Abstract
Background: Acne is commonplace in adolescents and can be difficult to manage. Providing an effective and well-tolerated treatment may lead to improved adherence, increased patient satisfaction, and improved clinical outcomes. Methods: A post hoc analysis of efficacy and cutaneous tolerability in 289 adolescents (age range, 12 to <18 years) with moderate-to-severe acne who had been enrolled in a multicenter study and were randomized to receive either clindamycin phosphate 1.2%/benzoyl peroxide 3.75% gel or vehicle once daily for 12 weeks. Results: Significantly superior reductions in lesion counts were observed in the clindamycin phosphate 1.2%/benzoyl peroxide 3.75% gel group compared to vehicle from Week 4, with mean percent reductions in inflammatory and noninflammatory lesions from baseline of 59.9 percent and 50.5 percent, respectively (both P<0.001 versus vehicle). One-third of patients treated with clindamycin phosphate 1.2%/benzoyl peroxide 3.75% gel achieved ≥2-grade improvement from baseline in their Evaluator’s Global Severity Score (compared to 8.5% with vehicle, P<0.001) and 35 percent of patients reported clear or almost clear skin at Week 12 (compared to 12.8% with vehicle, P<0.001). Cutaneous tolerability was excellent with all mean scores ≤0.2 at Week 12 (where 1=mild). Conclusions: Clindamycin phosphate 1.2%/benzoyl peroxide 3.75% gel is an effective, safe, well-tolerated treatment for adolescents with moderate-to-severe acne.
Acne vulgaris (acne) affects almost 85 percent of people aged 12 to 24 years.1 It is the most common dermatological condition encountered in adolescents, with teenagers comprising 36.5 percent of all patients seen with acne.2,3
While acne symptoms tend to be mild in the majority of adolescents,4 the impact of acne on any particular teenager is difficult to judge. Even mild acne can cause significant emotional distress for some, diminishing their quality of life and social functioning.5,6
It has been suggested that the majority of teenagers see acne as an unavoidable part of adolescence,4 and many are not seen by a physician or dermatologist as a result.4,7 However, acne can have profound psychosocial consequences and severe disease can leave permanent scarring, especially if left untreated.8–10
Acne is a chronic disease characterized by periods of exacerbations and remission, but generally not classified as a progressive disease.11 Adolescents’ perception of severity (considered severe in 16% of cases4) is similar to prevalence surveys suggesting acne is moderate to severe in about 15 to 20 percent of cases.5
Although it tends to resolve in the majority of patients by the third decade of life, up to 20 percent of adolescents can have acne that persists into adulthood, with a majority of these patients being female. It has been suggested that the prevalence of acne in adulthood is due to undertreated acne in adolescence, or new-onset acne in adulthood.
A number of studies have indicated that while many adolescents see acne as part of growing up, they do want to see it treated.4,12 Although they do not have a clear idea about the best treatment approach, they tend to prefer topical drugs.4 Also, patient education is seen as an important component of successful treatment.13
Recently, efficacy and tolerability data on a new fixed combination product, clindamycin phosphate 1.2% (Clin)/benzoyl peroxide (BP) 3.75% gel was reported.14 A post hoc analysis of efficacy and cutaneous tolerability was conducted in the adolescent patients with moderate-to-severe acne treated with Clin/BP 3.75% gel, or vehicle to gain insights into this important population.
METHODS
Study design. A post hoc analysis was conducted in 289 adolescent patients (age range, 12 to <18 years) with moderate-to-severe acne who were enrolled in a randomized, double-blind, multicenter study and were treated with either Clin/BP 3.75% gel or vehicle for 12 weeks. Patients were stratified by severity of acne (Evaluator’s Global Severity Score [EGSS], ranging from 0 [clear] to 5 [very severe]). They were dichotomized into a moderate (EGSS of 3) and a severe acne group (EGSS of 4).
Study population. The study group included male and female adolescents of any race or ethnicity who presented with 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 noninflammatory lesions (open and closed comedones), and 2 nodules or fewer. There was a washout period for patients using previous prescription and over-the-counter acne treatments.
Efficacy evaluation. Inflammatory and noninflammatory lesion counts as well as EGSS were evaluated at baseline and during treatment (Weeks 4, 8, and 12). Subject self-assessment (SSA) of acne severity was carried out at Weeks 2, 4, 8, and 12. Primary efficacy endpoints included absolute change in inflammatory and noninflammatory lesion counts and proportion of patients who achieved ≥2-grade reduction in EGSS from baseline to Week 12. Secondary efficacy endpoints included mean percent change in lesion counts and the proportion of patients who considered themselves “clear” or “almost clear” at Week 12 (≥90% clear skin).
Safety evaluation. Cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) were evaluated at each study visit on a scale of 0 (none) to 3 (severe). Adverse events (AEs) were evaluated throughout.
Statistical analysis. The intent-to-treat (ITT) population comprised all patients randomized and provided study drug. The safety population comprised all randomized patients presumed to have used study medication at least once and provided at least one post-baseline evaluation.
The investigator assessments (EGSS, lesion counts) were conducted independently of SSA. Statistical significance was based on 2-tailed tests of the null hypothesis resulting in P-values of 0.05 or less.
Adverse events were recorded and classified using Medical Dictionary for Regulatory Activities (MedDRA) terminology. Descriptive statistics were used to summarize cutaneous safety and tolerability scores at baseline and Weeks 4, 8, and 12.
RESULTS
Baseline characteristics. A randomized group of 289 patients received treatment with either Clin/BP 3.75% gel (N=155) or vehicle (N=134). Mean age was 15.0 years and 60.2 percent were male. Overall, mean inflammatory and noninflammatory lesion counts at baseline were 27.5 and 40.4, respectively, and 85.5 percent of patients had moderate acne (EGSS=3). There were no significant differences between the two treatment groups in terms of demographics or baseline characteristics (Table 1).
TABLE 1.
Subject baseline characteristics ITT population (aged 12 to <18 years)
| CLIN/BP 3.75% (N=155) | VEHICLE (N=134) | TOTAL (N=289) | P-VALUE | |
|---|---|---|---|---|
| AGE (YEARS) | ||||
| Mean (SD) | 14.8(1.47) | 15.1 (1.48) | 15.0(1.48) | 0.101 |
| GENDER | ||||
| Male | 95(61.3%) | 79 (59.0%) | 174(60.2%) | 0.592* |
| Female | 60 (38.7%) | 55(41.0%) | 115(39.8%) | |
| INFLAMMATORY LESION COUNT | ||||
| Mean (SD) | 27.7 (6.32) | 27.3 (6.38) | 27.5 (6.34) | 0.451 |
| NONINFLAMMATORY LESION COUNT | ||||
| Mean (SD) | 40.7(19.90) | 40.0(18.14) | 40.4(19.07) | 0.659 |
| EVALUATORS GLOBAL SEREVITY SCORE (EGSS) | ||||
| 3-Moderate | 132 (85.2%) | 115(85.5%) | 247 (85.5%) | 0.805* |
| 4-Severe | 23(14.8%) | 19(14.2%) | 42 (14.5%) | |
P-value from a Cochran-Mantel-Haenszel test of row means scores, stratified by analysis center
Efficacy. Lesion counts. At Week 4, the mean percent reduction in inflammatory and noninflammatory lesion counts was significantly superior to vehicle (P<0.001 and P=0.026, respectively).
By Week 12, the mean percent reduction in inflammatory and noninflammatory lesions was 59.9 and 50.5 percent, compared to 22.6 and 21.3 percent with vehicle (both P<0.001). Noticeably, there was no additional improvement seen with vehicle beyond the initial reduction in lesion counts seen at Week 4 (Figures 1A and Figures 1B).
Figure 1A.
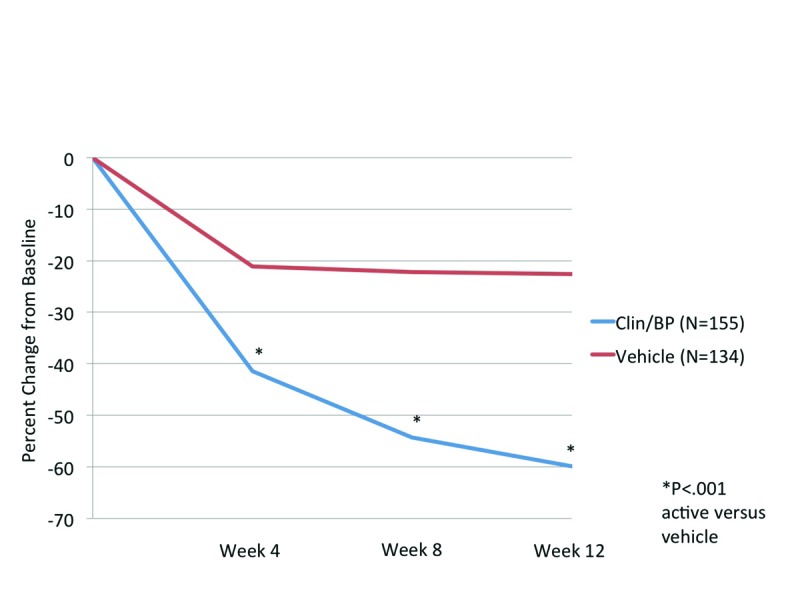
Inflammatory lesion counts (mean) at each evaluation, ITT population (aged 12 to <18 years)
Figure 1B.
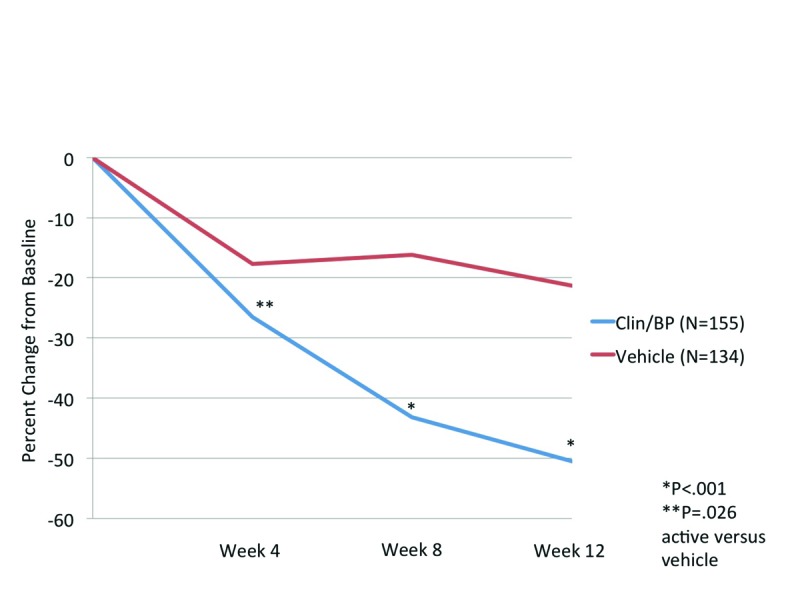
Noninflammatory lesion counts (mean) at each evaluation, ITT population (aged 12 to <18 years)
Evaluator’s Global Severity Score (EGSS). Overall treatments success (≥2-grade reduction in EGSS from baseline) with Clin/BP 3.75% gel was achieved in 33.1 percent of patients compared to 8.5 percent with vehicle (P<0.001, Figure 2). The difference between the two treatment arms was significant from Week 4.
Figure 2.
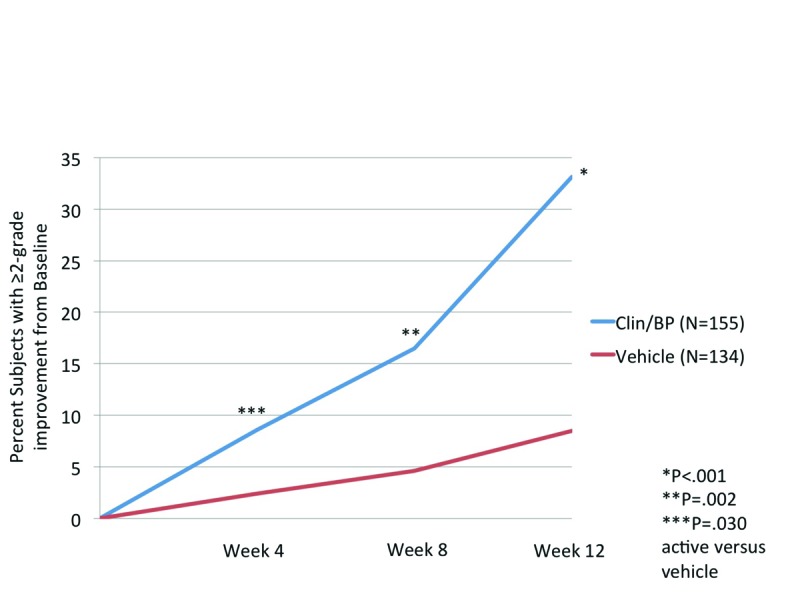
Evaluator’s Global Severity Score: Subjects achieving ≥2-grade improvement from baseline, ITT population (aged 12 to <18 years)
Subject Self Assessment (SSA). Patient’s perception of treatment success (clear or almost clear at Week 12) mirrored that of the investigator assessment, with 34.7 percent of patients clear/almost clear compared to 12.8 percent with vehicle (P<0.001, Figure 3). In almost 70 percent of patients, acne covered at least 50 percent of their face at baseline. By Week 2, marked improvement (75% clear or more) was seen in 32.4 percent of patients treated with Clin/BP 3.75%, increasing to 66 percent of patients by Week 12.
Figure 3.
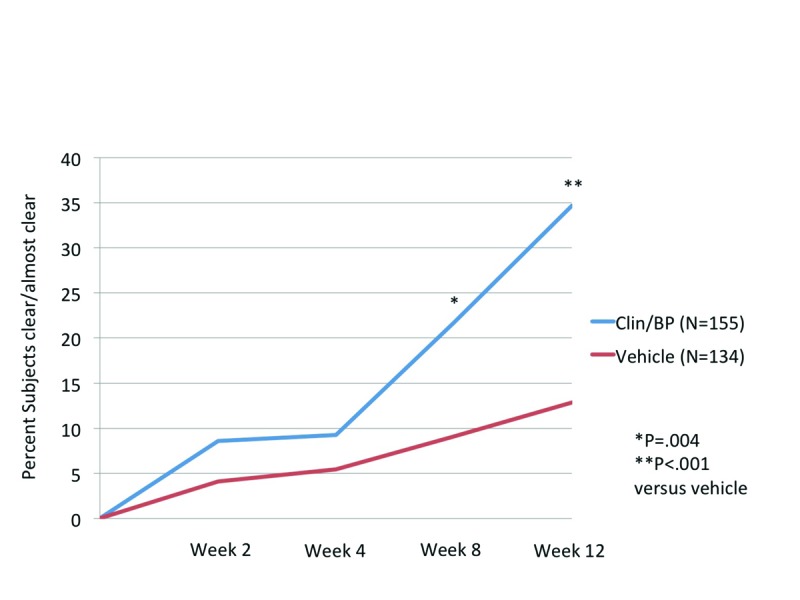
Subject Self-Assessment (SSA) Scores: Subjects achieving clear or almost clear skin (≥90%), ITT population (aged 12 to <18 years)
Safety. Cutaneous tolerability assessment. Overall mean scores for cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) at baseline and at each post-baseline visit were <1 (where 1=mild) and comparable between Clin/BP 3.75% gel and vehicle (Figure 4).
Figure 4.
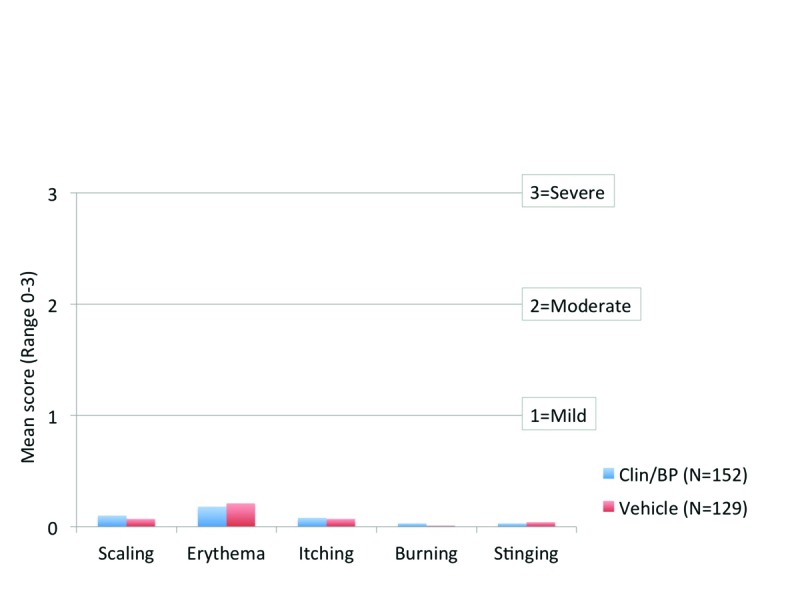
Cutaneous safety and tolerability mean scores at Week 12, ITT population (aged 12 to <18 years)
DISCUSSION
Acne in adolescents can be difficult to manage and affects young people at a time when they are undergoing significant psychological, social, and physical changes. Unrealistic expectations of therapy or poor tolerability can lead to low adherence and poor clinical outcomes.15,16 Effective therapies that can demonstrate both early treatment success or marked improvement, and are well-tolerated are important, coupled with time spent educating teenagers about their acne.13,17
Fixed combinations of clindamycin and benzoyl peroxide are widely used in the treatment of adolescent acne. This analysis revealed that adolescent patients with moderate-to-severe acne who were treated with Clin/BP 3.75% gel had statistically superior results in terms of lesion reduction and treatment success at Week 12. In addition, marked improvement in acne was seen in almost a third of patients as early as Week 2. Clin/BP 3.75% gel was well-tolerated with a similar AE profile to vehicle. Local signs and symptoms of erythema, scaling, itching, burning, or stinging were rare and generally mild when present.
While head-to-head study comparisons are limited by differences in study design, patient entry criteria, and investigators’ assessment biases, the results reported in this analysis are encouraging. Mean percent reductions of 59.9 and 50.5 percent in inflammatory and noninflammatory lesions were seen at Week 12. It is also of note that while lesion reduction with Clin-BO 3.75% gel increased throughout the study, any initial improvement in the vehicle group seen at Week 4 did not change over the duration of the study.
It has been reported that as many as 16 percent of adolescents with acne consider their condition to be severe.4 In this post hoc analysis, almost 15 percent of patients had severe acne. The data suggest that topical monotherapy may be more valuable than often assumed in this population and further analysis may provide additional insights.
Although many studies have shown that combination of clindamycin and BP is superior to each individual active ingredient,18 it is not possible to determine the contributions from the individual active ingredients in our analysis.
CONCLUSION
The fixed combination of Clin-BP 3.75% gel is an effective, safe, and well-tolerated topical treatment of adolescent patients with moderate-to-severe inflammatory and noninflammatory acne vulgaris, achieving early treatment success, marked improvement and good tolerability.
ACKNOWLEDGMENT
The author acknowledges Brian Bulley, MSc, of Inergy Limited for medical writing support. Valeant Pharmaceuticals funded Inergy’s activities pertaining to 11. this manuscript.
Footnotes
DISCLOSURE:Dr. Cook-Bolden was a principle investigator in the multicenter study and has been a consultant for Valeant Pharmaceuticals.
REFERENCES
- 1.Krowchuck DP. Managing Acne in Adolescent. Pediatr Clin North Am. 2000;47(4):841–857. doi: 10.1016/s0031-3955(05)70243-1. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander SF, Eichenfield LF, Fowler RF, et al. Acne epidemiology and pathophysiology. Semin Cutan Med Surg. 2010;29(2 Suppl 1):2–4. doi: 10.1016/j.sder.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Yentzer BA, Hick J, Reese EL, et al. Acne vulgaris in the United States: a descriptive epidemiology. Cutis. 2010;86(2):94–99. [PubMed] [Google Scholar]
- 4.Pearl A, Arroll B, Lello J, et al. The impact of acne: a study of adolescents’ attitudes, perception and knowledge. N Z Med J. 1998;111:269–271. [PubMed] [Google Scholar]
- 5.Poli F, Auffret N, Beylot C, et al. Acne as seen by adolescents: results of questionnaire study in 852 French individuals. Acta Derm Venereol. 2011;91:531–536. doi: 10.2340/00015555-1125. [DOI] [PubMed] [Google Scholar]
- 6.Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454–458. doi: 10.1001/archderm.134.4.454. [DOI] [PubMed] [Google Scholar]
- 7.Kilkenny M, Merlin K, Plunkett A, et al. The prevalence of common skin conditions in Australian school students: Acne vulgaris. Br J Dermatol. 1998;139:840–845. doi: 10.1046/j.1365-2133.1998.02510.x. [DOI] [PubMed] [Google Scholar]
- 8.Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity and severity risk factors of acne in high school pupils: a community-based study. J Invest Dermatol. 2009;129(9):2136–2141. doi: 10.1038/jid.2009.47. [DOI] [PubMed] [Google Scholar]
- 9.Purdy S, De Berker D. Acne. BMJ. 2006;333(7575):949–953. doi: 10.1136/bmj.38987.606701.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picardi A, Abeni D, Melchi CF, et al. Psychiatric morbidity in dermatological outpatients: an issue to be recognized. Br J Dermatol. 2000;143:983–991. doi: 10.1046/j.1365-2133.2000.03831.x. [DOI] [PubMed] [Google Scholar]
- 11.Thiboutot D, Gollnick H, Bettoli V, et al. Global alliance to improve outcomes in acne. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1–S50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Uslu G, Sendur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22:462–469. doi: 10.1111/j.1468-3083.2007.02497.x. [DOI] [PubMed] [Google Scholar]
- 13.Dreno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49:448–456. doi: 10.1111/j.1365-4632.2010.04416.x. [DOI] [PubMed] [Google Scholar]
- 14.Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13(9):611–617. [PubMed] [Google Scholar]
- 15.Niemeier V, Kupfer J, Gieler U. Acne vulgaris- psychosomatic aspects [in English, German] J Dtsch Dermatol Ges. 2006;4:1027–1036. doi: 10.1111/j.1610-0387.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 16.Krakowski AC, Stendardo S, Eichenfield LF. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatr Dermatol. 2008;25(Suppl 1):1–14. doi: 10.1111/j.1525-1470.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- 17.Serup J, Lindblad AK, Maroti M, et al. To follow or not to follow dermatological treatment-a review of the literature. Acta Derm Venereol. 2006;86:193–197. doi: 10.2340/00015555-0073. [DOI] [PubMed] [Google Scholar]
- 18.Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792–800. doi: 10.1016/j.jaad.2008.06.040. [DOI] [PubMed] [Google Scholar]


