Abstract
The dehydroformylation of aldehydes to generate olefins occurs during the biosynthesis of various sterols, including cholesterol in humans. Here, we implement a synthetic version that features the transfer of a formyl group and hydride from an aldehyde substrate to a strained olefin acceptor. A Rh(Xantphos)(benzoate) catalyst activates aldehyde C–H bonds with high chemoselectivity to trigger C–C bond cleavage and generate olefins at low loadings (0.3 to 2 mol%) and temperatures (22 to 80 °C). This mild protocol can be applied to various natural products and was used to achieve a three step synthesis of (+)-yohimbenone. A study of the mechanism reveals that the benzoate counterion acts as a proton-shuttle to enable transfer hydroformylation.
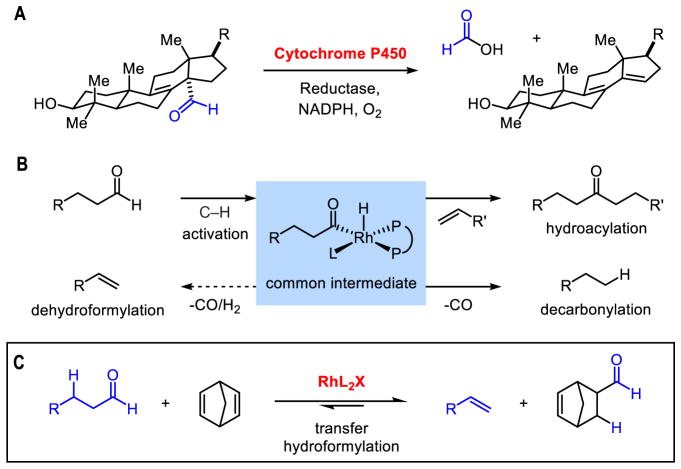
The cytochrome P450 enzymes have captured the imagination of chemists who seek to emulate their reactivity. For example, monooxygenases motivated the design of catalysts that epoxidize olefins and oxidize C–H bonds (1–4). This enzyme superfamily also includes various demethylases that break C–C bonds (5). In particular, lanosterol demethylase converts aldehydes to olefins by dehydroformylation during the biosynthesis of sterols in bacteria, algae, fungi, plants, and animals (6) (Figure 1A). Inspired by this step in biosynthesis, we sought a transition-metal catalyst for dehydroformylations in organic synthesis.
Fig. 1. Dehydroformylation in nature and organic synthesis. (A).
Dehydroformylation during sterol biosynthesis. (B) Reactivity of acyl-RhIII-hydrides. (C) Proposed transfer hydroformylation.
To this end, we aimed to trigger C–C bond cleavage (7–11) by chemoselective activation of aldehyde C–H bonds using Rh-catalysis (Figure 1B). Over the past fifty years, activating aldehyde C–H bonds with Rh has been thoroughly investigated (12); however, the resulting acyl–RhIII–hydrides have been trapped mainly by hydroacylation (13) and/or decarbonylation (14,15). This common intermediate is also implicated in hydroformylation, which is practiced on an industrial scale using syngas (16). Thus, we needed a strategy for diverting the acyl–RhIII–hydride towards dehydroformylation. To date, olefins generated by dehydroformylation have been observed in low-quantities during decarbonylations (15,17,18). One report describes the use of stoichiometric Ru for dehydroformylation of butyraldehyde (19), while another uses heterogeneous Rh or Pd catalysts for transforming steroidal aldehydes at 160–300 °C (20). In contrast, an Fe-peroxo complex cleaves aldehyde C–C bonds at room temperature, but this complex must be used in stoichiometric amounts and can lead to olefin epoxidation (21,22).
Given this challenge, we designed a strategy where dehydroformylation of an aldehyde substrate is driven by the concomitant hydroformylation of a strained olefin acceptor (Figure 1C) (23,24). This transfer hydroformylation avoids the accumulation of CO gas, which acts as a catalyst poison in related aldehyde dehomologations. Thus, formyl group transfer should proceed under mild conditions. Brookhart’s study on the linear-to-branched isomerization of aldehydes with Rh-catalysis supports the feasibility of this approach (25). Moreover, Morimoto developed hydroformylations of mono-substituted olefins using formaldehyde as a source of CO and H2 (26). Here, we report a Rh-catalyst for transfer hydroformylation that operates in the 22 to 80 °C temperature range, with loadings as low as 0.3 mol%. This mild protocol for dehydroformylation can be applied to a wide range of aldehydes, including those derived from alkaloid, terpene, steroid, and macrolide natural products.
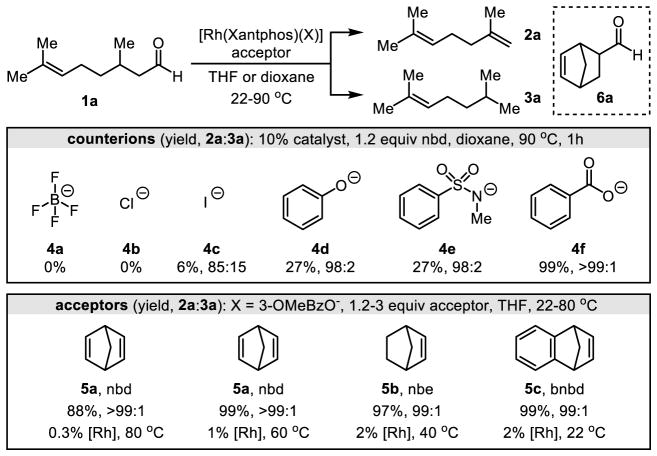
During initial studies, we obtained promising results by investigating non-traditional counterions for Rh(Xantphos) complexes (Figure 2). The Xantphos ligand was chosen given its success in related hydroacylations, hydroformylations, and decarbonylations (13,16). Using citronellal (1a) and norbornadiene (5a) as the model substrate and acceptor, respectively, we observed that typical counterions such as BF4− and Cl− yielded trace decarbonylation products, whereas a softer counterion, I−, led to mixed dehydroformylation and decarbonylation reactivity. An increase in reactivity and selectivity was obtained by switching to organic counterions such as phenolates and sulfonamidates. The use of a benzoate counterion provided a breakthrough in efficiency. Against expectations, further tuning of the counterion revealed few trends related to pKa, Hammett parameters, or coordinating ability. This observation suggests that the counterion plays a critical role in the mechanism (vide infra). 3-Methoxybenzoate provided a five-fold increase in initial rate compared to benzoate. We also identified 5-norbornene-2-carboxaldehyde (6a) as a stoichiometric product in each of these reactions indicating that a transfer hydroformylation mechanism operates.
Fig. 2. Effects of counterion structure and ring strain.
nbd = 1,5-norbornadiene, nbe = norbornene, bnbd = benzonorbornadiene; yields were determined by GC-FID analysis of the reaction mixtures using durene as an internal standard.
The choice of olefin acceptor influences both catalyst loading and reaction temperature (Figure 2). Because norbornadiene (5a) gave selectivity greater than 99:1 2a:3a, the catalyst loading could be lowered to 0.3 mol% at 80 °C or 1 mol% at 60 °C using this acceptor. The reaction temperature could be further reduced by using olefin acceptors that cannot chelate to Rh. For instance, norbornene (5b) displayed excellent reactivity at 40 °C, while a slightly more strained acceptor, benzonorbornadiene (5c), provided reactivity at ambient temperature. To examine the scope of this strategy, we chose norbornadiene (5a) as the acceptor because it afforded the highest chemoselectivity with the lowest catalyst loadings.
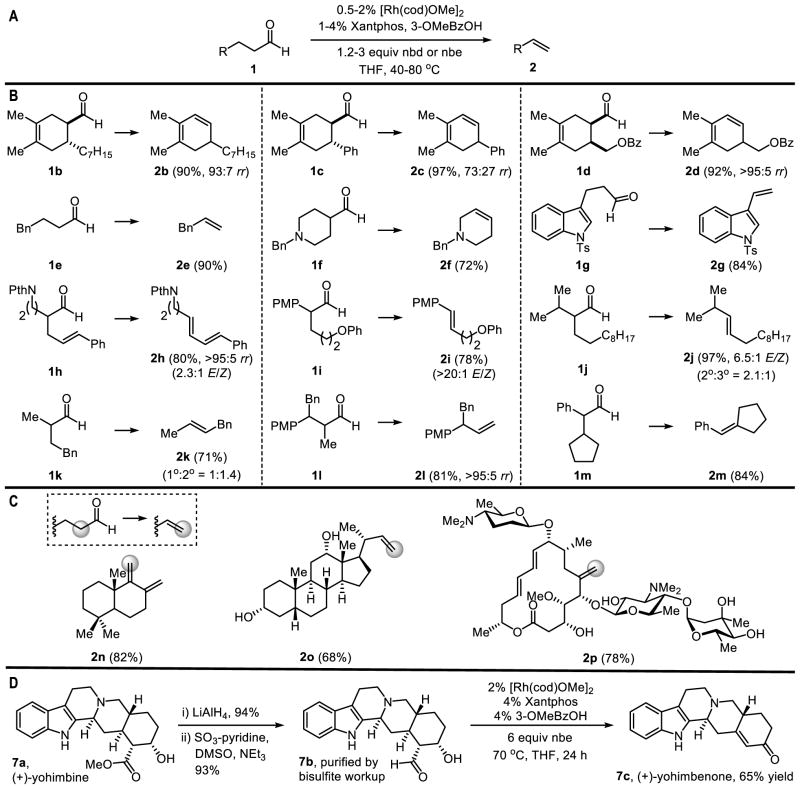
This transfer hydroformylation protocol enables access to olefins from a wide range of aldehyde precursors. The Diels-Alder cycloaddition was used to easily generate cyclohexene-4-carboxaldehdye substrates 1b through 1d. The trans adduct 1b underwent dehydroformylation to yield the conjugated 1,3-diene, whereas 1c gave a mixture of 1,3- and 1,4-dienes. The cis Diels-Alder adduct 1d yielded the 1,3-diene exclusively, most likely as a result of a syn-selective β-hydride elimination. We reason that the observed regioselectivities are controlled by kinetics because 4-phenylbutanal (1e) yields the terminal olefin (2e) without any isomerization to the styrene derivative. In general, Lewis basic functionality, such as ethers, esters, amines, phthalimides, and indoles, were tolerated (1f–1i, 1l). A vinylindole was derived by dehydroformylation of 1g, which was ultimately prepared from commercial indole and acrolein. Although 4-pentenals are prototypical substrates for intramolecular olefin hydroacylation, the α-allylated aldehyde 1h underwent chemoselective dehydroformylation to yield the conjugated diene. Disubstituted olefins enriched in the E-stereoisomer (>20:1 E/Z) were accessed from the corresponding α-arylated aldehydes (1i). Substrates that do not form conjugated products upon dehydroformylation were transformed with modest regioselectivities (1j and 1k); however, steric congestion favored terminal olefins over tri-substituted products (1l). Nonetheless, tri-substituted olefins were generated from substrates containing a single syn-β-hydrogen such as 1m.
Next, we applied this protocol to generate structurally complex olefins from natural products (Figure 3C). By dehydroformylation of a (+)-sclareolide derivative, we accessed a carbon-based scaffold 2n containing an exocyclic diene adjacent to a quaternary center. This product is a key intermediate in the synthesis of several terpenes. Furthermore, (+)-sclareolide is an inexpensive and readily available precursor, whereas typical precursors such as (+)-manool and (−)-polygodial have either been discontinued by commercial suppliers or are available only in milligram quantities (27).
Fig. 3. Applications of dehydformylation.
(A) General conditions for transfer hydroformylation. (B) Substrate scope. (C) Natural product derivatization. (D) Three step synthesis of (+)-yohimbenone. Yields are of isolated materials and mixtures of regioisomers where indicated; rr = regioisomeric ratio; rr values were determined by 1H NMR analysis of the reaction mixtures; the yields of 2e and 2k were determined by 1H NMR analysis of the reaction mixtures using durene as an internal standard; see the supplementary materials for details.
To study the chemoselectivity of dehydroformylation, we examined steroid and macrolide substrates (Figure 3C). Deoxycholic acid derivative 2o was prepared without protection of the hydroxyl groups, despite the potential for alcohol oxidation under Rh-catalysis (28,29). Thus, activation of the aldehyde C–H bond occurred with high chemoselectivity to initiate C–C bond cleavage. Smooth dehydroformylation of the antibiotic spiramycin I to generate macrolide 2p highlights the tolerance of this method to many functional groups, including dienes, amines, ethers, esters, and acetals. In this case, dehydroformylation introduced an exocyclic olefin that dramatically altered the topology of the macrolide.
The yohimbinoid family of indole alkaloids has often served as a testing ground for methodology (30). Padwa reported the de novo synthesis of racemic yohimbenone in eleven steps from methyl 3-indolylacetate (31). By using dehydroformylation as a key step, we prepared (+)-yohimbenone in three steps from commercially available and inexpensive (+)-yohimbine. Conversion of ester 7a to β-hydroxy aldehyde 7b was achieved in 87% yield by LiAlH4 reduction followed by Parikh-Doering oxidation, and the resulting aldehyde was purified by a simple workup with sodium bisulfite. This aldehyde contains both a syn- and an anti-β-hydrogen. Syn-selective dehydroformylation established the trisubstituted olefin at the ring-junction. To our surprise, however, the resulting allylic alcohol underwent transfer dehydrogenation in the same pot to yield (+)-yohimbenone in 65% yield. Because dehydroformylation is faster that the allylic alcohol oxidation, either the allylic alcohol or enone product could be selectively formed by controlling the reaction temperature and stoichiometry of the strained olefin acceptor (32).
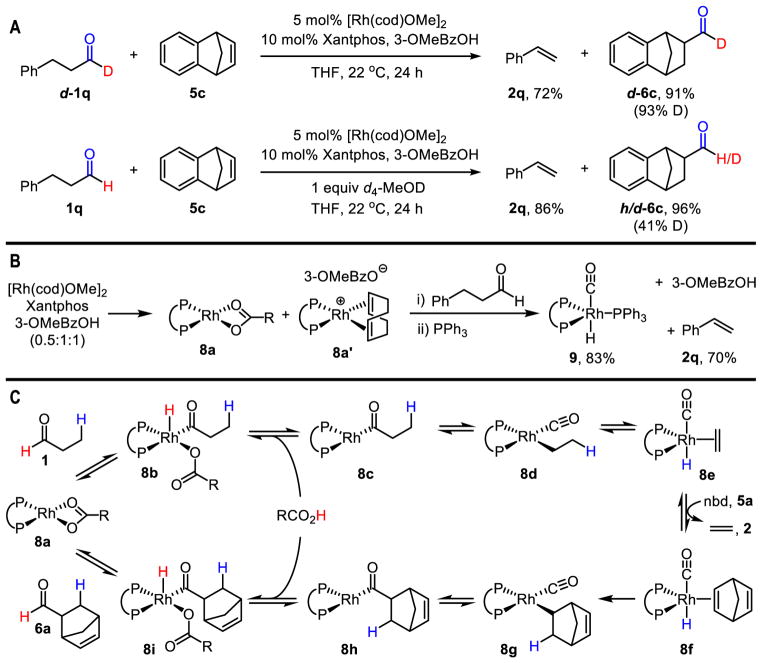
Through experiments designed to probe the mechanism, we obtained insight into why the counterion and strained acceptor are critical in diverting the acyl-RhIII-hydride intermediate along a unique pathway. Isotopic labeling studies revealed that the deuterium label of aldehyde d-1q is incorporated into the formyl group of the product d-6c. However, statistical scrambling occurred when protio-1q was subjected to transfer hydroformylation in the presence of deuterated methanol (Figure 4A). Together, these results suggest that the aldehyde proton is transferred to the product via the intermediacy of 3-methoxybenzoic acid, which can undergo proton-exchange with methanol. Experiments using stoichiometric Rh support this mechanistic scenario (Figure 4B). Combining the Rh-source, 3-methoxybenzoic acid, and phosphine ligand resulted in an equilibrium mixture of Rh-complexes 8a and 8a′, each with 3-methoxybenzoate counterions. Upon treatment of this mixture with hydrocinnamaldehyde (1q), we observed styrene (2q) in high yields along with the regeneration of the benzoic acid derivative (33). Subsequent addition of PPh3 enabled us to identify the organometallic product, Rh-hydrido-carbonyl 9, which is a catalyst for traditional hydroformylations (34). Although stoichiometric dehydroformylation takes place in the absence of the strained acceptor, our studies on the catalytic process revealed a correlation between the ring strain of the acceptor and the selectivity for dehydroformylation versus decarbonylation. Therefore, we propose that stoichiometric dehydroformylation in the absence of acceptor is thermodynamically downhill and reversible, but norbornadiene can irreversibly trap the Rh-hydrido-carbonyl intermediate to prevent decarbonylation and turn over the catalyst.
Fig. 4. Mechanistic studies.
(A) Deuterium labelling studies. (B) Isolation of organometallic intermediates. (C) Proposed catalytic cycle.
A proposed catalytic cycle for transfer hydroformylation is depicted in Figure 4C. The neutral Rh-complex 8a activates the aldehyde C–H bond to generate acyl-RhIII-hydride 8b. The 3-methoxybenzoate counterion can then undergo reductive elimination with the hydride ligand to generate acyl-RhI 8c and 3-methoxybenzoic acid (35). In contrast, most hydroacylations and decarbonylations typically employ innocent counterions such as Cl− and BF4−. De-insertion of CO and subsequent β-hydride elimination forges Rh-hydrido-carbonyl 8e. Exchange of the olefin product with nbd (5a) generates 8f, which irreversibly leads to the transfer hydroformylation product 6a through similar mechanistic steps in reverse order (Figure 4C). Thus, the ring-strain of the olefin acceptor and the ability of the counterion to act as a proton-shuttle by reversible redox processes afford high reactivity and selectivity.
Supplementary Material
Acknowledgments
The authors are grateful for financial support provided by the NIH General Medical Sciences (GM105938). S. K. M. is grateful for a Canada Graduate Scholarship from NSERC. We acknowledge Colin M. Rathbun for contributions during the optimization stage of reaction development.
Footnotes
References and Notes
- 1.Groves JT. The bioinorganic chemistry of iron in oxygenases and supramolecular assemblies. Proc Natl Acad Sci USA. 2003;100:3569–3574. doi: 10.1073/pnas.0830019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breslow R, Huang Y, Zhang X, Yang J. An artificial cytochrome P450 that hydroxylates unactivated carbons with regio- and stereoselectivity and useful catalytic turnovers. Proc Natl Acad Sci USA. 1997;94:11156–11158. doi: 10.1073/pnas.94.21.11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen MS, White MC. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science. 2010;327:566–571. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Loebach JL, Wilson SR, Jacobsen EN. Enantioselective epoxidation of unfunctionalized olefins catalyzed by (salen)manganes complexes. J Am Chem Soc. 1990;112:2801–2803. [Google Scholar]
- 5.Sigel A, Sigel H, Sigel RKO, editors. The ubiquitous roles of cytochrome P450 proteins: metal ions in life sciences. Vol. 3. Wiley; New York: 2007. [Google Scholar]
- 6.Lepesheva GI, Waterman MR. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim Biophysic Acta. 2007;1770:467–477. doi: 10.1016/j.bbagen.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dermenci A, Coe JW, Dong G. Direct activation of relatively unstrained carbon-carbon bonds in homogenous systems. Org Chem Front. 2014;1:567–584. doi: 10.1039/c4qo00053f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jun C–H. Transition metal catalyzed carbon-carbon bond activation. Chem Soc Rev. 2004;33:610–618. doi: 10.1039/b308864m. [DOI] [PubMed] [Google Scholar]
- 9.Ko HM, Dong G. Cooperative activation of cyclobutanones and olefins leads to bridged ring systems by a catalytic [4 + 2] coupling. Nat Chem. 2014;6:739–744. doi: 10.1038/nchem.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo Z, Ahneman D, Chu L, Terrett J, Doyle AG, MacMillan DWC. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science. 2014;345:437–440. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Kim MB, Herbert A, Fedorov KE, Grubbs RH, Stoltz BM. Palladium-catalyzed decarbonylative dehydration of fatty acids for the production of linear alpha olefins. Adv Synth Catal. 2014;356:150–156. doi: 10.1002/adsc.201301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garralda MA. Aldehyde C–H activation with late transition metal organometallic compounds. Formation and activity of acyl hydrido compounds. Dalton Trans. 2009:3635–3645. doi: 10.1039/b817263c. [DOI] [PubMed] [Google Scholar]
- 13.Willis MC. Transition metal catalyzed alkene and alkyne hydroacylation. Chem Rev. 2010;110:725–748. doi: 10.1021/cr900096x. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji J, Ohno K. Organic syntheses by means of noble metal compounds XXI. Decarbonylation of aldehydes using rhodium complex. Tetrahedron Lett. 1965;6:3969–3971. [Google Scholar]
- 15.Kreis M, Palmelund A, Bunch L, Madsen R. A general and convenient method for the rhodium-catalyzed decarbonylation of aldehydes. Adv Synth Catal. 2006;348:2148–2154. [Google Scholar]
- 16.Franke R, Selent D, Börner A. Applied hydroformylation. Chem Rev. 2012;112:5675–5732. doi: 10.1021/cr3001803. [DOI] [PubMed] [Google Scholar]
- 17.Iwai T, Fujihara Y, Tsuji T. The iridium-catalyzed decarbonylation of aldehydes under mild conditions. Chem Commun. 2008:6215–6217. doi: 10.1039/b813171f. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T, Akazome M, Tsuji Y, Watanabe Y. Ruthenium complex catalyzed intermolecular hydroacylation and transhydroformylation of olefins with aldehydes. J Org Chem. 1990;55:1286–1291. [Google Scholar]
- 19.Prince RH, Raspin KA. Olefin formation from saturated aldehydes and acids by reaction with ruthenium and rhodium complexes. Chem Commun. 1966:156–157. [Google Scholar]
- 20.McCombs CA, Foster CH Eastman Kodak Company. US Patent 4272444 A. Dehydroformylation of steroidal aldehydes. 1980 Aug 14;
- 21.Wertz DL, Sisemore MF, Selke M, Driscoll J, Valentine JS. Mimicking cytochrome P-450 2B4 and aromatase: aromatization of a substrate analogue by a peroxo Fe(III) porphyrin complex. J Am Chem Soc. 1998;120:5331–5332. [Google Scholar]
- 22.Goto Y, Wada S, Morishima I, Watanabe Y. Reactivity of peroxoiron(III) porphyrin complexes: models for dehydroformylation reactions catalyzed by cytochrome P-450. J Inorg Biochem. 1998;69:241–247. [Google Scholar]
- 23.Ahuja R, Punji B, Findlater M, Supplee C, Schinski W, Brookhart M, Goldman AS. Catalytic dehydroaromatization of n-alkanes by pincer-ligated iridium complexes. Nat Chem. 2011;3:167–171. doi: 10.1038/nchem.946. [DOI] [PubMed] [Google Scholar]
- 24.Phan DHT, Kou KGM, Dong VM. Enantioselective desymmetrization of cyclopropenes by hydroacylation. J Am Chem Soc. 2010;132:16354–16355. doi: 10.1021/ja107738a. [DOI] [PubMed] [Google Scholar]
- 25.Lenges CP, Brookhart M. Isomerization of aldehydes catalyzed by rhodium(I) olefin complexes. Angew Chem Int Ed. 1999;38:3533–3537. doi: 10.1002/(sici)1521-3773(19991203)38:23<3533::aid-anie3533>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Makado G, Morimoto T, Sugimoto Y, Tsutsumi K, Kagawa N, Kakiuchi K. Highly linear selective hydroformylation of 1-alkenes using formaldehyde as a syngas substitute. Adv Synth Catal. 2010;352:299–304. [Google Scholar]
- 27.Cortés M, Valderrama JA, Cuellar M, Armstrong V, Preite M. Synthesis of (+)-cyclozonarone and the absolute configuration of naturally occurring (−)-cyclozonarone. J Nat Prod. 2001;64:348–349. doi: 10.1021/np0004146. [DOI] [PubMed] [Google Scholar]
- 28.Imai H, Nishiguchi T, Fukuzumi K. Transfer hydrogenation and transfer hydrogenolysis. II. catalytic activity of some soluble complexes in hydrogen transfer from alcohol to olefins and the mechanism of the reaction catalyzed by hydridotetrakis(triphenylphosphine)rhodium(I) J Org Chem. 1974;39:1622–1627. [Google Scholar]
- 29.Jun C–H, Huh C–W, Na S–J. Direct synthesis of ketones from primary alcohols and 1-alkenes. Angew Chem Int Ed. 1998;37:145–147. [Google Scholar]
- 30.Lebold TP, Wood JL, Deitch J, Lodewyk MW, Tantillo DJ, Sarpong R. A divergent approach to the synthesis of the yohimbinoid alkaloids venenatine and alstovenine. Nat Chem. 2013;5:126–131. doi: 10.1038/nchem.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stearman CJ, Wilson M, Padwa A. Conjugate addition-dipolar cycloaddition cascade for the synthesis of benzo[a]quinolizine and indolo[a]quinolizine scaffolds: application to the total synthesis of (±)-yohimbenone. J Org Chem. 2009;74:3491–3499. doi: 10.1021/jo9003579. [DOI] [PubMed] [Google Scholar]
- 32.See the supplementary materials for detailed experimental conditions.
- 33.The peaks for the regenerated benzoic acid are very broad in the NMR spectra, possibly due to reversible reductive elimination. Analogous results were obtained using 4-fluorobenzoic acid as a surrogate, which enabled us to follow the reaction by 19F NMR and confirm the results.
- 34.Kranenburg M, van der Burgt YEM, Kamer PCJ, van Leeuwen PWNM, Goubitz K, Fraanje J. New diphosphine ligands based on heterocyclic aromatics inducing very high regioselectivity in rhodium-catalyzed hydroformylation: effect of the bite angle. Organometallics. 1995;14:3081–3089. [Google Scholar]
- 35.Manger M, Wolf J, Teichert M, Stalke D, Werner H. Syntheses, molecular structures, and reactivities of (π-allyl)rhodium(I) complexes containing bulky bis(phosphino)methanes R′2PCH2PiPr2 as ligands. Organometallics. 1998;17:3210–3221. [Google Scholar]
- 36.Kansal VK, Suhail A, Gupta A. U.S. patent # WO 2009155403. Processes for the preparation of varenicline and intermediates thereof. 2009 Jun 18;
- 37.Taarning E, Madsen R. Unsaturated aldehydes as alkene equivalents in the Diels–Alder reaction. Chem Eur J. 2008;14:5638–5644. doi: 10.1002/chem.200800003. [DOI] [PubMed] [Google Scholar]
- 38.Havis ND, Walters DR, Martin WP, Cook FM, Robin DJ. Synthesis and fungicidal activity of alicyclic diamines. J Agric Food Chem. 1996;44:2835–2838. [Google Scholar]
- 39.Higashino M, Ikeda N, Shinada T, Sakaguchi K, Ohfune Y. Stereoselective anti-SN2′ Mitsunobu reaction of a-hydroxy-a-alkenylsilanes. Tetrahedron Lett. 2011;52:422–425. [Google Scholar]
- 40.Gore S, Baskaran S, König B. Fischer indole synthesis in low melting mixtures. Org Lett. 2012;14:4568–4571. doi: 10.1021/ol302034r. [DOI] [PubMed] [Google Scholar]
- 41.Fridén-Saxin M, Pemberton N, da Silva Andersson K, Dyrager C, Friberg A, Grøtli M, Luthman K. Synthesis of 2-alkyl-substituted chromone derivatives using microwave irradiation. J Org Chem. 2009;74:2755–2759. doi: 10.1021/jo802783z. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahem I, Córdova A. Direct catalytic intermolecular α-allylic alkylation of aldehydes by combination of transition-metal and organocatalysis. Angew Chem Int Ed. 2006;45:1952–1956. doi: 10.1002/anie.200504021. [DOI] [PubMed] [Google Scholar]
- 43.Eriks JC, van der Goot H, Stark GJ, Timmerman H. Histamine H2-receptor agonists. Synthesis, in vitro pharmacology, and qualitative structure-activity relationships of substituted 4- and 5-(2-aminoethyl)thiazoles. J Med Chem. 1992;35:3239–3246. doi: 10.1021/jm00095a021. [DOI] [PubMed] [Google Scholar]
- 44.Leogane O, Lebel H. One-pot curtius rearrangement Processes from Carboxylic Acids. Synthesis. 2009;(11):1935–1940. [Google Scholar]
- 45.Fujita T, Watanabe S, Suga K, Nakayama H. The reaction of carboxylic acids with conjugated olefins using sodium naphthalenide in the presence of N,N,N′,N′-tetramethylethylenediamine. Synthesis. 1979;(4):310–11. [Google Scholar]
- 46.Gaspar B, Carreira EM. Cobalt catalyzed functionalization of unactivated alkenes: regioselective reductive C–C bond forming reactions. J Am Chem Soc. 2009;131:13214–13215. doi: 10.1021/ja904856k. [DOI] [PubMed] [Google Scholar]
- 47.Bailey WF, Gavaskar KV. Anionic cyclization of olefinic alkyllithiums: ring closure of terminally substituted 5-hexenyllithiums. Tetrahedron. 1994;50:5957–5970. [Google Scholar]
- 48.Boukouvalas J, Wang J-X, Marion O. Bruno Ndzi, Synthesis and stereochemistry of the antitumor diterpenoid (+)-zerumin B. J Org Chem. 2005;71:6670–6673. doi: 10.1021/jo0610154. [DOI] [PubMed] [Google Scholar]
- 49.Bhat S, Maitra U. Low molecular mass cationic gelators derived from deoxycholic acid: remarkable gelation of aqueous solvents. Tetrahedron. 2007;63:7309–7320. [Google Scholar]
- 50.Cresswell AJ, Davies SG, Lee JA, Morris MJ, Roberts PM, Thomson JE. Diastereodivergent hydroxyfluorination of cyclic and acyclic allylic amines: Synthesis of 4-deoxy-4-fluorophytosphingosines. J Org Chem. 2012;77:7262–7281. doi: 10.1021/jo301056r. [DOI] [PubMed] [Google Scholar]
- 51.Waser J, Gaspar B, Nambu H, Carreira EM. Hydrazines and azides via the metal-catalyzed hydrohydrazination and hydroazidation of olefins. J Am Chem Soc. 2006;128:11693–11712. doi: 10.1021/ja062355+. [DOI] [PubMed] [Google Scholar]
- 52.Kambe N, Moriwaki Y, Fujii Y, Iwasaki T, Terao J. Silver-catalyzed regioselective carbomagnesiation of alkynes with alkyl halides and Grignard reagents. Org Lett. 2011;13:4656–4659. doi: 10.1021/ol2018664. [DOI] [PubMed] [Google Scholar]
- 53.Kinoshita M, Ohtsuka M, Nakamura D, Akita H. First synthesis of (+)-a- and (+)-g-polypodatetraenes. Chem Pharm Bull. 2002;50:930–934. doi: 10.1248/cpb.50.930. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Liu JF, Ugrinov A, Pillai AFX, Sun ZM, Zhao P. Methoxy-directed aryl-to-aryl 1,3-rhodium migration. J Am Chem Soc. 2013;135:17270–17273. doi: 10.1021/ja409049t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.