Abstract
While reward-dependent facilitation of phasic dopamine signaling is well documented at both the cell bodies and terminals, little is known regarding fast dopamine transmission under aversive conditions. Exposure to aggressive confrontation is extremely aversive and stressful for many species including rats. The present study used fast-scan cyclic voltammetry and multiunit recording to determine if aggressive encounters and subsequent social defeat affect burst firing of ventral tegmental area (VTA) dopamine neurons and accumbal dopamine transients in defeated rats. Significant increases in the frequency of transient dopamine release were observed during interactions with an aggressive rat but not with a familiar cage mate. In agreement with voltammetric results, significant increases in burst frequency were detected in the VTA dopamine firing patterns during an aggressive confrontation; however, the number of spikes per burst remained unchanged. We found that neurons with lower burst rates under homecage conditions did not switch from non-bursting to bursting types, while neurons with higher burst levels showed amplified increases in bursting. This study demonstrates for the first time that aggressive confrontations in defeated rats are associated with increases in phasic dopamine transmission in the mesolimbic pathway.
Keywords: social defeat, stress, voltammetry, electrophysiology, dopamine
The mesolimbic dopamine pathway, which originates in the ventral tegmental area (VTA) and projects to terminal regions such as the nucleus accumbens, has been implicated in both natural and drug reinforcement as well as reward (Wise, 2006). Electrophysiological studies revealed that some abused drugs including alcohol and nicotine activate firing of VTA dopamine neurons in rats (Diana et al., 1993; Schilstrom et al., 2003). Positive reinforcers such as juice (Schultz et al., 1997) or cues that predict the availability of reward (Schultz, 1998) reliably elicit excitatory phasic responses in the VTA of monkeys. Moreover, during intracranial self-stimulation (ICSS), animals self-administer electrical current pulses directly to neurons in the VTA that activate dopaminergic impulse flow (Wise, 1996; Garris et al., 1999; Cheer et al., 2007a). The hypothetical link between dopaminergic activity in the VTA and positive reinforcement is frequently reiterated, although considerable evidence prompts a more qualified and differentiated view of the behavioral functions of mesolimbic dopamine activity (Berridge, 2007; Robbins and Everitt, 2007; Salamone et al., 2007).
There is convincing evidence that in contrast to positive reinforcers, noxious aversive stimuli induce a short-term, phasic inhibition of midbrain dopaminergic neurons. For example, Ungless and colleagues found that dopamine neurons are inhibited by a foot pinch in anesthetized rats (Ungless et al., 2004). Similar results were obtained during the application of foot shock (Coizet et al., 2006). At the same time, restraint which could be considered an aversive event increased the mean firing rate in all dopamine neurons, but preferentially increased burst firing in neurons with higher burst rates in the VTA of awake rats (Anstrom and Woodward, 2005). Therefore, it is important to determine whether electrophysiological activity patterns of VTA dopamine neurons are changed in same fashion under conditions which are aversive in character but different from temporally defined noxious stimuli (i.e. foot shock, pinch).
Subsecond fluctuations in striatal dopamine concentrations, or dopamine transients, are thought to result from burst firing of dopamine neurons and can be detected with fast-scan cyclic voltammetry (FSCV) (Rebec et al., 1997; Phillips et al., 2003; Robinson et al., 2003). It has been shown that various drugs with addiction liability including cocaine, alcohol and nicotine enhance dopamine transients in rat nucleus accumbens (Cheer et al., 2007b), which parallels increases in neuronal VTA burst firing (Diana et al., 1993; Schilstrom et al., 2003). The increased rate of dopamine transients in terminal regions was associated with the presentation, seeking, and anticipation of reward (Robinson et al., 2001; Phillips et al., 2003; Day et al., 2007; Cheer et al., 2007a,b). For example, enhancement in accumbal dopamine transients was tightly linked with a rat’s operant responses that were reinforced by intravenous cocaine as well as with cues predicting rewards including cocaine (Phillips et al., 2003), sucrose administration (Roitman et al., 2004; Day et al., 2007), and ICSS (Cheer et al., 2007a). An increase in the frequency of dopamine transients was also observed in the nucleus accumbens of male rats during the introduction of receptive females and subsequent appetitive sexual behaviors (Robinson et al., 2001). However, it is unknown whether striatal dopamine transient activity is changed by experiencing and reacting to aversive stimuli. Therefore, while reward-dependent facilitation of phasic dopamine signaling is well documented at both the cell bodies and terminals, little is known regarding fastdopamine transmission under relatively long-lasting aversive conditions in conscious animals.
Exposure to an aggressive confrontation even under controlled laboratory conditions is extremely aversive and stressful for many species including rats (Miczek et al., 2004, 2008). This state is physiologically and mentally more complex and long-lasting than that induced by shorter noxious stimuli such as a foot shock or pinch. During defeat in a brief aggressive episode a very large sympathetic and adrenocortical activation is evident (Tornatzky and Miczek, 1993; Miczek, 1999). While sympathetic and glucocorticoid activation are initially evident in both the winner and loser animal, they are more persistent in the defeated rat than in the winning opponent (Schuurman, 1980; Covington, III et al., 2005). In this study we have employed FSCV and multiunit recording techniques in freely moving rats to evaluate the effect of defeat stress on phasic dopamine transmission in the nucleus accumbens and VTA, respectively.
EXPERIMENTAL PPROCEDURES
Animals
Male Sprague-Dawley rats (300–350 g; Charles River, Raleigh, NC) were housed on a 12:12 light/dark cycle with food and water available ad libitum. Rats were group housed before surgery and singly housed after surgery. All protocols were approved by the Institutional Animal Care and Use Committee at Wake Forest University. To determine how exposure to aggression by the resident rat affected dopaminergic transmission, all experimental procedures were performed on the intruder. Resident pairs of male and female rats (n = 4 pairs) were housed for at least 45 days, and the aggressive behavior by the male resident was established in 5–6 confrontations with a male intruder, following earlier procedures (Miczek, 1979). Eighteen male rats, initially weighing 350–375 g and designated as intruders, were randomly assigned to one of three groups. The first group (n=8) had electrode arrays implanted in the midbrain to record neuronal activity during confrontation with an aggressive resident. The second and third groups (n = 5 each) were prepared for voltammetric recordings during confrontation with an aggressive resident rat or a familiar cage mate.
Defensive and submissive behavior in response to aggression
The male intruder was placed into the home cage of the resident male rat. For the duration of the confrontation between the resident and intruder, the resident female was removed. Importantly to notice, the intruder and aggressive resident were then introduced to each other for the first time. The latency of the first attack bite by the resident was within 1 min. The resident displayed the full repertoire of aggressive behavior which began with the naso-nasal and anogenital contact, aggressive allogrooming, sideways threats, pursuits and culminated in attack bites directed at the neck of the intruder. The intruder reacted with defensive upright postures to the sideways threats, assumed the submissive supine posture in reaction to the full aggressive posture of the resident, and displayed a crouch posture, when escape was not possible. These salient acts, postures and movements are illustrated and operationally defined, as previously established (Miczek, 1974; Miczek and de Boer, 2005).
Separate sets of experiments were carried out on male rats which interacted with each other without any confrontation. These rats were housed as cage mates for at least four weeks before testing. The recordings were performed in the shared home cage before (5 min) and during (5 min) the presentation of the cage mate. Behavioral elements such as allogrooming, biting attacks, aggressive postures and pursuit were not observed in the duration of interaction between these animals.
Electrophysiological recordings
After the three-day acclimation period, animals were handled daily for at least one week to habituate them to experimental manipulation and then implanted with microwire arrays (Biographics, Inc., Winston-Salem, NC). The rats were anesthetized with isoflurane, and a prophylactic dose of an antibiotic (genomyacin) was administered prior to surgery. Body temperature was maintained between 35–37°C and aseptic technique observed. After placing the rat in a stereotaxic apparatus, the scalp was shaved, swabbed with iodine and a central incision made to expose the skull. Small holes were drilled in the skull and two arrays of eight, stainless steel, Teflon-coated microwires (45–62 μm in diameter) were lowered bilaterally into the midbrain using the following coordinates: AP, + 3.5 mm from Lambda; ML ±1.8; DV, −8.3 from skull surface. Uncoated stainless steel ground wires were positioned 2–3 mm ventral to the cortical surface and wrapped around skull screws. The recording head stage was then secured to the cranium with dental cement using skull screws as anchors.
One day prior to recording sessions, a recording headset with sixteen unity-gain field effect transistors and cable were attached to the animal’s head stage to habituate it to being tethered to the recording system. Afterward, distributed neural activity was recorded in the midbrain using previously established protocols (Chang et al., 1994; Janak et al., 1999; Anstrom and Woodward, 2005).
On test days, animals were transported from the housing quarters into the experimental room. For each session, the home cage was placed on a table top. For the first segment of each recording session, animals remained in their home cage and neural activity was recorded for 15 minutes. This period is designated as “Home cage” activity. Animals were then placed in the resident’s cage on the same table while the resident was not present for another 5 minutes. The signals recorded during this period are designated as “Resident cage” activity. The resident was then returned to his home cage with the intruder. This period is designated as “Confrontation.” The duration of this period was at least five minutes and always included an aggressive confrontation (the first attack was observed within 1 min). Finally, the intruder was placed back into its home cage and neural activity was recorded for an extra five minutes (“Post-confrontation”).
Single neurons were isolated from background activity based on waveform criteria using movable windows based parameters (MNAP system, Plexon, Dallas, Texas) in multiple windows-based modules. Electric signals were amplified and filtered (0.5 and 5 kHz 3 dB cut-offs) via software control. A continuous flow of neural signals that were digitized (50 kHz per channel) by an array of digital signal processing units was sent to a computer where digital records were assigned a timestamp in reference to the start of the behavioral session. Temporal records of extracellular spike activity were superimposed on behavioral data using Magsort software (Biographics, Inc., Winston-Salem, NC) with a time resolution of 3 msec.
In this study, 137 neurons were recorded across 7 animals. One animal was excluded from the study because of electrode misplacement. Before including neural signals in a data set, autocorrelograms were calculated on all records to assure that single neurons were being recorded and were separated from background noise levels. Putative GABAergic and dopaminergic neurons recorded with microwires in awake rats were identified on the basis of electrophysiological parameters used in previous studies which include extracellular waveform shape and, firing rates and burst properties (Grace, 1988; Richards et al., 1997; Steffensen et al., 1998; Anstrom and Woodward, 2005; Grace et al., 2007). The extracellular spike waveform was calculated by measuring the length from the point at which the depolarization phase of the curve left a flat baseline to when the hyperpolarization phase returned to baseline and then comparing it to the length of a window of known duration.
Average firing rates and burst characteristics for midbrain neurons were determined for each experimental period (Home Cage, Resident Cage, Confrontation and Post) using a burst analysis (Nex software). For putative dopamine neurons, a burst was defined by a minimum of two successive spikes having an initial ISI less than or equal to 80 ms and ending with an ISI greater than 160 msec following previous convention (Grace and Bunney, 1984). These parameters were used to calculate percentage of spikes found in bursts, burst rates, and number of spikes in bursts.
For putative GABA neurons, the surprise method (Aldridge and Gilman, 1991) was used to calculate burst characteristics within each experimental period (Nex software). This method uses an algorithm that assumes that a series of at least three short-interval spikes, or a burst, would be evenly distributed within a random series of spikes. The method calculates how unlikely it would be to find bursts in a particular pattern and frequency within a collected data set. We have used a surprise value of 3 which has been determined to accurately identify burst firing within the basal ganglia (Wichmann and Soares, 2006). Significant differences in firing rates and burst characteristics in putative dopamine and GABA neurons were analyzed using one-way, repeated measures ANOVA with a Holm-Sidak post-hoc comparison.
Finally, previous studies have demonstrated that dopamine neurons with higher basal levels of burst firing have enhanced responses (increases in burst firing) to glutamatergic stimulation (Overton et al., 1996) or during restraint (Anstrom and Woodward, 2005). Putative dopamine neurons were subcategorized according to burst properties under resting conditions as in previous studies (Overton et al., 1996; Hyland et al., 2002; Anstrom and Woodward, 2005; Anstrom et al., 2007) in order to determine if different levels of basal activity could influence neural responses. To determine if changes in firing rate or burst levels during responses to aggression was influenced by different characteristics between dopamine neurons, a two-way, repeated measures ANOVA analysis with Holm-Sidak post-hoc comparisons was performed.
Histological localization of recording electrodes
To visualize electrode placement animals were deeply anaesthetized with pentobarbital (100 mg/kg) administered intraperitoneally. A 12-second anodal current of 10–15 μA was passed through at least four electrodes of an eight-electrode array. The animals were then perfused transcardially with a 0.9% saline wash followed by a 2% potassium ferrocyanide in 10% buffered formalin fixative to create a Prussian Blue spot marking electrode placement. The brains were removed and cryoprotected overnight in a 20% sucrose in 10% formalin solution at 4°C. 50 μm coronal sections were cut on a sliding microtome. Free-floating sections with Prussian blue marks were collected and stored serially in 0.1M phosphate buffer, pH 7.2. Sections were stained with neutral red, serially mounted on gelatin-coated slides, passed through an alcohol/xylene series and cover-slipped with Permount. Analysis and photomicroscopy were conducted using an Olympus BX60 microscope and a digital camera.
All recording sites were localized to the VTA as determined by immunohistochemical methods. Electrode arrays were arranged in either a two by three by three or a two by four configuration. These arrays spanned approximately 1 mm in an anterior-posterior dimension and approximately 0.5 mm in a medial-lateral dimension. Electrodes were localized from −5.2 to −6.0 mm AP; ± 0.3 to ± 0.5 mm ML and −7.5 to −7.8 mm DV from bregma. As in previous studies (Anstrom and Woodward, 2005), there were no clear regional differences in electrophysiological properties. Perhaps with greater numbers of neurons, significant differences in firing parameters corresponding to anatomical location would emerge.
Voltammetric recordings
Implantation and voltammetric recordings were carried out as previously described (Budygin et al., 2000; 2007, Phillips et al., 2003). Rats were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine hydrochloride (20 mg/kg, i.p.) and placed in a stereotaxic frame. A guide cannula (Bioanalytical Systems, West Lafayette, IL) was positioned above the nucleus accumbens core (+1.7 mm AP, +1.7 mm ML, with its tip −2.5 mm DV; all coordinates relative to bregma). An Ag/AgCl reference electrode was placed contralateral to the guide cannula. All items were secured to the scull with screws and cranioplastic cement. A detachable micromanipulator containing a carbon fiber electrode (80–130 μm length cylinder, T-650; Amoco, Greenville, SC) was inserted into the guide cannula, and the electrode was lowered into the nucleus accumbens core. A bipolar stimulating electrode was lowered to the VTA ipsilateral to the guide cannula at 5.2 mm posterior and 1.0 mm lateral to bregma. The stimulating electrode depth was optimized to evoke dopamine release in the nucleus accumbens (24 rectangular pulses, 60 Hz, 120 μA, 2 ms/phase, biphasic), monitored using a carbon fiber microelectrode. The stimulating electrode was then secured in place with cranioplastic cement, and the micromanipulator was removed and replaced with a stylet. The rats were individually housed and allowed to recover for 48 hrs.
On the day of the experiment, the intruder rat, while in its own home cage, was placed into a faradeic enclosure of a voltammetric system and a new carbon fiber electrode was lowered into the nucleus accumbens core. The carbon fiber and Ag /AgCl electrodes were connected to a head-mounted voltammetric amplifier attached to a commutator (Med Associates, St Albans, VT) located at the top of the test chamber. Voltammetric recordings were made every 100 ms by applying a triangular waveform (−0.4 to +1.3 V, 400 V/s), stored in a PC using software written in LabVIEW (National Instruments, Austin, TX). Dopamine release was optimized within the nucleus accumbens core by adjusting the vertical position of the working electrode (0.1 mm increments). All recording sites had electrically evoked (24 biphasic pulses, 60 Hz, 120 μA, 2 ms/phase) dopamine release of at least 0.5 μM. To optimize recording of transients, the electrode position was further adjusted to a location where spontaneous dopamine transients occurred with a frequency of > 1 per min (Cheer et al., 2007). The electrode was then locked in place, and the voltammetric waveform was applied. Following equilibration of the electrode, a 5 min recording was made before the rat was placed into the empty resident cage for the next recording session. In the next 5 min session, an aggressive resident confronted the intruder rat, after which the intruder was returned to its home cage. After the experiment, the VTA was again electrically stimulated to ensure that viable dopamine release could still be detected at the carbon fiber electrode. The carbon fiber electrode was then removed, cleaned, and calibrated with 1 μM dopamine in a flow injection analysis system (Budygin, 2007).
Spontaneous dopamine transients were identified using an algorithm written in LabVIEW (Robinson et al., 2002; Heien et al., 2005). The subtracted cyclic voltammogram for dopamine was chosen from the electrochemical response to the electrical stimulation. The software automatically scaled the current amplitude at the dopamine oxidation peak to the chosen template, subtracting the average of 10 scans before the scan under evaluation. The mean squared error between the two voltammograms was calculated, the inverse of this quantity was plotted, and a cutoff point was determined. To identify dopamine transients, the background-subtracted cyclic voltammograms at the targeted points were compared with those for dopamine. Criteria included peaks at approximately +0.6 and −0.2 V versus Ag/AgCl, their relative amplitude and the absence of extraneous peaks (Robinson et al., 2002).
Data analysis
Data were analyzed in GraphPad Prism (GraphPad Software, San Diego, CA). A t test, one-way repeated measures and two-way ANOVAs with Bonferroni post tests and Holm-Sidak post-hoc comparisons were performed to determine statistical significance. The data are presented as mean ± SEM and the criterion of significance was set at P < 0.05.
RESULTS
Voltammetry
After 10 min of adaptation, in the initial 5-min phase, baseline measures were collected (2.30±0.19 transients/min), and the rat was transferred into the empty resident’s cage. This did not significantly alter the frequency of dopamine transients during the second phase of 5-min recording. However, a two-fold increase in the frequency of dopamine transients was observed during the subsequent third phase when the aggressive resident was placed in its home cage and confronted the intruder (P<0.001; n=5, Bonferroni post test) (Fig. 1 and 2). Continuous behavioral observations indicated typical patterns of resident rat aggression, consisting of pursuits, threats and attacks resulting in defeat of the intruder, as defined by the intruder assuming a supine posture for at least 5 consecutive seconds. No significant correlation between specific elements of the aggressive behavior of residents and the appearance of dopamine transients in intruder brain was revealed. However, dopamine spikes were often coincident when the resident engaged in anogenital investigation, naso-nasal contact and random sniffing. Dopamine activity in the intruder’s nucleus accumbens significantly decreased during the next 5-min phase, when the aggressive resident was removed from cage (P<0.001; n=5, Bonferroni post test). It is noteworthy that the frequency of dopamine transients was still increased throughout this fourth phase compared to baseline recordings (3.62±0.24 vs 2.30±0.19 transients/min; P<0.005; n=5, Bonferroni post test). There was a significant main effect for the entire stress procedure (one-way repeated measures ANOVA; F=63.49; P<0.001, n=5).
Figure 1.
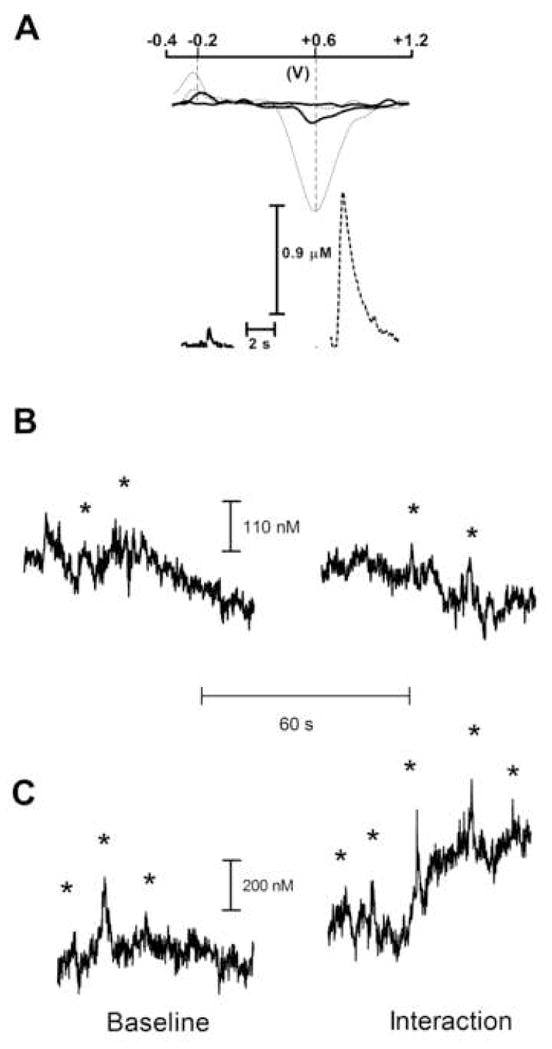
The oxidative (+0.6 V) and reductive (−0.2 V) peaks (upper panel) of the dopamine transient (solid line) are compared with those of dopamine observed during electrical stimulation of dopamine fibers (dotted line) (A). The correlation of cyclic voltammograms from electrically evoked (lower panel, right) and transient (lower panel, left) dopamine verifies that the changes in electrochemical signal during the transient (lower panel, left) are attributable to the oxidation of dopamine.
Representative traces of spontaneous dopamine transients in the nucleus accumbens of freely moving rats before and during presentation of cage mate (B) and aggressive resident (C). Cyclic voltammograms at the time of dopamine transients, which are indicated by asterisk, correlate with cyclic voltammograms at the time of electrically stimulated dopamine release (r2 ≥ 0.75).
Figure 2.
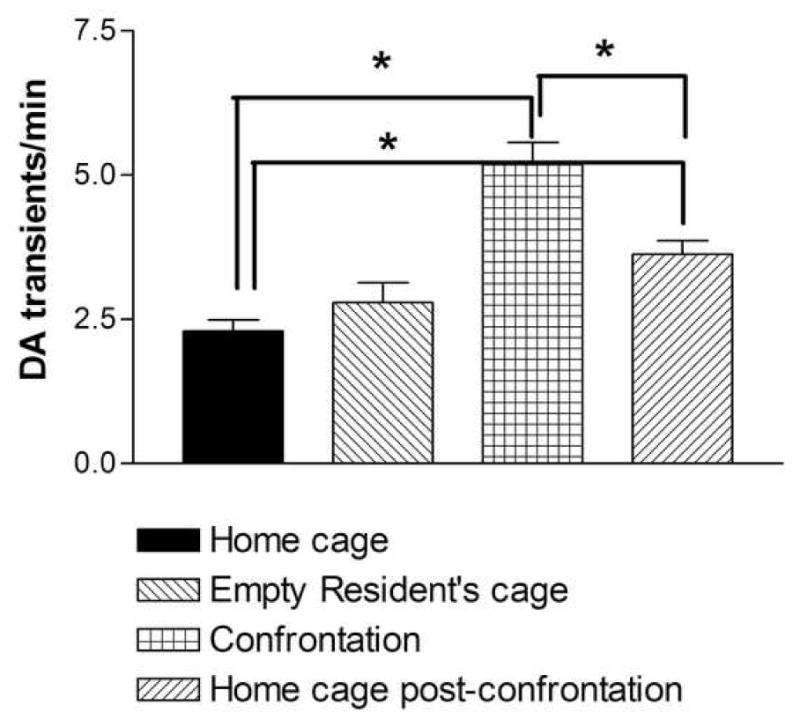
The effect of defeat stress on the frequency of dopamine transients in the nucleus accumbens of intruder rats. When the intruder rat is introduced to an aggressive resident environment, significant increases in dopamine transient activity are found during Confrontation and Post-confrontation periods. Data shown are mean ± SEM. *P<0.05.
In contrast to the results, which were obtained during the confrontation with an aggressive resident, no significant alteration in dopamine transient activity was found during the interactions of the intruder with a cage mate (before interaction:1.92±0.23 vs during interaction: 1.80±0.19 transients/min; P>0,05, n=5). No aggressive behavioral elements such as allogrooming, biting attacks, aggressive postures and pursuit were observed in this experiment.
Multiunit recording
Putative GABAergic populations of neurons were separated from putative dopaminergic neurons by electrophysiological criteria (Fig. 3A) used in previous studies which include long duration waveforms and average firing rates between 1–8 Hz (Grace, 1988; Marinelli et al., 2003; Anstrom and Woodward, 2005). The average duration of extracellular spike waveforms for putative dopamine neurons was 2.71 ± 0.02 msec, although these durations may be underestimated due to filtering conditions. Average firing rates for this population (n = 16) under resting conditions was 2.65 ± .47 Hz, ranged from 0.3–5.80 with a median of 2.7 Hz. Extracellular spike waveforms were at times triphasic (+/−/+) with variable positive domains and had prolonged negative phases. As a population, 10.17 ± 2.8 percent of spikes were found in bursts and each burst had an average of 3.80 ± 1.6 spikes per burst.
Figure 3.
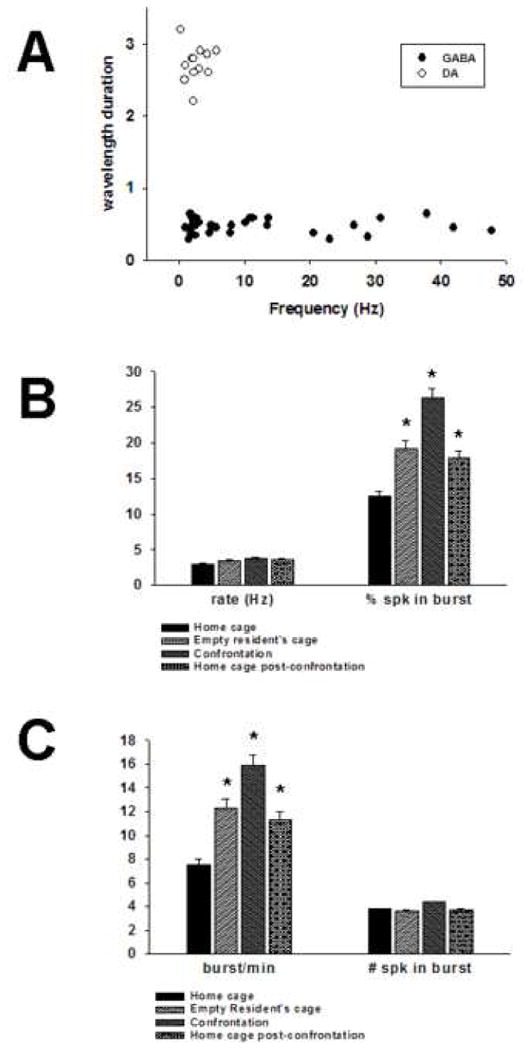
Separation of neuronal populations and responses during aggression and social defeat. As has been demonstrated in previous strudies, putative dopamine and GABA neurons recorded with stainless steel multielectrode arrays can be identified by waveform duration and firing rates (A). There was no significant difference in dopamine firing rate during any condition. However, significant increases in burst firing were detected (B). These changes were due to increases in burst frequency (burst/min) but not spikes per burst (C). *P<0.05.
Importantly, a recent report questioned the validity of electrophysiological criteria for VTA dopamine neurons, including the hyperpolarization-activated inwardly rectifying non-specific cation current, spike duration, and inhibition by dopamine D2 receptor agonists (Margolis et al., 2006). These widely accepted criteria were determined to be unreliable since they did not consistently predict the dopamine content of VTA neurons. The fact that the voltammetric data entirely support electrophysiological results in the current study suggests that the electrophysiological criteria used here are valid for identifying dopamine neurons.
With regard to putative GABAergic neurons, care was taken to record only activity clearly derived from single units and not presumed GABAergic “ensembles” (Kosobud et al., 1994). Single spike activity was confirmed by autocorrelational analysis. The average waveform duration for 32 representative presumed GABA neurons, identified by waveform, rate and burst characteristics, was 0.48 ± 0.004 msec. The waveforms were, in general, biphasic with consistent, well-defined negative phases. Under resting conditions, presumed GABA neurons (n=121, 7 animals) had an average firing rate of 9.05 ± 1.2 Hz with a median firing rate of 2.83 Hz.
Changes in putative dopaminergic firing rates and burst firing were examined during all four periods (Fig. 3B, C; Home cage, Resident’s cage, Confrontation and Post). Although there was an upward trend in firing rate, ANOVA determined that there were no significant increases (n = 16 neurons) in any experimental group (Resident’s cage, 3.25 ± 0.5 Hz; Confrontation, 3.58 Hz; Post, 3.28 ± 0.5 Hz) as compared to Home cage (2.65 ± 0.5 Hz), [F(df 3,15) = 2.12, P = 0.116].
Similarly to the voltammetric results, which were obtained during the interaction of the intruder with a cage mate, there were no significant differences in frequency when the rat was placed with the non-aggressive resident (2.91± 0.1 Hz) [F(df 2,4) = 0.88, P = 0.46]. Furthermore, no previously quiescent putative dopamine neurons were found to be activated. Importantly, no aggressive behavioral elements such as allogrooming, biting attacks, aggressive postures and pursuit were observed in this experiment.
There was, however, an overall effect of exposure to the resident’s cage and confrontation with the aggressive resident on dopaminergic burst firing (as defined by percentage of total spikes found in bursts) [F(df 3,15) = 4.94, P < 0.01). Post-hoc analysis showed that the only period where average percentage of spikes in bursts was significantly different from Home cage (10.17 ± 2.8) was the Confrontation period (23.28 ± 6.0, P < 0.01). No previously quiescent dopamine neurons were observed to be activated in any phase. The average burst frequency in the Home cage phase was 5.86 ± 2.1 bursts/minute, and the average number of spikes in a burst was 3.80 ± 0.15. Burst frequency increased during exposure to the Resident’s cage (10.61 ± 4.4) and peaked during the Confrontation (14.01 ± 4.3). The number of spikes per burst did not increase significantly in any phase (Resident’s cage, 3.46 ± 0.4; Confrontation, 4.27 ± 0.4; Post, 3.58 ± 0.4) as compared to the Home cage phase (3.8 ± 0.15) and even decreased during the phase in the Resident’s cage and in the Post phase.
To determine if defeat stress affected putative bursting vs non-bursting dopamine neurons differently, neurons were subclassified based on previously described burst firing patterns in awake animals (Hyland et al., 2002; Anstrom and Woodward, 2005). As a population, 10.17 ± 2.7 percent of spikes were found in bursts during a Home cage period, and of the 16 neurons, 2 were classified as pacemaker type. These neurons had regular firing patterns and an average of 0.63 ± 0.3 % of spikes within bursts and an average firing rate of 2.27 ± 0.8 Hz. Twelve putative dopamine neurons were classified as burst firing neurons with 16.0 ± 1.2 % of spikes within bursts and an average firing rate of 3.59 ± 0.4. The remaining two neurons were classified as “random” characterized by low baseline firing rates (0.7 ± 0.3 Hz) and lack of patterned firing or an interspike interval histogram with no clear peak (Fig 4A).
Figure 4.
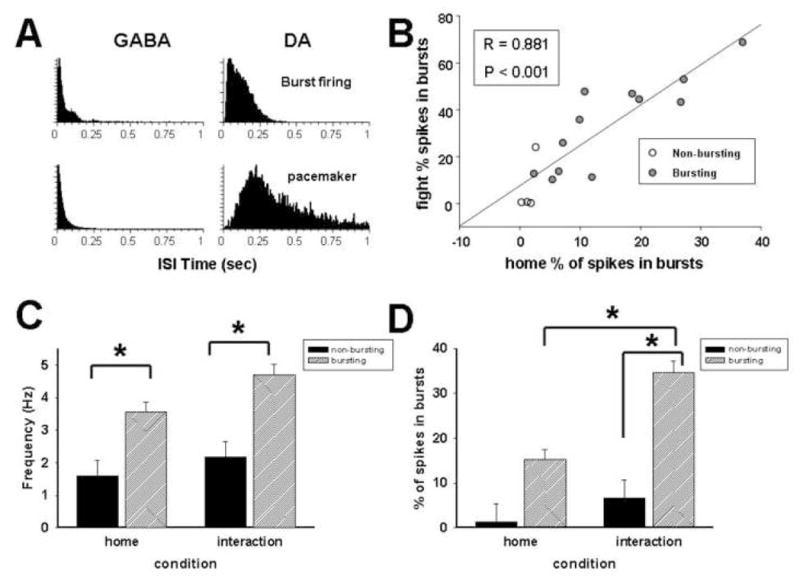
Responses of dopaminergic burst subtypes to aggressive confrontation. Representative interspike interval histograms demonstrate differences in burst profiles between GABA (left column) and dopamine neurons (right column) and between bursting and non-bursting dopamine neurons (A). Scatter plot shows that putative dopamine neurons with higher burst indices under homecage conditions show greater increases in bursting during confrontation with aggressive resident (B). First set of bars represents average ± SEM firing rates for bursting and non-bursting dopamine neurons. ANOVA analysis revealed significant effect of type only (C). Second set of bars represents average ± SEM burst firing levels in putative bursting and non-bursting dopamine neurons. There was a significant interaction between neuron type and condition.
To determine if the amount of change in burst firing depended on initial burst levels, a Pearson’s correlation between resting burst rates and bursts during the confrontation with an aggressive resident was performed. Initial burst rates significantly impacted how much a stressful confrontation increased burst firing levels (Fig. 4B; r = 0.88, P < 0.001). This result indicates that non-bursting, particularly pacemaker neurons, were unlikely to switch to burst profiles but that initial burst levels, perhaps conferred by intrinsic membrane properties, may influence response to social stress.
A two-way, repeated measures ANOVA analysis determined that there were significant interactions between conditions (Home cage vs Confrontation) and neuron type (bursting vs non-bursting) on dopaminergic burst firing levels (Fig. 4D) but not rate (Fig. 4C). Although there was a significant difference in average (F = 13.13, df (1,14), P< 0.01) firing rate between bursting and non-bursting neurons in each phase, there was no significant interaction between neuron type and condition (F = 0.43, df (2,14), P = 0.5). In contrast, there was a significant interaction between neuron type and condition (F = 4.60, df (2,14), P < 0.05) with respect to burst firing levels. Post-hoc comparisons demonstrated aggressive confrontations induced a significant change in mean percentage of spikes within bursts in burst neurons during confrontation (P < 0.001) but not in non-burst neurons. Burst firing levels were also significantly different during aggressive confrontations (P < 0.01) but not while animals were in the home cage.
DISCUSSION
In the present study, multiunit recording and FSCV were used to identify how episodic defeat affects dopaminergic firing patterns in the VTA and phasic dopamine release in the nucleus accumbens. Average firing rate, burst firing rate, and the frequency of dopamine transients were evaluated in intruder rats. Measurements were performed before (home cage and resident’s cage), during (resident’s cage), and after (home cage) confrontations with aggressive resident rats. Significant increases in transient frequency were found during intruder-resident confrontations but not when intruder rats were exposed to familiar cage mates, suggesting that dopaminergic increases are associated with events specific to defeat stress. Phasic release remained elevated after the confrontation terminated and the intruder was replaced in its home cage. There was a significant increase in burst firing, as indicated by increasing burst frequency, during confrontations between the aggressive resident and intruder rats. Increases in burst firing were most prominent in neurons with higher levels of bursting under home cage conditions. Furthermore, when bursting neurons were analyzed separately, a significant interaction between neuron type and condition was detected. Aggressive confrontations did not cause significant differences in firing rate, nor were any previously quiescent dopaminergic neurons detected, suggesting that increases in dopaminergic neurotransmission were due to increased frequency of burst activity at the cell bodies.
In intact animals, dopamine neurons have relatively slow single-spike firing patterns interspersed with periodic bursts. Burst activity, defined as transient rapid firing of dopaminergic cell bodies, is thought to be dependent on afferent input (Kitai et al., 1999; Floresco et al., 2003) and generates a high concentration of extracellular dopamine (or a dopamine transient) that lasts for brief periods (seconds). This activity occurs in both anesthetized and awake animals in the absence of salient stimuli and thus constitutes intrinsic dopaminergic activity (Grace, 1991; Hyland et al., 2002; Marinelli et al., 2003; Lee et al., 2004; Anstrom and Woodward, 2005). Excitatory phasic responses and subsequent dopamine transient activity can be elicited in response to a variety of stimuli which are associated with natural and drug rewards (Robinson et al., 2001; Schultz, 2001; Phillips and Wightman, 2004; Pan et al., 2005). On the other hand, some electrophysiological studies revealed that the activity of dopamine neurons may be inhibited by aversive stimuli including noxious inducements such as a foot pinch (Ungless et al., 2004) or foot shock (Coizet et al., 2006). Conversely, the inhibition of dopamine neurons may result in a negative affective state, leading presumably to negative affective encoding (Liu et al., 2008). The present electrophysiological results demonstrate that the increases in dopaminergic activity can take place during a highly aversive condition such as defeat stress. In fact, in contrast to the noxious stimuli, which were applied mostly in anesthetized rats (Ungless et al., 2004; Coizet et al., 2006), social defeat can be viewed as a more composted set of processing circuits that detect and respond to multiple stimuli. Importantly, these stimuli may not necessarily be limited by aversive incentives but may also be associated with processes of attentional switching and associative learning. Therefore, stressful encounters may impact signaling on the cell bodies of dopamine neurons in a different way than acute noxious stimuli.
The increases in burst frequency and not necessarily spikes per burst are consistent with an increased dopamine transient frequency in the nucleus accumbens that was found using voltammetry. The rise in accumbal extracellular dopamine in rats that were threatened by an aggressive opponent was found earlier by in vivo microdialysis and assays of post-mortem tissue (Mos and Van Valkenburg, 1979; Louilot et al., 1986; Tidey and Miczek, 1996). The dopamine concentration alterations detected in those experiments, which reflect a tonic dopamine activity, existed for an extended period. These changes may be the consequence of increased low-frequency tonic firing of dopamine neurons or glutamatergic actions at the level of dopamine terminals (Grace, 2000). However, the phasic dopamine increases observed in the present experiments may also contribute to the increased dialysis dopamine concentrations. The increase in dopamine transmission with consequent activation of dopamine receptors in striatal regions is critically important for behavioral excitation (Kelly and Iversen, 1976; Budygin et al., 2000; Sabeti et al., 2002). This relationship is more evident when dopamine uptake is inhibited by psychostimulants (Kuczenski et al., 1991; Sabeti et al., 2002; Budygin, 2007). For example, the time course of cocaine-induced stereotypy strictly parallels the time course of the dopamine uptake changes (Budygin et al., 2007). However, the increase in accumbal dopamine in defeated intruders is not correlated with motor activation (Tidey and Miczek, 1996). Moreover, the maximal dopamine response was observed when intruders displayed immobility. It is possible that the increase in accumbal dopamine during social defeat does not reach the threshold level that is necessary for locomotor activation. Indeed, in defeated intruders, extracellular dopamine levels were increased by only 160% of baseline (Tidey and Miczek, 1996), while psychomotor stimulants induce at least a 300 % increase when a marked psychomotor response is evident (Kuczenski et al., 1991). On the other hand, the possibility that other inhibitory mechanisms triggered by defeat stress suppress locomotor activity cannot be excluded.
There is now growing evidence that dopamine transients measured in the nucleus accumbens by FSCV are extremely sensitive to salient environmental events and may be coupled to specific behavioral acts. As mentioned above, accumbal dopamine transients, detected in rats, were tightly time locked to responding for intravenous cocaine during self-administration sessions and in response to cocaine-associated stimuli (Phillips et al., 2003). However, there was another source of dopamine transients, which was not time locked to explicit stimuli during cocaine self-administration (Stuber et al., 2005). In a different context, the dopamine transients in the nucleus accumbens of male rats were not exclusively associated with discrete events during sexual interactions with female rats (Robinson et al., 2001). Similarly, the dopamine transients reported here did not correlate with any specific defensive or submissive behavior. Most transients observed in the present study were associated with being the target of anogenital investigation and a sniffing during confrontation. It is important to note that under the present conditions increased transient activity was observed in situations when no rewarding events or reinforcement was evident. The current findings provide evidence for increases in phasic dopamine release that is not linked to rewarding stimuli. A further possibility associates the enhanced phasic dopamine signaling with switching attention to any salient stimulus. The data on the characterization of dopamine transients in the dorsal and ventral striatum of male rats during solitude and brief interaction with a conspecific (Robinson et al., 2002) would support this hypothesis. In this study dopamine transients were often followed by an increase in behaviors toward the conspecific (Robinson et al., 2002). Therefore, attending to salient stimuli and preparing for action are further possible interpretations for the increase in dopamine transmission that was observed in intruder brain during social defeat.
The hypothesis that dopamine could promote behavioral switching was previously discussed with respect to the general modulatory role of dopamine neuron input to striatal function (Oades, 1985; Redgrave et al., 1999). Moreover, it was suggested that the initial burst of dopaminergic neuron firing could represent an essential component in the process of switching attentional and behavioral selections to unexpected, behaviorally important stimuli, which are not necessarily aversive or rewarding (Redgrave et al., 1999). This switching response could be a crucial prerequisite for associative learning and might be a response that prepares the organism for an appropriate reaction to biologically significant events.
Alternatively, the increased phasic dopamine release may be specifically involved in the perception of the fearful experience, and may therefore be a reflection of aversive component in the social defeat. The fact that phasic release in the nucleus accumbens remained elevated when an intruder was replaced in its homecage agrees with this notion. Moreover, restraint, which is obviously highly aversive for conscious animals, also markedly increased the activity of VTA dopamine neurons and this alteration lasted for at least 24 hours (Anstrom and Woodward, 2005). However, this interpretation raises the issue of why the appearance of dopamine transients is not correlated with specific reactions to aggressive behaviors by the resident. Perhaps an efflux of specific pheromones, which may not coincide with aggressive postures and attack bites of the resident, can trigger the phasic dopamine response in the intruder’s brain. In fact, stress-induced odors can have powerful effects on the endocrine system including increased levels of stress hormones such as corticosterone and other glucocorticoids (Morrow et al., 2000; Apfelbach et al., 2005). Yet, both aggressive resident and defensive intruder rats are characterized by a rise in corticosterone during the initial phase of a confrontation (Schuurman, 1980; Covington, III and Miczek, 2001). Nevertheless, future studies are required to check the hypothesis that the pheromones of an aggressive animal may trigger the phasic dopamine response. This study demonstrates for the first time that aggressive confrontations in defeated rats are associated with increases in phasic dopamine neurotransmission. However, the exact role of this neurochemical response should be further elucidate.
Acknowledgments
The authors would like to thank Dr. Caroline Bass for helpful comments and Chad Collins for expert technical assistance. This work was supported by Wake Forest University Cross-Campus Collaborative Fund Award (EAB), Alcoholic Beverage Medical Research Foundation (KKA and EAB) and National Institutes of Health Grants DA021634 (EAB) and AA013983 (KAM).
Abbreviations
- VTA
ventral tegmental area
- ICSS
intracranial self-stimulation
- FSCV
fast-scan cyclic voltammetry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JW, Gilman S. The temporal structure of spike trains in the primate basal ganglia: afferent regulation of bursting demonstrated with precentral cerebral cortical ablation. Brain Res. 1991;543:123–138. doi: 10.1016/0006-8993(91)91055-6. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Cromwell HC, Woodward DJ. Effects of restraint and haloperidol on sensory gating in the midbrain of awake rats. Neuroscience. 2007;146:515–524. doi: 10.1016/j.neuroscience.2007.01.060. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429:55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan voltammetry. Neurosci Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- Chang JY, Sawyer SF, Lee RS, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci. 1994;14:1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007a;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007b;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. In vivo and in vitro intracellular recordings from rat midbrain dopamine neurons. Ann N Y Acad Sci. 1988;537:51–76. doi: 10.1111/j.1749-6632.1988.tb42096.x. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-derected behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Proc Natl Acad Sci USA. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res. 1999;817:172–184. doi: 10.1016/s0006-8993(98)01245-1. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine, and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci. 1991;9:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AE, Harris GC, Chapin JK. Behavioral associations of neuronal activity in the ventral tegmental area of the rat. J Neurosci. 1994;14:7117–7129. doi: 10.1523/JNEUROSCI.14-11-07117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Abercrombie ED, Tepper JM. Pallidal control of substantia nigra dopaminergic neuron firing pattern and its relation to extracellular neostriatal dopamine levels. Neuroscience. 2004;129:481–489. doi: 10.1016/j.neuroscience.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Shin R, Ikemoto S. Dual role of medial a 10 dopamine neurons in affective encoding. Neuropsychopharmacology. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louilot A, Le Moal M, Simon H. Differential reactivity of dopaminergic neurons in the nucleus accumbens in response to different behavioral situations. An in vivo voltammetric study in free moving rats. Brain Research. 1986;397:395–400. doi: 10.1016/0006-8993(86)90646-3. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology (Berl) 2003;168:84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Intraspecies aggression in rats: effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia. 1974;39:275–301. doi: 10.1007/BF00422968. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Aggressive and social stress responses in genetically modified mice: from horizontal to vertical strategy. Psychopharmacology (Berl) 1999;147:17–19. doi: 10.1007/s002130051132. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, III, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Boer SF. Aggressive, defensive, and submissive behavior. 2005:344–352. [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacology & Therapeutics. 2008 doi: 10.1016/j.pharmthera.2008.07.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000;864:146–151. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- Mos J, Van Valkenburg CFM. Specific effect on social stress and aggression on regional dopamine metabolism in rat brain. Neurosci Lett. 1979;15:325–327. doi: 10.1016/0304-3940(79)96134-2. [DOI] [PubMed] [Google Scholar]
- Oades RD. The role of noradrenaline in tuning and dopamine in switching between signals in the CNS. Neurosci Biobehav Rev. 1985;9:261–282. doi: 10.1016/0149-7634(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Overton PG, Tong ZY, Brain PF, Clark D. Preferential occupation of mineralocorticoid receptors by corticosterone enhances glutamate-induced burst firing in rat midbrain dopaminergic neurons. Brain Res. 1996;737:146–154. doi: 10.1016/0006-8993(96)00722-6. [DOI] [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005;25:6235–6242. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Wightman RM. Extrasynaptic dopamine and phasic neuronal activity. Nat Neurosci. 2004;7:199. doi: 10.1038/nn0304-199a. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology. 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Phillips PE, Budygin EA, Trafton BJ, Garris PA, Wightman RM. Sub-second changes in accumbal dopamine during sexual behavior in male rats. Neuroreport. 2001;12:2549–2552. doi: 10.1097/00001756-200108080-00051. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Rawal N, Mameli-Engvall M, Nomikos GG, Svensson TH. Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. Int J Neuropsychopharmacol. 2003;6:1–11. doi: 10.1017/S1461145702003188. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavior-related activity of primate dopamine neurons. Rev Neurol (Paris) 1994;150:634–639. [PubMed] [Google Scholar]
- Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schuurman T. Hormonal correlates of agonistic behavior in adult male rats. Prog Brain Res. 1980;53:415–420. doi: 10.1016/S0079-6123(08)60079-5. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiology and Behavior. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philosophical Transactions of the Royal Society B-Biological Sciences. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]


