronic health conditions, while 16% have 4 or more [19]. They are more likely to experience physical impairments and disability, poor quality of life, polypharmacy and mortality [20]. The development of comorbidities is thought to be due to a combination of genetic, environment, and behavioral factors [1]. Additionally, income, food security, and access to healthcare all contribute to the development and progression of chronic health conditions in the aged population [1].
Psychosocial Determinants
Psychosocial factors that contribute to decreased food intake include depression, social isolation, and loneliness, to name a few. Depression is and continues to be a prevalent condition in the older population; approximately 7% have major depressive disorders and up to 17% have clinically significant depressive symptoms [21]. Risk factors for late life depression include female gender, lower educational status, loss of a partner, cognitive decline, chronic health conditions, and decline in physical function [22]. The relationship between malnutrition and depression is multifaceted and complex. Physiological anorexia of aging is often seen in depressed older adults [23] and leads to a decline in macro- and micro-nutrient intake, while low serum levels of vitamin D [24], B6 and B12 [25, 26], and zinc [27] have all been linked to increased risk for depression. To further complicate this relationship, both depression and malnutrition are associated with functional impairments, coexisting medical illness, and mortality [28].
Social isolation (objective measure) and loneliness (subjective measure) are common among community-dwelling older adults who live alone, have functional impairments, lack transportation, low morale, and report limited social networks [29]. Furthermore, social isolation and loneliness are associated with chronic health conditions, cognitive impairment, poor self-reported health, and sleep disorders [30, 31]. Older adults who experience these psychosocial determinants are more likely to eat alone and often have chronically marginal nutrient intake, putting them at a greater risk for malnutrition [32]. Of particular concern are widowed or single older men. These individuals typically have fewer close relationships outside of their spouse, lack cooking skills, or are physically unable to prepare food [33, 34].
Economic and Environmental Determinants
Food insecurity, defined as “limited or uncertain availability of nutritionally adequate and safe foods or limited or uncertain ability to acquire acceptable foods in socially acceptable ways” [35] encompasses not only the lack of economic resources to obtain nutritionally adequate food, but also the inability to access and appropriately use food. According to the USDA, in 2013 the prevalence of food insecurity in the United States was 14.3% of households. Among older adults, 8.7% of households with an older adult and 9.0% of older adults living alone were food insecure [36]. Food insecure older adults are more likely to live below the poverty line, be African American or Hispanic, live alone or with grandchildren, be unemployed, disabled, have less than a high school education, and rent instead of own [13, 37].
Low socioeconomic status is a known cause of malnutrition in older adults, due in part to the limited resources for purchasing food; often, money goes toward less expensive and less nutritious foods [38]. Previous reviews highlight nutritional outcomes associated with food insecurity such as inadequate calorie consumption, low consumption of nutrient-dense foods, and fewer meals per day. [13, 39] Additionally, food insecurity has been linked to poorer self-reported heath [40], lower quality of life [39], cardiovascular disease [41], diabetes [42, 43], anemia [44], obesity [7, 45], functional impairment [7], anxiety and depression [46] [47], and cognitive function [49].
Current and future cohorts of older adults are electing to remain in their homes as long as possible, “aging in place”, rather than moving to a retirement community, assisted living facility or nursing home. Environmental factors such as food cost, availability, distance to obtain food, walkability, safety, and available transportation all influence dietary intake; when one of these factors is compromised, it can have a detrimental impact on nutritional status. Lee and Berthelot found that specific community covariates such as socioeconomic disadvantage, disability and social isolation, contribute to malnutrition-related mortality in older adults [50].
Nutritional Risks in the Hospital Setting and Care Transitions
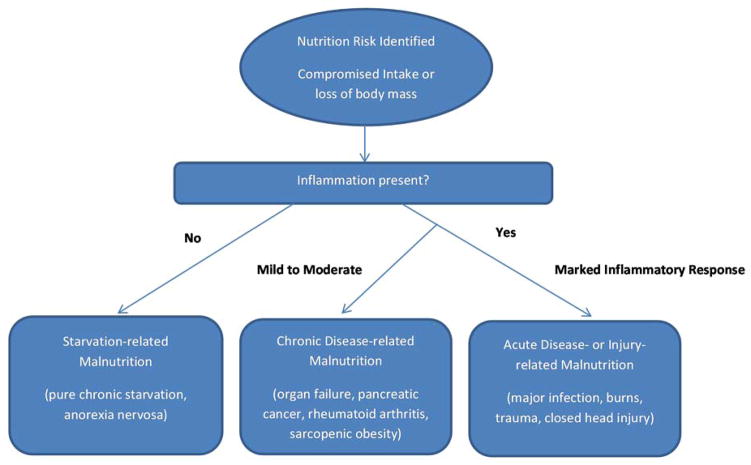
Shortcomings in past approaches used to assess nutritional status have hindered efforts to describe the extent and degree of malnutrition and nutritional risk for geriatric patients in clinical settings. The use of assessment tools like the Mini Nutritional Assessment (MNA) is rare in the clinical environment and serum albumin level, although heavily relied upon as a sole indicator of nutritional status, actually reflects primarily the inflammatory state rather than the true body store of essential nutrients.[51] Fortunately, as illustrated in Figure 1 and explained in detail elsewhere [52, 53], a new paradigm for describing malnutrition has been recently developed and codified. This approach more accurately reflects the close interrelationship between disease and malnutrition and has been widely endorsed as the best diagnostic criteria for assessing adult malnutrition in clinical practice. Its use is expected to substantially improve the accuracy of malnutrition diagnosis and lead to a better understanding of ways to surmount challenges to nutritional status going forward.
Figure 1.
Etiology-based malnutrition definitions. Adapted from White et al. [53]
Hospitalized = Nutritionally Vulnerable
Long recognized as a general concern, hospital malnutrition has received considerably more scientific attention in recent years, as evidenced by a number of recent studies and editorials [52, 54]. As of 2013, 14.1% of the population in the United States was over the age of 65 years [55], yet this relatively small cohort accounted for the greatest utilization of healthcare services. This portion made up 34.9% of all hospital stays, with a greater mean length of stay and cost per stay than any other age group [56]. This is because older adults have a higher number predisposing conditions with comorbid illnesses and disabilities and they are more vulnerable to adverse events during their hospitalization, such as falls, nosocomial infections, and adverse drug reactions [57–59] This combination of susceptibility and reduced ability to overcome stressors leads to poor long term outcomes and commonly results in loss of independence, which is a marker of frailty. The decline of all these physiological parameters can potentially be directly related to reduced energy intake. When energy intake is insufficient to meet the demands of the body either due to starvation, acute illness or chronic disease/disability, then malnutrition becomes the driver that leads to the further deterioration of functional abilities and inability to recover from disease. The prevalence of malnutrition is exceedingly high in hospital settings, with 39% of hospitalized older adults affected worldwide and an additional 47% at risk for malnutrition [60]. One prospective study suggests that under-nutrition precedes development of disease and hospitalization in adults older than 70 years [61].
Several recent publications addressing nutritional risk in older patients found that malnutrition in hospitalized geriatric patients is associated with an increased risk of death at three months. These papers have further illuminated the profile of malnutrition to include more clinical characteristics [62, 63], including eating difficulties and other functional limitations, depression, polypharmacy, pressure ulcers, and cognitive impairment, as well as social factors and hospital factors such as unnecessarily lengthy periods of medical orders requiring patients to have nothing by mouth (NPO), frequent meal interruptions and low acceptance of foods served [63]. It is imperative to recognize both malnutrition and risk of malnutrition as early as possible during the hospital admission process so that all of the medical, psychological, and functional factors related to malnutrition can be addressed by an interprofessional team focusing on the patient’s preferences regarding food choices, timing of meals, and/or self-feeding strategies within their individual social and environmental circumstances. We recommend using both the MNA (self or short forms) and the Nutrition Screening Initiative (NSI) Determine Checklist to most efficiently identify the greatest breadth of contributing nutritional risk factors.
Pre- and Post-surgical Concerns Reflect Nutritional Vulnerability
More than one-third of all surgical procedures are performed on individuals aged 65 years and older [64]; this population has higher rates of postoperative complications than for younger patients, resulting in permanent declines in cognitive and physical function [65]. Poor nutritional status presents an especially high risk situation for older adults undergoing surgery because it increases the risks for not only more complications [66] during hospitalization but also poor outcomes after discharge related to more infections [67], poor healing [68], and increased mortality [69, 70]. The subset of older adults undergoing elective surgery presents a unique opportunity for intervention because those with nutritional risk can ideally be identified with enough lead time to develop a viable nutritional support program that can be centered on the patient’s nutritional needs, food preferences, and type of surgery, to be sustained throughout the preoperative, hospitalization, and post-discharge settings. Targeted nutritional strategies with specific macronutrients or commercially available “immune enhancing” preparations, such as Abbott’s Ensure Immune Health or Nestle’s Oral Impact may even be able to mitigate some of the physiological stress of surgery and thus enhance resilience [66]. The biggest cause for alarm in older adults after surgery is insufficient action to fully address malnutrition and nutritional risk. While nurses screen for malnutrition in adults admitted into the hospital over 90 % of the time, as mandated by The Joint Commission, a known validated screening tool such as the MNA is used only 37.5 % of the time [71]. And the most concerning results of a recent national survey of nutritional screening practices in the United States revealed that fewer than 40% of those identified to be malnourished actually receive a clinical intervention. [71]. To address this, we wholeheartedly endorse implementing systematic models within institutions that address key principles as outlined by Tappenden, et al to: “(1) create an institutional culture where all stakeholders value nutrition, (2) redefine clinicians’ roles to include nutrition care, (3) recognize and diagnose all malnourished patients and those at risk, (4) rapidly implement comprehensive nutrition interventions and continued monitoring, (5) communicate nutrition care plans, and (6) develop a comprehensive discharge nutrition care and education plan” [52].
Vulnerability during Care Transitions
Another high-risk situation for older adults occurs when they are receiving transitional care, the care they need when they “move from one care site to another” [72]. When transitional care is mishandled, the result is often higher levels of complications and likelihood of hospital readmission [73]. Almost one fifth of Medicare patients discharged from a hospital require another hospitalization within 30 days due to an acute medical problem [74]. Those who are particularly vulnerable during transition hand-offs are the poor, the near-poor, and culturally diverse seniors, especially recent immigrants [72].
The goal of transitional care is “to provide coordination and continuity of health care” during the transfer [72]. Approximately 40% of patients of those aged 85 and older are discharged to a long-term care facility (skilled nursing homes, rehabilitation and assisted living facilities; hereafter LTC). Communication between the hospital and LTC team is critical during this handoff. For those discharged to home, discharge planning needs to include a realistic approach to assuring that adequate nutritional support is provided. Several recent studies have emphasized the need for special assistance to assure adequate nutrition during the early post-discharge period. Under-nourished older adults are more likely to experience adverse outcomes upon discharge and are more likely to be readmitted to hospital [75]. Having easy access to an adequate diet is an important component of recovery from surgery or illness. However, a commonly reported disconnect between the hospital-based health care system and community-based social services shows that many seniors who are eligible for food assistance post-hospital discharge are not receiving it [75–77]. Frequently, these services are being under-utilized by seniors, but in some locations, the services are under-funded, creating a waiting-list for services such as Meals on Wheels [75].
Threats to Nutritional Status in Long Term Care Settings
For more than a decade, the increased nutritional challenges faced by older adults living in LTC settings have been recognized and enumerated in studies from around the globe [60, 78–81]. This is not surprising because, for many older adults in LTC, aging brings a progressive physiological and medical decline that leads to nutritional vulnerability. Over time, those able to dine on their own may eventually progress to feeding dependency and even become altogether unable to consistently consume a nutritionally adequate diet. The brevity of this section does not reflect a lack of emphasis on this concern but rather the large body of literature already accumulated on this issue [79, 80, 82, 83].
Estimates of malnutrition prevalence in LTC vary widely, in part because of differing criteria used; there is no gold standard for nutrition screening tools in LTC [79, 83]. The evaluative criteria is almost always either a change in weight or BMI or a score on a validated nutritional assessment tool such the MNA, although MNA use is not common in U.S. nursing homes. In a recent systematic review of nutritional compromise in nursing home patients, malnutrition based on Minimum Data Set weight loss criteria of ≥ 5% in one month or ≥ 10% in 6 months was 6 to 15%, while rates of LTC residents at risk of malnutrition based on the MNA was 47 to 62% in the majority of studies [79]. In some regards, it seems surprising that these rates are not even higher, given the constellation of risk factors faced by older LTC residents [82]. As suggested by Cowan et al, [84] these factors can be categorized as individual and organizational, but there is no need for a strict categorization since much overlap can occur, especially in complicated patients. Leading causes of LTC malnutrition from the recent literature include depression, swallowing issues, eating/chewing difficulties, immobility, and impaired function, as well as insufficient staffing in the LTC facility [85]. Other facility factors shown to diminish oral intake include unappealing food delivery systems, lack of individualization in timing of menu selection and service, difficulty manipulating dishes, lids and food packages, and overly restrictive and unappetizing therapeutic diets [86].
Of course, the complicated co-morbidities faced by most LTC residents and the associated medications also play a role in increasing nutritional risk, along with concerns about oral health and about eating dependence. The aforementioned review of recent nursing home studies by Bell et al. documented a rate of total feeding dependency of 11 to 36% and a range of 8 to 87% of LTC residents needing some assistance at meals. A recent and unexpected nutrition-related concern in LTC is the challenge of dealing with obesity in these settings, both in terms of the facility (i.e. special equipment, extra staffing) and nutritional care planning (i.e. appropriate body weight and caloric intake targets). In fact, being obese increases the likelihood of institutionalization, although it can also be a reason for admission refusal by LTC facilities lacking the infrastructure to provide proper care for obese residents [87–89].
Finally, and perhaps most importantly, a very common reason for nutritional inadequacies in LTC is cognitive impairment; a large proportion of LTC residents (approximately 65%) are dealing with Alzheimer’s disease or other dementias that are moderate to severe [90]. The frailty and cognitive decline brought on as dementia progresses represents a serious threat to nutritional adequacy as the ability to self-feed, as well as to even recognize hunger, chew and swallow food, is lost. The adverse impact of chronically inadequate food intake due to on-going mealtime difficulties is profoundly felt on both physical health and quality of life for the person with dementia [90]. The stage of the disease process, when advanced, will dictate that the goals of care emphasize end-of-life management, as further discussed in the next section.
Feeding in Advanced Dementia and at the End of Life
End-of-life care constitutes another important situation of extreme nutritional vulnerability for older adults. Feeding decisions in late stage dementia often provoke wrenching moral and ethical questions for family members regarding whether or not to continue hand feeding or opt for tube feeding placement. Ideally, these decisions will be based on sound medical evidence and efforts to honor the wishes of the patient [91–93]. It is important for both patients and their families to understand that placing a feeding tube does not necessarily decrease a person’s risk of suffering. Controlled observational studies have shown that instituting a tube feeding does not improve survival, aspiration, or wound healing. However, decision-makers are often not made aware of this information [94–96]. Disadvantages of feeding tubes placed in persons with dementia include agitation, discomfort, diarrhea, abdominal pain, and increased risk of local complications [90]. When even the most dedicated feeding care strategies do not achieve nutritional adequacy, the overall goal becomes maintaining comfort and quality of life. Careful hand feeding is a viable and often preferable alternative to tube feeding as an intervention for persons with advanced dementia [90, 93]. This recommendation was recently supported by both the American Geriatrics Society and the American Academy of Hospice and Palliative Medicine [93, 97].
In the case of a terminal health condition (expected survival time of weeks to months); there is also a situation of extreme nutritional vulnerability. In this case, the focus shifts away from meeting nutrient requirements to emphasize a different type of nourishment, one that promotes comfort, dignity, and quality of life. While the family may view the provision of fluids and food as a basic need, in fact case law in the U.S. treats artificial nutrition (enteral and parenteral) as a medical treatment. This means that the law recognizes the right that a patient has to refuse artificial nutrition [98]. Feeding at the end of a terminal illness, as in advanced dementia, challenges both the family and the physician and involves concerns that go beyond the scope of this brief discussion. As addressed more completely in other literature [93, 98, 99], including wishes about artificial nutrition as part of Advance Directives/end of life planning is vitally important. The type of nutritional support chosen in end-of-life care should be consistent with treatment goals and emphasize comfort and the patient’s preferences.
Conclusions
Suboptimal nutrition contributes to the disablement process of older adults, wherein they become progressively more frail and dependent upon health care resources, leading to increased hospital and nursing home admissions as well as increased emergency department use [89, 100]. While some contributory causes of malnutrition in older adults are immutable, many others are amenable to change. Frail older adults may become less vulnerable with strong, consistent nutritional support. Care plans for these individuals must take their nutritional needs into account to optimize resiliency in the face of acute and/or chronic health challenges. Future investigations should emphasize interventions targeting modifiable determinants of nutritional vulnerability in the home, hospital, and long term care settings.
Acknowledgments
The authors acknowledge the support of the National Institutes of Health via training grant support for KPS (T32 AG000029) and the Claude D. Pepper Older Americans Independence Center (P30 AG028716).
References
- 1.Bernstein M, Munoz N. Position of the Academy of Nutrition and Dietetics: food and nutrition for older adults: promoting health and wellness. J Acad Nutr Diet. 2012;112(8):1255–77. doi: 10.1016/j.jand.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013;76(4):296–302. doi: 10.1016/j.maturitas.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Soenen S, Chapman IM. Body weight, anorexia, and undernutrition in older people. J Am Med Dir Assoc. 2013;14(9):642–8. doi: 10.1016/j.jamda.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Gielen E, Verschueren S, O’Neill TW, Pye SR, O’Connell MD, Lee DM, et al. Musculoskeletal frailty: a geriatric syndrome at the core of fracture occurrence in older age. Calcif Tissue Int. 2012;91(3):161–77. doi: 10.1007/s00223-012-9622-5. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 6.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12(2):719–36. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Brewer DP, Catlett CS, Porter KN, Lee JS, Hausman DB, Reddy S, et al. Physical limitations contribute to food insecurity and the food insecurity-obesity paradox in older adults at senior centers in Georgia. J Nutr Elder. 2010;29(2):150–69. doi: 10.1080/01639361003772343. [DOI] [PubMed] [Google Scholar]
- 8.Penn DM, Fischer JG, Sun Lee J, Hausman DB, Johnson MA. High BMI and waist circumference are associated with a high prevalence of comorbidities in older Americans Act programs in Georgia senior centers. J Nutr Health Aging. 2009;13(9):827–32. doi: 10.1007/s12603-009-0220-9. [DOI] [PubMed] [Google Scholar]
- 9.Porter KN, Johnson MA. Obesity is more strongly associated with inappropriate eating behaviors than with mental health in older adults receiving congregate meals. J Nutr Gerontol Geriatr. 2011;30(4):403–15. doi: 10.1080/21551197.2011.623960. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MA, Fischer JG, Park S. Vitamin D deficiency and insufficiency in the Georgia Older Americans Nutrition Program. J Nutr Elder. 2008;27(1–2):29–46. doi: 10.1080/01639360802059704. [DOI] [PubMed] [Google Scholar]
- 11.Quigley KK, Hermann JR, Warde WD. Factors associated with Oklahoma Older Americans Act Nutrition Program participants ability to shop, cook, and feed themselves. J Nutr Elder. 2005;25(2):69–82. doi: 10.1300/j052v25n02_05. [DOI] [PubMed] [Google Scholar]
- 12.Porter Starr K, Fischer JG, Johnson MA. Eating behaviors, mental health, and food intake are associated with obesity in older congregate meal participants. J Nutr Gerontol Geriatr. 2014;33(4):340–56. doi: 10.1080/21551197.2014.965375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JS, Fischer JG, Johnson MA. Food insecurity, food and nutrition programs, and aging: experiences from Georgia. J Nutr Elder. 2010;29(2):116–49. doi: 10.1080/01639366.2010.480895. [DOI] [PubMed] [Google Scholar]
- 14.Mathus-Vliegen EM. Obesity and the elderly. J Clin Gastroenterol. 2012;46(7):533–44. doi: 10.1097/MCG.0b013e31825692ce. [DOI] [PubMed] [Google Scholar]
- 15.Porter Starr KN, McDonald SR, Bales CW. Obesity and Physical Frailty in Older Adults: A Scoping Review of Lifestyle Intervention Trials. J Am Med Dir Assoc. 2014 doi: 10.1016/j.jamda.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker AE. Multiple chronic diseases and quality of life: patterns emerging from a large national sample, Australia. Chronic Illn. 2007;3(3):202–18. doi: 10.1177/1742395307081504. [DOI] [PubMed] [Google Scholar]
- 17.Seals DR, Melov S. Translational geroscience: emphasizing function to achieve optimal longevity. Aging (Albany NY) 2014;6(9):718–30. doi: 10.18632/aging.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10:E65. doi: 10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parekh AK, Goodman RA, Gordon C, Koh HK. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126(4):460–71. doi: 10.1177/003335491112600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luppa M, Sikorski C, Luck T, Ehreke L, Konnopka A, Wiese B, et al. Age- and gender-specific prevalence of depression in latest-life – Systematic review and meta-analysis. Journal of Affective Disorders. 2012;136(3):212–21. doi: 10.1016/j.jad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand. 2006;113(5):372–87. doi: 10.1111/j.1600-0447.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 23.Cabrera MAS, Mesas AE, Garcia ARL, de Andrade SM. Malnutrition and Depression among Community-dwelling Elderly People. Journal of the American Medical Directors Association. 2007;8(9):582–4. doi: 10.1016/j.jamda.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Williams JA, Sink KM, Tooze JA, Atkinson HH, Cauley JA, Yaffe K, et al. Low 25-Hydroxyvitamin D Concentrations Predict Incident Depression in Well-Functioning Older Adults: The Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gougeon L. Nutritional predictors of depression in a cohort of community-dwelling elderly Canadians: NuAge cohort. Appl Physiol Nutr Metab. 2014;39(12):1412. [Google Scholar]
- 26.Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. The American Journal of Clinical Nutrition. 2010;92(2):330–5. doi: 10.3945/ajcn.2010.29413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL. Zinc in Depression: A Meta-Analysis. Biological Psychiatry. 2013;74(12):872–8. doi: 10.1016/j.biopsych.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Taylor WD. Depression in the Elderly. New England Journal of Medicine. 2014;371(13):1228–36. doi: 10.1056/NEJMcp1402180. [DOI] [PubMed] [Google Scholar]
- 29.Coyle CE, Dugan E. Social Isolation, Loneliness and Health Among Older Adults. Journal of Aging and Health. 2012;24(8):1346–63. doi: 10.1177/0898264312460275. [DOI] [PubMed] [Google Scholar]
- 30.Dickens AP, Richards SH, Greaves CJ, Campbell JL. Interventions targeting social isolation in older people: a systematic review. BMC Public Health. 2011;11:647. doi: 10.1186/1471-2458-11-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):5797–801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero-Ortuno R, Casey AM, Cunningham CU, Squires S, Prendergast D, Kenny RA, et al. Psychosocial and functional correlates of nutrition among community-dwelling older adults in Ireland. The journal of nutrition, health & aging. 2011;15(7):527–31. doi: 10.1007/s12603-010-0278-4. [DOI] [PubMed] [Google Scholar]
- 33.Hughes G, Bennett KM, Hetherington MM. Old and alone: barriers to healthy eating in older men living on their own. Appetite. 2004;43(3):269–76. doi: 10.1016/j.appet.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Holwerda TJ, Beekman AT, Deeg DJ, Stek ML, van Tilburg TG, Visser PJ, et al. Increased risk of mortality associated with social isolation in older men: only when feeling lonely? Results from the Amsterdam Study of the Elderly (AMSTEL) Psychol Med. 2012;42(4):843–53. doi: 10.1017/S0033291711001772. [DOI] [PubMed] [Google Scholar]
- 35.Bickel G, Nord M, Prince C, Hamilton W, Cook J. U.S. Department of Agriculture FaNS, editor. Guide to measruing household food security revised. Alexandria, VA: 2000. [Google Scholar]
- 36.Coleman-Jensen A, Nord M, Singh A. Household food security in the United States in 2012, ERR-155. U.S. Department of Agriculture, Food and Nutrition Service, Economic Research Service; 2013. [Google Scholar]
- 37.Ziliak J, Gundersen C. The State of Senior Hunger in America 2012: An Annual Report. National Foundation to End Senior Hunger; 2014. [Google Scholar]
- 38.Cook JT, Frank DA. Food security, poverty, and human development in the United States. Ann N Y Acad Sci. 2008;1136:193–209. doi: 10.1196/annals.1425.001. [DOI] [PubMed] [Google Scholar]
- 39.Buys DR, Locher JL. Food insecurity and hunger among older adults. In: Bales C, Locher J, Saltzman E, editors. Handbook of Clinical Nutrition and Aging. 3. New York: Springer Science+Business Media; 2014. pp. 147–59. [Google Scholar]
- 40.Alley DE, Soldo BJ, Pagán JA, McCabe J, deBlois M, Field SH, et al. Material Resources and Population Health: Disadvantages in Health Care, Housing, and Food Among Adults Over 50 Years of Age. American journal of public health. 2009;99(Suppl 3):S693–S701. doi: 10.2105/AJPH.2009.161877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seligman HK, Laraia BA, Kushel MB. Food Insecurity Is Associated with Chronic Disease among Low-Income NHANES Participants. The Journal of Nutrition. 2010;140(2):304–10. doi: 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JS, Gundersen C, Cook J, Laraia B, Johnson MA. Food Insecurity and Health across the Lifespan. Advances in Nutrition: An International Review Journal. 2012;3(5):744–5. doi: 10.3945/an.112.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holben DH, Pheley AM. Diabetes Risk and Obesity in Food-Insecure Households in Rural Appalachian Ohio. Preventing Chronic Disease. 2006;3(3):A82. [PMC free article] [PubMed] [Google Scholar]
- 44.Klesges LM, Pahor M, Shorr RI, Wan JY, Williamson JD, Guralnik JM. Financial difficulty in acquiring food among elderly disabled women: results from the Women’s Health and Aging Study. American Journal of Public Health. 2001;91(1):68–75. doi: 10.2105/ajph.91.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinour LM, Bergen D, Yeh MC. The food insecurity-obesity paradox: a review of the literature and the role food stamps may play. J Am Diet Assoc. 2007;107(11):1952–61. doi: 10.1016/j.jada.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Kim K, Frongillo EA. Participation in food assistance programs modifies the relation of food insecurity with weight and depression in elders. J Nutr. 2007;137(4):1005–10. doi: 10.1093/jn/137.4.1005. [DOI] [PubMed] [Google Scholar]
- 47.Stuff JE, Casey PH, Szeto KL, Gossett JM, Robbins JM, Simpson PM, et al. Household food insecurity is associated with adult health status. J Nutr. 2004;134(9):2330–5. doi: 10.1093/jn/134.9.2330. [DOI] [PubMed] [Google Scholar]
- 48.Kim J, Park SK, Lim YJ. Analysis of the factors affecting the success of weight reduction programs. Yonsei Med J. 2007;48(1):24–9. doi: 10.3349/ymj.2007.48.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao X, Scott T, Falcon LM, Wilde PE, Tucker KL. Food insecurity and cognitive function in Puerto Rican adults. The American Journal of Clinical Nutrition. 2009;89(4):1197–203. doi: 10.3945/ajcn.2008.26941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee MR, Berthelot ER. Community Covariates of Malnutrition Based Mortality Among Older Adults. Annals of Epidemiology. 2010;20(5):371–9. doi: 10.1016/j.annepidem.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Jensen GL. Inflammation as the key interface of the medical and nutrition universes: a provocative examination of the future of clinical nutrition and medicine. JPEN J Parenter Enteral Nutr. 2006;30(5):453–63. doi: 10.1177/0148607106030005453. [DOI] [PubMed] [Google Scholar]
- 52.Tappenden KA, Quatrara B, Parkhurst ML, Malone AM, Fanjiang G, Ziegler TR. Critical Role of Nutrition in Improving Quality of Care: An Interdisciplinary Call to Action to Address Adult Hospital Malnutrition. Journal of the Academy of Nutrition and Dietetics. 2013;113(9):1219–37. doi: 10.1016/j.jand.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 53.White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) J Acad Nutr Diet. 2012;112(5):730–8. doi: 10.1016/j.jand.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A, Miller S, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186–95. doi: 10.1177/0148607113512154. [DOI] [PubMed] [Google Scholar]
- 55.US Bureau. [Accessed December 12, 2014];US Census Bureau: State and County QuickFacts. 2014 http://quickfacts.census.gov/qfd/states/00000.html.
- 56.Weiss AJ, Elixhauser A. HCUP Statistical Brief #180. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Overview of Hospital Stays in the United States, 2012. [PubMed] [Google Scholar]
- 57.Col N, Fanale JE, Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Archives of internal medicine. 1990;150(4):841–5. [PubMed] [Google Scholar]
- 58.Creditor MC. Hazards of Hospitalization of the Elderly. Annals of Internal Medicine. 1993;118(3):219–23. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez HM, Callahan KE, Likourezos A, Leipzig RM. House staff member awareness of older inpatients’ risks for hazards of hospitalization. Archives of internal medicine. 2008;168(4):390–6. doi: 10.1001/archinternmed.2007.87. [DOI] [PubMed] [Google Scholar]
- 60.Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734–8. doi: 10.1111/j.1532-5415.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 61.Mowe M, Bøhmer T, Kindt E. Reduced nutritional status in an elderly population (> 70 y) is probable before disease and possibly contributes to the development of disease. The American journal of clinical nutrition. 1994;59(2):317–24. doi: 10.1093/ajcn/59.2.317. [DOI] [PubMed] [Google Scholar]
- 62.Cerri AP, Bellelli G, Mazzone A, Pittella F, Landi F, Zambon A, et al. Sarcopenia and malnutrition in acutely ill hospitalized elderly: Prevalence and outcomes. Clin Nutr. 2014 doi: 10.1016/j.clnu.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 63.Heersink JT, Brown CJ, Dimaria-Ghalili RA, Locher JL. Undernutrition in hospitalized older adults: patterns and correlates, outcomes, and opportunities for intervention with a focus on processes of care. J Nutr Elder. 2010;29(1):4–41. doi: 10.1080/01639360903574585. [DOI] [PubMed] [Google Scholar]
- 64.Buie VC, Owings MF, DeFrances CJ, Golosinskiy A. National hospital discharge survey: 2006 annual summary. Vital Health Stat. 2010;13(168):1–79. [PubMed] [Google Scholar]
- 65.Inoue M, Kinoshita K, Sano F, Kobayashi M, Yasuda S, Sowa T, et al. Perioperative nutritional support with immune-enhancing diet for surgical closure of open thoracic window. Kyobu Geka. 2012;65(7):559–62. [PubMed] [Google Scholar]
- 66.Martindale RG, McClave SA, Taylor B, Lawson CM. Perioperative Nutrition: What Is the Current Landscape? Journal of Parenteral and Enteral Nutrition. 2013;37(5 suppl):5S–20S. doi: 10.1177/0148607113496821. [DOI] [PubMed] [Google Scholar]
- 67.Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: Relationship to postoperative wound complications. Journal of Arthroplasty. 1991;6(4):321–5. doi: 10.1016/s0883-5403(06)80183-x. [DOI] [PubMed] [Google Scholar]
- 68.Guo JJ, Yang H, Qian H, Huang L, Guo Z, Tang T. The effects of different nutritional measurements on delayed wound healing after hip fracture in the elderly. J Surg Res. 2010;159(1):503–8. doi: 10.1016/j.jss.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Foster MR, Heppenstall RB, Friedenberg ZB, Hozack WJ. A prospective assessment of nutritional status and complications in patients with fractures of the hip. Journal of orthopaedic trauma. 1990;4(1):49–57. doi: 10.1097/00005131-199003000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Cerri AP, Bellelli G, Mazzone A, Pittella F, Landi F, Zambon A, et al. Sarcopenia and malnutrition in acutely ill hospitalized elderly: Prevalence and outcomes. Clinical Nutrition. 2014 doi: 10.1016/j.clnu.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 71.Patel V, Romano M, Corkins MR, DiMaria-Ghalili RA, Earthman C, Malone A, et al. Nutrition Screening and Assessment in Hospitalized Patients A Survey of Current Practice in the United States. Nutrition in Clinical Practice. 2014;29(4):483–90. doi: 10.1177/0884533614535446. [DOI] [PubMed] [Google Scholar]
- 72.Graham CL, Ivey SL, Neuhauser L. From hospital to home: assessing the transitional care needs of vulnerable seniors. Gerontologist. 2009;49(1):23–33. doi: 10.1093/geront/gnp005. [DOI] [PubMed] [Google Scholar]
- 73.Boling PA. Managing posthospital care transitions for older adults: challenges and opportunities. Jama. 2014;312(13):1303–4. doi: 10.1001/jama.2014.12360. [DOI] [PubMed] [Google Scholar]
- 74.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–2. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Locher JL, Wellman NS. “Never the twain shall meet:” dual systems exacerbate malnutrition in older adults recently discharged from hospitals. J Nutr Gerontol Geriatr. 2011;30(1):24–8. doi: 10.1080/01639366.2011.545039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sahyoun NR, Akobundu U, Coray K, Netterville L. Challenges in the delivery of nutrition services to hospital discharged older adults: the community connections demonstration project. J Nutr Elder. 2009;28(2):127–42. doi: 10.1080/01639360902950133. [DOI] [PubMed] [Google Scholar]
- 77.Sahyoun NR, Anyanwu UO, Sharkey JR, Netterville L. Recently hospital-discharged older adults are vulnerable and may be underserved by the Older Americans Act Nutrition Program. J Nutr Elder. 2010;29(2):227–40. doi: 10.1080/01639361003772608. [DOI] [PubMed] [Google Scholar]
- 78.Kayser-Jones J. Malnutrition, dehydration, and starvation in the midst of plenty: the political impact of qualitative inquiry. Qual Health Res. 2002;12(10):1391–405. doi: 10.1177/1049732302238750. [DOI] [PubMed] [Google Scholar]
- 79.Bell CL, Tamura BK, Masaki KH, Amella EJ. Prevalence and measures of nutritional compromise among nursing home patients: weight loss, low body mass index, malnutrition, and feeding dependency, a systematic review of the literature. J Am Med Dir Assoc. 2013;14(2):94–100. doi: 10.1016/j.jamda.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Pauly L, Stehle P, Volkert D. Nutritional situation of elderly nursing home residents. Z Gerontol Geriatr. 2007;40(1):3–12. doi: 10.1007/s00391-007-0430-x. [DOI] [PubMed] [Google Scholar]
- 81.Serrano-Urrea R, Garcia-Meseguer MJ. Malnutrition in an elderly population without cognitive impairment living in nursing homes in Spain: study of prevalence using the Mini Nutritional Assessment test. Gerontology. 2013;59(6):490–8. doi: 10.1159/000351763. [DOI] [PubMed] [Google Scholar]
- 82.Sloane PD, Ivey J, Helton M, Barrick AL, Cerna A. Nutritional issues in long-term care. J Am Med Dir Assoc. 2008;9(7):476–85. doi: 10.1016/j.jamda.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 83.Bell CL, Lee AS, Tamura BK. Malnutrition in the nursing home. Curr Opin Clin Nutr Metab Care. 2015;18(1):17–23. doi: 10.1097/MCO.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 84.Cowan DT, Roberts JD, Fitzpatrick JM, While AE, Baldwin J. Nutritional status of older people in long term care settings: current status and future directions. Int J Nurs Stud. 2004;41(3):225–37. doi: 10.1016/S0020-7489(03)00131-7. [DOI] [PubMed] [Google Scholar]
- 85.Tamura BK, Bell CL, Masaki KH, Amella EJ. Factors associated with weight loss, low BMI, and malnutrition among nursing home patients: a systematic review of the literature. J Am Med Dir Assoc. 2013;14(9):649–55. doi: 10.1016/j.jamda.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 86.Messinger-Rapport BJ, Gammack JK, Little MO, Morley JE. Clinical update on nursing home medicine: 2014. J Am Med Dir Assoc. 2014;15(11):786–801. doi: 10.1016/j.jamda.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Powell LS, Felix HC, Bradway C, Miller E, Heivly A, Fleshner I. Additional research on the cost of caring for obese nursing home residents is critical to maintaining adequate resources in the long-term care industry. J Am Med Dir Assoc. 2010;11(3):222. doi: 10.1016/j.jamda.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 88.Felix HC. Personal care assistance needs of obese elders entering nursing homes. J Am Med Dir Assoc. 2008;9(5):319–26. doi: 10.1016/j.jamda.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 89.Buys DR, Roth DL, Ritchie CS, Sawyer P, Allman RM, Funkhouser EM, et al. Nutritional risk and body mass index predict hospitalization, nursing home admissions, and mortality in community-dwelling older adults: results from the UAB Study of Aging with 8.5 years of follow-up. J Gerontol A Biol Sci Med Sci. 2014;69(9):1146–53. doi: 10.1093/gerona/glu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aselage M, Amella EJ, Rose SB, Bales CW. Dementia-related mealtime difficulties: assessment and management in the long-term care setting. In: Bales CW, Locher JL, Saltzman ES, editors. Handbook of Clinical Nutrition and Aging. 3. Springer; 2014. [Google Scholar]
- 91.Heuberger RA. Artificial nutrition and hydration at the end of life. J Nutr Elder. 2010;29(4):347–85. doi: 10.1080/01639366.2010.521020. [DOI] [PubMed] [Google Scholar]
- 92.Galanos AN, Neff EC, Heuberger RA. What is “optimal nourishment” for older adults at the end of life? A conversation. Interview by Connie W. Bales. J Nutr Elder. 2010;29(4):386–92. doi: 10.1080/01639366.2010.528330. [DOI] [PubMed] [Google Scholar]
- 93.Fischberg D, Bull J, Casarett D, Hanson LC, Klein SM, Rotella J, et al. Five things physicians and patients should question in hospice and palliative medicine. J Pain Symptom Manage. 2013;45(3):595–605. doi: 10.1016/j.jpainsymman.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Hanson LC, Carey TS, Caprio AJ, Lee TJ, Ersek M, Garrett J, et al. Improving decision-making for feeding options in advanced dementia: a randomized, controlled trial. J Am Geriatr Soc. 2011;59(11):2009–16. doi: 10.1111/j.1532-5415.2011.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Candy B, Sampson EL, Jones L. Enteral tube feeding in older people with advanced dementia: findings from a Cochrane systematic review. Int J Palliat Nurs. 2009;15(8):396–404. doi: 10.12968/ijpn.2009.15.8.43799. [DOI] [PubMed] [Google Scholar]
- 96.Teno JM, Mitchell SL, Kuo SK, Gozalo PL, Rhodes RL, Lima JC, et al. Decision-making and outcomes of feeding tube insertion: a five-state study. J Am Geriatr Soc. 2011;59(5):881–6. doi: 10.1111/j.1532-5415.2011.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Committee AGSECaCPaMoC. American Geriatrics Society feeding tubes in advanced dementia position statement. J Am Geriatr Soc. 2014;62(8):1590–3. doi: 10.1111/jgs.12924. [DOI] [PubMed] [Google Scholar]
- 98.Yukawa M, Seel Ritchie C. Nutrition at the End of Life. In: Bales CW, Locher JL, Saltzman E, editors. Handbook of Clinical Nutrition and Aging. 3. New York, NY: Springer Science+Business Media; 2014. pp. 303–12. [Google Scholar]
- 99.Del Rio MI, Shand B, Bonati P, Palma A, Maldonado A, Taboada P, et al. Hydration and nutrition at the end of life: a systematic review of emotional impact, perceptions, and decision-making among patients, family, and health care staff. Psychooncology. 2012;21(9):913–21. doi: 10.1002/pon.2099. [DOI] [PubMed] [Google Scholar]