Elevations in parathyroid hormone (PTH) level have been associated with adverse clinical outcomes, including cardiovascular disease and mortality.1,2 Loop diuretics have been linked to increased PTH levels in patients with chronic kidney disease (CKD),3,4 but this association has not been investigated in the general population. We used data from the National Health and Nutrition Examination Survey (NHANES)5,6 to test the hypothesis that loop diuretic use associates with elevated PTH in adults with preserved renal function.
Methods
We studied participants from NHANES 2003 to 20045 and 2005 to 20066 because PTH measurements were available in these years. Participants were excluded for age younger than 18 years, estimated glomerular filtration rate (eGFR) lower than 60 mL/min/1.73 m2 (using the CKD-EPI [Chronic Kidney Disease Epidemiology Collaboration] equation), or missing PTH value. “Loop users” had prescriptions for furosemide, bumetanide, or torsemide.
Baseline characteristics of loop users and nonusers were compared using t tests or χ2 tests. A multivariable linear regression model was constructed to test the association between loop use and natural log-transformed PTH level. Covariates were age, sex, body mass index (BMI), history of congestive heart failure (CHF); smoking; dietary calcium and phosphorus intake; eGFR; and levels of serum 25-(OH) vitamin D, calcium, phosphorus, uric acid, albumin, and alkaline phosphatase. Least-square means were used to estimate natural log-transformed PTH levels of loop users and nonusers. These values were exponentiated and the adjusted geometric means of PTH level from the final model are reported. Multivariable logistic regression was used to test the association between loop use and odds of a PTH level higher than 65 pg/mL.
Results
Of 20 470 participants, 9287 were younger than 18 years; 2217 had an eGFR lower than 60 mL/min/1.73 m2, and 3 were missing a PTH value. The remaining 8963 participants were included in the analysis. After application of survey weights, 1.8% of participants were loop users.
Loop users were more likely to be female, older, nonsmokers, and have a higher BMI (Table). They had significantly higher uric acid and alkaline phosphatase levels but lower eGFR, 25-(OH) vitamin D, and albumin levels. Loop users had a lower dietary intake of calcium and phosphorus and were more likely to have a history of CHF. Race and serum calcium and phosphorus levels were similar between groups.
Table.
Characteristics of Non–Loop Users and Loop Users
| Characteristic | Median (Quartiles 1–3) | P Valuea | |
|---|---|---|---|
| Non–Loop Users (n = 8801) | Loop Users (n = 162) | ||
| Age, y | 41.7 (29.9–53.1) | 65.7 (55.7–74.9) | <.001 |
| Male, % | 48.7 | 36.4 | <.001 |
| African American, % | 22.8 | 25.3 | .15 |
| BMI | 27.1 (23.7–31.5) | 31.4 (27.2–38.7) | <.001 |
| Current smoker, %b | 21.3 | 13.6 | .01 |
| Hyperparathyroidism, %c | 6.5 | 19.8 | <.001 |
| History of CHF, % | 1.4 | 32.1 | <.001 |
| eGFR, mL/mind | 96.5 (82.8–109.9) | 76.3 (67.6–88.7) | <.001 |
| Calcium level, mg/dL | 9.5 (9.3–9.7) | 9.4 (9.2–9.6) | .08 |
| Phosphorus level, mg/dL | 3.7 (3.4–4.1) | 3.7 (3.4–4.1) | .35 |
| Uric acid level, mg/dL | 5.2 (4.2–6.1) | 5.6 (4.9–6.7) | <.001 |
| Albumin level, g/dL | 0.42 (0.40–0.45) | 0.40 (0.38–0.42) | <.001 |
| Alkaline phosphatase level, U/L | 64.2 (53.1–78.3) | 77.1 (65.4–99.3) | <.001 |
| PTH level, pg/mL | 37.4 (28.2–49.0) | 51.5 (41.3–69.4) | <.001 |
| Total 25 (OH) vitamin D level, ng/mL | 22.7 (16.8–28.7) | 18.3 (13.1–23.6) | <.001 |
| Dietary calcium level, mg/d | 413.1 (268.8–631.3) | 330.7 (237.4–511.2) | <.001 |
| Dietary phosphorus level, mg/d | 667.4 (473.1–912.8) | 515.4 (375.6–705.8) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHF, congestive heart failure; CKD, chronic kidney disease; cr, creatinine; eGFR, estimated glomerular filtration rate; EPI, Epidemiology Collaboration; GFR, glomerular filtration rate; PTH, parathyroid hormone.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; to convert alkaline phosphatase to microkatals per liter, multiply by 0.0167; to convert calcium to millimoles per liter, multiply by 0.25; to convert 25-hydroxyvitamin D to nanomoles per liter, multiply by 2.496; to convert uric acid to micromoles per liter, multiply by 59.485.
Bold font indicates statistical significance (P < .05).
Defined as having smoked more than 100 cigarettes in one’s lifetime and still smoking some or most days of the week.
Defined as PTH level greater than 65 pg/mL.
Calculated using CKD-EPI (GFR = 141 × min (Scr/κ, 1)α × max (Scr/κ, 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where Scr indicates serum creatinine and κ is a correction factor, defined as follows: →0.7 if female and →0.9 if male.
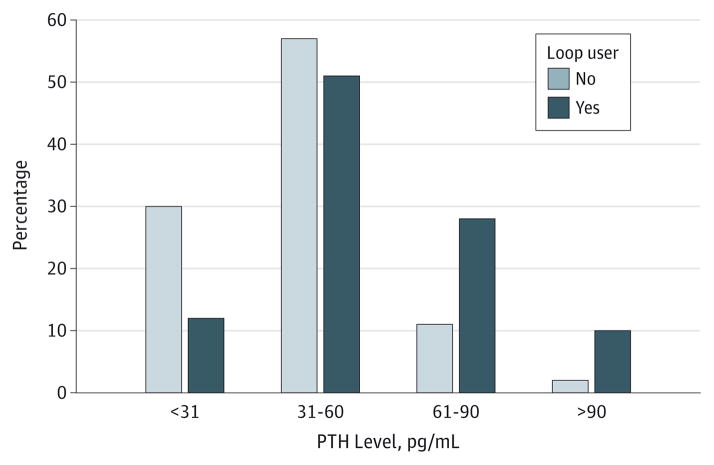
The Figure depicts the range of PTH levels among loop users and nonusers. Using the final multivariable model, the adjusted geometric mean PTH level in loop users was significantly higher than in nonusers (43.4 pg/mL compared with 38.8 pg/mL; P < .001). Using multivariable logistic regression, loop use was associated with significantly increased odds of a PTH level higher than 65 pg/mL (odds ratio, 1.83 [CI, 1.16–2.88]; P = .01).
Figure. The Range of Parathyroid Hormone (PTH) Levels Among Loop Users and Nonusers.
Levels are depicted in increments of 30 pg/mL. More loop users have PTH values above the upper limit of normal.
Discussion
Our results demonstrate that loop use associates with higher PTH, even after adjustment for potential confounders. While previous studies have found similar associations in CKD, we demonstrate that this relationship extends to patients with preserved kidney function, having an impact on a considerably larger population. Clinicians often monitor for electrolyte changes after initiation of loop diuretics but may not measure PTH level. Many loop users are elderly or have CHF, both of which contribute to bone loss. Use of a different diuretic class, repletion of vitamin D, or increased dietary calcium intake may combat a rise in PTH and reduce the risk of adverse outcomes.
Conclusions
This study included a large, representative sample of adults. Many variables known to influence PTH level were included in the analysis. Missing data limited our ability to examine the relationship between loop use and clinical outcomes, and while we tried to control for potential confounders, residual confounding may exist. Prospective studies are needed to define the direct effect of loop use on PTH level. Nonetheless, our work suggests that loop diuretic use may lead to elevation in PTH level and consequently other adverse clinical effects.
Acknowledgments
Funding/Support: This study was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases Program at the National Institutes of Health (NIH) (training grant T32DK007540).
Role of the Sponsor: The NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentation: Data from this research were presented in poster form at the American Society of Nephrology Annual Meeting; November 10, 2013; Atlanta, Georgia.
Author Contributions: Dr Corapi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Corapi, McMahon, Seifter, Bhan.
Acquisition, analysis, or interpretation of data: Corapi, McMahon, Wenger, Bhan.
Drafting of the manuscript: Corapi, Wenger.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Corapi, Wenger.
Administrative, technical, or material support:
Study supervision: McMahon, Wenger, Bhan.
References
- 1.Yu N, Donnan PT, Leese GP. A record linkage study of outcomes in patients with mild primary hyperparathyroidism: the Parathyroid Epidemiology and Audit Research Study (PEARS) Clin Endocrinol (Oxf) 2011;75(2):169–176. doi: 10.1111/j.1365-2265.2010.03958.x. [DOI] [PubMed] [Google Scholar]
- 2.Hagström E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119(21):2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 3.Isakova T, Anderson CA, Leonard MB, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Diuretics, calciuria and secondary hyperparathyroidism in the Chronic Renal Insufficiency Cohort. Nephrol Dial Transplant. 2011;26(4):1258–1265. doi: 10.1093/ndt/gfr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichel H, Deibert B, Geberth S, Schmidt-Gayk H, Ritz E. Frusemide therapy and intact parathyroid hormone plasma concentrations in chronic renal insufficiency. Nephrol Dial Transplant. 1992;7(1):8–15. [PubMed] [Google Scholar]
- 5.National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2003–2004. [Google Scholar]
- 6.National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005–2006. [Google Scholar]