INTRODUCTION
The limbic system is involved in some of the most challenging neurobehavioral disorders known to medicine, including disorders of mood and anxiety such as depression and posttraumatic stress disorder (PTSD), substance abuse and dependence, and disorders of cognition and memory such as Alzheimer disease. Advances in surgical neuromodulation of the limbic circuitry underlying these disorders offer a new hope for treatment. This article reviews limbic neuromodulation as it applies to addiction, PTSD, and memory.
THE LIMBIC SYSTEM
The definition of the limbic system has evolved over time, but most investigators would agree that affective processing is a central component of this system. Structures commonly included in the limbic system are the hippocampus, cingulate gyrus, amygdala, septal nuclei, hypothalamus, ventral striatum, ventral tegmentum, and prefrontal cortical regions. A full list of the structures and pathways is given in Table 1. The concept of the “greater limbic system” involves the role of memory and affect in orchestrating behavior to ensure the survival of the organism and species. This concept incorporates not only affective processing but also the association between memory, affect, and goal-directed behavior. This definition of the greater limbic system provides a key relationship that can help guide research on neuromodulation for addiction, PTSD, and disorders of memory and cognition.
Table 1.
Brain nuclei and fiber tracts of the limbic system
| Nucleus | Tract |
|---|---|
| Anterior nucleus of the thalamus | Mammillothalamic tract |
| Amygdala | Stria terminalis |
| Cingulate gyrus | Cingulum, internal capsule |
| Dentate gyrus | — |
| Entorhinal cortex | — |
| Habenula | Stria medullaris |
| Hippocampus | Fornix |
| Hypothalamus | — |
| Mammillary bodies | Mammillothalamic tract |
| Mediodorsal nucleus of the thalamus | Internal capsule |
| Nucleus accumbens | Medial forebrain bundle |
| Prefrontal cortex | Internal capsule |
| Subiculum | — |
| Septal nuclei | Anterior commissure |
| Ventral tegmental area | Medial forebrain bundle |
ADDICTION
Addiction is a major global medical, social, economic, and public health challenge. Approximately 25% of all deaths in Western industrial nations are directly or indirectly attributed to the consumption of addictive substances.1 Alcohol is the most frequently abused substance in the world, and in the United States, 1 in 6 patients in community-based practice has a problem with alcohol consumption.2 Other frequently abused substances include opioids, cocaine, and tobacco products. The cost for treatment of the addiction and, more importantly, from the loss of productivity is invaluable. The National Institute of Drug Abuse has estimated the annual cost of substance use disorders to the United States at over half a trillion dollars. A large body of evidence over the last several decades has shown that several components of the limbic system play a major role in addiction.
The nucleus accumbens (NAc) is one of the principal nuclei involved in the neural circuitry underlying reward and motivation, and is one of the main targets of the mesocorticolimbic reward pathway. A large body of evidence from several species, including humans, has implicated this pathway in reward processing, addiction, and goal-directed behavior.
The NAc is located in the ventral portion of the striatum, and its principal neuronal subtype is the γ-aminobutyric acid (GABA)ergic medium spiny inhibitory neuron. Single-neuron recordings from the NAc during self-administration of drug reinforcement have shown a population of neurons that exhibit increasing firing rates while the animals are working toward receiving a drug reward, and are quiescent immediately after reward acquisition.3,4 Ablation of the NAc may result in a decrease in reward-seeking behavior, and certain investigators have indicated that this has potential as a treatment for severe intractable drug addiction.
Few studies have investigated the role of ablation of the NAc in humans in drug-seeking behavior. Gao and colleagues5 performed bilateral ablation of the NAc in 28 patients addicted to various opioids. Although complete remission was reported in only 7 patients, the investigators reported decreased withdrawal symptoms in the remaining patients and concluded that bilateral ablation of the NAc is a safe and effective treatment for opioid addiction. These results were extended to a cohort of 12 patients with alcohol dependence who underwent bilateral NAc ablation. In this study there was also a significant reduction in dependence and craving in the majority of patients.6
Although these ablative studies confirm the important role of the NAc in drug-seeking in humans, ablation has the disadvantage of being irreversible and, therefore, potentially damaging to normal reward processing. From this perspective, deep brain stimulation (DBS) carries less risk. However, human studies of DBS of the NAc specifically to evaluate drug-seeking behavior are limited to case reports or case series. These reports suggest that DBS of the NAc decreases craving for nicotine, alcohol, and heroin.7
The NAc can be subdivided into two anatomically and functionally distinct regions known as the core and shell. Evidence suggests that the shell may be selectively involved in limbic processing, whereas the core may be considered an interface between limbic and motor networks.8 Thus, the shell may mediate the desire to use a substance and, through its connectivity with the core, translate this desire into action. One could hypothesize that DBS for addiction should target the shell and not the core, as the latter could produce undesired motor suppression. The role of the core in nonspecific motor response has also been described in animal studies.9
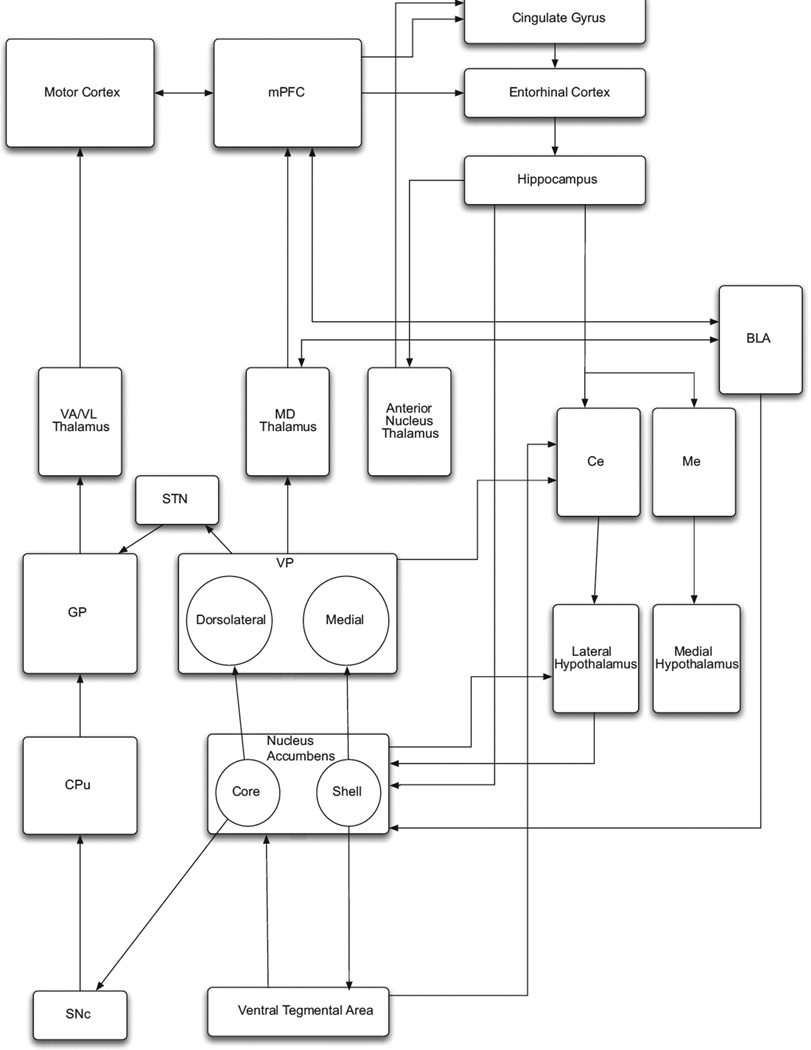
Just as the activity of striatal neurons is modulated by dopaminergic input from the substantia nigra pars compacta, analogous input from the ventral tegmental area (VTA) provides dopaminergic modulation of accumbal neurons. This mesoaccumbens pathway, located within the medial forebrain bundle (MFB), has long been known to be a powerful substrate for electrical self-stimulation that mimics addictive drug-seeking behavior.10,11 Thus, other nuclei and pathways in this reward network such as the VTA, MFB, ventral pallidum, mediodorsal thalamic nucleus, and cortical regions such as the cingulate, prefrontal, and orbitofrontal cortices, are potential targets of DBS for addiction (Fig. 1). However, neuromodulation of most of these regions specifically for addiction has not been described in humans with the exception of the accumbens and cingulate gyrus. Inadvertent DBS of the MFB has been shown to cause hypomania.12 Thus, stimulation of the MFB supports the role of the NAc in goal-directed behavior in humans. Hypomania in MFB stimulation seems to confirm the role of the NAc in goal-directed motor activity in humans.
Fig. 1.
Nuclei within the limbic system and their connectivity to motor output systems. BLA, basolateral nucleus of amygdala; Ce, central nucleus of amygdala; CPu, caudate-putamen; GP, globus pallidus; MD, mediodorsal nucleus; Me, medial nucleus of amygdala; mPFC, medial prefrontal cortex; SNc, substantia nigra pars compacta; VA/VL, ventral anterior/ventral lateral nuclei. Note that the ventral striatum/nucleus accumbens projects to the motor striatum via its core subdivision. In addition, the hypothalamus has efferents to the brainstem and spinal cord. These pathways provide a route for the limbic system to influence behavioral output.
The cingulate gyrus is another major limbic region with connectivity to the medial prefrontal cortex, mediodorsal thalamus, amygdala, hippocampus, and NAc.13 Neuromodulation of the cingulate gyrus has been shown to be effective for pain and obsessive-compulsive disorder (OCD).14 Early clues as to role of this region in addiction came from cingulotomies performed in patients to treat intractable pain. Most of these patients had narcotic dependence that improved following cingulotomy.15 Since then, several studies have reported successful cingulotomy for narcotic dependence.16,17 More recently, a Russian group reported the successful use of bilateral cryocingulotomies for the treatment of intractable heroin addiction in 348 patients. Follow-up was reported in 187 of these patients, 62% of whom were found to be completely free of opioid use after 2 years, while another 13% showed partial improvement.18
In addition to the NAc and the cingulate gyrus, other nuclei in the limbic reward network that may be potential targets of neuromodulation for drug addiction include the hypothalamus, basolateral amygdala, lateral habenula, substantia innominata, and subthalamic nucleus (STN).19–24
Witjas and colleagues25 reported on 2 patients with a history of addiction who underwent DBS of the STN for the treatment of Parkinson disease. In 1 patient, premorbid excessive alcohol intake was greatly reduced after stimulation was initiated.1 The STN is divided into motor, associative, and limbic regions. Rouaud and colleagues26 proposed that high-frequency stimulation of the STN decreases incentive motivation for cocaine while inducing the opposite effect for food.3,4
The ventromedial nucleus of the hypothalamus has also been tested as a target for alcohol addiction. Unfortunately, all patients showed side effects including reduction of sexual drive, decreased impulsivity, amnesia, visual disturbances, and vegetative crisis.5,23 Application of electrical stimulation to the lateral hypothalamus has been shown to potentially lead to weight loss in patients with intractable morbid obesity.6,27 In obese patients, functional neuroimaging studies have shown that food activates the ventral striatum in the same way that drugs do in patients with substance dependence.7,28
The amygdala has also been proposed as a target of DBS for drug addiction, based on evidence showing that inactivation of the basolateral amygdala in rats reduces cue-induced reinstatement of cocaine-seeking and activation of the amygdala during presentation of drug-associated cues.8,29
DBS of the lateral habenula at low frequency also results in increasing target activity and increasing self-administration of cocaine, whereas high-frequency stimulation had no effect. Unconventional stimulation alternating between 10 and 100 Hz decreased self-administration of cocaine; however, it also decreased general motivation.9,30
DBS for the treatment of addiction is still in its infancy, and is based largely on the studies of neuromodulation in rodent models. This brings us to an interesting and unique limitation and ethical dilemma. Animal models of addiction do not represent the full complexity of the disorder. Furthermore, there are differences in anatomic brain regions between rodents and humans, and many technical aspects of DBS in rodents differ significantly from human clinical parameters. Additional caveats for studying the effects of DBS in animal models include the difficulties in replicating the social and environmental factors associated with different clinical disease states, the multitude of cognitive and physiologic domains that characterize psychiatric disorders, and the differences in structural homology among rodents, nonhuman primates, and humans.12,31 Because of these neuroanatomic and pathophysiologic differences between rodent models and human patients, Müller and colleagues7 suggest that it is necessary to carefully continue to study the effects of DBS in severely addicted individuals in addition to the translational studies in animal models.13 These investigators further state that in their opinion, enough is known to ethically justify continuing to analyze the risk versus benefit of DBS, comparing the enormous medical and socioeconomic burden of the disease with the risks of DBS and its potential to alleviate disease severity and even prevent relapses.7,14 A recent cost analysis was conducted by Stephen and colleagues32 to evaluate the cost of DBS in comparison with that of medical treatment of opiate addiction. The study revealed that the threshold success rate, which is defined as the percentage of patients remaining heroin-free after 6 months of treatment, at which DBS would be as cost-effective as methadone is estimated at only 49%. This group concluded that a theoretical course of DBS would need a success rate of 36.5% to match the current methadone treatment, but a success rate of 49% to be cost-effective.15
It is therefore imperative that DBS for addiction can only be considered when the highest medical ethical standards are applied, given the nature of the conditions that are being treated.16,17,33 Furthermore, patients should be carefully screened and followed by an interdisciplinary team that includes the neurosurgeon, psychiatrists, neurologists, social workers and support groups, because DBS is an intensive procedure that requires extensive follow-up and careful observation of symptoms and possible side effects. As Luigjes and colleagues34 point out, DBS should be restricted to chronically addicted, treatment-refractory patients stable enough to comply with an intensive period of treatment and research.18
POSTTRAUMATIC STRESS DISORDER
PTSD is an anxiety disorder that develops following a life-threatening or an integrity-threatening traumatic event, and often includes perceptual, cognitive, affective, physiologic, and psychological features. PTSD is characterized by hyperarousal, intrusive vivid reliving of memories, and persistent avoidance of trauma-associated stimuli.19–24,35 The estimated lifetime prevalence of PTSD in the United States is approximately 6.8%, and the 12-month prevalence is about 3.8%.36,37 Unfortunately, 30% of patients still suffer from PTSD at least 10 years after the initial trauma despite the best current medical therapy.38
Functional neuroimaging studies in veterans indicate that the amygdala may play a critical role in the development of PTSD. In fact, PTSD patients subjected to provocative testing have shown increased activity in the amygdala on functional magnetic resonance imaging or positron emission tomography (PET)/computed tomography.39–43 The intensity of the amygdala activity seen on imaging also correlates with the severity of PTSD symptoms.41,44 Of note, veterans with injury to the amygdala never develop PTSD.45 Koenigs and Grafman46 showed that 40% of veterans who suffered brain injury in combat develop PTSD unless the amygdala is injured. The amygdala seems to be responsible for the encoding and retrieval of the memories associated with the traumatic events. In this sense, it is responsible for the symptoms and suffering associated with PTSD.
If it is assumed that DBS can functionally reduce the activity of a cerebral target and that activity in the amygdala seems to be responsible for PTSD development, DBS of the amygdala may treat the symptoms of PTSD. Langevin and colleagues47 tested this hypothesis using a rodent model of PTSD in which rats were given inescapable shock in the presence of an unfamiliar object. The rats then developed a tendency to bury the object when reexposed to it several days later. This behavior mimics the symptoms of PTSD. Ten rats underwent placement of an electrode in the right basolateral nucleus of the amygdala (BLn). The rats were then subjected to a session of inescapable shocks while being exposed to an object. Five rats received high-frequency DBS treatment while the other 5 rats did not. Rats that were treated with BLn DBS spent on average 13-fold less time burying the ball than the control rats, and thus the behavior of treated rats was nearly normalized. More recently, Stidd and colleagues48 demonstrated that the effects of BLn DBS in this rat model of PTSD were preserved even when DBS was initiated late after the establishment of the PTSD behavior.
Alternative methods of neuromodulation in the treatment of PTSD, such as vagus nerve stimulation (VNS), have also been studied. In VNS, electrical pulses are applied to the vagus nerve in the neck to activate afferents. VNS does not provide direct stimulation to the cortex but acts broadly by activating the parasympathetic nervous system. The Food and Drug Administration approved VNS in 1997 for epilepsy and in 2005 for treatment-resistant depression. George and colleagues49 performed an open-label study of adjunctive VNS treatment for anxiety, and suggested that this treatment was well tolerated and possibly efficacious in patients with a range of anxiety disorders, including OCD, panic disorder, and PTSD. The group speculated that the effects likely derived from downstream modulation by the vagus nerve afferents on the locus coeruleus, orbitofrontal cortex, insula, hippocampus, and amygdala.22
Unfortunately, human investigations of neuromodulation for the treatment of PTSD are limited. There are currently no human studies involving amygdala DBS. A longitudinal study in humans is needed to evaluate the effects of DBS of the amygdala on PTSD.
MEMORY
Alzheimer disease is the most common form of dementia. It is a progressive disease, and currently there is no cure. Pharmacologic agents such as acetylcholinesterase inhibitors and N-methyl-d-aspartate receptor antagonists are used to delay the progression, but they are not effective in all patients and carry significant side-effect profiles. Alzheimer disease is usually diagnosed in people older than 65 years. Early onset is less frequent but is also possible. In 2006, there were 26.6 million patients with Alzheimer globally, and the number is expected to reach 1 in 85 by 2050.5 Therefore, it is imperative to develop a different strategy in Alzheimer treatment. This section reviews the role of limbic neuromodulation in the treatment of dementia.
At the heart of the limbic system since its earliest conceptualization lie the mesial temporal lobe structures and their targets, including the hippocampal formation and fornix. The role of this region in the formation of memory is well established. Abnormalities of these regions have been linked to disorders of cognition and memory such as Alzheimer disease and other forms of dementia. For example, hippocampal atrophy and volume have been used to predict the onset of dementia.50–52 Other regions with connectivity to the hippocampus and fornix include the mammillary bodies, anterior nucleus of the thalamus, cingulate gyrus, and basal forebrain cholinergic nuclei.
The anterior nucleus of the thalamus (AN) has already been studied as a target of DBS in the treatment of intractable epilepsy. The AN receives afferent fibers from mammillary bodies via the mammillothalamic tract and from the subiculum via the fornix. Efferent fibers from the AN project to the cingulate gyrus. The AN is an integral part of the limbic system that seems to play a role in modulating alertness, and is involved in learning and memory. Oh and colleagues53 studied DBS of the AN in the treatment of patients with intractable epilepsy, and found that not only did it result in seizure control but was also associated with improvements in both verbal recall and oral information processing. The investigators proposed that the effect on memory could be related to the bilateral activation of a thalamacortical circuit following DBS surgery. These results suggest that DBS of the AN and functionally related regions may be used to enhance memory. Indeed, high-frequency stimulation of the AN in rats has been shown to increase hippocampal neurogenesis and to reverse experimentally suppressed hippocampal neurogenesis. These findings suggest that DBS not only alters neuronal activity but also produces long-term neuronal changes that may potentially enhance memory formation.54
High-frequency stimulation to isolated hippocampal slices also increases synaptic plasticity in the CA1 region and results in a 2-fold increase of nonamyloidogenic α-secretase activity, in comparison with controls stimulated at low frequency.55 DBS treatment has also been shown to facilitate acquisition of object-recognition memory in rats in comparison with the baseline.55 Stone and colleagues56 took this idea one step further and showed that stimulation of the entorhinal cortex in rats influences cognitive function via activity-dependent regulation of hippocampal neurogenesis. Acute stimulation of entorhinal cortex transiently promoted proliferation in the dentate gyrus. New cells generated as a consequence of stimulation eventually differentiated into neurons, survived for at least several weeks, and acquired normal dentate granule cell morphology. After maturation, these neurons integrated into hippocampal circuits supporting memory.56 Another group confirmed this finding by showing that DBS of the fornix in rats reverses the memory-impairing effects of scopolamine when compared with sham.57 Furthermore, they reported that the fornix is not sensitive to the frequency but rather the current and no side effects on anxiety or general motor activity were encountered.57
Human studies have corroborated these results in a single patient who underwent DBS of the hypothalamus for obesity. Hamani and colleagues58 discovered that DBS in this region surprisingly evoked detailed autobiographical memories. Electroencephalographic source localization in this patient identified activity in mesial temporal lobe structures in response to DBS of the hypothalamus. The investigators hypothesized that they were actually stimulating fibers of the fornix within the hypothalamus, which resulted in activation of mesial temporal lobe structures.58
Based on this initial result, the investigators from the previous study conducted a phase I clinical trial of DBS of the fornix/hypothalamus in patients with mild Alzheimer disease, using PET imaging to illustrate an early and striking reversal of the impaired glucose utilization in the temporal and parietal lobes that was maintained after 12 months of continuous stimulation. Stimulation of the fornix/hypothalamus drove neural activity in the memory circuit, including the entorhinal and hippocampal areas, and activated the brain’s default mode network. At 6 and 12 months follow-up, evaluations suggested possible improvements and/or slowing in the rate of cognitive decline. In addition, there were no serious adverse events reported.59
Fontaine and colleagues60 also conducted a prospective study in a single patient with Alzheimer disease who underwent bilateral forniceal DBS. Electrodes were placed in the fornix within the hypothalamus bilaterally; at 1-year follow-up, the patient’s memory score stabilized in comparison with baseline, with an increased metabolism in the mesial temporal lobes. No complications were reported.
Whereas the aforementioned human studies indirectly stimulated the mesial temporal lobe by purportedly stimulating forniceal fibers in the hypothalamus, Suthana and colleagues61 studied the effect of direct entorhinal stimulation in patients without dementia. This study involved stimulation of the entorhinal cortex in patients implanted with depth electrodes for the purpose of seizure monitoring. In this study, 7 subjects underwent stimulation of the entorhinal cortex while learning a visuospatial memory task involving reaching locations by using landmarks. The subjects who underwent stimulation during the learning phase reached their target locations more quickly and by shorter routes. Remarkably, this study suggests that direct stimulation of mesial temporal lobe structures may enhance learning and memory in patients without baseline dementia.61
Other targets have been tested and studied for possible impact on memory and treatment of dementia. Freund and colleagues62 reported a single patient with dementia treated with DBS of the nucleus basalis of Meynert (nbM). Stimulation of the nbM resulted in markedly improved cognitive function. Improvement in attention, concentration, alertness, drive, and spontaneity resulted in the patient’s renewed enjoyment of former interests and enhanced social communication. While the exact mechanism for the success of nbM DBS in memory improvement remains unknown, many hypotheses have been proposed, including enhancement of the synthesis of nerve growth factor and possible facilitating/resetting of neural oscillation.63
Costa and colleagues64 reported experimental investigations into the effect of low-frequency electrical stimulation (25 Hz) of the pedunculopontine (PPN) area on working memory in patients with Parkinson disease. Patients showed a consistent decrease in response time on both the verbal and visual-object tasks when going from the “Off” to the “On” condition. However, the accuracy score did not significantly differ between the two experimental conditions. Costa proposed that stimulation of the PPN area may facilitate the speed of processing of information in the content of working memory, or the memory system that keeps multiple pieces of information in a place where they can be manipulated.
In conclusion, our understanding of the neural substrates underlying memory is still in its infancy, despite important discoveries over the last few years. DBS as a means to modulate and augment memory and cognition shows promise in the treatment of conditions characterized by cognitive impairment. Learning and recall are highly dynamic processes. Therefore, dynamic neuromodulatory devices and technologies are likely to be required to modulate these functions.65 The initial goal of neuromodulation in memory is to help patients suffering from cognitive difficulties such as dementia. However, the remote possibility of cognitive enhancement using neuromodulation may raise ethical dilemmas in the future.
SUMMARY
The limbic system lies at the center of some of the most intractable neuropsychiatric disorders such as addiction, PTSD, and dementia. Successful treatment of these disorders has eluded physicians and scientists for decades. Preliminary results show that DBS to specific nuclei within the limbic system may be at least as successful as traditional pharmacologic therapies. In addition, advances in limbic neuromodulation are increasing our understanding of how the limbic system works in healthy people. There remains a mix of healthy skepticism and misunderstanding among the public and physicians outside of neurosurgery regarding the safety and efficacy of DBS for neuropsychiatric disorders. The ethical and legal challenges to the widespread application of this technology offer new opportunities for education and increasing social awareness, and will require the cooperative effort of multidisciplinary treatment teams consisting of neurosurgeons, neurologists, psychiatrists, and other specialties.
KEY POINTS.
The nucleus accumbens is a potential target of deep brain stimulation (DBS) for drug addiction.
The amygdala is a potential DBS target for posttraumatic stress disorder and anxiety.
The entorhinal area, hippocampus, and fornix are potential DBS targets for dementia.
Neuromodulation for neuropsychiatric disorders poses ethical challenges.
REFERENCES
- 1.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–2212. [PubMed] [Google Scholar]
- 2.Xu F, et al. Surveillance for certain health behaviors among States and selected local areas—United States, 2010. MMWR Surveill Summ. 2013;62:1–247. [PubMed] [Google Scholar]
- 3.Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiol Behav. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- 4.Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–296. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- 5.Gao G, et al. Clinical study for alleviating opiate drug psychological dependence by a method of ablating the nucleus accumbens with stereotactic surgery. Stereotact Funct Neurosurg. 2003;81:96–104. doi: 10.1159/000075111. [DOI] [PubMed] [Google Scholar]
- 6.Wu HM, et al. Preliminary findings in ablating the nucleus accumbens using stereotactic surgery for alleviating psychological dependence on alcohol. Neurosci Lett. 2010;473:77–81. doi: 10.1016/j.neulet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Müller UJ, et al. Deep brain stimulation of the nucleus accumbens for the treatment of addiction. Ann N Y Acad Sci. 2013;1282:119–128. doi: 10.1111/j.1749-6632.2012.06834.x. [DOI] [PubMed] [Google Scholar]
- 8.Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- 9.Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell sub-region of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 10.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 11.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 12.Coenen VA, et al. Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery. 2009;64:1106–1114. doi: 10.1227/01.NEU.0000345631.54446.06. [discussion: 1114–5]. [DOI] [PubMed] [Google Scholar]
- 13.Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 14.Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery. 2007;61:1–11. doi: 10.1227/01.neu.0000279719.75403.f7. [discussion: 11–3]. [DOI] [PubMed] [Google Scholar]
- 15.Foltz EL, White LE. Pain ‘relief’ by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 16.Kanaka TS, Balasubramaniam V. Stereotactic cingulumotomy for drug addiction. Appl Neurophysiol. 1978;41:86–92. doi: 10.1159/000102404. [DOI] [PubMed] [Google Scholar]
- 17.Balasubramaniam V, Kanaka TS, Ramanujam PB. Stereotaxic cingulumotomy for drug addiction. Neurol India. 1973;21:63–66. [PubMed] [Google Scholar]
- 18.Medvedev SV, Anichkov AD, Poliakov II. Physiological mechanisms of the effectiveness of bilateral stereotactic cingulotomy in treatment of strong psychological dependence in drug addiction. Fiziol Cheloveka. 2003;29:117–123. [in Russian]. [PubMed] [Google Scholar]
- 19.Knight G. Chronic depression and drug addiction treated by stereotactic surgery. Nurs Times. 1969;65:583–586. [PubMed] [Google Scholar]
- 20.Müller D, Roeder F, Orthner H. Further results of stereotaxis in the human hypothalamus in sexual deviations. First use of this operation in addiction to drugs. Neurochirurgia (Stuttg) 1973;16:113–126. doi: 10.1055/s-0028-1090504. [DOI] [PubMed] [Google Scholar]
- 21.Langevin JP. The amygdala as a target for behavior surgery. Surg Neurol Int. 2011;2:7. doi: 10.4103/2152-7806.91609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu L, Wang X, Kosten TR. Stereotactic neurosurgical treatment of drug addiction. Am J Drug Alcohol Abuse. 2009;35:391–393. doi: 10.3109/00952990903312478. [DOI] [PubMed] [Google Scholar]
- 23.Stelten BM, Noblesse LH, Ackermans L, et al. The neurosurgical treatment of addiction. Neurosurg Focus. 2008;25:E5. doi: 10.3171/FOC/2008/25/7/E5. [DOI] [PubMed] [Google Scholar]
- 24.Pelloux Y, Baunez C. Deep brain stimulation for addiction: why the subthalamic nucleus should be favored. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.02.016. http://dx.doi.org/10.1016/j.conb.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Witjas T, et al. Addiction in Parkinson’s disease: impact of subthalamic nucleus deep brain stimulation. Mov Disord. 2005;20:1052–1055. doi: 10.1002/mds.20501. [DOI] [PubMed] [Google Scholar]
- 26.Rouaud T, et al. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A. 2010;107:1196–1200. doi: 10.1073/pnas.0908189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiting DM, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg. 2013;119:56–63. doi: 10.3171/2013.2.JNS12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 29.Langevin JP. The amygdala as a target for behavior surgery. Surg Neurol Int. 2012;3:40–46. doi: 10.4103/2152-7806.91609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman A, et al. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59:452–459. doi: 10.1016/j.neuropharm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- 32.Stephen JH, et al. Deep brain stimulation compared with methadone maintenance for the treatment of heroin dependence: a threshold and cost-effectiveness analysis. Addiction. 2012;107:624–634. doi: 10.1111/j.1360-0443.2011.03656.x. [DOI] [PubMed] [Google Scholar]
- 33.Carter A, Hall W. Proposals to trial deep brain stimulation to treat addiction are premature. Addiction. 2011;106:235–237. doi: 10.1111/j.1360-0443.2010.03245.x. [DOI] [PubMed] [Google Scholar]
- 34.Luigjes J, et al. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry. 2012;17:572–583. doi: 10.1038/mp.2011.114. [DOI] [PubMed] [Google Scholar]
- 35.Novakovic V, et al. Brain stimulation in posttraumatic stress disorder. Eur J Psychotraumatol. 2011;2 doi: 10.3402/ejpt.v2i0.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 37.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breslau N. Outcomes of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):55–59. [PubMed] [Google Scholar]
- 39.Protopopescu X, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Semple WE, et al. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- 41.Shin LM, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 42.Shin LM, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 43.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armony JL, Corbo V, Clément MH, et al. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 45.Koenigs M, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langevin JP, De Salles AA, Kosoyan HP, et al. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res. 2010;44:1241–1245. doi: 10.1016/j.jpsychires.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 48.Stidd DA, Vogelsang K, Krahl SE, et al. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul. 2013 doi: 10.1016/j.brs.2013.05.008. http://dx.doi.org/10.1016/j.brs.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 49.George MS, et al. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul. 2008;1:112–121. doi: 10.1016/j.brs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Laakso MP, et al. Hippocampal volumes in Alzheimer‘ s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 51.Junqué C, et al. Amygdalar and hippocampal MRI volumetric reductions in Parkinson’s disease with dementia. Mov Disord. 2005;20:540–544. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- 52.Aybek S, et al. Hippocampal atrophy predicts conversion to dementia after STN-DBS in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:521–524. doi: 10.1016/j.parkreldis.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Oh YS, et al. Cognitive improvement after long-term electrical stimulation of bilateral anterior thalamic nucleus in refractory epilepsy patients. Seizure. 2012;21:183–187. doi: 10.1016/j.seizure.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Toda H, Hamani C, Fawcett AP, et al. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg. 2008;108:132–138. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- 55.Arrieta-Cruz I, Pavlides C, Pasinetti GM. Deep brain stimulation in midline thalamic region facilitates synaptic transmission and short-term memory in a mouse model of Alzheimer’s disease. Transl Neurosci. 2010;1:188–194. doi: 10.2478/v10134-010-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stone SS, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hescham S, et al. Deep brain stimulation of the forniceal area enhances memory functions in experimental dementia: the role of stimulation parameters. Brain Stimul. 2013;6:72–77. doi: 10.1016/j.brs.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Hamani C, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63:119–123. doi: 10.1002/ana.21295. [DOI] [PubMed] [Google Scholar]
- 59.Laxton AW, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 60.Fontaine D, et al. Symptomatic treatment of memory decline in Alzheimer’s disease by deep brain stimulation: a feasibility study. J Alzheimers Dis. 2013;34:315–323. doi: 10.3233/JAD-121579. [DOI] [PubMed] [Google Scholar]
- 61.Suthana N, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freund HJ, et al. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol. 2009;66:781–785. doi: 10.1001/archneurol.2009.102. [DOI] [PubMed] [Google Scholar]
- 63.Hardenacke K, et al. Stimulate or degenerate: deep brain stimulation of the nucleus basalis Meynert in Alzheimer dementia. World Neurosurg. 2012 doi: 10.1016/j.wneu.2012.12.005. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 64.Costa A, et al. Effects of deep brain stimulation of the pedunculopontine area on working memory tasks in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:64–67. doi: 10.1016/j.parkreldis.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Hu R, Eskandar E, Williams Z. Role of deep brain stimulation in modulating memory formation and recall. Neurosurg Focus. 2009;27:E3. doi: 10.3171/2009.4.FOCUS0975. [DOI] [PMC free article] [PubMed] [Google Scholar]