Abstract
Background
The purpose of this study was to evaluate the safety and efficacy of thymosin beta 4 ophthalmic solution (RGN-259; Tβ4) in subjects with moderate to severe dry eye using the CAE™ model.
Methods
This single-center, prospective, double-masked, placebo-controlled Phase II study randomized 72 qualifying subjects 1:1 to receive either 0.1% Tβ4 or placebo treatment for a total of 28 days. The study consisted of six visits over a 32-day period, including a screening visit (day –1), controlled adverse environment challenge (CAE) visits (day 1, day 28), and follow-up visits (days 14, 29, and 30). The primary efficacy endpoints were ocular discomfort scores and inferior corneal staining measured at visit 5 on day 29. Secondary endpoints included central and superior corneal staining, conjunctival staining, conjunctival redness, tear-film break-up time, and daily symptom scores recorded over the course of the study. Safety measures included visual acuity, slit-lamp evaluation, conjunctival redness, tear film break-up time, intraocular pressure, dilated funduscopy, and corneal sensitivity.
Results
Neither of the primary endpoints, ie, ocular discomfort or inferior corneal staining, showed a significant difference between treatment and control groups at visit 5. Despite this, significant differences between treatment groups were observed for a number of secondary endpoints. The discomfort scores in the CAE on day 28 were reduced by 27% in 0.1% Tβ4-treated subjects compared with the placebo group (P=0.0244). Subjects in the 0.1% Tβ4 treatment group also showed statistically significant improvements in central and superior corneal staining compared with staining scores in the control group (P=0.0075 and P=0.0210). No adverse events were observed.
Conclusion
This study confirms the efficacy of 0.1% Tβ4 as a topical treatment for relief of signs and symptoms of dry eye. Significant improvements in both signs and symptoms of dry eye were observed, and the treatment exhibited a large safety window, with no adverse events reported by any subjects enrolled in the study.
Keywords: thymosin beta 4, RGN-259, controlled adverse environment, dry eye
Introduction
Dry eye is a common ocular disorder that can range from a minor discomfort to a significant disease; in the most severe cases, it can lead to corneal ulcers, infections, and serious visual impairment.1 Conventional treatments using artificial tears, ointments, serum tears, or anti-inflammatories provide limited relief, and there is a real need for improved treatments. A therapeutic agent that could reduce inflammation and accelerate ocular surface healing would reduce the risk of permanent injury, improve vision, and provide improved comfort for those with dry eye.
Thymosin beta 4 (Tβ4) is a 43-amino acid peptide that is a major constituent protein of platelets, macrophages, and polymorphonuclear cells, where it acts as a G-actin binding molecule and regulator of actin polymerization.2–5 These cell types function in trauma response and wound repair, and Tβ4 is among the first genes to be upregulated in the integrated physiological response to tissue trauma.2,4 Endogenous or exogenous Tβ4 promotes wound repair in dermal, ocular, cardiac, and central nervous system animal models,6–13 and accelerates dermal repair in several animal models.7,14 Tβ4 is a pleotropic signaling molecule that works in part by downregulating nuclear factor kappa B-mediated transcription of inflammatory chemokines, cytokines, and metalloproteinases.2,4,15 It also acts to upregulate expression of laminin-5, and promotes cell migration, cell survival, and recruitment and maturation of stem cells.6–8,12,14–16 This spectrum of activity makes Tβ4 an attractive molecule as a potential therapeutic agent for inflammatory or traumatic conditions.
The effect of Tβ4 on corneal ocular surface healing has been examined in both rats and mice following corneal injury.8,10,11,18 Tβ4 promoted corneal ocular surface healing, increased corneal epithelial cell migration, and decreased proinflammatory cytokine levels in multiple rodent models of corneal injury.10,11,18 Mouse corneas topically treated with Tβ4 after alkali injury demonstrated accelerated re-epithelialization at all time points and decreased polymorphonuclear infiltration at 7 days post-injury compared with vehicle-treated controls.11 Other murine model studies demonstrated that Tβ4 promoted improved corneal epithelial intercellular adhesion following corneal dry eye injury.20 The results of these studies show that Tβ4 reduced corneal staining more than positive controls and demonstrated a statistically significant reduction in staining compared with vehicle control.
The clinical potential of topical 0.1% Tβ4 for treatment of corneal inflammatory conditions was demonstrated in a recent study testing treatment of neurotrophic keratitis, a condition marked by persistent, non-healing corneal defects that are typically unresponsive to available anti-inflammatory agents.21 This was a physician-sponsored compassionate use trial using the RGN-259 formulation of 0.1% Tβ4, and a complete clearing of defects was observed in six of nine subjects. Another physician-sponsored study examined the efficacy of 0.1% Tβ4 in subjects with severe dry including several with graft versus host disease.22 Subjects in this study showed significant improvements in both ocular discomfort and total corneal fluorescein staining.22 These studies established the potential of topical Tβ4 as a therapeutic approach to ocular surface disorders. The safety of topical Tβ4 formulations has been demonstrated, both in dermal preparations and in the preservative-free formulation that has been used in the eye. In a Phase I clinical trial, an injectable solution of Tβ4 administered for 14 consecutive days at four escalating dose levels was deemed safe and well tolerated.23
The established anti-inflammatory and tissue repair actions of Tβ4, together with evidence from preclinical and clinical studies, suggest that Tβ4 may represent an important new therapy for treatment of dry eye. Here, we describe a Phase II study in subjects with moderate to severe dry eye, exacerbated by a controlled adverse environment (CAE™). The use of the CAE allows for a greater standardization of environmental conditions that are a key component of dry eye, while at the same time providing a greater therapeutic window to assess therapy efficacy.24
Materials and methods
This study employed a single-center, randomized, double-masked, placebo-controlled protocol with equal randomization of subjects into two treatment groups, ie, 0.1% Tβ4 and placebo, in 72 subjects. The demographics of the subjects are shown in Table 1. The study was conducted in compliance with good clinical practices, including International Conference on Harmonisation guidelines, and consistent with the 1989 version of the Declaration of Helsinki. The institutional review board, Alpha IRB, San Clemente, CA, USA, approved the protocol.
Table 1.
Patient demographic characteristics
| Characteristic | 0.1% Tβ4 (n=36) | Placebo (n=36) | Total ITT, safety population (n=72) |
|---|---|---|---|
| Mean age, years (SD) | 57.1 (12.07) | 55.3 (12.76) | 56.2 (12.37) |
| Female, na (%) | 29 (80.6%) | 25 (69.4%) | 54 (75.0%) |
| Male, na (%) | 7 (19.4%) | 11 (30.6%) | 18 (25.0%) |
| White, na (%) | 33 (91.7%) | 34 (94.4%) | 67 (93.1%) |
| Asian, na (%) | 2 (5.6%) | 1 (2.8%) | 3 (4.2%) |
| Black or African American, na (%) | 0 (0.0%) | 1 (2.8%) | 1 (1.4%) |
| Other, na (%) | 1 (2.8%) | 0 (0.0%) | 1 (1.4%) |
| Non-Hispanic, na (%) | 34 (94.4%) | 36 (100.0%) | 70 (97.2%) |
| Iris color, nb (%) | |||
| Black | 4 (5.6%) | 0 (0.0%) | 4 (2.8%) |
| Blue | 28 (38.9%) | 30 (41.7%) | 58 (40.3%) |
| Brown | 28 (38.9%) | 26 (36.1%) | 54 (37.5%) |
| Hazel | 6 (8.3%) | 8 (11.1%) | 14 (9.7%) |
| Green | 6 (8.3%) | 8 (11.1%) | 14 (9.7%) |
| Other | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Notes: na, number of subjects; nb, number of eyes.
Abbreviation: ITT, intention to treat.
Solid phase chemical synthesis was used to prepare the Tβ4; this was followed by reversed-phase high performance liquid chromatographic purification and lyophilization of the acetate salt. The test agents were formulated as preservative-free, sterile solutions, with the active containing 0.1% (w/w) Tβ4. Both active and placebo were packaged in 8 mL squeeze bottles that contained approximately 2 mL solution. The placebo formulation was composed of the same excipients as the active, and was identical to the active in color, consistency, and odor. Each bottle of test agent delivered the same amount per drop, and had to be used within 6 hours of opening; therefore, two bottles were needed for each day of dosing.
Study design
Enrolled subjects were aged 18 years or older, of any race and sex, with a history of dry eye syndrome in both eyes, and use or desire to use artificial tear substitutes within the previous 6 months. All had corneal staining of ≥2 in any corneal surface segment (at least one eye), and a conjunctival redness score of ≥1 (at least one eye) prior to CAE at visit 1. Subjects also had a best corrected visual acuity of 0.7 logMAR or better in each eye, and had to demonstrate a positive response to the CAE as defined in the protocol at visit 1 for inclusion. All participants provided informed consent. The study comprised six visits conducted over a period of approximately 31 days (Figure 1 and Table 1).
Figure 1.
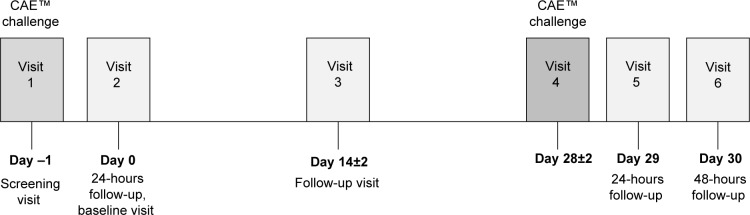
Study timeline.
Abbreviation: CAE, controlled adverse environment.
Scales for corneal staining were based upon a 0–4 rating where 0= no staining and 4= confluent staining. Conjunctival redness was measured using a similar 0–4 scale, where 0= none and 4= severe. In both cases, 0.5 incremental scores were allowed. Ocular discomfort was graded on a 0–4 scale with no half units; for this scale 0= no discomfort and 4= constant discomfort.
At visit 1 (day –1) prospective subjects had their signs and symptoms assessed before and after CAE exposure. Ocular discomfort scores were recorded throughout the CAE challenge. Subjects initially qualifying for study entry were instructed to discontinue all ophthalmic medications and instilled a commercially available sterile irrigating (balanced salt) solution (ie, the run-in solution) twice daily until visit 2, day 0. The first dose of this run-in solution was administered in-office by a trained technician. Subjects were dispensed a daily diary with which to enter the details of dosing and symptomatology. At visit 2, the 24-hour follow-up visit, subjects were reassessed and those who met entry criteria for discomfort and corneal staining were randomized to receive either 0.1% Tβ4 ophthalmic solution (RGN-259; RegeneRx Pharmaceuticals Inc, Rockville, MD, USA) or placebo, for the 28-day duration of test agent treatment. Subjects received their first dose of randomized test agent in-office at visit 2, and were then instructed to instill 1–2 drops of study drug (0.1% Tβ4 or placebo) into each eye twice a day for a total of 28 days.
Subjects returned to the clinic for visit 3 (day 14±2) to have their signs and symptoms assessed, and to receive additional supplies of test agent. At visit 4 (day 28±2) subjects underwent a second CAE exposure; on the following two days, visits 5 and 6, subjects underwent additional assessments of signs and symptoms.
The coprimary endpoints for this study were the differences at visit 5 between the active and placebo mean values for inferior corneal staining (primary sign) and for ocular discomfort score (primary symptom). Secondary endpoints included differences at visit 5 in corneal and conjunctival staining, conjunctival redness, and tear film break-up time; differences at visit 4 in ocular discomfort during CAE, corneal and conjunctival staining, conjunctival redness, and tear film break-up time.
Statistical analysis
The quantitative variables were summarized using summary statistics (n, mean, median, standard deviation, minimum, and maximum). Qualitative variables were summarized using counts and percentages. Inferior corneal fluorescein staining score at visit 5 (primary sign) and ocular discomfort at visit 5 (primary symptom) were summarized using descriptive statistics (number of observations, mean, standard deviation, median, minimum, and maximum). Active treatment was compared with placebo using a two-sample t-test assuming unequal variances, assessed at the level of α=0.05. The primary efficacy variables were also analyzed using a nonparametric Wilcoxon rank sum test for sensitivity, as well as an analysis of covariance (ANCOVA) model adjusted for baseline (visit 1 pre-CAE) score. The change from baseline to visit 5 was also analyzed.
The continuous secondary efficacy variables collected at each visit were also summarized statistically (n, mean, standard deviation, median, minimum, and maximum), and analyzed similarly to the primary efficacy endpoints. All visit-based data were analyzed at each time point (pre- and post-CAE) if applicable, as well as the change from pre-CAE to post-CAE. Finally, a repeated-measures mixed model was generated to assess the environmental treatment effect, including data from visit 3, visit 4 (pre-CAE), visit 5, and visit 6. Baseline (visit 1 pre-CAE) was included as a covariate, and the model was used to obtain least squares estimates and test for treatment effects at each visit and overall. Ocular discomfort in the chamber was measured every 5 minutes after the patient entered the CAE. The area under the curve was calculated for each patient using the trapezoidal rule, and compared between treatment groups using a two-sample t-test assuming unequal variances, as well as an ANCOVA model adjusted for baseline (visit 1 area under the curve). Each symptom collected in the diary was summarized by day and time point, and compared between groups using a mixed model accounting for repeated measures within each subject.
Safety analysis
The quantitative safety variables were summarized using descriptive statistics (n, mean, standard deviation, minimum, median, and maximum). The qualitative safety variables were summarized by frequencies and percentages. Adverse events were coded using the MedDRA dictionary, version 13.1. Frequencies and percentages were provided by treatment group of subjects with treatment-emergent adverse events (TEAEs), serious TEAEs, and TEAEs causing premature discontinuation. An adverse event was treatment-emergent if it occurred or worsened after the first dose of study drug up through visit 6.
Safety endpoints were analyzed for both eyes. For efficacy endpoints, the unit of analysis was the “worst eye”. Eyes were eligible for analysis if they met all “inclusion criteria”. In the event that both eyes were eligible for analysis, the worst eye was chosen as the eye with the greater increase of inferior corneal staining from pre-CAE at visit 1 to visit 2. If both eyes had an equal increase in inferior corneal staining from pre-CAE at visit 1 to visit 2, the eye with greater ocular discomfort at visit 2 was chosen as the worst eye. If both eyes had an equal increase in inferior corneal staining from pre-CAE at visit 1 to visit 2 and equal ocular discomfort at visit 2, then the right eye was chosen as the worst eye.
Results
A total of 72 subjects were screened. Of these, 72 were enrolled in the study, with 36 subjects assigned to each group, ie, 0.1% Tβ4 ophthalmic solution or placebo. Sixty-nine subjects completed the trial. Two subjects in the placebo group and one in the 0.1% Tβ4 did not complete the study and five subjects had major protocol deviations and were not included in the per-protocol analysis. Subject demographics are summarized in Table 2. All subjects received the study medication or vehicle on day 0 (visit 2).
Table 2.
Schedule of procedures
| Procedure | Visit 1 (day -1)
|
Visit 2 (day 0)
|
Visit 3 (day 14±2)
|
Visit 4 (day 28±2)
|
Visit 5 (day 29)
|
Visit 6 (day 30)
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAE challenge
|
24-hour follow-up | Day 14 follow-up | CAE challenge
|
24-hour follow-up | 48-hour follow-up | |||||
| Pre-CAE | CAE | Post-CAE | Pre-CAE | CAE | Post-CAE | |||||
| Informed consent | × | |||||||||
| Medical history | × | × | × | × | × | × | ||||
| Pregnancy test | × | × | ||||||||
| Randomization | × | |||||||||
| Dispense study drug | × | × | × | × | ||||||
| Dispense 1-day diary | × | × | × | |||||||
| Collect 1-day diary | × | × | × | |||||||
| Dispense 2-week diary | × | × | ||||||||
| Collect 2-week diary | × | × | ||||||||
| 4-symptom evaluation | × | × | × | × | × | × | ||||
| VA | × | × | × | × | × | × | ||||
| Slit-lamp examination | × | × | × | × | × | × | × | × | ||
| Conjunctival redness | × | × | × | × | × | × | × | × | ||
| TBUT | × | × | × | × | × | × | × | × | ||
| Corneal staining | × | × | × | × | × | × | × | × | ||
| Conjunctival staining | × | × | × | × | × | × | × | × | ||
| Ocular discomfort | × | × | × | × | × | × | × | × | ||
| IOP, dilated funduscopy | × | × | ||||||||
| Corneal sensitivity | × | × | ||||||||
| Adverse event query | × | × | × | × | × | × | × | |||
| Study exit | × | |||||||||
Abbreviations: CAE, controlled adverse environment; VA, visual acuity; TBUT, tear break-up time; IOP, intraocular pressure.
The primary efficacy measures were inferior fluorescein corneal staining and ocular discomfort in the worst eye at visit 5. Mean inferior fluorescein corneal staining was 2.08 in 0.1% Tβ4-treated subjects and 1.90 in placebo-treated subjects. This difference (0.18) was not statistically significant (P=0.2586, Wilcoxon rank sum test). The ANCOVA with visit 2 values as the covariate provided least square means of 2.06 in 0.1% Tβ4-treated subjects and 1.92 in placebo-treated subjects. This difference (0.14) was also not significant (P=0.3452). Mean ocular discomfort at visit 5 was 1.6 in 0.1% Tβ4-treated eyes and 1.3 in placebo-treated eyes. This difference (0.3) was not statistically significant (P=0.2210). The ANCOVA with visit 2 as the covariate provided a least square mean of 1.5, again with a difference (0.2) that was not statistically significant (P=0.4901). Despite these findings, when measures were adjusted for baseline in several ad hoc statistical analyses, several significant differences between active and placebo groups were identified.
To address the possible impact of baseline staining, we compared superior corneal staining scores from the 2 days of CAE exposure (visits 1 and 4), before or after 28 days of treatment with 0.1% Tβ4 or placebo; the results of this analysis are shown in Figure 2. Mean superior corneal staining after CAE was reduced after 28 days of 0.1% Tβ4 treatment, while those in the placebo group showed a modest (insignificant) increase in mean staining. Comparison of the change in staining demonstrated a statistically significant difference between the two treatment groups (P=0.0210).
Figure 2.
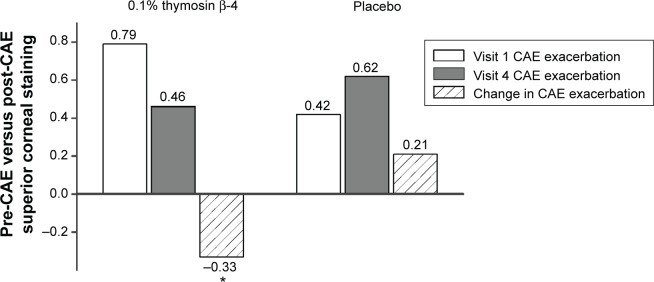
Reduction of superior corneal fluorescein staining by Tβ4 between visit 1 and visit 4. Mean values for staining exacerbation in the CAE were reduced in Tβ4-treated subjects when compared with placebo-treated subjects.
Note: *P=0.021.
Abbreviation: CAE, controlled adverse environment.
A similar comparison of central corneal staining after recovery from the CAE exposure (visits 2 and 5) also showed a significant reduction in fluorescein staining (Figure 3). The mean difference in staining for the 0.1% Tβ4-treated subjects was decreased by 0.37, while the mean for subjects in the placebo group increased by 0.16; this difference was statistically significant (P=0.0075). Peripheral (the mean superior and inferior cornea together) corneal staining also was significantly less in 0.1% Tβ4-treated eyes after 28 days of treatment in two separate analyses. The change from visit 1 post-CAE to visit 4 post-CAE was –0.19 in the 0.1% Tβ4-treated eyes and +0.13 in the placebo-treated eyes (P=0.0379). A summary of the corneal staining data is provided in Table 3.
Figure 3.
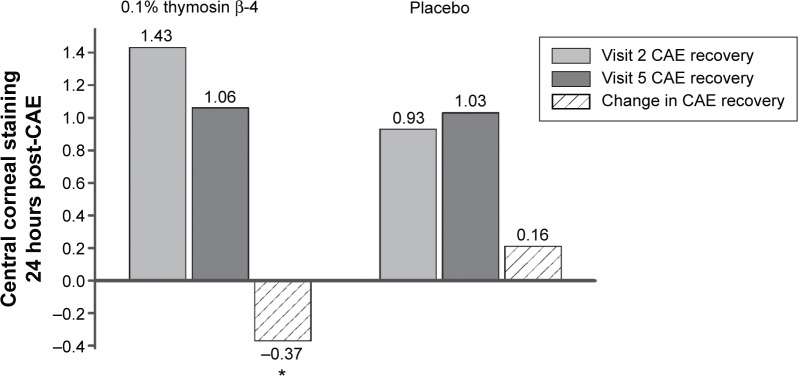
Reduction of central corneal fluorescein staining by Tβ4 between visit 2 (baseline after CAE) and visit 5 (24-hour follow up after CAE). The data show a statistically significant improvement by Tβ4 in healing and protection from exacerbation.
Note: *P=0.0075.
Abbreviation: CAE, controlled adverse environment.
Table 3.
Corneal staining by region
| Region | Superior | Inferior | Peripheral | Central | |
|---|---|---|---|---|---|
| Treatment comparison | |||||
| Mean Change (SD) post-CAE | Placebo | +0.32 (0.815) | −0.06 (0.886) | +0.13 (0.691) | +0.65 (0.942) |
| Visit 1 to Visit 4 | 0.1% Tβ4 | −0.14 (0.648) | −0.24 (0.826) | −0.19 (0.575) | +0.53 (1.09)c |
| Mean Difference | −0.47 | −0.18 | −0.33 | −0.12 | |
| P-valuea | 0.0109 | 0.3755 | 0.0379 | 0.6304 | |
| P-valueb | 0.0181 | 0.3478 | 0.0741 | 0.7322 | |
| Mean Change (SD) 24 hour post-CAE | Placebo | – | −0.4 (1.23) | – | 0.16 (0.682) |
| Visit 2 to Visit 5 | 0.1% Tβ4 | – | −0.4 (1.08) | – | −0.36 (0.871) |
| Mean Difference | 0.00 | −0.52 | |||
| P-valuea | 1.0000 | 0.0075 | |||
| P-valueb | 0.9186 | 0.0066 | |||
Notes:
P-value calculated using a two-sample t-test assuming unequal variances comparing Tβ4 to placebo.
P-value calculated using a Wilcoxon rank sum test comparing Tβ4 to placebo.
For central staining, comparison is Visit 1 pre-CAE to Visit 4 post-CAE. Bold values indicate. Significant differences between the placebo and 0.1% Tβ4 are highlighted in bold.
Abbreviations: CAE, controlled adverse environment; SD, standard deviation.
As expected, ocular discomfort scores increased over the course of the CAE exposures for all treatment groups. Following the 28-day treatment with 0.1% Tβ4, however, there was a distinction between the active and placebo treatment groups. From the beginning to the end of the CAE, discomfort scores rose from 1.3 to 3.5 in the placebo group but only rose from 1.7 to 3.3 in the 0.1% Tβ4-treated group; comparison of the changes show a significantly lower increase for 0.1% Tβ4-treated subjects (P=0.0224, Figure 4).
Figure 4.
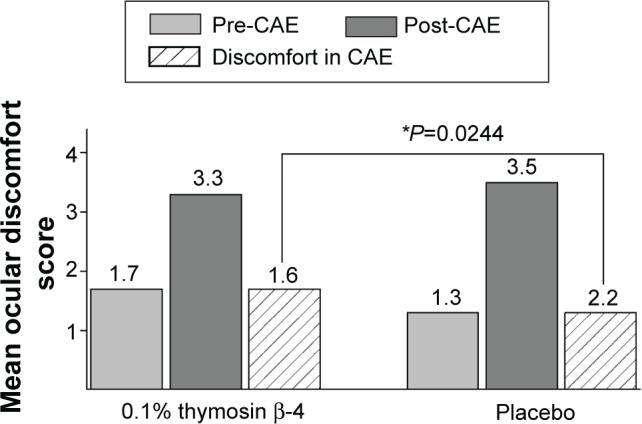
Reduction in CAE-associated ocular discomfort following 28 day treatment with 0.1% Tβ4. The change in mean discomfort scores in Tβ4-treated subjects at visit 4 was statistically reduced when compared to mean placebo scores (P=0.0244).
Abbreviation: CAE, controlled adverse environment.
No safety issues arose in this adverse environment challenge study with the use of 0.1% Tβ4 or its vehicle over the duration of treatment. No clinically significant changes in visual acuity, slit-lamp, and funduscopic assessments or intraocular pressure and corneal sensitivity measurements were observed. There was also a very low rate of TEAEs; only two of these were suspected to be related to study treatment, which was placebo in both cases. Only one subject was withdrawn from the study due to an adverse event, ie, moderately severe vomiting, suspected to be unrelated to the study treatment.
The total number of subjects with at least one ocular TEAE was two (5.6%) in the 0.1% Tβ4-treated group and five (13.9%) in the placebo group. Both of the 0.1% Tβ4 events were classified as mild (5.6%), while three were mild (8.3%) and two moderate (5.6%) in the placebo group. When broken down, one incidence of mild eye pain (2.8% each) occurred in each of the 0.1% Tβ4 and placebo groups. One mild blurred vision (2.8%) was reported in the 0.1% Tβ4 group, and one mild eye irritation in the placebo group (2.8%). A mild decrease in visual acuity was reported once (2.8%) in the placebo-treated group, and a mild hordeolum was reported once (2.8%) in the Tβ4-treated group. Instillation site pain was reported twice in the placebo-treated group (5.6%), one of which was mild (2.8%) and one was moderate (2.8%). A moderate instillation site reaction was also reported in the placebo-treated group once (2.8%).
Discussion
In the present Phase II clinical trial, a 28-day course of 0.1% Tβ4 ophthalmic formulation elicited significant positive effects on ocular discomfort and on corneal staining in subjects with dry eye. Results suggest that Tβ4 has a protective effect, reducing the extent of corneal staining exhibited by subjects following exposure to a controlled adverse environment. In addition, significant differences between active and placebo groups at 24 hours post-CAE are consistent with an acceleration in healing for subjects treated with Tβ4.
A number of potential mechanisms may underlie these effects.3,4 Tβ4 has been shown to reduce production of inflammatory mediators and infiltration of inflammatory cells; these actions may reduce local concentrations of reactive oxygen species, thereby reducing local tissue damage and attenuating apoptotic activation.3,19,25 All of these effects would act to protect cells exposed to adverse stimuli, and would also potentially reduce activity in neural pathways associated with pain sensation. Tβ4 has also been shown to regulate cellular actin dynamics and may enhance the cell motility, cell-matrix interactions, and cell-matrix remodeling that occurs during the course of wound healing.2,12
Another important finding from this study was the affirmation that therapeutic use of Tβ4 in a topical formulation is safe and well tolerated by users. No significant adverse events were reported, and no subjects withdrew from the study due to any adverse effect of the study formulation. Dry eye is a chronic disease, and future studies will need to address the long-term efficacy of Tβ4 as well as its long-term safety profile.
Two central features of dry eye that have made the development of new therapeutics challenging are the lack of correlation between signs and symptoms and the high degree of individual patient variability.1 This variability may contribute to the failure of comparisons between treatment groups to reach significance. Despite this, the findings of significant improvement for both a symptom (ocular discomfort) and a sign (corneal fluorescein staining), along with a lack of any drug-related adverse events, establish that Tβ4 has the potential to be a new, potent, and useful treatment for dry eye. Optimization of treatment regimens and study protocols going forward should allow future studies to confirm and extend these positive preliminary findings.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Report of the International Dry Eye Workshop (DEWS) Ocul Surf. 2007;5:65–204. doi: 10.1016/s1542-0124(12)70086-1. No authors listed. [DOI] [PubMed] [Google Scholar]
- 2.Crockford D, Turjman N, Allan C, Angel J. Thymosin beta4: structure, function, and biological properties supporting current and future clinical applications. Ann N Y Acad Sci. 2010;1194:179–189. doi: 10.1111/j.1749-6632.2010.05492.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein AL, Hannappel E, Sosne G, Kleinman HK. Thymosin beta 4: a multifunctional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther. 2012;12:37–51. doi: 10.1517/14712598.2012.634793. [DOI] [PubMed] [Google Scholar]
- 4.Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. Beta-thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2010;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 5.Low TL, Goldstein AL. Chemical characterization of thymosin beta 4. J Biol Chem. 1982;257:1000–1006. [PubMed] [Google Scholar]
- 6.Bock-Marquette I, Saxena A, White MD, et al. Thymosin β4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 7.Malinda KM, Sidhu GS, Mani H, et al. Thymosin β4 accelerates wound healing. J Invest Dermatol. 1999;113:364–368. doi: 10.1046/j.1523-1747.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- 8.Sosne G, Siddiqi A, Kurpakus-Wheater M. Thymosin-β4 inhibits corneal epithelial cell apoptosis after ethanol exposure in vitro. Invest Ophthalmol Vis Sci. 2004;45:1095–1100. doi: 10.1167/iovs.03-1002. [DOI] [PubMed] [Google Scholar]
- 9.Morris D, Chopp M, Zhang L, et al. Tβ4 Improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010;169:674–682. doi: 10.1016/j.neuroscience.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosne G, Hafeez S, Greenberry A, 2nd, Kurpakus-Wheater M. Thymosin beta 4 promotes human conjunctival epithelial cell migration. Curr Eye Res. 2002;24:268–273. doi: 10.1076/ceyr.24.4.268.8414. [DOI] [PubMed] [Google Scholar]
- 11.Sosne G, Sliter EA, Barrett R, et al. Thymosin β4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–299. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- 12.Sosne G, Xu L, Prach L, et al. Thymosin β4 stimulates laminin-5 production independent of TGF-beta. Exp Cell Res. 2004;293:175–183. doi: 10.1016/j.yexcr.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Zhang ZG, Morris D, et al. Neurological function recovery after Tβ4 treatment in mice with experimental auto encephalomyelitis. Neuroscience. 2009;164:1887–1893. doi: 10.1016/j.neuroscience.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philp D, Badamchian M, Scheremeta B, et al. Thymosin beta 4 and a synthetic peptide containing its actin-binding domain promote dermal wound repair in db/db diabetic mice and in aged mice. Wound Repair Regen. 2003;11:19–24. doi: 10.1046/j.1524-475x.2003.11105.x. [DOI] [PubMed] [Google Scholar]
- 15.Sosne G, Qiu P, Christopherson PL, Wheater MK. Thymosin β4 suppression of corneal NFκB. A potential anti-inflammatory pathway. Exp Eye Res. 2007;84:663–669. doi: 10.1016/j.exer.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinkel R, El-Aouni C, Olson T, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–2240. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smart N, Risebro CA, Melville AA, et al. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 18.Sosne G, Chan CC, Thai K, et al. Thymosin beta 4 promotes corneal wound healing and modulates inflammatory mediators in vivo. Exp Eye Res. 2001;72:605–608. doi: 10.1006/exer.2000.0985. [DOI] [PubMed] [Google Scholar]
- 19.Sosne G, Christopherson PL, Barrett RP, Friedman R. Thymosin-beta4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Invest Ophthalmol Vis Sci. 2005;46:2388–2395. doi: 10.1167/iovs.04-1368. [DOI] [PubMed] [Google Scholar]
- 20.Allan CB, Cockcroft D, Crawford KS, Belen LJ. Effects of thymosin β4 in a murine CAE™ model of experimental dry eye. Invest Ophthalmol Vis Sci. 2011;52:E3782. [Google Scholar]
- 21.Dunn SP, Heidemann DG, Chow CY, et al. Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin β4. Ann NY Acad Sci. 2010;1194:199–206. doi: 10.1111/j.1749-6632.2010.05471.x. [DOI] [PubMed] [Google Scholar]
- 22.Sosne G, Dunn S, Crockford D, Kim C, Dixon E. Thymosin beta 4 eye drops significantly improve signs and symptoms of severe dry eye in a physician-sponsored phase-2 clinical trial. Invest Ophthalmol Vis Sci. 2013;54:E6033. [Google Scholar]
- 23.Ruff D, Crockford D, Girardi G, Zhang Y. A randomized, placebo-controlled, single and multiple dose study of intravenous thymosin beta4 in healthy volunteers. Ann N Y Acad Sci. 2010;1194:223–229. doi: 10.1111/j.1749-6632.2010.05474.x. [DOI] [PubMed] [Google Scholar]
- 24.Abelson R, Lane KJ, Rodriguez J, et al. A single-center study evaluating the effect of the controlled adverse environment (CAE™) model on tear film stability. Clin Ophthalmol. 2012;6:1865–1872. doi: 10.2147/OPTH.S33905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson SE, Mohan RR, Mohan RR, et al. The corneal wound healing response; cytokine mediated interaction of the epithelium, stroma and inflammatory cells. Prog Retin Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]


