Abstract
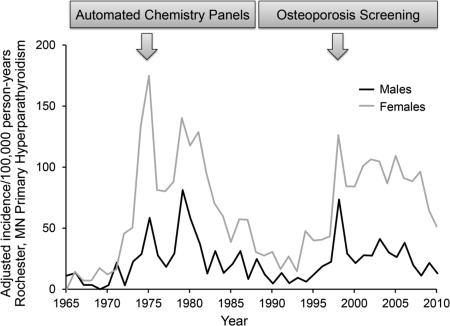
Introduction of automated serum calcium measurements in the 1970s resulted in a sharp rise in primary hyperparathyroidism (PHPT) incidence. However, recent investigations suggest a significant rise in PHPT incidence for unclear reasons. Our objective was to update our population-based secular trends in PHPT incidence, to determine if there has been a significant rise in PHPT incidence as suggested by others, and, if possible, to identify changes in clinical practice that might be responsible. Rochester, Minnesota, residents who met criteria for PHPT from 2002 through 2010 were identified through the medical records-linkage system of the Rochester Epidemiology Project and added to the historical cohort beginning in 1965. Incidence rates were adjusted to the 2010 US white population. Altogether, 1142 Rochester residents have been diagnosed with PHPT since 1965, including 341 in 2002-2010. Over time, two periods of increased PHPT incidence occurred, one beginning in 1974 (121.7 per 100,000 person-years) and a second peak (86.2 per 100,000 person-years) starting in 1998. The median age of PHPT subjects has increased significantly from 55 years in 1985-1997 to 60 years of age in 1998-2010 and more patients (36%) had a parathyroidectomy in 1998-2010. Although serum calcium measurement has declined since 1996, there was a progressive increase in parathyroid hormone testing between 1994 and 2008. There was also a rise in orders for bone mineral density measurements in women since 1998, which peaked in 2003-2004. A second sharp rise in PHPT incidence occurred in our community in 1998, simultaneously with the introduction of national osteoporosis screening guidelines, Medicare coverage for bone density measurement, and new medications for the treatment of osteoporosis. Case ascertainment bias from targeted PHPT screening in patients being evaluated for osteoporosis is the most likely explanation.
Keywords: Primary hyperparathyroidism, hypercalcemia, osteoporosis, epidemiology, incidence
Graphical Abstract
Introduction
Primary hyperparathyroidism (PHPT) is the third most common endocrine disorder and the most common cause of hypercalcemia in the outpatient setting.1,2 We observed a significant increase in PHPT incidence among Rochester, Minnesota residents in 1974 that was associated with the introduction of automated serum chemistry panels and attributed to identification of previously unrecognized prevalent cases of PHPT with asymptomatic hypercalcemia.3 After the initial rise in PHPT diagnoses, the incidence fell to 27.7 per 100,000 person-years, which was thought to represent the true rate in an environment of automated measurement of serum calcium.3 PHPT clinical characteristics also changed in the era of routine calcium measurement, from symptomatic disease with more severe hypercalcemia to asymptomatic PHPT with mild hypercalcemia that was more common in older women who are also at risk for osteoporosis.1-7
Throughout the 1980's, the incidence of PHPT in Rochester steadily decreased, and this lower rate persisted throughout most of the 1990's.7,8 However, a second sharp increase in PHPT incidence was noted in 1998, which suggested that another important change in the epidemiology of PHPT might be occurring. Furthermore, three recent studies have suggested that the incidence of PHPT was higher than previously reported. 4,5,9 The goal of the current study was to update our population-based secular trends in PHPT incidence, to determine if there has been a significant rise in PHPT incidence as suggested by recent investigations, and, if possible, to identify changes in clinical practice that might be responsible. Availability of the population-based medical records-linkage system of the Rochester Epidemiology Project offered a unique opportunity to address this issue.10,11
Methods
Most endocrinologic care in this community is provided by the Mayo Clinic, which has maintained a common medical record with its two hospitals for over 100 years. The diagnoses and surgical procedures recorded in these records are indexed, as are the medical records of the other providers who serve the local population.11 After approval from the Institutional Review Boards of Mayo Clinic and the Olmsted Medical Center, we used this comprehensive medical records-linkage system (the Rochester Epidemiology Project) to identify all Rochester residents first diagnosed with PHPT from 2002 through 2010. This was accomplished by screening the complete records of all those with an outpatient clinic or hospital diagnosis of hyperparathyroidism, parathyroid adenoma, osteitis fibrosa cystica, familial benign hypercalcemia, malignant hypercalcemia, or hypercalcemia not otherwise specified. We also ascertained all patients undergoing parathyroid surgery, those with an autopsy/death certificate diagnosis of hyperparathyroidism, and any patients with a tissue registry diagnosis of hyperparathyroidism during the time period.
In addition, all Rochester residents with serum calcium levels exceeding 2.52 mmol/L at least twice between 2002 and 2010 were identified directly from Mayo's Laboratory Information System. Although the methods used to measure serum calcium levels changed over time, the normal range remained unchanged since instrumentation was calibrated against atomic absorption spectrophotometry according to certified references from the National Bureau of Standards. We also identified all residents with a parathyroid hormone (PTH) level (two-site immunochemiluminometric assays) above the upper normal range as follows: 1.0 - 5.2 pmol/L by manual bead immunoassay (in-house assay) in December 1974 to December 2, 2003; 10 - 55 pg/mL (1.1 to 5.8 pmol/L) by Nichols Advantage (Nichols Institute Diagnostics, San Juan Capistrano, CA U.S.A.) in December 3, 2003 to May 1, 2006; 10 - 67 pg/mL (1.1 to 7.1 pmol/L) by Diagnostics Products Corporation assay (Diagnostics Products Corporation, Los Angeles, CA U.S.A.) in March 31, 2005 to July 15, 2007; and 15 to 65 pg/mL (1.59 to 6.90 pmol/L) performed on the Roche Cobas 6000 (Roche Diagnostics GmbH, Mannheim, Germany) since July 16, 2007.
For each potential case identified, the complete (inpatient and outpatient) medical record was reviewed by one of the investigators (MLG). Mayo Clinic records contain the details of every inpatient hospitalization at its two hospitals, every outpatient office or clinic visit, all emergency room and nursing home care, as well as all radiographic reports and pathology reports, including autopsies.11 This information was supplemented by that available from the other providers of care to local residents, most notably the Olmsted Medical Center and its associated hospital.10
Inclusion criteria for patients for PHPT were consistent with our previous reports.7,8 Patients were accepted as having “definite” PHPT if they met one or more of the following criteria: i) histopathologic proof of parathyroid adenoma or hyperplasia; ii) hypercalcemia (calcium level >2.52 mmol/L) with an elevated serum immunoreactive PTH level; or iii) hypercalcemia persisting for more than one year for which no other cause (e.g., thiazide diuretics, cancer, creatinine level >176.8 mmol/L, or lithium therapy) was evident. Two groups of patients were identified as having “possible” hyperparathyroidism: i) patients with at least two elevated serum calcium levels from at least three determinations who were followed for less than one year; ii) patients with at least two elevated serum calcium levels and in at least two different years that were followed by three or more normal calcium values. Patients with familial benign hypocalciuric hypercalcemia were excluded. To assure comparability with the earlier studies, patients must have established residency in Rochester for at least one year before the initial elevated serum calcium level was observed.
The updated cohort from 2002-2010 was combined with the previously identified Rochester PHPT cases.3,7,8 PHPT occurrence was analyzed in four time periods based on periods of incidence rate change identified within our population: Pre-chemistry panel era, 1965 to June 30, 1974; early chemistry panel era, July 1, 1974 through 1984; late chemistry panel era, 1985 through 1997; and osteoporosis screening era, 1998 through 2010.3,7,8 Patient characteristics evaluated included reason for diagnosis, sex, age, maximum serum calcium level, initial management (surgery, surgery recommended but refused, surgery recommended but patient too ill, observation or uncertain) and presentation (symptoms, abnormal serum calcium, other biochemical or radiologic abnormality, uncertain). In the current update (2002-2010), we also identified osteoporosis based on measurement of bone mineral density (BMD) by dual-energy xray absorptiometry (DXA) that led to the diagnosis of PHPT.
Incidence rates for PHPT were based on the date of the initial elevated serum calcium level consistent with previous studies.3,7,8 Age- and sex-specific incidence rates were estimated using the number of patients in each age (<45, 45-54, 55-64, 65-74, 75+) and sex group as the numerator, with corresponding age- and sex-specific person-years as denominators.12 Person-years were estimated by decennial census data for the Rochester population with interpolation for intercensal years. The rates from our study, including previous reports were directly age- and sex-adjusted to the U.S. white population in 2010. For direct comparison, recently published incidence rates by others were adjusted to this same standard population regardless of the inherent racial distribution.9 Ninety-five percent confidence intervals (95% CIs) for the incidence rates were calculated under the Poisson distribution. In an attempt to identify changes in clinical practice, rates of calcium, PTH, and BMD measurement for local residents were also estimated using the number of unique patients with each test per year, derived from actual test results for calcium and PTH and order placement for the BMD measurements. In order to compare time periods (e.g., 1985-1997 vs. 1998-2010) in terms of clinical and demographic characteristics, Pearson chi-square tests for categorical variables and Mann-Whitney statistics for continuous variables were used.
Results
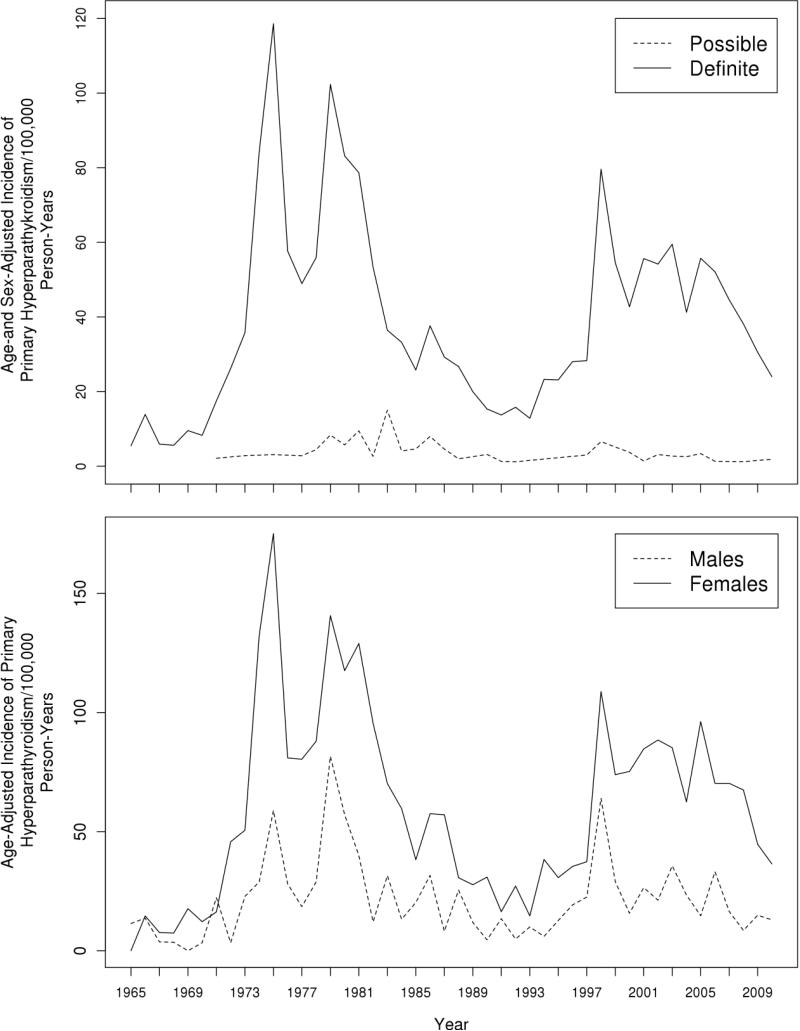
Altogether, 341 Rochester residents (269 females, 72 males) were newly diagnosed with PHPT in 2002 through 2010 for a cohort total of 1142 PHPT patients from 1965 through 2010, of whom the majority had definite PHPT (94%). As shown in Figure 1, however, two periods of increased PHPT incidence occurred, one beginning in 1974 (121.7 per 100,000 person-years, 95% CI: 88.4 – 154.9, re-adjusted to the 2010 U.S. white population) associated with the introduction of automated serum calcium measurement which persisted until 1984 when the incidence rate decreased to 37.3 per 100,000 person-years. A second peak (86.2 per 100,000 person-years, 95% CI: 64.0 – 108.3) started in 1998 and continued until 2007, when the incidence rate dropped to 31.3 per 100,000 person-years. Additionally, overall PHPT incidence of 50.4 per 100,000 person-years in 1998-2010 was nearly twice the 1985-1997 rate of 27.9 per 100,000 person-years (Table 1). When comparing PHPT incidence rates in 1985-1997 to those in 1998-2010, a greater than three-fold rise in women age 65-74 and more than a two-fold increase in women ≥ 75 years old occurred. Similarly, since 1998, the highest PHPT incidence rate was observed in men ≥ 75 years, whereas no men in this age-group were seen in 1985-1997.
Figure 1.
Age-adjusted (to 2010 U.S. whites) incidence of definite (solid line) and possible (dashed line) primary hyperparathyroidism (upper panel) and women (solid line) and men (dashed line) with primary hyperparathyroidism (lower panel), among Rochester, Minnesota, residents, 1965-2010.
Table 1.
Incidence (per 100,000 Person-Years) of Primary Hyperparathyroidism among Rochester, Minnesota, Residents in 1965-2010, by Time Periods Based on Incidence Rate Change
| Time Period | Men | Women | Both Sexes | |||
|---|---|---|---|---|---|---|
| Age-groups | n | Incidence | n | Incidence | n | Incidence |
| 1965-June 1974 (Pre-chemistry panel era) | ||||||
| < 45 | 11 | 6.6 | 9 | 4.8 | 20 | 5.6 |
| 45-54 | 4 | 19.4 | 8 | 34.0 | 12 | 27.2 |
| 55-64 | 1 | 6.5 | 15 | 70.4 | 16 | 43.6 |
| 65-74 | 2 | 20.4 | 9 | 53.5 | 11 | 41.3 |
| ≥ 75 | 0 | 0.0 | 6 | 42.5 | 6 | 29.3 |
| Total | 18 | 9.2 * | 47 | 24.5 * | 65 | 17.4† |
| July 1974-1984 (Early chemistry panel era) | ||||||
| < 45 | 37 | 17.9 | 42 | 18.4 | 79 | 18.2 |
| 45-54 | 11 | 42.7 | 63 | 226.6 | 74 | 138.2 |
| 55-64 | 18 | 88.5 | 82 | 334.2 | 100 | 222.8 |
| 65-74 | 16 | 128.2 | 62 | 290.7 | 78 | 230.7 |
| ≥ 75 | 3 | 35.3 | 34 | 155.7 | 37 | 122.0 |
| Total | 85 | 41.1* | 283 | 123.5* | 368 | 84.1† |
| 1985-1997 (Late chemistry panel era) | ||||||
| < 45 | 25 | 7.8 | 23 | 7.0 | 48 | 7.4 |
| 45-54 | 13 | 28.6 | 34 | 69.9 | 47 | 50.0 |
| 55-64 | 11 | 35.2 | 36 | 101.3 | 47 | 70.3 |
| 65-74 | 7 | 32.4 | 22 | 74.5 | 29 | 56.7 |
| ≥ 75 | 0 | 0.0 | 23 | 61.7 | 23 | 43.6 |
| Total | 56 | 16.0* | 138 | 38.3 * | 194 | 27.9† |
| 1998-2010 (Osteoporosis screening era) | ||||||
| < 45 | 30 | 7.4 | 36 | 9.0 | 66 | 8.4 |
| 45-54 | 32 | 37.3 | 87 | 96.9 | 119 | 67.8 |
| 55-64 | 21 | 36.5 | 108 | 177.0 | 129 | 112.1 |
| 65-74 | 19 | 54.9 | 97 | 246.0 | 116 | 156.6 |
| ≥ 75 | 15 | 57.5 | 70 | 161.4 | 85 | 122.3 |
| Total | 117 | 23.2* | 398 | 74.6* | 515 | 50.4† |
Incidence per 100,000 person-years directly adjusted for age according to the population distribution of white persons in the United States in 2010.
Incidence per 100,000 person-years directly adjusted for age and sex according to the population distribution of white persons in the United States in 2010.
The median age of PHPT recognition in the 1998-2010 cohort was older than noted in 1985-1997, at 60.4 years (p < 0.001) (Table 2). Women (77%) were still more likely to have PHPT than men in 1998-2010, but the female to male sex-ratio demonstrated a nonsignificant increase from 2.5 in 1985-1997 to 3.4 in 1998-2010 (p = 0.093). The majority of patients in recent time periods were categorized with definite PHPT (92% in 1985-1997 and 96 % in 1998-2010). Maximum serum calcium levels were similar throughout the different cohort periods. Only 7% of PHPT patients had symptoms at presentation during the prior time period which was not significantly different from 11% of PHPT subjects with symptoms in 1998-2010 (p = 0.071). The most common symptoms leading to the PHPT diagnosis in 1998-2010 were as follows: osteoporotic fracture (n = 10), nephrolithiasis (n =22), fatigue (n = 17), anxiety/depression (n = 3), cognitive impairment (n = 1), hypercalcemic crisis (n = 1), and other (n = 3). Five PHPT cases (1%) in 1998-2010 were identified initially due to an incidental radiologic detection of a parathyroid adenoma. Significantly more patients had surgery in 1998-2010 (36%) than in 1985-1997 (26%) (p < 0.001). Histology was consistent with a parathyroid adenoma in the majority of subjects in both 1998-2010 (96%) and 1985-1997 (89%) (p = 0.249). Also, 47 asymptomatic patients with BMD-defined osteoporosis which led to the diagnosis of PHPT were identified in 2002-2010. Of these patients, 22 (47%) underwent parathyroidectomy.
Table 2.
Demographic and Clinical Characteristics of Rochester, Minnesota, Residents with a First Diagnosis of Primary Hyperparathyroidism (PHPT) in 1965-2010, by Time Periods Based on Incidence Rate Change
| Characteristic | 1965-June 1974 Pre-chemistry Panel Era (n=65) | July 1974-1984 Early Chemistry Panel Era (n=368) | 1985-1997 Late Chemistry Panel Era (n=194) | 1998-2010 Osteoporosis Screening Era (n=515) | p-valve* |
|---|---|---|---|---|---|
| Age, Years | <0.001 | ||||
| Median | 55.5 | 57.3 | 55.1 | 60.4 | |
| (25th - 75th percentiles) | (43.6-65.4) | (47.6-67.9) | (45.1-66.4) | (52.0-71.0) | |
| Presentation, n (%) | |||||
| Symptom | 13 (20.0) | 25(6.8) | 13 (6.7) | 57 (11.0) | 0.071 |
| Abnormal serum Calcium | 50 (77.0) | 339 (92.0) | 177 (91.2) | 453 (88.0) | |
| Other biochemical or radiological abnormality | 1 (1.5) | 1 (0.3) | 4 (2.1) | 5 (1.0) | |
| Autopsy | 0 (0.0) | 3 (1.0) | 0 (0.0) | 0 (0.0) | |
| Uncertain | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Sex, n (%) | 0.093 | ||||
| Female | 47 (72.3) | 283 (76.9) | 138 (71.1) | 398 (77.3) | |
| Male | 18 (27.7) | 85 (23.1) | 56 (28.9) | 117 (22.7) | |
| Mode of Diagnosis, n (%) | |||||
| Histologic Evidence | 23 (35.4) | 94 (25.5) | 50 (25.8) | 183 (35.5) | <0.001 |
| Inappropriate parathyroid hormone level | 27 (41.5) | 161 (43.8) | 80 (41.2) | 257 (50.0) | |
| Hypercalcemia > 1 year | 13 (20.0) | 87 (23.6) | 48 (24.8) | 53 (10.2) | |
| “Possible” PHPT | 2 (3.1) | 26 (7.1) | 16 (8.2) | 22 (4.3) | |
| Maximum serum calcium level, mg/dL | 0.015 | ||||
| Median | 10.8 | 10.7 | 10.7 | 10.8 | |
| (25th - 75th percentiles) | (10.6-11.3) | (10.5-11.1) | (10.4-10.9) | (10.6-11.1) | |
| Range | 10.2-12.5 | 10.2-16 | 10.2-13.1 | 9.4**-14.7 | |
| Initial Management | |||||
| Surgery | 18 (27.7) | 73 (19.8) | 37 (19.1) | 179 (34.8) | < 0.001 |
| Surgery recommended but refused | 6 (9.2) | 12 (3.3) | 4 (2.1) | 12 (2.3) | |
| Surgery recommended but patient too ill | 2 (3.1) | 15 (4.1) | 1 (0.5) | 10 (1.9) | |
| Decision to observe | 378 (57.0) | 268 (72.8) | 152 (78.4) | 314 (61.0) | |
| Uncertain | 2 (3.1) | 0 (0) | 0 (0) | 0 (0) |
P-values comparing characteristics of PHPT patients diagnosed in 1998-2010 to those diagnosed in 1985-1997.
Includes 2 patients with normocalcemic PHPT with hypercalciuria, kidney stones, and parathyroid adenomas pathologically confirmed after parathyroidectomy.
Discussion
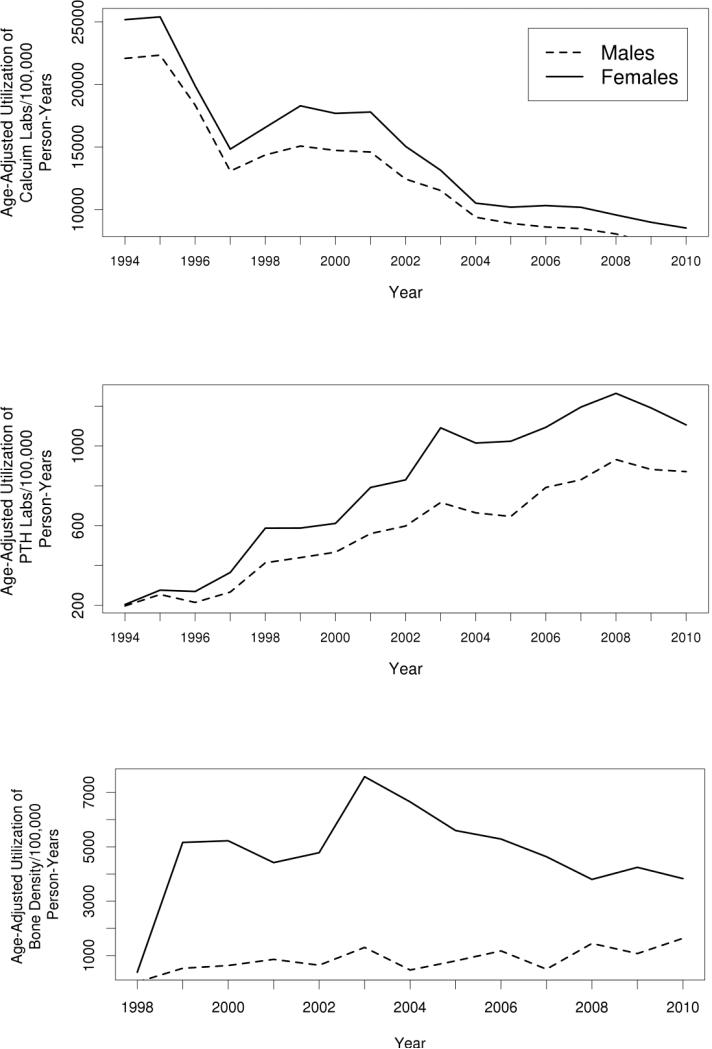
We have identified a second sharp rise in the incidence of PHPT in Rochester in 1998 similar to that observed in 1974 after the introduction of automated chemistry panels.3 The first peak in PHPT incidence was attributed to “sweeping” the population for previously unrecognized PHPT cases through routine serum calcium testing. However, the most recent increase occurred despite a reduction in serum calcium measurements (Figure 2) that accompanied a regulatory change on June 13, 1996, that eliminated the use of automated chemistry panels and necessitated individual orders for serum calcium measurements.8
Figure 2.
Utilization of serum calcium (panel A) and PTH (panel B) measurements from 1994 to 2010 and bone mineral density unique patient orders (panel C) from 1998 to 2010 at Mayo Clinic Rochester for all Olmsted County, Minnesota, residents.
Several other investigators have reported higher recent PHPT incidence rates. In Tayside, Scotland, Yu et al. estimated PHPT incidence rates of 95.7, 51.9, and 75.5 per 100,000 person-years in 1998-2006 for females, males, and both sexes combined (adjusted to the U.S. 2010 white population) compared, respectively, to 85.3, 29.6, and 59.1 per 100,000 person-years in Rochester in 1998-2006.4 Similarly, Abood et al. evaluated the incidence of PHPT in Denmark in 1977-2010 and demonstrated a progressive rise in rates between 1990 and 2010, including a 3-fold increase in incidence between 1998 and 2010.5 Most recently, Yeh and colleagues reported the incidence of PHPT from the Kaiser Permanente Southern California database for 1995-2010 and showed that the incidence of PHPT was also higher than prior estimates.9 Incidence rates (adjusted to the U.S. 2010 white population) were 68.6, 24.5, and 48.3 per 100,000 person-years in 1998-2006, respectively, for females, males, and both sexes combined, although a significant amount of that population was non-white and PHPT incidence is greater among blacks.9
There are several potential causes for the recent increase in PHPT incidence. One possible contributing factor is heightened awareness of osteoporosis, resulting in serum calcium and PTH screening as part of an evaluation for secondary causes of osteoporosis.13,14 Indeed, there was a progressive increase in PTH testing between 1994 and 2008 at our institution (Figure 2). In addition, measurement of serum PTH and calcium was more common in women and increased with age (Appendix Figures A and B), consistent with measures targeted to those most likely to have PHPT. In 1998, the National Osteoporosis Foundation's first clinical practice guidelines for osteoporosis were published; and the Medicare Bone Mass Measurement Coverage Standardization Act, adding BMD testing to the list of procedures for which Medicare will pay, went into effect.15 Screening for reduced BMD was recommended for all women ≥ 65 years of age in the absence of other risk factors, and at menopause if fractures or osteoporosis risk factors were present. As a consequence, a sharp increase in BMD measurement has occurred since 1992 16, with the largest growth between 1995 and 1996 when specific treatments for osteoporosis became widely available 17, including the U.S. Food and Drug Administration approval of osteoporosis treatment for men in 2000. BMD measurement has increased most dramatically in postmenopausal women, who are also at the greatest risk of having PHPT.1 We also saw a rise in BMD measurement orders in women, which peaked in 2003-2004 (Figure 2). Taken together, these findings suggest a role for ascertainment bias due to osteoporosis-related testing being performed in the patients most likely to have PHPT.
There are additional factors that could have contributed to a rise PHPT incidence since 1998. Estrogen use has been shown to lower serum calcium levels in postmenopausal women with PHPT 18, but a dramatic decline in estrogen use in postmenopausal women accompanied the release of the Women's Health Initiative (WHI) report in June 2002.19-21 However, increases in the incidence of PHPT in both older women and men, combined with the increase in PHPT incidence before the landmark WHI study was published, argue against this as a plausible factor. Another consideration is the epidemic of obesity, which has been associated with PHPT.22 However, greater obesity would have been expected to cause a steady increase in PHPT incidence over time rather than a sharp rise. Other possibilities include population changes in oral calcium and vitamin D intake.23-28 However, calcium intake from dietary sources in U.S. adults has not changed significantly in the recent decade, and the use of calcium and vitamin D supplements has remained common, especially with advancing age.29,30 Finally, the incidental detection of parathyroid adenomas through increased imaging, such as neck ultrasonography introduced in the 1980's, might lead to an increase in PHPT incidence similar to that observed for thyroid cancer 31,32, but only 5 Rochester PHPT cases were identified this way in the most recent time period.
The median age of PHPT subjects has increased significantly from 55 years in 1985-1997 to 60 years in 1998-2010. Patients with symptoms at PHPT presentation were not significantly different in 1985-1997 (7%) compared to the most recent period (11%), and these levels remain below those observed in the pre-chemistry panel era (20%). In addition, the number of patients initially recommended for parathyroidectomy in 1998-2010 has increased (39%) and is now similar to the 1965-June 1974 time period (40%), when patients often were identified due to symptomatic disease rather than biochemical detection.3 This is, in part, likely the result of the 2002 changes (from Z- to T-scores) in BMD guidelines for parathyroidectomy in asymptomatic PHPT33, combined with increased detection of PHPT through BMD screening. Indeed, a significant number of subjects had osteoporosis based on BMD measurement that led to the PHPT diagnosis in 2002-2010, of whom approximately half underwent parathyroidectomy.
Our study has several inherent limitations given its retrospective design. In addition, the population of Rochester is primarily white, which limits the application of study results to more ethnically diverse populations.10 This is especially relevant since PHPT appears to be more common among blacks compared to other races.9 We were also unable to capture orders for BMD testing at our institution prior to 1998. In addition, there is no clear explanation for the decline in PHPT incidence observed in the last 2 years of our study. However, reductions in Medicare reimbursement for BMD testing in the nonhospital setting, initiated in January 2007, has attenuated the prior growth observed in such testing and may have reduced targeted screening for secondary causes of osteoporosis.34 We observed a similar reduction in BMD orders at Mayo Clinic Rochester after 2006 as shown in Figure 2. A similar sharp decline was also seen in PTH levels measured, particularly in women, after 2008.
Conclusions
The epidemiology of PHPT has exhibited significant changes over the last 5 decades, which appear to largely reflect alterations in medical practice. Clinical medicine is dynamic, with constant innovations in patient care, introduction of new medications and treatment guidelines, and variations in reimbursement paradigms. The clinical spectrum of PHPT first shifted from a symptomatic disease with multiple complications to uncomplicated, asymptomatic PHPT in older individuals with the advent of automated chemistry panels. Subsequently, the introduction of osteoporosis screening guidelines and multiple new medications for the treatment of osteoporosis may have contributed to a second surge in PHPT incidence. In addition, patients are now significantly older and more likely to have a parathyroidectomy than in the previous 2 decades. The effect of clinical practice shifts will be important to track as the epidemiology of PHPT is updated, and may help us better understand the differences in PHPT incidence observed in various locations around the world. More importantly, the impact of practice changes on PHPT epidemiology emphasizes how influential clinical medicine can be on the expression of a disease.
Supplementary Material
Highlights.
A second sharp increase in the incidence rate of primary hyperparathyroidism has occurred since 1998.
The greatest increase in primary hyperparathyroidism incidence was seen in women ≥ 65 and men ≥ 75 years.
Since 1998, patients with primary hyperparathyroidism are older and more likely to undergo parathyroidectomy.
Ascertainment bias associated with osteoporosis-related testing is the most likely cause of the increased incidence of primary hyperparathyroidism since 1998.
ACKNOWLEDGEMENTS
The authors thank Mary G. Roberts for help in preparing the manuscript as well as Philip I. Haigh, MD, MSc, FRCSC, FACS from Kaiser Permanente Los Angeles Medical Center and Dr. Ning Yu from Population Heath Sciences at the University of Dundee for providing summary data from their respective cohorts to allow comparison of their incidence rates to Rochester, Minnesota.
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging (R01AG034676). The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
Abbreviations
- BMD
bone mineral density
- PHPT
primary hyperparathyroidism
- PTH
parathyroid hormone
- WHI
Women's Health Initiative
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors state that they have no conflicts of interest.
REFERENCES
- 1.Melton LJ., 3rd The epidemiology of primary hyperparathyroidism in North America. JBone Miner Res. 2002;17(Suppl 2):N12–17. [PubMed] [Google Scholar]
- 2.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 3.Heath H, 3rd, Hodgson SF, Kennedy MA. Primary hyperparathyroidism. Incidence, morbidity, and potential economic impact in a community. New Engl J Med. 1980;302:189–193. doi: 10.1056/NEJM198001243020402. [DOI] [PubMed] [Google Scholar]
- 4.Yu N, Donnan PT, Murphy MJ, Leese GP. Epidemiology of primary hyperparathyroidism in Tayside, Scotland, UK. Clin Endocrinol. 2009;71:485–493. doi: 10.1111/j.1365-2265.2008.03520.x. [DOI] [PubMed] [Google Scholar]
- 5.Abood A, Vestergaard P. Increasing incidence of primary hyperparathyroidism in Denmark. Dan Med J. 2013;60:A4567. [PubMed] [Google Scholar]
- 6.Jorde R, Bonaa KH, Sundsfjord J. Primary hyperparathyroidism detected in a health screening. The Tromso study. J Clin Epidemiol. 2000;53:1164–1169. doi: 10.1016/s0895-4356(00)00239-0. [DOI] [PubMed] [Google Scholar]
- 7.Wermers RA, Khosla S, Atkinson EJ, Hodgson SF, O'Fallon WM, Melton LJ., 3rd. The rise and fall of primary hyperparathyroidism: a population-based study in Rochester, Minnesota, 1965-1992. Ann Intern Med. 1997;126:433–440. doi: 10.7326/0003-4819-126-6-199703150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wermers RA, Khosla S, Atkinson EJ, et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J bone Miner Res. 2006;21:171–177. doi: 10.1359/JBMR.050910. [DOI] [PubMed] [Google Scholar]
- 9.Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98:1122–1129. doi: 10.1210/jc.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stafford RS, Drieling RL, Hersh AL. National trends in osteoporosis visits and osteoporosis treatment, 1988-2003. Arch Intern Med. 2004;164:1525–1530. doi: 10.1001/archinte.164.14.1525. [DOI] [PubMed] [Google Scholar]
- 14.Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87:4431–4437. doi: 10.1210/jc.2002-020275. [DOI] [PubMed] [Google Scholar]
- 15.National Osteoporosis Foundation . Physician's guide to prevention and treatment of osteoporosis. Washington, DC: 1998. pp. 1–29. [Google Scholar]
- 16.Jaglal SB, McIsaac WJ, Hawker G, Jaakkimainen L, Cadarette SM, Chan BT. Patterns of use of the bone mineral density test in Ontario, 1992-1998. CMAJ. 2000;163:1139–1143. [PMC free article] [PubMed] [Google Scholar]
- 17.Farley JF, Blalock SJ. Trends and determinants of prescription medication use for treatment of osteoporosis. Am J Health Syst Pharm. 2009;66:1191–1201. doi: 10.2146/ajhp080248. [DOI] [PubMed] [Google Scholar]
- 18.Marcus R, Madvig P, Crim M, Pont A, Kosek J. Conjugated estrogens in the treatment of postmenopausal women with hyperparathyroidism. Ann Intern Med. 1984;100:633–640. doi: 10.7326/0003-4819-100-5-633. [DOI] [PubMed] [Google Scholar]
- 19.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 21.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 22.Bolland MJ, Grey AB, Gamble GD, Reid IR. Association between primary hyperparathyroidism and increased body weight: a meta-analysis. J Clin Endocrinol Metab. 2005;90:1525–1530. doi: 10.1210/jc.2004-1891. [DOI] [PubMed] [Google Scholar]
- 23.Khosla S, Ebeling PR, Firek AF, Burritt MM, Kao PC, Heath H., 3rd Calcium infusion suggests a “set-point” abnormality of parathyroid gland function in familial benign hypercalcemia and more complex disturbances in primary hyperparathyroidism. J Clin Endocrinol Metab. 1993;76:715–720. doi: 10.1210/jcem.76.3.8445032. [DOI] [PubMed] [Google Scholar]
- 24.Insogna KL, Mitnick ME, Stewart AF, Burtis WJ, Mallette LE, Broadus AE. Sensitivity of the parathyroid hormone-1,25-dihydroxyvitamin D axis to variations in calcium intake in patients with primary hyperparathyroidism. New Engl J Med. 1985;313:1126–1130. doi: 10.1056/NEJM198510313131805. [DOI] [PubMed] [Google Scholar]
- 25.Tohme JF, Bilezikian JP, Clemens TL, Silverberg SJ, Shane E, Lindsay R. Suppression of parathyroid hormone secretion with oral calcium in normal subjects and patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1990;70:951–956. doi: 10.1210/jcem-70-4-951. [DOI] [PubMed] [Google Scholar]
- 26.Rao DS, Honasoge M, Divine GW, et al. Effect of vitamin D nutrition on parathyroid adenoma weight: pathogenetic and clinical implications. J Clin Endocrinol Metab. 2000;85:1054–1058. doi: 10.1210/jcem.85.3.6440. [DOI] [PubMed] [Google Scholar]
- 27.Grey A, Lucas J, Horne A, Gamble G, Davidson JS, Reid IR. Vitamin D repletion in patients with primary hyperparathyroidism and coexistent vitamin D insufficiency. J Clin Endocrinol Metab. 2005;90:2122–2126. doi: 10.1210/jc.2004-1772. [DOI] [PubMed] [Google Scholar]
- 28.Paik JM, Curhan GC, Taylor EN. Calcium intake and risk of primary hyperparathyroidism in women: prospective cohort study. BMJ. 2012;345:e6390. doi: 10.1136/bmj.e6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangano KM, Walsh SJ, Insogna KL, Kenny AM, Kerstetter JE. Calcium intake in the United States from dietary and supplemental sources across adult age groups: new estimates from the National Health and Nutrition Examination Survey 2003-2006. J Am Diet Assoc. 2011;111:687–695. doi: 10.1016/j.jada.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–822. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973- 2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 32.Leenhardt L, Bernier MO, Boin-Pineau MH, et al. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur J Endocrinol. 2004;150:133–139. doi: 10.1530/eje.0.1500133. [DOI] [PubMed] [Google Scholar]
- 33.Bilezikian JP, Potts JT, Jr., Fuleihan Gel H, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Bone Miner Res. 2002;17(Suppl 2):N2–11. [PubMed] [Google Scholar]
- 34.Yoo JW, Nakagawa S, Kim S. Effect of reimbursement reductions on bone mineral density testing for female Medicare beneficiaries. J Womens Health (Larchmt) 2012;21:1144–1148. doi: 10.1089/jwh.2012.3517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.