Abstract
Angiotensin II (AngII) is an octapeptide hormone that plays a central role in regulation of sodium balance, plasma volume, and blood pressure. Its role in the pathogenesis of hypertension is highlighted by the wide use of inhibitors of the renin-angiotensin system (RAS) as the first-line antihypertensive therapy. However, despite intensive investigation, the mechanism of AngII-induced hypertension is still incompletely understood. Although diverse pathways are likely involved, increasing evidence suggests that the activation of intrarenal RAS may represent a dominant mechanism of AngII-induced hypertension. (Pro)renin receptor (PRR), a potential regulator of intrarenal RAS, is expressed in the intercalated cells of the collecting duct (CD) and induced by AngII, in parallel with increased renin in the principal cells of the CD. Activation of PRR elevated PGE2 release and COX-2 expression in renal inner medullary cells whereas COX-2-derived PGE2 via the EP4 receptor mediates the upregulation of PRR during AngII infusion, thus forming a vicious cycle. The mutually stimulatory relationship between PRR and COX-2 in the distal nephron may play an important role in mediating AngII-induced hypertension.
Introduction
The RAS has been known for more than a century as one of most important hormonal systems that regulate blood pressure, cardiovascular function, renal hemodynamics and tubular sodium reabsorption [1]. AngII is the major effector hormone in this system and produces vasoconstrictive, pro-inflammatory, anti-natriuretic, and anti-diuretic effects. Over the years, AngII has been shown to play important roles in the pathogenesis of hypertension, heart failure, cardiac remodeling, chronic kidney disease, diabetes, etc. [2]. Inhibition of AngII production with angiontensin converting enzyme inhibitor (ACEi) or AngII action with AT1 blockers is used as the first-line antihypertensive therapy. Despite the intensive investigation, the mechanism of AngII-induced hypertension is still incompletely understood.
(Pro)renin receptor (PRR) is a new member of the RAS and it binds renin and its inactive precursor, prorenin, with almost equal affinity; this interaction elevates the catalytic activity [3]. Due to its ubiquous expression pattern, PRR is considered to play an important role in regulation of tissue renin activity thereby controlling the activity of local RAS [3]. In recent years, there is increasing recognition of local RAS as an important contributor of hypertension, cardiovascular disease, and kidney diseases [4,5]. Within the kidney, PRR expression is found in glomerular mesangial cells [3], the subendothelium of renal arteries [3], and the distal nephron [6]. Chronic infusion of AngII in rats increased renal PRR transcript levels and augmented the PRR activity in renal medullary tissues, which may contribute to increased renin activity in the CD during AngII hypertension [7]. Increased expression of CD PRR is also observed in 2K1C Goldblatt hypertensive model [8]. The activation of renal medullary PRR may serve as an important mechanism triggering the local renin response that may participate in regulation of blood pressure and fluid metabolism during AngII hypertension [7].
The renal medulla is a major site of production and action of prostaglandins (PGs).
Cyclooxygenase-2 (COX-2) is abundantly expression in the renal medulla where COX-2-derived products exert complex roles in regulation of fluid balance and blood pressure. Evidence is emerging to suggest that PRR and COX-2 stimulate the expression of each other in the renal medullary cells [9•,10••,11•]. This review will focus on recent findings regarding the mutually stimulatory relationship between the two mediators in the renal medulla and discuss its implication in the control of intrarenal RAS and blood pressure during AngII-induced hypertension.
The role of intrarenal RAS in AngII-induced hypertension
In recent years, a new paradigm has emerged that the activation of local RAS in the kidney (termed intrarenal RAS) serves as an important mechanism of AngII-induced hypertension [12•]. The existence of intrarenal RAS was first described over 20 years ago, where the level of renal interstitial and tubular fluid AngII was much higher than in plasma [13,14]. The existence of intrarenal RAS is further highlighted by the discovery of renin expression in the connecting tubules and cortical and medullary collecting ducts (CDs) [15,16] and angiotensinogen expression in the proximal tubule [17], the two key elements of paracrine tubular RAS. The regulation of intrarenal RAS by AngII is distinct from that of systemic RAS. AngII infusion elevates de novo AngII generation in the kidney due to augmentation of angiontensinogen [18,19] and renin in the collecting duct (CD) [20,21], indicating a positive feedback regulation of intrarenal RAS by AngII. This is completely opposite to the well-established view of the negative feedback regulation of juxtaglomerular renin by AngII. Subsequent functional studies using pharmacological and genetic approaches examine the role of intrarenal RAS during AngII-induced hypertension. Experiments in rats infused with Val5-Ang II, an isoform of AngII that can be separated from endogenous AngII (Ile5-Ang II) by high-performance liquid chromatography, demonstrated that the chronic Val5-Ang II (exogenous AngII) infusion induces renal Ile5-Ang II (endogenous Ang II) synthesis [22]. In another study, when endogenous AngII production was reduced by ACE inhibition, AngII-infused mice became normotensive [23,24]. The genetic absence of kidney ACE substantially blunts the hypertension induced by AngII infusion [25]. In experiments involving kidney cross-transplantation between global AT1 KO mice and wild-type controls, AngII is shown to cause hypertension through stimulation of AT1 receptors in the kidney [26]. Lastly, overexpression of renin in the CD causes spontaneous hypertension [27] and CD-specific deletion of renin attenuates AngII-induced hypertension [28••]. Together, these results represent compelling evidence for an essential role of intrarenal RAS in the pathogenesis of hypertension at least during AngII infusion. This mechanism may have a broad implication since activation of intrarenal RAS has been linked to salt-sensitive hypertension [29].
Role of PRR in regulation of COX-2 expression in the CD
To study the potential interaction between PRR and COX-2, Kaneshiro et al. assessed the expression and function of renal COX-2 in rats with transgenic expression of human PRR receptor [30]. In this study, the transgene expression was driven by a cytomegalovirus immediate early gene enhancer and rabbit β-actin gene terminator sequences. The transgenic rats with global overexpression of human PRR exhibited increased COX-2 expression in the macula densa and decreased renal cortical blood flow following COX-2 inhibition with NS-398 whereas this treatment was ineffective in wild-type rats [30]. In the transgenic rats, phospho-ERK was elevated in the cortex but tissue AngII levels were unaffected, suggesting that PRR may stimulate COX-2 expression via ERK-dependent and AngII-independent mechanism [30]. It is unclear why the global increase in PRR expression leads to the selective upregulation of COX-2 expression in the macula densa. This study focuses on macula densa as a site of COX-2 regulation by PRR. At this point, the effects of PRR activation on COX-2 expression in the renal medulla remain unclear.
Within the kidney, PRR is predominantly expressed in the intercalated cells of the CD. Renal medulla is a rich source of PG synthesis, where COX-2 is abundantly expressed at baseline and induced by various physiological stimuli including high salt loading [31] and water deprivation [32,33]. To exmaine the possible interaction between PRR and COX-2 in the renal medulla, Gonzalez et al. tested the effect of PRR activation on COX-2 expression in primary rat inner medullary (IM) cells [11•]. In this study, PRR and COX-2 were colocalized in intercalated and interstitial cells whereas principal cells did not express PRR or COX-2 [11]. Exposure of rat IM cells to rat recombinant prorenin (100 nmol/L) increased ERK 1/2 phosphorylation and COX-2 expression. Prorenin-induced COX-2 expression was blunted by siRNA-mediated knockdown of PRR, ERK1/2 inhibition but not AT1R blockade. These results suggest that PRR activation increases COX-2 expression in the renal medullary cells via ERK-dependent and AngII-independent pathway. Prorenin binding to PRR is known to induce two different signaling pathways, the activation of ERK and the non-proteolytic activation of prorenin. It is evident that the activation of ERK but not the enzymatic activity of prorenin mediates the increased COX-2 expression.
Renal medullary COX-2 expression has been detected in various cell types including interstitial cells [34,35], vasa recta and medullary capillaries [36], and epithelial cells [21]. Gonzalez et al. for the first time describe that COX-2 is colocalized with PRR to the intercalted cells of the CD in the rat [11•]. In recent years, increasing evidence suggests an important role of intercalacted cells in the overal control of fluid metabsoslsim and blood pressure besides the regulation of urine adidification [37–41]. In response to increased urine flow, β-intercalated cells (β-ICs) produces ATP that triggers the erelease of PGE2 that acts in a parachrine fashion to inhibit ENaC in the principalcells of the CD. The PGE2-mediated communciation between ICs and pinciple cells of the CD contributes to the development of the hydroelectrolytic imbalance associated with distal renal tubular acidosis (dRTA) [42••]. The enzymatic sources of PGE2 and its possible interaction with PRR in the ICs remain to be determined in future studies.
Role of COX-2/EP4 pathway in regulation of renal medullary PRR expression during AngII-induced hypertension
In the renal cortex, COX-2 is expressed in the macula densa and mediates the expression and renin release in response to salt depletion or ACE inhibition [31,43–46]. Evidence is emerging that COX-2 exerts influence on intrarenal RAS by regulating PRR expression. This possibility is first suggested by the observation that transgenic overexpression of COX-2 in podocytes leads to albuminuria and glomerular injury accompanied with increased PRR expression [47]. Treatment with COX-2 inhibitor in the transgenic mice abrogates PRR upregulation and improves renal pathologies [47].
We recently examined the role of COX-2-derived metabolites in regulation of renal medullary PRR expression during AngII-induced hypertension [9•] and further determined the EP subtypes involved [10••]. In cultured primary rat IMCD cells, AngII treatment induced parallel increases in PRR expression, renin activity, and COX-2 expression [9•]. The induction of COX-2 expression at 4 h proceed that of the full-length PRR expression at 12 h, suggesting a causal role of COX-2-derived products in regulation of PRR [9•]. In this study, renin activity was assayed at a single time point of 12 h and was expected to be determined by the PRR level [9•]. Inhibition of COX-2 with NS-398 blocked AngII-induced PRR expression and renin activity. This phenomenon in cell culture has been recapitulated by animal experiments where COX-2 inhibition nearly completely abolished the upregulation of renal medullary PRR expression after 14-day AngII infusion and partially attenuated the hypertension in Sprague-Dawley rats [9•]. On the contrary to the suppressed plasma renin levels, urinary renin levels were elevated after AngII infusion and reduced by COX-2 inhibitor [9•]. These results suggest that COX-2 plays a key role in determining the activation of intrarenal RAS during AngII-induced hypertension. More recently, Gonzalez et al. report that renal medullary PRR and COX-2 expression in Sprague-Dawley rats is increased at day 3 but not day 14 of AngII infusion [48•]. It remains unclear why the time courses of PRR and COX-2 upregulation are different between Gonzalez’s study and ours. Despite the discrepancy, both studies observe a similar antihypertensive effect of COX-2 inhibition at day 14 of AngII infusion.
COX-2 can exert a complex role in regulation of blood pressure depending on the type of hypertension. A series of previous studies support the view that renal medullary COX-2 functions a natriuretic and antihypertensive factor during high salt loading. In this regard, renal medullary COX-2 expression is increased in response to chronic salt loading [31] and intramedullary delivery of NS-398 induces salt-sensitive hypertension in rats [34,49]. However, increasing numbers of studies using COX-2 deficiency mice or COX-2 inhibitors demonstrate prohypertensive action of COX-2 in rodent models of AngII-induced hypertension [9•,48•,50–52] although COX-2-derived products may exhibit vasodilatory and antihypertensive actions during acute AngII infusion [53]. It remains uncertain how renal medullary COX-2 exerts distinct actions in blood pressure regulation during AngII infusion and chronic salt loading. It is possible that renal medullary COX-2 exerts prohypertensive action during AngII infusion through the activation of intrarenal RAS [9•,48•] but exhibit antihypertensive action during chronic salt loading via PGE2-meidated natriuresis [54–56].
COX-2-derived prostanoids include PGE2, PGF2α, PGD2, PGI2, and thromboxane A2 with PGE2 being the dominant one in the kidney [57,58]. Two lines of evidence point to PGE2 as a major regulator of PRR expression in ICMD cells [9•]. First, exposure of the cells to exogenous PGE2 increased PRR expression. Second, COX-2 inhibition attenuated AngII-induced PRR expression, which was completely reversed by addition of exogenous PGE2 [9•]. The biologic action of PGE2 is mediated by G-protein-coupled E-prostanoid receptors designated EP1, EP2, EP3 and EP4 [59]. These four subtypes of EP receptor couple to distinct signaling pathways. Among the four EP subtypes, the EP4 receptor plays a dominant role in regulation of renin release from the juxtaglomerular apparatus [60,61]. Using pharmacological inhibitors and activators of the EP4 receptor, we demonstrated an essential role of this EP subtype in mediating AngII-induced PRR expression both in vitro and in vivo [10••]. The in vivo EP4 antagonism also attenuated AngII-induced hypertension and urinary renin level [10••]. The detailed signaling pathway downstream of the EP4 receptor in the CD is not known. This EP subtype typically signals through the Gs protein, which elevates intracellular cAMP. It seems reasonable to speculate that cAMP pathway may be involved in EP4-depenent stimulation of PRR expression during AngII hypertension. In addition, in vitro evidence suggests a potential role of the EP1 receptor in mediating AngII- induced PRR expression in cultured IMCD cells [10••]. This finding needs to be validated by in vivo studies.
Summary
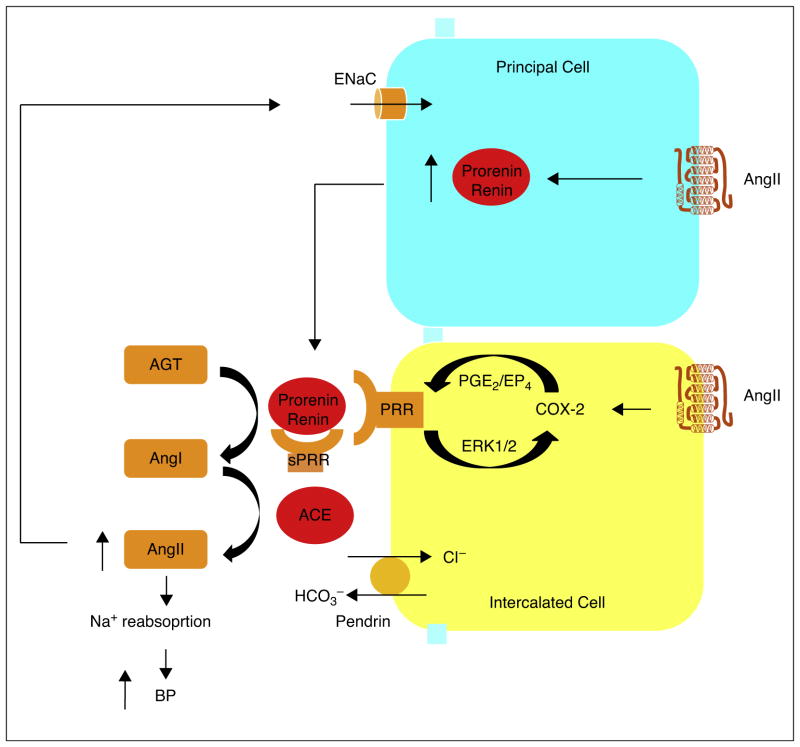
Transgenic overexpression of PRR increases COX-2 expression in the kidney. In cultured renal medullary cells, overexpression of PRR directly stimulates COX-2 expression via ERK1/2, independently of the activity of RAS. On the other hand, the COX-2/EP4 pathway mediates the upregulation of PRR in the renal medulla during AngII-induced hypertension. The mutually stimulatory relationship between renal medullary PRR and PGs suggests existence of a vicious cycle that may play an important role in amplifying the local renin response to increased circulating AngII level (Figure 1). A thorough understanding of this vicious cycle will provide insight into the molecular mechanism of AngII-induced hypertension and contribute to the development of pharmacological approaches and discovery of new drugs for hypertension as well as kidney diseases.
Figure 1.
Illustration of the interaction of PRR and PGs in the renal medulla during AngII-induced hypertension. Renin is expressed in the principal cells of the CD and its expression is induced by AngII. Prorenin and renin, released from the principalcells, bind PRR on the surface of intercalated cells or soluble PRR (sPRR) in the lumen to increase their catalytic activity. In the intercalated cells, AngII induces the expression of PRR that increases COX-2 expression via ERK1/2 and in turn COX-2-derived PGE2 via the EP4 receptor stimulates PRR expression, thus forming a vicious cycle in order to achieve the sustained activation of intrarenal RAS. This leads to heightened luminal AngII level that increases Na+ reabsorption possibly via increased ENaC activity, eventually leading to elevated blood pressure.
Acknowledgments
Sources of funding
This work was supported by National Natural Science Foundation of China Grants No. 91439205 and No. 31330037 and, National Institutes of Health Grant DK094956, National Basic Research Program of China 973 Program 2012CB517600 (No. 2012CB517602), and VA Merit Review. T. Yang is Research Career Scientist in Department of Veterans Affairs.
We thank Aihua Lu (Sun Yatsen University) and Hong Wang (Sun Yatsen University) for their technical and administrative assistance.
Footnotes
Conflict of interest
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Hall JBBM. Intrarenal and circulating angiotensin ii and renal function. In: Robertson J, Nicholls MG, editors. The Renin-Angiotensin system. Vol. 26. New York, NY: Gower Medical Publishing; 1993. pp. 1–26.43. [Google Scholar]
- 2.Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin ii production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (pro)renin receptor: Site-specific and functional linkage to the vacuolar h+-atpase in the kidney. Hypertension. 2009;54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension. 2011;57:859–864. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieto MC, Botros FT, Kavanagh K, Navar LG. Prorenin receptor in distal nephron segments of 2-kidney, 1-clip goldblatt hypertensive rats. The Ochsner Journal. 2013;13:26–32. [PMC free article] [PubMed] [Google Scholar]
- 9•.Wang F, Lu X, Peng K, Zhou L, Li C, Wang W, Yu X, Kohan DE, Zhou SF, Yang T. Cox-2 mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol Renal Physiol. 2014 doi: 10.1152/ajprenal.00548.2013. The authors demonstrate that COX-2 expression is induced in the renal medullary cells in response to AngII treatment and its inhibition attenuates AngII-induced hypertensive response accompanied with suppressed (pro)renin receptor expression and renin levels in the renal medulla. This study further defines PGE2 as the prostanoid responsible for the action of COX-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin e-prostanoid4 receptor mediates angiotensin ii-induced (pro)renin receptor expression in the rat renal medulla. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.03654. Employing the EP4 receptor agonists and antagonists, the authors demonstrate that the EP4 receptor plays a major role in determining the renal medullary (pro) renin receptor expression and local renin levels in the renal medulla. This is a major progress in understanding the PGE2 signaling in regulation of intrarenal RAS activity during AngII-induced hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (pro)renin receptor in rat renal inner medullary cells. Hypertension. 2013;61:443–449. doi: 10.1161/HYPERTENSIONAHA.112.196303. The authors demonstrate that overexpression of (pro)renin receptor in renal medullary cells stimulates PGE2 release and COX-2 expression via ERK1/2 but not the activity of RAS. This study contributes to understanding the interaction between (pro)renin receptor and COX-2 in the distal nephron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Zhuo JL, Ferrao FM, Zheng Y, Li XC. New frontiers in the intrarenal renin-angiotensin system: a critical review of classical and new paradigms. Front Endocrinol. 2013;4:166. doi: 10.3389/fendo.2013.00166. This is a comprehensive review of current knowledge in intrarenal renin-agiotensin system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins i and ii in anesthetized rats. J Am Soc Nephrol. 1994;5:1153–1158. doi: 10.1681/ASN.V541153. [DOI] [PubMed] [Google Scholar]
- 14.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–1357. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 16.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingelfinger JR, Pratt RE, Ellison K, Dzau VJ. Sodium regulation of angiotensinogen mrna expression in rat kidney cortex and medulla. J Clin Invest. 1986;78:1311–1315. doi: 10.1172/JCI112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin ii and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol. 2011;11:180–186. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mrna and protein in angiotensin ii-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin ii-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin ii stimulates renin in inner medullary collecting duct cells via protein kinase c and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57:594–599. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin ii levels in val5-ang ii-infused rats. Am J Physiol Renal Physiol. 2009;296:F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ang ii-induced hypertension and ace inhibition. Am J Physiol Renal Physiol. 2010;298:F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin ii formation during angiotensin ii-induced hypertension. Hypertension. 2009;53:351–355. doi: 10.1161/HYPERTENSIONAHA.108.124511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ace protects against hypertension. J Clin Invest. 2013;123:2011–2023. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin ii causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramkumar N, Ying J, Stuart D, Kohan DE. Overexpression of renin in the collecting duct causes elevated blood pressure. Am J Hypertens. 2013;26:965–972. doi: 10.1093/ajh/hpt071. [DOI] [PubMed] [Google Scholar]
- 28••.Ramkumar N, Stuart D, Rees S, Hoek AV, Sigmund CD, Kohan DE. Collecting duct-specific knockout of renin attenuates angiotensin ii-induced hypertension. Am J Physiol Renal Physiol. 2014;307:F931–F938. doi: 10.1152/ajprenal.00367.2014. This is the first report on the generation of collecting duct-specific deletion of renin. The null mice exhibit blunted hypertensive response to AngII infusion. This is probably the most compelling evidence for a functional role of collecting duct renin in AngII-induced hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70:641–646. doi: 10.1038/sj.ki.5001627. [DOI] [PubMed] [Google Scholar]
- 31.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol. 1998;274:F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 32.Yang T, Schnermann JB, Briggs JP. Regulation of cyclooxygenase-2 expression in renal medulla by tonicity in vivo and in vitro. Am J Physiol. 1999;277:F1–F9. doi: 10.1152/ajprenal.1999.277.1.F1. [DOI] [PubMed] [Google Scholar]
- 33.Yang T. Regulation of cyclooxygenase-2 in renal medulla. Acta Physiol Scand. 2003;177:417–421. doi: 10.1046/j.1365-201X.2003.01102.x. [DOI] [PubMed] [Google Scholar]
- 34.Ye W, Zhang H, Hillas E, Kohan DE, Miller RL, Nelson RD, Honeggar M, Yang T. Expression and function of cox isoforms in renal medulla: evidence for regulation of salt sensitivity and blood pressure. Am J Physiol Renal Physiol. 2006;290:F542–F549. doi: 10.1152/ajprenal.00232.2005. [DOI] [PubMed] [Google Scholar]
- 35.Campean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis — site-specific expression in rodent kidney (rat, mouse) Am J Physiol Renal Physiol. 2003;285:F19–F32. doi: 10.1152/ajprenal.00443.2002. [DOI] [PubMed] [Google Scholar]
- 36.Therland KL, Stubbe J, Thiesson HC, Ottosen PD, Walter S, Sorensen GL, Skott O, Jensen BL. Cycloxygenase-2 is expressed in vasculature of normal and ischemic adult human kidney and is colocalized with vascular prostaglandin e2 ep4 receptors. J Am Soc Nephrol. 2004;15:1189–1198. doi: 10.1097/01.asn.0000124673.79934.24. [DOI] [PubMed] [Google Scholar]
- 37.Kleyman TR, Satlin LM, Hallows KR. Opening lines of communication in the distal nephron. J Clin Invest. 2013;123:4139–4141. doi: 10.1172/JCI71944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wall SM, Pech V. Pendrin and sodium channels: relevance to hypertension. J Nephrol. 2010;23(Suppl 16):S118–S123. [PubMed] [Google Scholar]
- 39.Wall SM. Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens. 2005;14:480–484. doi: 10.1097/01.mnh.0000168390.04520.06. [DOI] [PubMed] [Google Scholar]
- 40.Wall SM, Weinstein AM. Cortical distal nephron cl(-) transport in volume homeostasis and blood pressure regulation. Am J Physiol Renal Physiol. 2013;305:F427–F438. doi: 10.1152/ajprenal.00022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wall SM, Pech V. The interaction of pendrin and the epithelial sodium channel in blood pressure regulation. Curr Opin Nephrol Hypertens. 2008;17:18–24. doi: 10.1097/MNH.0b013e3282f29086. [DOI] [PubMed] [Google Scholar]
- 42••.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal beta-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123:4219–4231. doi: 10.1172/JCI63492. Increasing evidence suggests an important role of renal intercalated cells in regulation of function of the principal cells of the collecting duct. In the present study, the authors generated a mouse model of type I distal tubular acidosis (dRTA) via inactivation of the B1 proton pump subunit (ATP6V1B1). This model displayed renal loss of NaCl, K+, and water. Inhibition of PGE2 synthesis restored renal ENaC expression and attenuated salt-wasting phenotype. This study suggests that ATP-triggered PGE2 production in the intercalated cells acts in paracrine fashion to inhibit sodium and water transport in the principal cells of the collecting duct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Renin expression in cox-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol. 2000;279:F819–F825. doi: 10.1152/ajprenal.2000.279.5.F819. [DOI] [PubMed] [Google Scholar]
- 44.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin e2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 45.Harris RC, Wang JL, Cheng HF, Zhang MZ, McKanna JA. Prostaglandins in macula densa function. Kidney Int Suppl. 1998;67:S49–S52. doi: 10.1046/j.1523-1755.1998.06710.x. [DOI] [PubMed] [Google Scholar]
- 46.Harris RC. Cox-2 and the kidney. J Cardiovasc Pharmacol. 2006;47(Suppl 1):S37–S42. doi: 10.1097/00005344-200605001-00007. [DOI] [PubMed] [Google Scholar]
- 47.Cheng H, Fan X, Moeckel GW, Harris RC. Podocyte cox-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. J Am Soc Nephrol. 2011;22:1240–1251. doi: 10.1681/ASN.2010111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Gonzalez AA, Green T, Luffman C, Bourgeois CR, Gabriel Navar L, Prieto MC. Renal medullary cyclooxygenase-2 and (pro)renin receptor expression during angiotensin ii-dependent hypertension. Am J Physiol Renal Physiol. 2014;307:F962–F970. doi: 10.1152/ajprenal.00267.2014. The authors demonstrate that AngII infusion elevates renal medullary expression of (pro)renin receptor and COX-2 at day 3 preceding the significant increase in blood pressure, supporting the causal relationship between the mediators and the hypertension development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zewde T, Mattson DL. Inhibition of cyclooxygenase-2 in the rat renal medulla leads to sodium-sensitive hypertension. Hypertension. 2004;44:424–428. doi: 10.1161/01.HYP.0000140924.91479.03. [DOI] [PubMed] [Google Scholar]
- 50.Martinez-Revelles S, Avendano MS, Garcia-Redondo AB, Alvarez Y, Aguado A, Perez-Giron JV, Garcia-Redondo L, Esteban V, Redondo JM, Alonso MJ, Briones AM, Salaices M. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxidants Redox Signal. 2013;18:51–65. doi: 10.1089/ars.2011.4335. [DOI] [PubMed] [Google Scholar]
- 51.Quilley J. Cox-2 and angiotensin ii-induced hypertension and oxidative stress. Am J Hypertens. 2011;24:1188. doi: 10.1038/ajh.2011.135. [DOI] [PubMed] [Google Scholar]
- 52.Wu R, Duchemin S, Laplante MA, De Champlain J, Girouard H. Cyclo-oxygenase-2 knockout genotype in mice is associated with blunted angiotensin ii-induced oxidative stress and hypertension. Am J Hypertens. 2011;24:1239–1244. doi: 10.1038/ajh.2011.137. [DOI] [PubMed] [Google Scholar]
- 53.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin ii. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stokes JB, Kokko JP. Inhibition of sodium transport by prostaglandin e2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977;59:1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stokes JB. Effect of prostaglandin e2 on chloride transport across the rabbit thick ascending limb of henle. Selective inhibitions of the medullary portion. J Clin Invest. 1979;64:495–502. doi: 10.1172/JCI109487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan Y, Zhang Y, Breyer RM, Fowler B, Davis L, Hebert RL, Breyer MD. Prostaglandin e2 inhibits renal collecting duct na+ absorption by activating the ep1 receptor. J Clin Invest. 1998;102:194–201. doi: 10.1172/JCI2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang T, Du Y. Distinct roles of central and peripheral prostaglandin e2 and ep subtypes in blood pressure regulation. Am J Hypertens. 2012;25:1042–1049. doi: 10.1038/ajh.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 59.Breyer MD, Breyer RM. Prostaglandin e receptors and the kidney. Am J Physiol Renal Physiol. 2000;279:F12–F23. doi: 10.1152/ajprenal.2000.279.1.F12. [DOI] [PubMed] [Google Scholar]
- 60.Nusing RM, Treude A, Weissenberger C, Jensen B, Bek M, Wagner C, Narumiya S, Seyberth HW. Dominant role of prostaglandin e2 ep4 receptor in furosemide-induced salt-losing tubulopathy: a model for hyperprostaglandin e syndrome/antenatal bartter syndrome. J Am Soc Nephrol. 2005;16:2354–2362. doi: 10.1681/ASN.2004070556. [DOI] [PubMed] [Google Scholar]
- 61.Facemire CS, Nguyen M, Jania L, Beierwaltes WH, Kim HS, Koller BH, Coffman TM. A major role for the ep4 receptor in regulation of renin. Am J Physiol Renal Physiol. 2011;301:F1035–F1041. doi: 10.1152/ajprenal.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]