Abstract
Sepsis mortality varies dramatically in individuals of variable immune conditions, with poorly defined mechanisms. This phenomenon complements the hypothesis that innate immunity may adopt rudimentary memory, as demonstrated in vitro with endotoxin priming and tolerance in cultured monocytes. However, previous in vivo studies only examined the protective effect of endotoxin tolerance in the context of sepsis. In sharp contrast, we report herein that pre-conditioning with super-low or low dose endotoxin lipopolysaccharide (LPS) cause strikingly opposite survival outcomes. Mice pre-conditioned with super-low dose LPS experienced severe tissue damage, inflammation, increased bacterial load in circulation, and elevated mortality when they were subjected to cecal-ligation and puncture (CLP). This is in contrast to the well-reported protective phenomenon with CLP mice pre-conditioned with low dose LPS. Mechanistically, we demonstrated that super-low and low dose LPS differentially modulate the formation of neutrophil extracellular trap (NET) in neutrophils. Instead of increased ERK activation and NET formation in neutrophils pre-conditioned with low dose LPS, we observed significantly reduced ERK activation and compromised NET generation in neutrophils pre-conditioned with super-low dose LPS. Collectively, our findings reveal a mechanism potentially responsible for the dynamic programming of innate immunity in vivo as it relates to sepsis risks.
Keywords: Innate immunity, Pre-conditioning dynamics, Neutrophil, Polymicrobial sepsis, Super-low dose endotoxin
Highlights
-
•
Super-low dose endotoxin pre-conditioning exacerbates, while higher dose endotoxin alleviates sepsis mortality.
-
•
Super-low dose endotoxin reduces, while higher dose endotoxin facilitates neutrophil extracellular trap (NET) formation.
-
•
Super-low dose endotoxin suppresses, while higher dose endotoxin induces ERK activation required for NET formation.
1. Introduction
Sepsis is a life-threatening syndrome commonly experienced by patients in critical care units. Sepsis syndrome poses a particular concern for people with prior health conditions, a phenomenon often referred as “second-hit” or “pre-conditioning” (Rocksen et al., 2004, Wang et al., 1998). Prior immune condition may have a critical impact on the clinical outcome of sepsis. Despite extensive past studies, mechanisms contributing to the alteration of sepsis risks remain poorly understood.
A classic example of innate immunity pre-conditioning is manifested in the endotoxin tolerance of innate leukocytes (Morris and Li, 2012). Most studies regarding cellular responses to endotoxin lipopolysaccharide (LPS) utilized tolerant dosages of LPS (often referred as “low dose” in the literature) (> 10 ng/mL in vitro culture, > 1 μg/mouse or > 50 μg/kg body weight in vivo injection). Low dose LPS (L-LPS) causes robust induction of pro-inflammatory mediators in monocytes/macrophages through the Toll-Like-Receptor 4 (TLR4) pathway (Kawai and Akira, 2007). Shortly after the initial wave of expression, host leukocytes develop a state of “endotoxin tolerance”, in which the expressions of pro-inflammatory mediators are suppressed (Henricson et al., 1993, Li et al., 2000, West and Heagy, 2002). Endotoxin tolerance serves as a compensatory mechanism for the resolution of inflammation (Jacinto et al., 2002, Medvedev et al., 2002). Based on the tolerance concept, endotoxin has been employed experimentally to elicit a protective mechanism for subsequent inflammatory conditions that include ischemia injury and sepsis (Kopanakis et al., 2013, Meng et al., 1997).
Mechanistically, the prior conditioning with L-LPS not only reduces tissue inflammation through inducing tolerance, but also boosts bacterial killing activities of neutrophils partly through increasing the formation of neutrophil extra-cellular trap (NET) (Landoni et al., 2012). NETs released by neutrophils not only traps and clears bacteria, but may also degrade inflammatory mediators and therefore limit further exacerbation of inflammation (Campbell et al., 2012). Indeed, individuals with impaired NETosis demonstrate excessive production of inflammatory mediators from neutrophils and persistent inflammation (Schauer et al., 2014). NET-deficient mice also develop exacerbated and chronic inflammation that can be reduced by adoptive transfer of aggregated NETs (Schauer et al., 2014). In the context of sepsis, depletion of neutrophil NETs in vivo results in hyper susceptibility to polymicrobial sepsis (Meng et al., 2012). Mechanistically, NET formation in neutrophils may depend upon the activation of the extracellular signal-regulated kinase (ERK) (Hakkim et al., 2011, Yoo et al., 2014), and selective inhibition of ERK has been demonstrated to be a potent suppressor of NETosis (Hakkim et al., 2011). ERK is known to be activated by LPS and may serve as a compensatory anti-inflammatory signal and contribute to endotoxin tolerance in other innate immune cells (Maitra et al., 2011).
Although LPS tolerance may explain the beneficial and protective effect of L-LPS pre-conditioning in the context of sepsis, it may not reconcile increased sepsis mortality often observed in patients with prior health conditions. In sharp contrast, emerging studies hint at an intriguing connection between subclinical super-low levels of circulating bacterial endotoxin lipopolysaccharide (SL-LPS) (< 100 pg/mL) in humans with adverse health conditions and/or life styles such as chronic infections, aging, chronic smoking and drinking (Ancuta et al., 2008, Cani et al., 2008, Goto et al., 1994, Lira et al., 2010, Rao, 2009, Szeto et al., 2008, Wiedermann et al., 1999). Compromised mucosal barriers, altered commensal microbiota, and vasculature leakage collectively contribute to the mild elevation of plasma endotoxin in these individuals. We and others have previously shown that super-low dose LPS (SL-LPS, ~ 1–100 pg/mL in vitro, 100 pg–10 ng/mouse or 5 ng–0.5 μg/kg body weight in vivo injection) “primes” monocytes/macrophages for a more robust response to a secondary LPS challenge, a phenomenon known as the “Shwartzman reaction” (Deng et al., 2013, Henricson et al., 1993, Hirohashi and Morrison, 1996, Zhang and Morrison, 1993a). At the molecular levels, we demonstrate that SL-LPS preferentially clears away the compensatory anti-inflammatory signals in monocytes/macrophages, in sharp contrast to the effect of low dose LPS (Deng et al., 2013, Maitra et al., 2012). The dynamic pathway-switching in monocytes/macrophages induced by varying dosages of LPS may underlie the “pre-conditioning” of innate immunity and “rudimentary” innate memory (Fu et al., 2012).
Despite this intriguing connection, pre-conditioning with SL-LPS in the context of sepsis mortality has never been studied. Thus, we tested the hypothesis that SL-LPS pre-conditioning may exacerbate, instead of alleviate sepsis mortality in vivo. We employed the classical cecal ligation and puncture (CLP) model of polymicrobial sepsis, and examined mortality, tissue inflammation, and neutrophil bacterial killing in mice pre-conditioned with either low dose (50 μg/kg body weight) or super-low dose (5 ng/kg body weight) LPS. Another feature of this study involves the emerging concept of innate leukocyte programming. Although emerging studies reported the differential programming of monocytes/macrophages by SL-LPS and L-LPS (Morris et al., 2015), no data is available with regard to whether neutrophil, a key innate leukocyte in the context of sepsis, could be dynamically programmed by SL-LPS and L-LPS. Our study reveals that pre-conditioning with super-low dose LPS significantly increased CLP sepsis mortality and tissue inflammation, through reducing neutrophil NETosis and bacterial killing. Counter-intuitively, we observed that SL-LPS significantly suppressed ERK activation in neutrophils, instead of activating ERK. Taken together, our study reveals programming dynamics of innate immunity in vivo by SL-LPS.
2. Materials and Methods
2.1. Mice
Inbred C57 BL/6mice between the ages of 8 and 12 weeks were used for experimentation. The mice were bred and housed in our pathogen-free, limited access, ultra-barrier facility with a 12-h light-dark cycle. All experiments described were performed in adherence to the National Institutes of Health guidelines on the use of experimental animals, and approval was obtained from the Institutional Animal Care and Use Committee (IACUC) of Virginia Polytechnic Institute and State University.
2.2. Reagents
Fluorescein isothiocyanate (FITC) conjugated anti-mouse Ly6G/Ly6C (Gr-1) antibody and Streptavidin conjugated anti-mouse CXCR2 antibody were from Biolegend (San Diego, CA). Anti-citrullinated histone H3 antibody was from Abcam (Cambridge, MA) and the anti-citrullinated Histone H4 antibody was from Millipore (Billerica, MA).
2.3. Cecal Ligation and Puncture (CLP)
CLP were performed as previously described (Toscano et al., 2011). Briefly, anesthetized mice (male, 8 weeks old) with approved protocol from the institutional IACUC committee were operated at the abdomen, with the cecum being identified, exposed, and ligated with a 3-0 silk suture at its base below the ileocecal junction. The antimesenteric border of the cecum was punctured once with a 21-gauge needle, and the cecum was gently squeezed to extrude a small amount of stool. The cecum was returned to the abdomen, which was closed in two layers with 3–0 silk by means of running sutures. Sham controls were performed with ligation but without puncture. After surgery, mice were resuscitated with 1.0 mL of saline including Buprenorphine (0.05 mg/kg in 1 mL saline) given by subcutaneous injection. The mice were monitored and recorded every 4 h for mortality for four days in a blinded fashion by observers.
2.4. Bacterial Counts in Blood
The bacterial count was determined as previously described (Godshall et al., 2002). In brief, mice were sacrificed at various days post-CLP. Aliquots of serial dilutions were placed on agar dishes (Difco Laboratories) and incubated at 37 °C. Colony Forming Units (CFU) were analyzed after 24 h. The results were expressed as log of CFU per mL or CFU × 103/mL of blood.
2.5. Pathology
For routine histologic analysis, frozen livers (1 mm × 5 mm), lungs (left lobe) and kidneys (whole kidney) were sectioned, stained with H&E. PMN were counted in a blinded and standardized fashion by light microscopy (Axiovert 40, Zeiss, Goettingen, Germany). Briefly, a micrometer ocular (× 20) was used to count PMN in 6 different visual fields of each section. The histologic grading of liver injury was evaluated by the following score scale of values: 1). Infiltration of inflammatory cells (numbers/field): 1: 0–9; 2: 10–19; 3: 20–29; 4: 30–39; 5: 40 and over; 2). Hepatocyte degeneration (%): 1: 0–9; 2: 10–29; 3: 30–49; 4: 50–69; 5: 70 and over; and 3). Hepatocyte necrosis (%): 1: 0–9; 2: 10–19; 3: 20–29; 4: 30 and over. For the scoring of lung damage, infiltration of inflammatory cells, vascular congestion and interstitial edema were evaluated. For kidney damage, infiltration of inflammatory cells, necrosis, degeneration of tubular cells and glomerulus damage were evaluated. All parameters were evaluated by the following score scale of values: 0, absent; 1, mild; 2, moderate; and 3, severe (Meng et al., 1997). All histopathological evaluations were done in a blinded fashion by an independent pathologist.
2.6. NET Formation Through Flow Cytometry and Immunofluorescence
For in vivo analyses, mice were administrated with super-low dose (5 ng/kg body weight) or low dose (50 μg/kg body weight) LPS through i.p. injection. After 24 h, splenocytes were harvested and stimulated with PMA (50 nM) for 3 h. Cells were fixed and permeabilized with BD Phosflow™ buffer (BD Biosciences, San Jose, CA). Anti-citrullinated Histone H4 antibody (Millipore, Billerica, MA) was labeled with Alexa Fluor 647 using Zenon™ labeling kit (Life Technologies, Grand Island, NY), and then was used to stain splenocytes together with anti-Ly6G and anti-CD11b antibodies (BioLegend, San Diego, CA). For in vitro studies, bone marrow cells from C57 BL/6 mice were cultured with G-CSF (100 ng/mL) in the presence of super-low dose (100 pg/mL) or higher dose (1 μg/mL) LPS for 3 days, with fresh LPS added to the cell cultures every 24 h. In some experiments, TUDCA (0.5 mM) was also added to the cell culture. After stimulation with Phorbol myristate acetate (PMA, 50 nM) for 3 h, cells were fixed, permeabilized and stained with Alexa Fluor 647 labeled anti-Histone H4 (citrulline 3) antibody together with anti-Ly6G and anti-CD11b antibodies. The samples were then analyzed by FACSCanto II (BD Biosciences). The data were processed by FACSDiva (BD Biosciences), or Flow Jo (Tree Star, Ashland, OR). For immunofluorescence analyses, peritoneal cells were harvested from PBS or LPS conditioned-mice at indicated time point post-CLP, and spun on glass slides through cytospin. Neutrophils were co-stained with antibodies against Ly6G and citrullinated Histone H3. DAPI was used to stain cell nucleus. Samples were analyzed with fluorescence microscopy.
2.7. Determination of Protein Phosphorylation
Bone marrow cells from C57 BL/6 mice were cultured with G-CSF (100 ng/mL) in the presence of super-low dose (100 pg/mL) or higher dose (1 μg/mL) LPS, with fresh LPS added to the cell culture every 24 h. In some experiments, Tauroursodeoxycholic acid (TUDCA, 0.5 mM) was also added to the cell cultures. After 3 h or 3 days, cells were then fixed, permeabilized and stained with either anti-pERK1/2 (T204/Y202, eBioscience, San Diego, CA) or anti-pJNK (T183/Y185, BD Biosciences) antibody together with anti-Ly6G and anti-CD11b antibodies. The samples were analyzed by FACSCanto II. The data were processed by FACSDiva, or Flow Jo. Whole protein lysates were also prepared for Western blot analyses.
2.8. ELISA of Cytokines
Plasma samples were collected from the peripheral blood. TNF-α and CXCL1/KC/GROα levels were measured by enzyme-linked immunosorbent assay (ELISA) using ELISA Kit from eBioscience and RayBiotec, respectively.
2.9. Chemotaxis Assays
Chemotaxis of neutrophils was measured with 48-well micro-chambers and polycarbonate filters (5 μm pore size) (NeuroProbe, Cabin John, MD) as described (Tancevski et al., 2014). The results were expressed as the mean ± SEM of the chemotaxis index (CI), representing the fold increase in the number of migrated cells in response to chemo-attractants over spontaneous cell migration (to control medium).
2.10. Statistical Analysis
Statistical analysis was performed with Prism software (GraphPad Software, La Jolla, CA). Values were expressed as means ± SEM. The significance of the differences was assessed by Student’s t test or one-way ANOVA where appropriate. p < 0.05 was considered statistically significant. The mortality studies were assessed with log rank test with p < 0.05 considered as significant.
3. Results
3.1. Pre-conditioning With Super-low LPS Exacerbates Sepsis Mortality
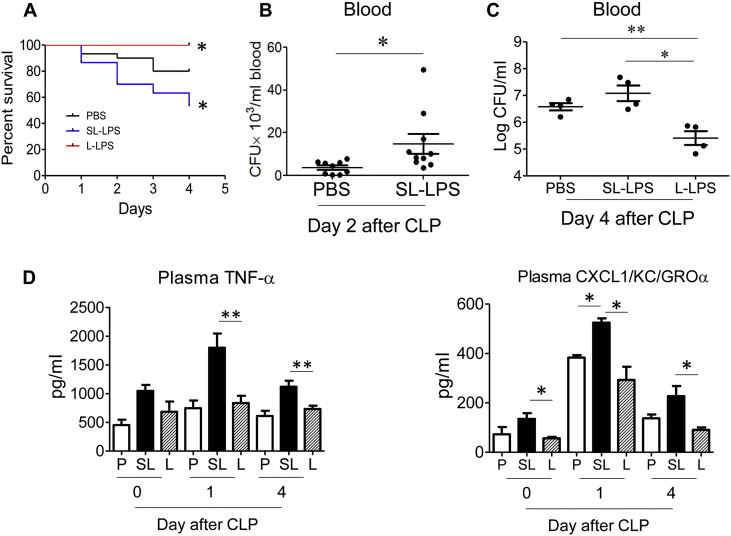
To examine the impact of super-low LPS pre-conditioning on sepsis mortality, we used the murine model of cecal ligation and puncture (CLP). As a control, we simultaneously studied the effect of low dose LPS. In consistent with previous reports (Kopanakis et al., 2013, Landoni et al., 2012), mice injected i.p. with L-LPS (50 μg/kg body weight) 24 h before CLP exhibited significantly improved survival as compared to sham mice or mice injected with PBS controls (Fig. 1A). In sharp contrast, we documented that mice injected with SL-LPS (5 ng/kg body weight) displayed significantly reduced survival as compared to PBS controls (Fig. 1A).
Fig. 1.
Opposite sepsis outcomes in mice pre-conditioned with super-low and low dose LPS. (A). WT mice (male, 8 weeks old) were pre-conditioned through i.p. injection of either PBS (30 mice), super-low dose (SL-LPS, 5 ng/kg) LPS (30 mice), or low-dose (L-LPS, 50 μg/kg) LPS (30 mice). CLP were performed 1 d post-pre-conditioning, and mortality was closely monitored every 4 h for 4 d. *p < 0.05. (B). Blood samples were collected from mice 2 d post-CLP. Bacteria were cultured, counted and plotted. The results were expressed as colony-forming units (CFU)/mL. *p < 0.05, N = 11 mice per group. (C). Blood samples were collected from mice 4 d post-CLP. Bacteria were cultured, counted and plotted. The results were expressed as log of colony-forming units (CFU)/mL. *p < 0.05, **p < 0.01. N = 4 mice per group, the data were representative from 3 independent experiments. (D). Blood collected at days 0, 1, and 4 post-CLP were used to measured plasma levels of TNF-α and KC through ELISA. P: PBS; SL: SL-LPS; L: L-LPS *p < 0.05; **p < 0.01. N = 4 mice per group, the data were representative from 3 independent experiments.
Next, we examined bacterial loads in the blood after CLP. As shown in Fig. 1B, bacterial counts were significantly increased in the peripheral blood of CLP mice pre-conditioned with 5 ng/kg body weight SL-LPS. In contrast, bacterial counts were significantly reduced in the peripheral blood of CLP mice pre-conditioned with 50 μg/kg body weight L-LPS (Fig. 1C).
We further examined selected inflammatory markers in circulation. In consistent with previous findings (Kopanakis et al., 2013, Landoni et al., 2012), CLP mice pre-conditioned with tolerant dose (50 μg/kg body weight) L-LPS had significantly reduced levels of plasma TNF-α and KC as compared to CLP mice pre-conditioned with either PBS or priming-dose (5 ng/kg body weight) SL-LPS (Fig. 1D). In sharp contrast, we documented that CLP mice pre-conditioned with priming SL dose (5 ng/kg body weight) LPS had a significant and persistent elevation of plasma levels of TNF-α and KC (CXCL1) as compared to other two groups. Collectively, these data reveal that the mice pre-conditioned with SL-LPS (5 ng/kg body weight) displayed significantly reduced survival, increased blood bacteria counts and increased systemic inflammatory responses upon septic challenge. This is sharp contrast to the protective effects observed in mice pre-conditioned with tolerant L-LPS.
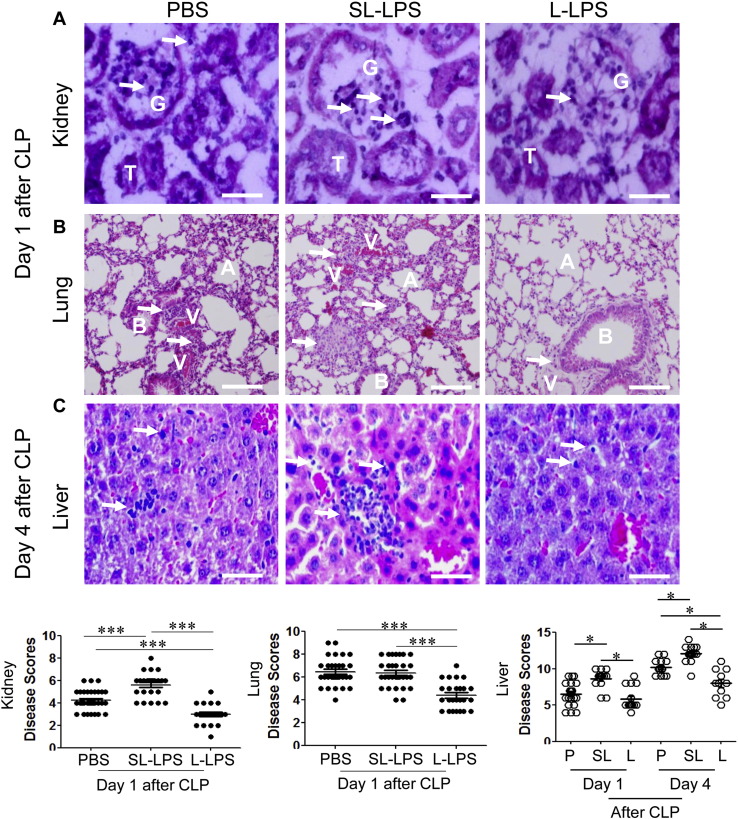
3.2. Elevated Tissue Injuries in CLP Mice Pre-conditioned With Super-low LPS
Given the opposite survival outcomes, we further examined tissue damage and inflammation in vital organs. Consistent to previous reports, the disease scores and neutrophil infiltrations in kidney, liver, and lung were significantly lower in CLP mice pre-conditioned with tolerant L-LPS (Fig. 2A–C). In contrast, we observed significantly elevated disease scores and neutrophil infiltrations in kidney, liver and lung from CLP mice pre-conditioned with SL-LPS (Fig. 2A–C). The elevated neutrophil levels in vital organs are consistent with our above data that the circulating chemokine CXCL1/KC levels are highest in the CLP mice pre-conditioned with SL-LPS. On the other hand, the reduced neutrophil levels and reduced tissue damages in CLP mice pre-conditioned with tolerant L- LPS are correlated with reduced CXCL1/KC levels, and in line with previous studies (Kopanakis et al., 2013, Landoni et al., 2012).
Fig. 2.
Differential modulation of tissue injuries in CLP mice pre-conditioned with super-low or low dose LPS. Vital organs were collected from CLP mice pre-conditioned with either PBS, 5 ng/kg LPS, or low dose (50 μg/kg) LPS. (A) Frozen kidney sections were stained with H&E. G: glomerulus; T: tubular cells; arrow: PMN. Scale bar = 50 μm. (B) Frozen lung sections (5 μm) were stained with H&E. A: Alveolus; B: Bronchiole; V: Blood vessels; arrow: PMN. Scale bar = 50 μm. (C) Frozen liver sections (5 μm) were stained with H&E. Arrow: PMN. Scale bar = 50 μm. Disease scores were evaluated and plotted. *p < 0.05. ***p < 0.001. Data were pooled from three independent experiments.
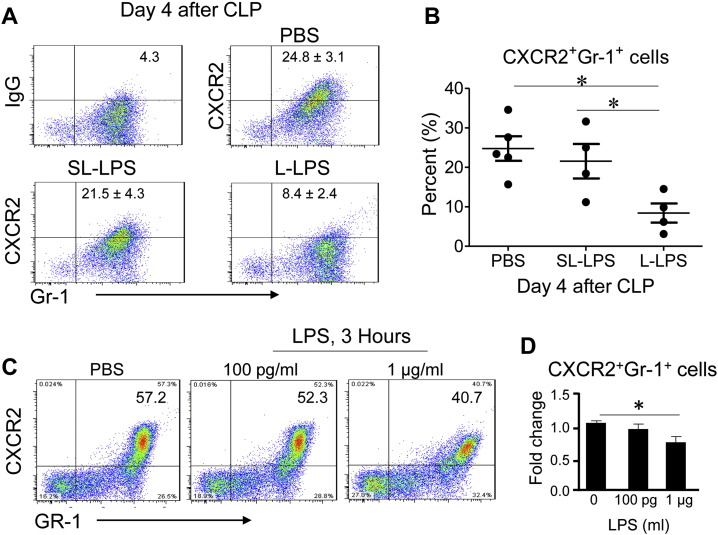
3.3. Differential Modulation of Neutrophil CRXCR2 Expression by Super-low and Low Dose LPS
Neutrophil infiltrations in vital organs are not only affected by the circulating levels of inflammatory chemokines such as CXCL1/KC, but also by the surface expression levels of chemokine receptors such as CXCR2. Indeed, CXCR2 was shown to play a critical role in CLP-related organ injury and mortality, and that tolerant dose LPS potently leads to a dramatic reduction in cellular levels of CXCR2 in neutrophils, correlating with reduced neutrophil infiltration in vital organs (Ness et al., 2003, Sabroe et al., 2003). We confirmed this finding as shown in Fig. 3. The levels of CXCR2 expression on peritoneal neutrophils harvested from CLP mice pre-conditioned with L-LPS were significantly reduced as compared to CLP mice pre-conditioned with PBS (Fig. 3A–B). In contrast, we observed that SL-LPS failed to significantly reduce the expression levels of CXCR2 (Fig. 3A–B). We further tested the in vitro modulation of CXCR2 by LPS. As shown in Fig. 3C–D, only L-LPS led to significant reduction of CXCR2 expression in cultured neutrophils as measured by flow cytometry. In sharp contrast, SL-LPS failed to significantly alter the expression levels of CXCR2. Coupled with the fact the SL-LPS pre-conditioning leads to a primed induction of CXCL1/KC, the CXCR2 ligand, these results may explain the increased infiltration of neutrophils in vital tissues such as liver and kidney in CLP mice pre-conditioned with SL-LPS.
Fig. 3.
Differential modulation of neutrophil migration by super-low and low dose LPS. (A) CXCR2 expression on the Gr-1+ cell population isolated from the peritoneal lavages of mice 4 d post-CLP was analyzed by flow cytometry. Mice were pre-conditioned with either 5 ng/kg (SL-LPS) or 50 μg/kg (L-LPS) 1 d prior to the CLP procedure. (B) Cumulative percentages of CXCR2+Gr-1+ cells from the peritoneal lavage 4 d post-CLP. Results were expressed as the mean ± SEM, N = 4–5 mice per group as indicated in the figure. Data were representative of 3 independently performed experiments. *p < 0.05. All mice used were male and 8–10 weeks old. (C) CXCR2 expression on neutrophils in vitro was analyzed by flow cytometry after stimulation of indicated LPS concentration. Bone marrow cells were stimulated with either PBS, 100 pg/mL or 1 μg/mL LPS for 3 h. The expression levels of CXCR2 on neutrophils were quantified through flow cytometry. *p < 0.05. (D) Cumulative fold change of CXCR2+Gr-1+ cells in vitro after stimulation with LPS. Data represent 3 independently performed experiments. Results were expressed as the mean ± SEM. *p < 0.05.
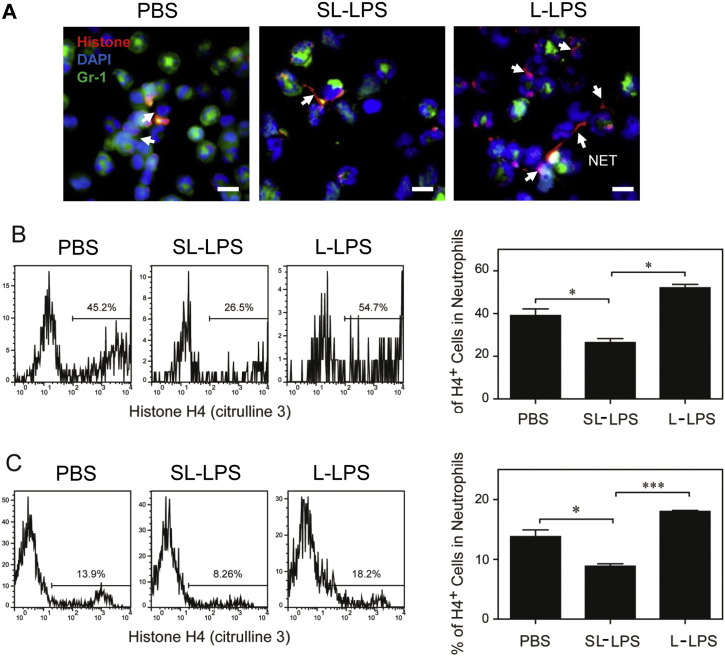
3.4. Reduction of Neutrophil NET Formation by Super-low LPS
In addition to the differential modulation of inflammatory responses, we also observed differential bacterial loads in the circulating blood of CLP mice pre-conditioned with super-low and low dose LPS. Our data suggest that the bacterial killing ability of neutrophils may be altered in mice pre-conditioned with varying dosages of LPS. Indeed, it has been reported that pre-conditioning with L-LPS facilitates neutrophil NET formation, thus favoring bacteria clearance (Landoni et al., 2012). NET is also implicated in the clearance of inflammatory mediators and the reduction of inflammation (Campbell et al., 2012). Thus, we focused our current study to examine the NET formation. First, we stained the citrullinated Histones in peritoneal neutrophils, a well-defined marker for NET formation. Consistent with previous findings, we observed elevated NET formation in peritoneal neutrophils from CLP mice pre-conditioned with L-LPS (Fig. 4A). In contrast, we observed that peritoneal neutrophils from CLP mice pre-conditioned with SL-LPS failed to have noticeable elevation in NET formation, as observed under microscopy.
Fig. 4.
Differential regulation of NET formation by super-low and low dose LPS. (A) The PMN isolated from the peritoneal lavage 1 d after CLP from the mice pre-conditioned with either PBS, low dose LPS (L-LPS), or super low dose LPS (L-LPS) were spun on the slides. Neutrophils were stained with the anti-mouse Gr-1 antibody followed by biotinylated anti-Rat Ig antibody and Streptavidin-FITC (green). NETs were stained with the anti-citrullinated Histone H3 antibody followed by the biotinylated goat anti-rabbit Ig antibody and Streptavidin-PE (red). DNA was stained with DAPI (blue). White arrow: NET. Scale bar = 30 μm. (B) C57 BL/6 mice were i.p. injected with super-low dose (5 ng/kg) or low dose (50 μg/kg) LPS. After 24 h, splenocytes were harvested and re-stimulated with PMA (50 nM) for 3 h. The levels of histone H4 (citrulline 3) within CD11b+/Ly6G+ neutrophils were analyzed by flow cytometry. The plotted data represent three independent experiments. (C) BM cells from C57 BL/6 mice were cultured with G-CSF (100 ng/mL) in the presence of either super-low dose (100 pg/mL) or low dose (1 μg/mL) LPS for 3 days, and fresh LPS was added to the cell cultures every 24 h. After stimulation with PMA (50 nM) for 3 h, the levels of histone H4 (citrulline 3) within CD11b+/Ly6G+ neutrophils were analyzed by flow cytometry. Quantitative data were shown as means ± SEM from three independently treated samples. Asterisks indicate statistically significant differences compared between indicated groups (*, p < 0.05; ***, p < 0.001).
To better examine and quantitate the neutrophil potential of generating NET, we used flow cytometry to measure neutrophils containing citrullinated Histones. As shown in Fig. 4B, significantly higher percentages of neutrophils harvested from mice pre-conditioned with L-LPS were positive for citrullinated Histones upon ex vivo PMA challenge, as compared to neutrophils from PBS pre-conditioned mice. In contrast, significantly lower percentages of neutrophils from mice pre-conditioned with SL-LPS were positive for citrullinated Histones upon ex vivo challenge with Phorbol myristate acetate (PMA).
We further examined NET formation in cultured neutrophils conditioned with varying dosages of LPS in vitro. Consistent with our in vivo and ex vivo data, we observed that G-CSF cultured neutrophils together with SL-LPS exhibited reduced NET formation triggered by PMA (Fig. 4C, D). In sharp contrast, cultured neutrophils with L-LPS had significantly elevated NET formation upon further PMA challenge. Taken together, our data reveal that SL-LPS pre-conditioning dampens, while L-LPS pre-conditioning potentiates the NET forming potential of neutrophils both in vitro and in vivo.
3.5. Differential Modulations of JNK and ERK by Super-low and Low Dose LPS Contribute to Dynamic Modulation of Neutrophil Function
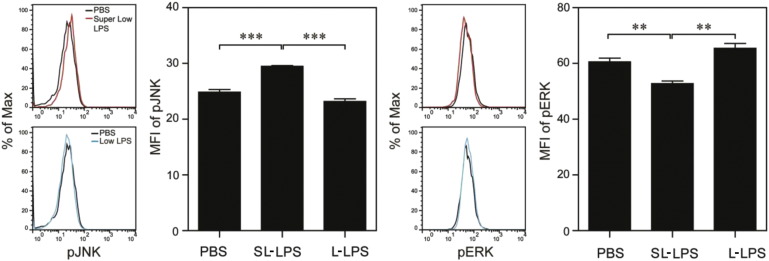
Next, we focused on examining the molecular mechanisms responsible for the opposite regulation of NET formation in neutrophils pre-conditioned by super-low and low dose LPS. Studies from other systems suggest that ERK is responsible for NET formation (Hakkim et al., 2011, Yoo et al., 2014), while recent studies suggest that the c-Jun N-terminal kinase (JNK) and ERK may form a mutually competitive signaling circuit (Arany et al., 2004, Bakal et al., 2008, Junttila et al., 2008, Shen et al., 2003). However, no published study is available with regard to the potential regulation and involvement of ERK/JNK in NET formation in neutrophils challenged with SL-LPS. Based on these clues, we first tested the activation status of JNK and ERK in neutrophils via flow cytometry. As shown in Fig. 5A and consistent with previous studies, we observed that neutrophils cultured with 1 μg/mL tolerant dose L-LPS had significantly elevated levels of p-ERK. In sharp contrast, we observed that SL-LPS selectively activated p-JNK accompanied. Counter-intuitively, we observed a significant reduction of p-ERK in cells treated with SL-LPS. Our data provides evidence that SL-LPS and L-LPS differentially skew the dynamic signaling circuit within neutrophils.
Fig. 5.
Differential modulations of JNK and ERK phosphorylation by super-low and low dose LPS contribute to dynamic regulation of NET formation. Bone marrow (BM) cells from C57 BL/6 mice were cultured with G-CSF (100 ng/mL) in the presence of either super-low dose (100 pg/mL) or low dose (1 μg/mL) LPS. The phosphorylation levels of JNK (T183/Y185) or ERK1/2 (T204/Y202) within CD11b+/Ly6G+ neutrophils were analyzed by flow cytometry after 3 d or 3 h respectively. Quantitative data were shown as means ± SEM from three independently treated samples. Asterisks indicate statistically significant differences compared between indicated groups (**, p < 0.01; ***, p < 0.001).
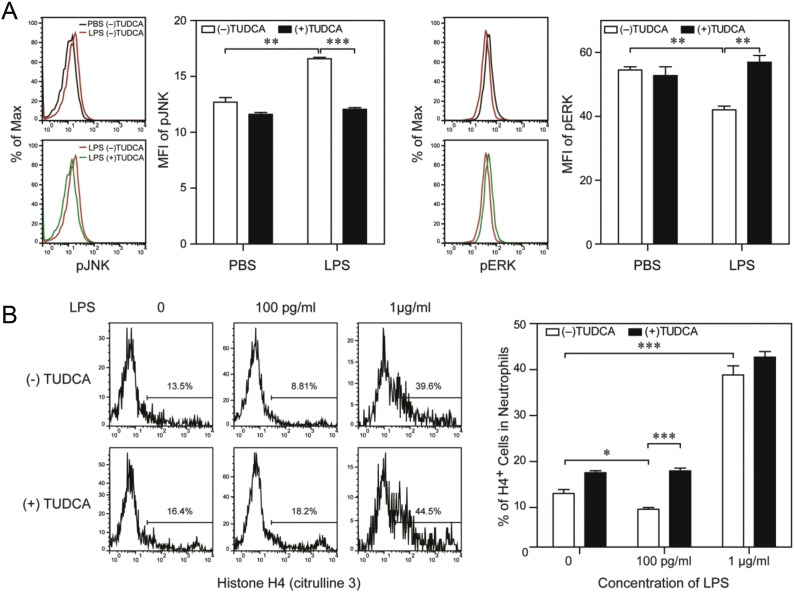
We further explored a potential intervention strategy in re-balancing neutrophil responses. Among published studies with existing small chemical compounds, we noticed the intriguing effects of Tauroursodeoxycholic acid (TUDCA), a bile acid derivative from the Chinese traditional medicine (Engin et al., 2013). TUDCA was shown to have remarkable beneficial effects in treating wound and injury, as well as other inflammatory conditions (Colak et al., 2008, Engin et al., 2013). At the biochemical level, TUDCA was shown to suppress JNK and activate ERK in other cellular systems (Engin et al., 2013, Schliess et al., 1997). We hypothesize that TUDCA may be able to intervene the harmful effects of SL-LPS in neutrophils. Indeed, we observed that co-stimulation of cultured neutrophils with TUDCA and SL-LPS restored ERK activation and suppressed JNK activation as compared to neutrophils treated with SL-LPS alone (Fig. 6A).
Fig. 6.
TUDCA restores ERK phosphorylation, NET formation in neutrophils treated with super-low dose LPS. (A) BM cells were cultured with G-CSF (100 ng/mL) in the presence of either super-low dose (100 pg/mL) or low dose (1 μg/mL) LPS. Some cells were also treated with TUDCA (0.5 mM). The phosphorylation levels of JNK (T183/Y185) or ERK1/2 (T204/Y202) within CD11b+/Ly6G+ neutrophils were analyzed by flow cytometry after 3 d or 3 h respectively. (B) BM cells were cultured with G-CSF (100 ng/mL) in the presence of either super-low dose (100 pg/mL) or low dose (1 μg/mL) LPS for 3 days, and some cells were also treated with TUDCA (0.5 mM). After stimulation with PMA (50 nM) for 3 h, the levels of histone H4 (citrulline 3) within CD11b+/Ly6G+ neutrophils were analyzed by flow cytometry. Quantitative data were shown as means ± SEM from three independently treated samples. Asterisks indicate statistically significant differences compared between the indicated groups (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Finally, given the promising effects of TUDCA on JNK and ERK in neutrophils, we further tested its effects on neutrophil NET formation. As shown in Fig. 6B, co-stimulation of neutrophils with TUDCA and SL-LPS restored the NET-forming ability of neutrophils as assessed by flow cytometry. Taken together, our data reveal that SL-LPS reduces neutrophil NET formation through suppressing ERK activation. Further, TUDCA may serve as an effective intervention compound in restoring neutrophil NET formation, through augmenting ERK activation in neutrophils challenged with SL-LPS.
4. Discussion
Collectively, our current study provides a first systematic analysis of in vivo relevance of dynamic innate pre-conditioning in the context of sepsis (Fig. 7). Mortality risks for sepsis vary dramatically in humans, and patient conditions prior to septic insult may be a critical contributing factor. However, previous studies solely examined the potential beneficial effects of “tolerant” dose L-LPS in animal models of sepsis (Kopanakis et al., 2013, Landoni et al., 2012). These studies are consistent with in vitro mechanistic studies that tolerant dose L-LPS tempers the pro-inflammatory cytokine storm (Morris and Li, 2012). Intriguingly, recent studies in humans and mice reveal the presence of subclinical super-low dose SL-LPS in circulation, potentially responsible for non-resolving chronic inflammation (Sunil et al., 2007, Terawaki et al., 2010). We and others reported that innate leukocytes can be dynamically pre-conditioned to opposite functional states in vitro, with super-low dose LPS selectively “primes” the expression of pro-inflammatory cytokines (Deng et al., 2013, Zhang and Morrison, 1993b).
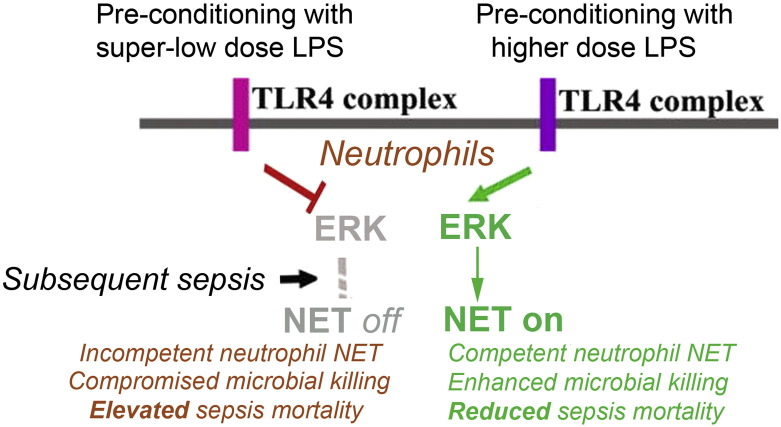
Fig. 7.
An illustrative diagram summarizing the dynamic programming of neutrophils by super-low dose LPS. Low dose LPS activates ERK and neutrophil NET formation. Together with other tolerant effects in other cells such as monocytes/macrophages, low dose LPS pre-conditioning offers protection to subsequent sepsis challenge. In contrast, this work reveals that super-low dose LPS significantly suppresses ERK and NET formation in neutrophils. This dynamic switch in signaling may exacerbate sepsis mortality. Restoration of this switch may serve as a potential future therapeutic target for the prevention or treatment of sepsis.
Despite the emerging significance of dynamic innate immunity preconditioning or “training”, no existing study is available to address the pre-conditioning effect of super-low LPS in the context of sepsis. We have aimed at an integrated study that compares and reconciles the pre-conditioning effects of both super-low and low dose LPS in septic mice. Consistent with previous studies, we found that CLP-mice pretreated with low dose LPS showed reduced mortality, reduced inflammatory responses, diminished injury of vital organs (liver, lung and kidney), and increased NET formation as well as effective bacterial clearance. In sharp contrast, we demonstrated that CLP-mice pre-conditioned with super-low dose LPS displayed increased mortality, elevated plasma levels of TNF-α and KC, increased neutrophil infiltration in vital tissues, attenuated ability of neutrophil NET formation and increased circulating levels of bacteria.
Our data provide much-needed evidence to support the pathological relevance of super-low dose endotoxemia, and extend the intriguing dynamics of innate priming and tolerance to an in vivo model of sepsis. Emerging in vitro studies reveal that innate monocytes may adopt distinct phenotypes by prior conditioning or “training” with varying dosages of innate stimulants (Deng et al., 2013, Netea et al., 2011, Quintin et al., 2012). For example, pre-conditioning with super-low dose LPS “primes” the expression of selected pro-inflammatory mediators such as TNF-α and IL-12, while suppressing the expression of iNOS (Hirohashi and Morrison, 1996). In contrast, pre-conditioning with elevated low dose LPS “tolerizes” the expression of selected genes (Hirohashi and Morrison, 1996). In consistent with these in vitro studies, we confirmed the “priming” and “tolerance” effects in vivo, and demonstrated that pre-conditioning with super-low dose LPS exacerbates the levels of TNF-α and KC in CLP-mice. Studies also suggest that the innate responses to varying dosages of LPS may not fit into the simple paradigm of priming and tolerance, and hint at more complex adaptation profiles (Foster et al., 2007). On a separate note, the adaptation phenomenon to varying dosages of LPS is different as compared to the traditional M1/M2 concept, in which distinct agonists are required for the differential activation of M1 (by IFNγ) or M2 (through IL-4) (Martinez et al., 2008). Similar adaptation paradigm to varying signal strength may also exists in other immune cell types. For instance, although T helper cells are well known to adopt different functional states (Th1, Th2, Th17, Treg) when challenged with distinct combination of cytokines (Hong et al., 2011), emerging recent study reported that T helper cell differentiation can also be achieved through varying the signal strength of the TCR signal (Salmond et al., 2014, van Panhuys et al., 2014). Collectively, these studies draw serious attention to the issue of dynamic responses of immune cells when challenged with varying dosages of the same stimulant.
Our study reveals additional aspects of innate pre-conditioning and adaptation in neutrophils. Neutrophil is a key innate leukocyte, and its modulation bears critical relevance to the outcome of sepsis (Stearns-Kurosawa et al., 2011). Neutrophils have multiple functions that include cytokine expression, degranulation, suppression of T cells, and NET formation (Mayadas et al., 2014). Although the dynamic pre-conditioning of monocytes by varying dosages of LPS is recently noticed, no information is available regarding the dynamic responses of neutrophils in this context. Our current study fills this critical void and reveals intriguing dynamics of neutrophils when challenged with varying dosages of LPS. We particularly focused on neutrophil NET and revealed opposing behaviors of NET generation in neutrophils pre-conditioned with super-low and low dose LPS. Our current work offers a glimpse of neutrophil dynamics pre-conditioned with varying dosages of LPS, and begets future systems studies about the complex programming of neutrophils in health and disease. Furthermore, or data suggest that differential pre-conditioning of neutrophils may be harnessed for the effective treatment of sepsis.
In terms of underlying molecular mechanisms, our study eludes to the functional significance of mutually inhibitory circuits in fine-tuning leukocyte responses. Traditional studies overly simplified the signaling systems into cascades of events that by no means can reconcile the complex dynamics of living systems. Instead, emerging studies from other systems emphasize the necessity of mutually inhibitory circuits required for complex cellular outcomes (Tyson et al., 2001). This may enable organisms highly coordinated adaptation to changing environments. We reported dynamic circuits that are responsible for the priming and tolerance phenotypes in monocytes/macrophages challenged with varying dosages of LPS (Fu et al., 2012, Maitra et al., 2012). Similarly-wired circuits also exist in T Helper cells, responsible for the dynamic differentiation into a plethora of T helper cells (Hong et al., 2011). JNK and ERK may constitute a mutually inhibitory circuits based on previous studies (Arany et al., 2004, Bakal et al., 2008, Junttila et al., 2008, Shen et al., 2003). In this current study, we observed that super-low dose LPS selectively suppresses ERK and favors JNK activation in neutrophils. Furthermore, suppression of ERK by super-low dose LPS correlates with suppressed neutrophil NET formation and reduced bacterial killing activity. In contrast, higher dose LPS may flip this circuit, induces ERK and NET formation. Mechanisms responsible for the competitive regulation of ERK and JNK are not clear, and warrant future studies. One possible scenario may be the competitive regulation of opposing MAP kinase phosphatases (MKPs). A recent study indicates that the varying signal strength of CD40 ligand may differentially activate either MKP1 (a selective JNK and p38 phosphatase) or MKP3 (a selective EKR phosphatase) (Srivastava et al., 2011). Further comprehension of the dynamic circuits within innate leukocytes is clearly warranted. Capitalizing on this initial finding, we attempted at re-balancing the skewed circuit in neutrophils with a promising drug TUDCA. We demonstrate that TUDCA can restore ERK activation in the presence of super-low dose LPS, as well as the NET formation in neutrophils. This attempt raises an intriguing potential for the future treatment of sepsis through re-balancing network dynamics in leukocytes. Supporting this concept, a recent study suggests that pre-conditioning with an un-conventional TLR2 agonist may render a broader protection toward viral infection (Shirey et al., 2013).
Taken together, our data reveal dynamics of innate pre-conditioning by SL-LPS in the context of sepsis, and provide a mechanism for differential modulation of neutrophil NET formation by SL-LPS. Our work also serves as a prelude to a daunting task in further defining the emerging concept of innate immunity programming and memory in health and disease. At the translational level, our study suggests that strategies aimed at re-balancing the innate immunity dynamics may hold promise in the prevention and/or treatment of severe sepsis.
Declaration of Interests
The authors declare no conflicts of financial interests.
Author Contributions
K.C., S. G., R.Y., N.D., and Z.U. performed the experiments. L.L. conceived and designed the study. K.C., S,G. and L.L. analyzed the data and wrote the manuscript.
Funding
This work is supported in part by grants from the National Institute of Health R01 HL115835 and R56 AI108264 to L.L. The funding agency has no role in the actual experimental design, analysis, or writing of this manuscript.
References
- Ancuta P., Kamat A., Kunstman K.J., Kim E.Y., Autissier P., Wurcel A., Zaman T., Stone D., Mefford M., Morgello S. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany I., Megyesi J.K., Kaneto H., Tanaka S., Safirstein R.L. Activation of ERK or inhibition of JNK ameliorates H(2)O(2) cytotoxicity in mouse renal proximal tubule cells. Kidney Int. 2004;65:1231–1239. doi: 10.1111/j.1523-1755.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- Bakal C., Linding R., Llense F., Heffern E., Martin-Blanco E., Pawson T., Perrimon N. Phosphorylation networks regulating JNK activity in diverse genetic backgrounds. Science. 2008;322:453–456. doi: 10.1126/science.1158739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A.M., Kashgarian M., Shlomchik M.J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 2012;4:1–7. doi: 10.1126/scitranslmed.3004801. 157ra141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Colak A., Kelten B., Sagmanligil A., Akdemir O., Karaoglan A., Sahan E., Celik O., Barut S. Tauroursodeoxycholic acid and secondary damage after spinal cord injury in rats. J. Clin. Neurosci. 2008;15:665–671. doi: 10.1016/j.jocn.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Deng H., Maitra U., Morris M., Li L. Molecular mechanism responsible for the priming of macrophage activation. J. Biol. Chem. 2013;288:3897–3906. doi: 10.1074/jbc.M112.424390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F., Yermalovich A., Nguyen T., Hummasti S., Fu W., Eizirik D.L., Mathis D., Hotamisligil G.S. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci. Transl. Med. 2013;5:1–14. doi: 10.1126/scitranslmed.3006534. 211ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster S.L., Hargreaves D.C., Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Fu Y., Glaros T., Zhu M., Wang P., Wu Z., Tyson J.J., Li L., Xing J. Network topologies and dynamics leading to endotoxin tolerance and priming in innate immune cells. PLoS Comput. Biol. 2012;8:e1002526. doi: 10.1371/journal.pcbi.1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godshall C.J., Scott M.J., Peyton J.C., Gardner S.A., Cheadle W.G. Genetic background determines susceptibility during murine septic peritonitis. J. Surg. Res. 2002;102:45–49. doi: 10.1006/jsre.2001.6319. [DOI] [PubMed] [Google Scholar]
- Goto T., Eden S., Nordenstam G., Sundh V., Svanborg-Eden C., Mattsby-Baltzer I. Endotoxin levels in sera of elderly individuals. Clin. Diagn. Lab. Immunol. 1994;1:684–688. doi: 10.1128/cdli.1.6.684-688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkim A., Fuchs T.A., Martinez N.E., Hess S., Prinz H., Zychlinsky A., Waldmann H. Activation of the Raf–MEK–ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- Henricson B.E., Manthey C.L., Perera P.Y., Hamilton T.A., Vogel S.N. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect. Immun. 1993;61:2325–2333. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi N., Morrison D.C. Low-dose lipopolysaccharide (LPS) pretreatment of mouse macrophages modulates LPS-dependent interleukin-6 production in vitro. Infect. Immun. 1996;64:1011–1015. doi: 10.1128/iai.64.3.1011-1015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T., X., J., Li L., Tyson J. A mathematical model for the reciprocal differentiation of T helper 17 cells and induced regulatory T cells. PLoS Comput. Biol. 2011;7:1–13. doi: 10.1371/journal.pcbi.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto R., Hartung T., McCall C., Li L. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor-associated kinase. J. Immunol. 2002;168:6136–6141. doi: 10.4049/jimmunol.168.12.6136. [DOI] [PubMed] [Google Scholar]
- Junttila M.R., Li S.P., Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. TLR signaling. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kopanakis K., Tzepi I.M., Pistiki A., Carrer D.P., Netea M.G., Georgitsi M., Lymperi M., Droggiti D.I., Liakakos T., Machairas A. Pre-treatment with low-dose endotoxin prolongs survival from experimental lethal endotoxic shock: benefit for lethal peritonitis by Escherichia coli. Cytokine. 2013;62:382–388. doi: 10.1016/j.cyto.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Landoni V.I., Chiarella P., Martire-Greco D., Schierloh P., van-Rooijen N., Rearte B., Palermo M.S., Isturiz M.A., Fernandez G.C. Tolerance to lipopolysaccharide promotes an enhanced neutrophil extracellular traps formation leading to a more efficient bacterial clearance in mice. Clin. Exp. Immunol. 2012;168:153–163. doi: 10.1111/j.1365-2249.2012.04560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Cousart S., Hu J., McCall C.E. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J. Biol. Chem. 2000;275:23340–23345. doi: 10.1074/jbc.M001950200. [DOI] [PubMed] [Google Scholar]
- Lira F.S., Rosa J.C., Pimentel G.D., Souza H.A., Caperuto E.C., Carnevali L.C., Jr., Seelaender M., Damaso A.R., Oyama L.M., de Mello M.T. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 2010;9:82. doi: 10.1186/1476-511X-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Gan L., Chang S., Li L. Low-dose endotoxin induces inflammation by selectively removing nuclear receptors and activating CCAAT/enhancer-binding protein {delta} J. Immunol. 2011;186:4467–4473. doi: 10.4049/jimmunol.1003300. [DOI] [PubMed] [Google Scholar]
- Maitra U., Deng H., Glaros T., Baker B., Capelluto D.G., Li Z., Li L. Molecular mechanisms responsible for the selective and low-grade induction of proinflammatory mediators in murine macrophages by lipopolysaccharide. J. Immunol. 2012;189:1014–1023. doi: 10.4049/jimmunol.1200857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F.O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev A.E., Lentschat A., Wahl L.M., Golenbock D.T., Vogel S.N. Dysregulation of LPS-induced toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 2002;169:5209–5216. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- Meng X., Ao L., Brown J.M., Meldrum D.R., Sheridan B.C., Cain B.S., Banerjee A., Harken A.H. LPS induces late cardiac functional protection against ischemia independent of cardiac and circulating TNF-alpha. Am. J. Physiol. 1997;273:H1894-1902. doi: 10.1152/ajpheart.1997.273.4.H1894. [DOI] [PubMed] [Google Scholar]
- Meng W., Paunel-Gorgulu A., Flohe S., Hoffmann A., Witte I., Mackenzie C., Baldus S.E., Windolf J., Logters T.T. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit. Care. 2012;16:R137. doi: 10.1186/cc11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M., Li L. Molecular mechanisms and pathological consequences of endotoxin tolerance and priming. Arch. Immunol. Ther. Exp. (Warsz) 2012;60:13–18. doi: 10.1007/s00005-011-0155-9. [DOI] [PubMed] [Google Scholar]
- Morris M., Gilliams E., Li L. Innate immune programming by endotoxin and its pathological consequences. Front. Immunol. 2015;5(680):1–8. doi: 10.3389/fimmu.2014.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness T.L., Hogaboam C.M., Strieter R.M., Kunkel S.L. Immunomodulatory role of CXCR2 during experimental septic peritonitis. J. Immunol. 2003;171:3775–3784. doi: 10.4049/jimmunol.171.7.3775. [DOI] [PubMed] [Google Scholar]
- Netea M.G., Quintin J., van der Meer J.W. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Quintin J., Saeed S., Martens J.H., Giamarellos-Bourboulis E.J., Ifrim D.C., Logie C., Jacobs L., Jansen T., Kullberg B.J., Wijmenga C. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocksen D., Koch B., Sandstrom T., Bucht A. Lung effects during a generalized Shwartzman reaction and therapeutic intervention with dexamethasone or vitamin E. Shock. 2004;22:482–490. doi: 10.1097/01.shk.0000142254.38630.36. [DOI] [PubMed] [Google Scholar]
- Sabroe I., Prince L.R., Jones E.C., Horsburgh M.J., Foster S.J., Vogel S.N., Dower S.K., Whyte M.K. Selective roles for toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J. Immunol. 2003;170:5268–5275. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- Salmond R.J., Brownlie R.J., Morrison V.L., Zamoyska R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat. Immunol. 2014;15:875–883. doi: 10.1038/ni.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer C., Janko C., Munoz L.E., Zhao Y., Kienhofer D., Frey B., Lell M., Manger B., Rech J., Naschberger E. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- Schliess F., Kurz A.K., vom Dahl S., Haussinger D. Mitogen-activated protein kinases mediate the stimulation of bile acid secretion by tauroursodeoxycholate in rat liver. Gastroenterology. 1997;113:1306–1314. doi: 10.1053/gast.1997.v113.pm9322526. [DOI] [PubMed] [Google Scholar]
- Shen Y.H., Godlewski J., Zhu J., Sathyanarayana P., Leaner V., Birrer M.J., Rana A., Tzivion G. Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J. Biol. Chem. 2003;278:26715–26721. doi: 10.1074/jbc.M303264200. [DOI] [PubMed] [Google Scholar]
- Shirey K.A., Lai W., Scott A.J., Lipsky M., Mistry P., Pletneva L.M., Karp C.L., McAlees J., Gioannini T.L., Weiss J. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N., Sudan R., Saha B. CD40-modulated dual-specificity phosphatases MAPK phosphatase (MKP)-1 and MKP-3 reciprocally regulate Leishmania major infection. J. Immunol. 2011;186:5863–5872. doi: 10.4049/jimmunol.1003957. [DOI] [PubMed] [Google Scholar]
- Stearns-Kurosawa D.J., Osuchowski M.F., Valentine C., Kurosawa S., Remick D.G. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil V.R., Patel K.J., Nilsen-Hamilton M., Heck D.E., Laskin J.D., Laskin D.L. Acute endotoxemia is associated with upregulation of lipocalin 24p3/Lcn2 in lung and liver. Exp. Mol. Pathol. 2007;83:177–187. doi: 10.1016/j.yexmp.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto C.C., Kwan B.C., Chow K.M., Lai K.B., Chung K.Y., Leung C.B., Li P.K. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2008;3:431–436. doi: 10.2215/CJN.03600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancevski I., Nairz M., Duwensee K., Auer K., Schroll A., Heim C., Feistritzer C., Hoefer J., Gerner R.R., Moschen A.R. Fibrates ameliorate the course of bacterial sepsis by promoting neutrophil recruitment via CXCR2. EMBO Mol. Med. 2014;6:810–820. doi: 10.1002/emmm.201303415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki H., Yokoyama K., Yamada Y., Maruyama Y., Iida R., Hanaoka K., Yamamoto H., Obata T., Hosoya T. Low-grade endotoxemia contributes to chronic inflammation in hemodialysis patients: examination with a novel lipopolysaccharide detection method. Ther. Apher. Dial. 2010;14:477–482. doi: 10.1111/j.1744-9987.2010.00815.x. [DOI] [PubMed] [Google Scholar]
- Toscano M.G., Ganea D., Gamero A.M. Cecal ligation puncture procedure. JoVE. 2011;51:e2860. doi: 10.3791/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson J.J., Chen K., Novak B. Network dynamics and cell physiology. Nat. Rev. Mol. Cell Biol. 2001;2:908–916. doi: 10.1038/35103078. [DOI] [PubMed] [Google Scholar]
- van Panhuys N., Klauschen F., Germain R.N. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization in vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.C., Klein R.D., Wahl W.L., Alarcon W.H., Garg R.J., Remick D.G., Su G.L. Tissue coexpression of LBP and CD14 mRNA in a mouse model of sepsis. J. Surg. Res. 1998;76:67–73. doi: 10.1006/jsre.1998.5290. [DOI] [PubMed] [Google Scholar]
- West M.A., Heagy W. Endotoxin tolerance: a review. Crit. Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- Wiedermann C.J., Kiechl S., Dunzendorfer S., Schratzberger P., Egger G., Oberhollenzer F., Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J. Am. Coll. Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- Yoo D.G., Winn M., Pang L., Moskowitz S.M., Malech H.L., Leto T.L., Rada B. Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation. J. Immunol. 2014;192:4728–4738. doi: 10.4049/jimmunol.1301589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Morrison D.C. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J. Exp. Med. 1993;177:511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Morrison D.C. Lipopolysaccharide structure–function relationship in activation versus reprogramming of mouse peritoneal macrophages. J. Leukoc. Biol. 1993;54:444–450. doi: 10.1002/jlb.54.5.444. [DOI] [PubMed] [Google Scholar]