Abstract
This review provides an updated perspective on rapidly proliferating efforts to harness extracellular vesicles (EVs) for therapeutic applications. We summarize current knowledge, emerging strategies, and open questions pertaining to clinical potential and translation. Potentially useful EVs comprise diverse products of various cell types and species. EV components may also be combined with liposomes and nanoparticles to facilitate manufacturing as well as product safety and evaluation. Potential therapeutic cargoes include RNA, proteins, and drugs. Strategic issues considered herein include choice of therapeutic agent, means of loading cargoes into EVs, promotion of EV stability, tissue targeting, and functional delivery of cargo to recipient cells. Some applications may harness natural EV properties, such as immune modulation, regeneration promotion, and pathogen suppression. These properties can be enhanced or customized to enable a wide range of therapeutic applications, including vaccination, improvement of pregnancy outcome, and treatment of autoimmune disease, cancer, and tissue injury.
Keywords: exosomes, extracellular RNA, microvesicles, drug delivery, liposomes, gene medicine, gene therapy, mesenchymal stem cells, nanoparticles
INTRODUCTION
Secretion and exchange of cellular contents via extracellular vesicles (EVs) is emerging as a universal feature of cellular life, potentially decorating every branch of the tree of life. Early progress in harnessing EVs for therapeutic applications has engendered great interest; promising results and the rapidly unfolding picture of EV biogenesis have been extensively discussed elsewhere (1–5). In this review, we first contextualize the therapeutic potential of EVs within the broader emerging view of EVs as universal components of cellular biology and then provide an updated perspective on outstanding questions pertaining to the translation of EV-based therapies from preclinical demonstrations to viable clinical products. In each of the following sections, we summarize contemporary understanding and accomplishments within the EV field and conclude by offering open questions, the investigation of which could further the ultimate goal of harnessing EVs for therapeutic applications.
NATURALLY OCCURRING VESICLES
EV Sources and Biogenesis
EVs have evolved conceptually from being considered debris in mammalian cell culture to comprising a vast empire of cellular satellites with a multitude of missions. Recognition of the reality of EVs has derived in part from increased microscopic resolution. These membrane-bound structures vary in size from 50 nm to 2 μm in diameter and appear to be both a mode of communication among cells through transfer of protein, nucleic acids, and lipids and a means of disposal of unwanted substances from cells. Essentially all cells appear to release vesicles, and each cell type may release a heterogeneous composite of vesicle subtypes. Vesicles are released by mammalian cells (6), gram-positive and -negative bacteria (7), fungi (8), yeast (9), single-cell parasites such as the malaria-causing protozoa (10), plant cells (11, 12), nematodes (13), and flies (14). EV functions include delivery of virulence factors, modulation of inflammation and immune responses, and transfer of developmental and physiologically modulating signals in multicellular organisms. In some organisms, it is unclear how these membrane-derived vesicles pass through the cell wall–a process that may be facilitated by cell wall lipids or hydrolytic enzymes. The multitude of modes by which EVs are produced and loaded with specific cargo by different organisms provides an ensemble of mechanisms that may be harnessed for applications in biotechnology.
Ever-expanding research on mammalian EVs has increased understanding of their complexity. EVs comprise a mixture of membrane vesicles originating, for example, by fusion of endosomally derived multivesicular bodies with the plasma membrane releasing exosomes, or by direct budding from the plasma membrane generating ectosomes or microvesicles. “Exosome” is a confusing term in that it has also been used to describe a complex of 3′ to 5′ exonucleases in cells (15). In fact, the terminology used to describe the heterogeneous array of vesicles released by cells is continually expanding and is based primarily on vesicle size and cell of origin, with various types of vesicles distinguished by density, biochemical constituents, and ultrastructural appearance (16). For example, very large vesicles (1–2 μm) released from tumor cells have been termed oncosomes (17), and some tumor cells also release retrovirus-like particles due to expression of human endogenous retroviral sequences (18). In this review, we use the collective term EVs to encompass all types of secreted vesicles, as different subtypes are not well defined and there is not yet consensus on descriptive terminology. For terms and relationships between vesicles, see the International Society for Extracellular Vesicles at http://www.isev.org, the American Society for Exosomes and Microvesicles at http://www.asemv.org, Vesiclepedia at http://microvesicles.org (19), and the exRNA Research Portal at http://exrna.org.
EV biogenesis involves various cellular mechanisms but is generally thought to involve ubiquitination and the endosomal sorting complex required for transport (ESCRT), with ectosome budding associated with lipid rafts, higher-ordered oligomerization of membrane proteins, and segregation into ceramide-rich microdomains (for a review, see Reference 20). Cells also release apoptotic blebs and autophagy-derived vesicles (21). EV generation is intimately related to certain biological processes, for example, the biogenesis of viruses. Infected cells release vesicles even prior to release of membrane-bound virions, including herpes simplex virus type 1, vaccinia virus, and Epstein Barr virus (EBV) (for a review, see Reference 22), and retrovirus biogenesis mechanisms parallel those of ectosomes (23). Cell membrane dynamics, such as the formation of plasma membrane ruffles, lamellipodia, filopodia, protrusions, blebs, cilia, and nanotubules, can all be accompanied by pinching off of cellular contents into EVs (13, 24, 25).
EV composition is determined largely by the cell type and physiological state of the producer cells. Typically, EV membranes are enriched in glycosphingolipids (26), cholesterol (26, 27), and phosphatidylserine (PS) (27, 28). EVs exhibit an overall lipid profile that is similar to, but distinguishable from, that of the cell of origin (29). For a review of lipidomic studies of EVs, see Reference 30. The proteomic content of the EVs is multifactorial: Some proteins are present in most EVs, including HSP70, Alix, tetraspanins [cluster of differentiation 63 (CD63), CD81, CD9], and major histocompatibility complex class II (MHCII) proteins (31, 32), and other proteins are associated with specific EV subsets. As with lipids and nucleic acids, the ensemble of proteins incorporated into EVs is related to, but distinct from, the overall protein pool in the cell of origin, suggesting the possible existence of EV sorting mechanisms. Incorporated proteins include receptors and other membrane proteins that confer various functions. The nucleic acid content of vesicles is also variable and includes DNA, ribosomal RNA, mRNA, and noncoding RNAs such as microRNAs (miRNAs). The extent to which these incorporated RNAs are intact and functional or represent inactive fragments is an area of active investigation.
EVs and recipient cells interact via a range of mechanisms that are still under study. Interactions include ligand docking on the vesicle surface to receptors on cells, potentially triggering a signaling response; transfer of membrane proteins from vesicles to cell membranes; fusion of the vesicle and cell membranes; vesicle uptake through endocytotic processes, including clathrin-coated pits, pinocytosis, caveolae, macropinocytosis, and phagocytosis; and extrusion through a vesicle-cell channel (33, 34). The fate of vesicular components in recipient cells may depend on the mode of uptake, with processing through the endosomal pathway potentially leading to degradation of EV contents, even though this is the primary route of entry (35). Although the mechanisms of information transfer remain to be elucidated, natural EVs exert diverse and potent effects on recipient cells. For example, prokaryotes can transmit virulence factors (36) such as cholesterol-binding toxins (37), HIV can transfer receptors that make a previously noninfectable cell infectable (38), cytotoxic CD4+ T cells release costimulatory vesicles (39), tumor cells can transfer oncoproteins [e.g., EGFRvIII (40)], and miRNAs in vesicles derived from EBV-infected cells can alter the immune response of recipient cells (41). As discussed below, this diversity of mechanisms by which EVs are generated and confer effects provides both opportunities and challenges for developing EV-based therapeutics.
Open Questions
How many types of naturally occurring EVs are there and how do they differ among producer cell type and physiological state? Many methods are used to isolate EVs, and EV contents and properties overlap with those of the cells of origin and other EV types. Formalizing EV nomenclature and defining attributes is a work in progress. There is a pressing need for useful standards to enable cross-lab comparisons and reproduction of results.
What is the fate and function of EV content in recipient cells? The mechanisms of EV uptake and content delivery (or degradation) vary among EV types and recipient cell types. Elucidating and understanding these processes is critical for harnessing EVs as therapeutic delivery vehicles.
To what extent does natural EV-mediated transfer of biomolecules modulate recipient cell state in vivo? Multiple lines of evidence indicate that EVs can transfer biomolecules to modulate recipient cell state in vivo, for example, following bolus injection of purified or concentrated EVs. However, the extent to which such processes naturally shape cellular function and intercellular communication, particularly under homeostatic conditions, remains poorly understood. Moreover, we do not understand the relative importance of EV-mediated transfer between proximal cells, for example, when diffusional barriers lead to local accumulation of secreted EVs rather than transfer of EVs via the circulation, where EV concentrations may be lower.
THERAPEUTIC USES OF EVS
Intrinsic Therapeutic Activity
EVs from various cell sources have therapeutic potential. Vesicles derived from mesenchymal stem cells (MSCs) appear particularly useful for enhancing recovery from various injuries. For example, injection of EVs derived from mouse MSCs suppressed hypoxia-induced inflammation and hypertension in mice (42). MSC-derived EVs also reduced myocardial infarct size in mice following myocardial ischemia/reperfusion injury (43). MSC EVs may exert a neuroprotective effect after brain injury (44). Similarly, EVs from oligodendrocytes promote remyelination of the central nervous system (CNS) (45). EVs from mouse MSCs delivered miR-16 and other molecules to mouse breast cancer cells, conferring downregulated expression of vascular endothelial growth factor and decreased tumor growth (46).
EVs can also mediate therapeutic benefits by alternatively attenuating or promoting immune responses and inflammation (as summarized in Reference 16, figure 4). For instance, vesicles derived from dendritic cells (DCs) overexpressing interleukin-4 (IL-4), transforming growth factor-β, or IL-10 are potently immunosuppressive and protective in many inflammatory conditions, including collagen-induced arthritis, delayed type hypersensitivity (DTH), and drug-induced colitis (for reviews, see Reference 47 and Reference 48, figures 2 and 3). Placental EVs inhibit maternal immune rejection of the fetus through display of ligands (i.e., Fas ligand and tumor necrosis factor–related apoptosis-inducing ligand) that inhibit T cell signaling and induce lymphocyte apoptosis (49, 50). EVs from tolerized immune cells suppress a wide variety of inflammatory diseases in mice, including contact sensitivity (51), DTH, and collagen-induced arthritis (52–54). Colitis is prevented in mice by exogenous EVs, such as Bacteroides fragilis outer membrane vesicles (OMVs) containing polysaccharide A (55) or grape-derived EV-like nanoparticles (12). EVs can also be harnessed as antiviral therapeutics by activating specific types of immune function. Placental EVs protect nonplacental cells from viral infection by upregulating autophagy through transfer of miRNAs (56). EVs derived from interferon-α (IFN-α)-treated macrophages or liver sinusoidal cells deliver antiviral RNAs and proteins to hepatocytes, which decreases replication of hepatitis B virus (57). How various naturally occurring EVs promote these diverse responses remains to be elucidated.
Vaccination Against Infectious Disease
One of the first therapeutic uses of EVs was vaccination against infectious disease (58). Such vaccination typically uses vesicles with proinflammatory properties. For example, EVs generated by bone marrow–derived macrophages primed with lipopolysaccharide (LPS) and adenosine triphosphate (ATP) include vesicles containing IL-1β, caspase-1, and inflammasome components (59). EVs derived from antigen-pulsed macrophages or DCs induce immune responses when introduced into naïve animals. In some cases, the immune response induced by EVs is more effective than that induced by protein subunit–based vaccines. EV vaccines often induce T helper 1 (Th1)-type immune responses and cell-mediated immunity, which is most effective for clearing viral and bacterial infections. For example, EVs derived from DCs pulsed with diphtheria toxoid and from macrophages treated with Mycobacterium tuberculosis proteins both induced immune responses with strong Th1 biases, whereas comparable subunit–based vaccines induced Th2-type immune responses, which favor antibody-mediated immunity (60, 61). Such differences impact vaccine efficacy–EV vaccination conferred decreased growth of M. tuberculosis in mouse lungs compared to antigen-based vaccines. In cases in which no effective antigen-based vaccine exists, EV-based vaccines also provide a new therapeutic strategy. For example, chickens vaccinated with EVs derived from Eimeria tenella antigen-pulsed chicken DCs developed stronger antibody responses and had increased survival after challenge compared to antigen-vaccinated chickens (62). EV-based vaccines administered during pregnancy may prevent diseases in newborns. Vaccination of pregnant mice with EVs from DCs pulsed with Toxoplasma gondii–derived antigens increased survival in pups subsequently challenged with T. gondii (63).
Microbe-derived vesicles can also be used as vaccines. Mice vaccinated with OMVs from Bordetella pertussis controlled infection following challenge with several strains of that bacterium (64). Like EV vaccines, OMV vaccines promoted a Th1-type immune response, whereas the comparable antigen-based vaccine favored a Th2-type response. Indeed, the OMV-based vaccines MenBVac and MeNZB have proven efficacious in protecting humans against serogroup B meningococcal disease (65). Following this success, a second-generation OMV-based vaccine, in which a Neisseria meningitidis strain was engineered to express increased levels of the protein antigens and less toxic forms of lipid A and LPS, proved both safe and effective in humans (66).
Vaccination to Treat Cancer
EV vaccines have potential for treating cancer. Treatment of mice bearing ovalbumin (OVA)-expressing melanoma with DC-derived EVs [containing OVA and α-galactosylceramide, an invariant natural killer T cell (iNKT) immune cell ligand] increased antitumor CD8+ T cell infiltration and decreased tumor growth (67). Vaccination with vesicles derived by homogenization and sonication of melanomas decreased tumor growth and metastasis in mice (68). These results have motivated the production of EV vaccines that are now in clinical trials.
EV-Mediated Delivery of Exogenous Therapeutic Biomolecules
EVs display characteristics of ideal delivery vehicles, including a lipid composition that enhances vesicle stability in circulation (28) and proteins that slow EV clearance, such as inhibitors of complement and phagocytosis (69, 70). Furthermore, EVs can deliver therapeutic biomolecules ranging from nucleic acids to small molecules. For example, delivery of the immunosuppressive drug curcumin (which has poor bioavailability) was enhanced by loading it into EVs derived from mouse lymphoma cells (EL-4) (71). This formulation conferred protection in a mouse model of septic shock. Intratumoral injection of HEK293-derived EVs loaded with mRNA (and protein) encoding a prodrug-converting enzyme combination conferred reduced growth of a schwannoma xenograft when the nontoxic prodrug (5-fluorocytosine) was administered systemically (72).
Targeting EVs to specific cell types can reduce off-target effects and enhance specific uptake by target recipient cells. EVs derived from HEK293 cells overexpressing let-7a miRNA and targeted to epidermal growth factor receptor (EGFR) via a phage display–derived peptide decreased growth of breast cancer xenografts in a manner that required the targeting ligand (73). Doxorubicin loaded into EVs displaying an αv integrin–specific arginine-glycine-asparagine (RGD) peptide trafficked selectively to breast cancer xenografts in mice and significantly reduced tumor growth, whereas untargeted EVs went primarily to the liver and spleen without reducing tumor growth (74). EVs may also enable transport across the blood-brain barrier (BBB). Intranasal delivery of EVs derived from EL-4 cells delivered curcumin across the BBB to microglial cells and attenuated experimental autoimmune encephalomyelitis (EAE) in mice (75). Systemic delivery of EVs displaying rabies virus glycoprotein (RVG) peptide and carrying small interfering RNA (siRNA) downregulated proteins associated with Alzheimer’s disease in the mouse brain (76).
In sum, there is mounting preclinical evidence that EV-based therapeutics are promising and perhaps uniquely well suited to some applications. Moving these approaches to the clinic will require focus on translational questions, including the following and the practical considerations discussed in subsequent sections of this review.
Open Questions
How will EV source affect recipient immune response? Because EVs display MHC (77) and carry a wide variety of allogeneic proteins, it may be necessary to derive therapeutic EVs from an autologous source, which would greatly impact the scalability and ultimate cost of EV therapies. This question has not been directly investigated, but some evidence indicates that allogeneic EVs (from other individuals of the same species) are tolerated in immune-competent animals. BALB/c EVs did not induce maturation of splenic DCs upon intravenous injection into B10 mice, although B10 splenic DCs were able to mature upon incubation with an agonistic CD40 antibody, suggesting that BALB/c EVs neither induced nor inhibited immune responses in B10 mice (27). Additionally, T cells isolated from B10 mice after intravenous injection of BALB/c EVs were not stimulated in a mixed leukocyte reaction with BALB/c antigen-presenting cells, which suggests that allogeneic EVs can even have a tolerizing effect (78). Furthermore, xenogenic EVs may also be tolerated in vivo. EVs derived from human MSCs were tolerated and functional in immune-competent mice (43). However, robust immune profiling following repeated injection of nonsyngeneic EVs has not been investigated and constitutes a central question that could guide translation of EV therapies to various clinical applications.
Do tumor-derived EVs have oncogenic potential? Tumor-derived EVs promote angio-genesis, metastasis, and immune suppression (79–87). These EVs contain retrotransposon elements, oncogenic mRNA and miRNA, and transcription factors that can potentially alter the genome and the transcriptome of recipient cells, favoring proliferation and transformation (40, 88–90). Thus, although tumor-derived EVs can be harnessed as cancer vaccines, their potential cancer-promoting effects must be considered and monitored in therapeutic contexts.
How should dosing of EVs be evaluated? EV-mediated signaling is dose-dependent (91), so the tuning of EV dose may enable the balancing of potential deleterious and therapeutic effects of EV administration. Understanding the role of EV dose is also important for achieving therapeutic efficacy. For example, at a vaccination rate of once a week for 3 weeks, the Escherichia coli OMV dose needed to provide 100% protection against lethal E. coli challenge was 1 μg, whereas 0.5 μg provided only 80% protection (92). Thus, intermediate therapeutic benefits may result from suboptimal vesicle dosing.
LOADING THERAPEUTICS INTO VESICLES
EV Loading Strategies
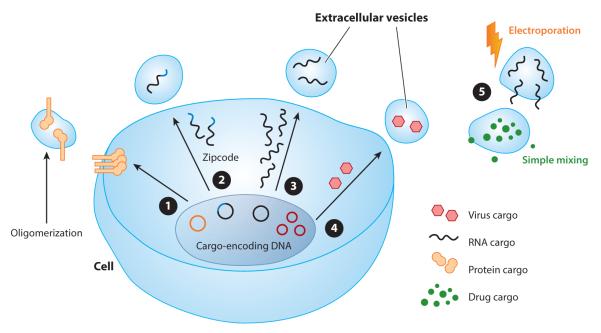
Strategies for loading therapeutically active cargo molecules into EVs can be divided into ex vivo and in vitro strategies (Figure 1). In the former, EVs are purified and then loaded with therapeutic cargo molecules. In the latter, cargo molecules are incorporated into EVs during vesicle biogenesis. Each methodology can be applied to different types of cargo molecules and has both advantages and disadvantages.
Figure 1.
Strategies for incorporating therapeutic agents into extracellular vesicles (EVs). EVs can carry DNA, RNA, proteins, lipids, or drugs, which are incorporated via various mechanisms. ❶ Directing highly oligomeric proteins to EVs using plasma membrane anchors (100). ❷ Including zipcode-like sequences in the 3′ untranslated region to facilitate mRNA loading into EVs (105). ❸ Overexpressing mRNA to drive incorporation into EVs by passive loading. ❹ Delivering an adeno-associated virus expression vector termed a vexosome (114). ❺ Loading EVs with cargo by physical methods, including mixing (e.g., for curcumin) (71) and electroporation for drugs (74) or RNA (76).
The most broadly applied ex vivo loading strategy is electroporation of molecules into EVs. This approach has been used to load small-molecule drugs (71, 74, 75, 93) and siRNA (76, 94). It is not clear, however, if RNA cargo molecules such as siRNA, miRNA, and mRNA are effectively loaded into EVs by electroporation (73). Commonly used electroporation conditions may induce siRNA precipitation and yield low siRNA incorporation into EVs (95). It may be possible to reduce aggregation of EVs and cargo RNA by optimizing electroporation conditions, as demonstrated using a trehalose-containing pulse medium (96). Even so, the efficiency with which EVs can be loaded by electroporation will be limited by the small volume occupied by EVs compared to the volume of the medium in which they are typically suspended. It is not yet clear to what extent the concentration of EVs and cargo molecules can be increased to improve EV loading before aggregation of either component becomes problematic during electroporation.
Strategies for loading EVs in vitro may be further subdivided into passive and active loading approaches. Passive loading relies on overexpression of the therapeutic cargo molecule (typically a protein or RNA) and uses either native trafficking mechanisms or mass action to achieve loading during EV biogenesis. The sorting of proteins into EVs during biogenesis is increasingly well understood (97–99). One strategy to enhance cargo protein loading uses a natural mechanism through which oligomeric, membrane-anchored proteins traffic to EVs by fusing a cargo protein to two additional domains that promote aggregation and membrane localization (e.g., via myristoylation) (100). The rules governing the sorting of RNAs into EVs are more elusive. Multiple proteins and ribonucleoproteins (RNPs) have been implicated in active RNA sorting into EVs (101–104). Three sorting mechanisms have been implicated in facilitating or limiting the loading of RNAs into EVs: (a) Zipcode mRNA sequences may interact with proteins to promote mRNA loading into EVs (105); (b) heterogeneous nuclear RNP A2B1 binding motifs can increase loading of miRNAs into EVs (104); and (c) post-transcriptional modifications may impair loading of miRNA into EVs (106). Passive loading into EVs has been used to incorporate natural miRNAs (73, 107, 108), miRNAs chemically modified prior to transfection into EV-producing cells (109), small hairpin RNAs (shRNAs) (107), and mRNAs (72, 110). In the case of mRNA cargo, it is often difficult to distinguish between effects caused by the mRNA itself or the mRNA-encoded protein, as both may be incorporated into EVs and transferred to recipient cells (72). In either case, EVs loaded with such cargo molecules would modulate the function of recipient cells. The efficiency of passive loading also presents challenges, as it may enable loading of undesired cargo species from the EV-producing cell, including proteins, small regulatory RNAs, retroviral genomes (23), and host cell–derived mRNA and miRNA.
Active loading may increase the concentration of cargo molecules within EVs, potentially impacting the potency and efficacy of EV-based therapeutics. The most commonly employed method for cargo protein loading is expression of a genetic fusion between the cargo and a protein that natively localizes into EVs. The N termini of the lactadherin C1C2 domain or the lysosomal protein Lamp2 have been fused to proteins (111) or peptides (76, 112) to display cargo proteins on the EV surface; a similar approach for peptide display has used the platelet-derived growth factor receptor (73, 113). Active loading of cargo proteins into the EV lumen has not yet been described.
Another approach for loading nucleic acids into EVs is to exploit viral packaging strategies. Many viruses hijack cellular membranes for efficient propagation, and virus-EV hybrid particles (termed vexosomes, vector-exosomes, or retrovirus-like particles) represent novel gene therapy vehicles. Nonenveloped viruses such as adeno-associated virus (AAV) (114) and hepatitis A virus (115) can be incorporated into EVs during propagation. AAV is particularly interesting, as it is a commonly used viral vector for gene therapy in clinical trials. Vexosomes contain AAV vectors within the EVs and combine the advantages of both components: The EV component is potentially less immunogenic and may confer enhanced penetration across biological barriers through genetic modification of the producer cells, and the AAV component is effective for gene delivery with long-term stability in nondividing cells.
Open Questions
To what extent are cargo molecules intact when incorporated into EVs? This concern is especially relevant for RNA cargo loaded by producer cells, for which degradation in the cytoplasm or incorporation of nucleases may decrease integrity of the cargo molecule. Typical profiles of RNA contained in EVs reveal primarily small RNAs in the 200 bp range, with a low level of longer RNAs (up to about 5 kb) (88). It remains unknown whether natural mechanisms for selectively packaging RNA into EVs via RNP association may be harnessed to protect the integrity and stability of longer RNAs such as translatable mRNAs.
How do various loading strategies impact potency and heterogeneity of EV-based delivery vehicles? To date, quantitative characterization of EV-mediated delivery vehicles is generally lacking. To evaluate the feasibility of EV-mediated delivery, compare EVs to comparable synthetic delivery vehicles (such as liposomes), and identify potential opportunities for improving EV-based delivery, the following questions require investigation: What is the maximum loading capacity of EVs for different types of cargo with various types of EVs? How heterogeneous are EVs loaded by passive or active means, and do EVs loaded by either approach represent only a subset of EVs produced? How does loading efficiency and composition within EVs impact EV-mediated cargo molecule delivery and efficacy?
Is it possible to selectively exclude some molecules from EVs during biogenesis? Packaging of some cell-derived proteins, RNA, and lipids may be undesirable in an EV-based therapeutic product. If the offending component(s) cannot be removed from the producer cell stock entirely (e.g., by genome editing of a producer cell line), alternative strategies may be required to ensure that such components are minimally loaded into EVs. EV-based products may need to be monitored for such contamination.
DELIVERY AND FATE OF THERAPEUTIC VESICLES
Biodistribution
Although the pharmacokinetics of injected liposomes and nanoparticles have been well studied, only recently have such properties of injected EVs been characterized. By labeling EVs with luciferase, Lai et al. (113, 116) used bioluminescence to track the fate of EVs in mice after intravenous injection. Thirty min after injection, most of the signal was detected in the spleen, liver, lung, and kidney, with some signal detectable in the brain, heart, and muscle. High EV levels in the spleen resulted from its high vascularity. The decay of the signal in blood followed a two-phase exponential decline, with a short half-life of 20 min and a longer half-life of more than 3 h. The first phase is likely attributed to a rapid redistribution of EVs to organs (mainly liver and lung), whereas the second phase probably represents the final elimination of the vesicles from the body. The authors suggested mechanisms of renal and hepatic clearance of the labeled vesicles, with a peak signal in the urine at ~60 min postinjection. Most importantly, intravenously injected EVs trafficked to a tumor, presumably because of the enhanced permeability and retention effect and leaky vasculature of the tumor.
The organ distribution of EVs after in vivo administration has also been evaluated using membrane dyes such as PKH or XenoLight DiR. PKH-labeled vesicles were found in the liver, spleen, lung, and bone marrow (90), whereas XenoLight DiR–labeled vesicles were found in the liver, lung, spleen, kidney, brain, intestines, and tumor tissue (73). The reports with dyes, however, indicate a relatively long retention time for EVs in the body (24 h), in contrast to the bioluminescent imaging data (6 h). This difference could be explained by the relatively long half-life of membrane-labeling dyes, which may be retained in recipient cells and metabolized more slowly than the reporter proteins in or on vesicles. Therefore, the dye-labeling strategy is effective in tracking the early fate of vesicles, but protein-based bioluminescent imaging may provide more accurate temporal data over longer times.
Some cell types bind to intravenously or subcutaneously administered vesicles. In one study, biotinylated B cell–derived EVs were primarily taken up by hepatic and splenic macrophages5 min after systemic administration (117). Intriguingly, this study reported rapid kinetics for the elimination of EVs from the circulation (half-life of 2 min), which may be attributed to the use of B cell–derived EVs being efficiently taken up by the spleen and liver.
In contrast to systemic administration, locally administered EVs may achieve very high concentrations at target cells. Intranasally administered EVs containing curcumin and JSI124 (a STAT3 inhibitor) were taken up by microglial cells in the brain and alleviated inflammation in an LPS-induced brain inflammation model and in a myelin oligodendrocytes glycoprotein–induced EAE model; these drug-loaded EVs also reduced the growth of gliomas in the brain (75). The exact route of EV entry into the brain was not addressed, but the kinetics suggest transfer through the olfactory and the trigeminal nerves as well as the vasculature (118). Therefore, intranasal delivery of EVs may circumvent the BBB and lead to sufficient drug accumulation in certain regions of the CNS. The fate of EVs administered using other routes into the CNS (e.g., intrathecal, intracerebral, or intraventricular) has not been reported.
Targeting Recipient Cells
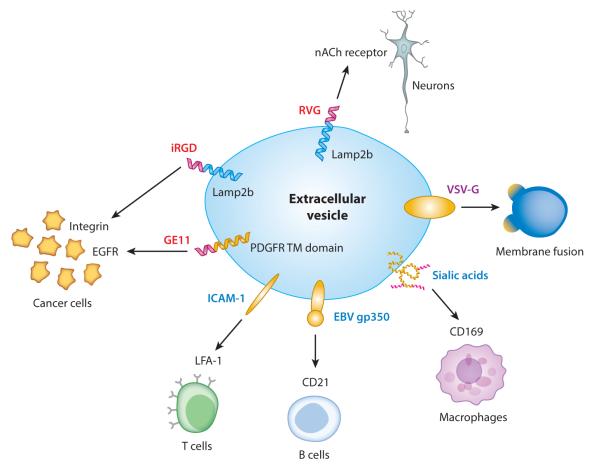
Targeting EVs to specific recipient cell types may be mediated by natural EV components or bioengineered moieties on the EV surface (Figure 2). Native EV proteins reflect the donor cell type, so selection of optimal donor-recipient cell combinations may facilitate specific uptake of therapeutic EVs. For example, EVs from T cell lines are efficiently taken up by myeloid cells such as macrophages and microglial cells (75), whereas mature DC–derived EVs are efficiently internalized by activated T cells (119). EBV-infected B cell–derived vesicles bind very efficiently to other B cells (120). In general, however, the attributes needed for efficient and specific uptake (i.e., EV uptake by target cells compared to various other cell types in vivo) have not been systematically and comprehensively evaluated.
Figure 2.
Bioengineering extracellular vesicles (EVs) for therapeutic delivery. Targeting EVs to specific recipient cells may be achieved by expressing proteins in EV-producing cells, including natural proteins such as EBV gp350 [selectively binds to B cells (121)], ICAM-1 [binds to T cells (119)], or sialic acid residues [promote uptake by macrophages (117)] (blue text) and engineered proteins such as EV transmembrane proteins that display peptides on the EV surface [e.g., GE11 (73), iRGD (74), and RVG (76)] (red text). Expression of other exogenous proteins may facilitate other steps of EV-mediated delivery, including fusion of EVs and donor cells [e.g., VSV-G (127)] (purple text). Other abbreviations: EBV, Epstein Barr virus; EGFR, epidermal growth factor receptor; ICAM-1, intercellular adhesion molecule-1; iRGD, internalizing arginine-glycine-asparagine; LFA-1, lymphocyte function–associated antigen-1; nACh, nicotinic acetylcholine; PDGFR TM domain, platelet-derived growth factor receptor, transmembrane domain; RVG, rabies virus glycoprotein; VSV-G, vesicular stomatitis virus glycoprotein.
Identifying ligand-receptor combinations involved in cell type–associated EV uptake may also inform EV engineering. For example, EBV-infected B cell–derived vesicles present the viral gp350 peptide, which binds the CD21 surface receptor on B cells (120). Expression of the gp350 peptide in HEK293T cells leads to its incorporation into EVs, which B cells internalize more effectively. Interestingly, EV-mediated delivery of CD40 ligand and gp350 peptide to B cells from patients with B chronic lymphocytic leukemia caused EBV-specific T cells to target these B cells in vitro (121). Thus, EV-mediated targeting could represent a novel type of immunotherapy for B cell malignancies. Similar strategies might target EVs to T cells, which bind DC-derived vesicles via interactions between intercellular adhesion molecule-1 (ICAM-1) and lymphocyte function–associated antigen-1 (LFA-1) (119).
An alternative to harnessing natural targeting mechanisms is to engineer EVs to display targeting moieties on the vesicle surface (Figure 2). Fusion of the RVG peptide, which targets neurons in the CNS, to the EV marker Lamp2b promoted EV transport across the BBB and release of functional cargo (siRNA) in the brain (76). EVs displaying an αv integrin–specific internalizing RGD (iRGD) peptide (74) or EGFR-specific GE11 peptide (73) fused to Lamp2b exhibited potent tumor cell targeting in cell culture and in vivo. Notably, doxorubicin-loaded, iRGD-targeted EVs exhibited less cardiac toxicity in vivo than did control EVs loaded with this drug, the latter being more similar to commercially available liposomal doxorubicin (74).
EV Uptake by Recipient Cells
EV binding is mediated by receptors that interact with either universal EV molecules, such as lipids and carbohydrates, or specific peptides present on subsets of EVs. In the spleen, EV binding to macrophages is dependent on CD169 (sialoadhesin), which interacts with α2,3-linked sialic acids on vesicles, although CD169 does not play a role in EV uptake by Kuppfer cells in the liver (117). Surface glycosaminoglycans such as heparan sulfate have also been implicated in vesicle uptake. Soluble heparin, which mimics heparan sulfate, inhibits EV uptake by recipient cells via interaction with heparin-binding sites on both the cells and EVs (122, 123). Chinese hamster ovary cells deficient in cell surface heparan sulfate proteoglycans take up fewer EVs than do wild-type cells, whereas enzymatic depletion of heparan sulfate chains on EVs do not abrogate the cellular uptake of these EVs by wild-type cells (123), suggesting that cell surface heparan sulfate proteoglycans bind to as-yet unidentified EV components. Receptor-ligand interactions specific for vesicles of particular cell origins also play an important role in the uptake of EVs into tissues (124). Common EV-associated molecules that have been associated with uptake include integrins, tetraspanins, milk-fat globules, EGFVIII protein, and PS.
Following initial binding, cells internalize EVs by processes that include receptor-mediated phagocytosis or endocytosis via receptors that include T cell immunoglobulin- and mucin-domain-containing molecule-4 (TIM4), which binds to PS on EVs; scavenger receptors; integrins; and complement receptors (124). How EV cargo is released into the cytoplasm after entry into recipient cells is unclear. Possible mechanisms include membrane fusion in cells, EV membrane degradation, and selective cargo release. Membrane fusion requires similar fluidities of the EV and target cell membranes, which are mismatched at neutral pH but are more similar at acidic pH (~5) and may then promote fusion (28). This low pH can occur in the endocytic compartment, but EV fusion with the plasma membrane is unlikely, except in acidic milieus such as the tumor microenvironment (125). Release of cargo in cells may require breakdown of the endosomal membrane, as occurs, for example, with AAV virions (126). In many cases, EV cargo can be degraded by recipient cells, thereby inhibiting therapeutic delivery but limiting the impact of off-target delivery.
The identification of natural communication routes between cells (i.e., donor-recipient cell combinations exhibiting functional modulation) may help to identify mechanisms for enhancing functional cargo delivery through EV engineering. For example, expressing the vesicular stomatitis virus glycoprotein (VSV-G) on the EV surface may promote EV–plasma membrane fusion and enhance the delivery of EV cargo (127). However, AAV vexosomes incorporating VSV-G were inferior to those lacking VSV-G in mediating transduction, indicating that EV–plasma membrane fusion does not universally lead to increased cargo delivery (114).
Open Questions
What processes limit or promote the functional delivery of cargo biomolecules to recipient cells? Uptake of cargo into a cell is not equivalent to cargo functionality. For instance, EVs may potentially pass through cells within the multivesicular body compartment, which could explain how EVs cross the BBB (i.e., via a transendothelial route). Endocytotic mechanisms must circumvent the lysosomal degradative pathway, and direct fusion between the EV and target cell plasma membrane or endocytotic membrane does not always ensure functionality of the contents. In general, the fate of EVs within the body and cells remains poorly understood and requires additional investigation to elucidate how these processes impact functional EV-mediated delivery.
How can functional EV-mediated delivery be enhanced? The rate-limiting steps restricting EV-mediated delivery have not been characterized quantitatively, in contrast to drug and gene delivery. Moreover, it is not clear whether functional delivery by EVs derived from different sources or targeting different cell types is limited by similar or distinct steps in the overall delivery process described above. Advancing EV-based therapies to the clinic will require quantitative, mechanism-driven analyses of EV-mediated delivery.
MANUFACTURING VESICLES FOR THERAPEUTIC USE
Producer Cell Type Choice
Vesicle biogenesis is a universal cellular process, and thus the menu of potential producer cells types is lengthy (see above). Mammalian vesicles can be produced in cell lines or primary cells. Immortalized cell lines [e.g., HeLa (128) or EL-4 mouse lymphoma lines (71)] typically produce more vesicles than do primary cells and can be cultured indefinitely. However, the function of cell line–derived EVs is less well characterized, and as discussed above, these vesicles carry some risk of oncogenic potential. In contrast, primary cell–derived vesicles often have well-characterized functions (see above for MSC- or DC-derived EVs) that may complement the action of exogenously loaded therapeutic agents. Autologous primary cell–derived EVs may also reduce the risk of immunological rejection. Disadvantages of primary cells include lower vesicle yield and limiting passage numbers, making it harder to generate a cell bank. Nonmammalian cells, including bacterial, yeast, and plant cells, can also be used to produce vesicles for delivery. For example, grape EVs were internalized by both mammalian hematopoietic and nonhematopoietic cells in culture and exhibited excellent bioavailability in vivo without any apparent toxic effects (12). However, other than in vaccine applications, the clinical potential of these nonmammalian sources has not been evaluated.
Physical Methods to Generate Lipid Nanoparticles
Physical methods can be used to derive biological nanovesicles from cells that may recapitulate some features of secreted EVs. Such physical processes include extrusion of cells through filters, resulting in cellular fragmentation, and generation of vesicles that preserve the orientation of the plasma membrane (93). In mice with colon adenocarcinoma, such vesicles targeted the endothelium of tumors through LFA-1 on the vesicle surface and delivered doxorubicin that had antitumor activity. These nanovesicles were similar in size, morphology, and protein content to endogenously produced EVs. The yield of physically generated nanovesicles (in terms of protein content and total particle counts) was 100-fold higher from the same number of cells. Another method to generate artificial vesicles is to extrude cells through a microfluidic chamber (129). These fabricated vesicles are similar to endogenous EVs in size, shape, and composition and can deliver RNA molecules to recipient cells. Vesicles can potentially also be formed by sonication, lysis, electroporation, and freeze-thawing of cells. In general, however, the membrane protein and lipid composition of such physically derived vesicles will reflect that of the entire cell, which is different than naturally secreted EVs. Whether this impacts delivery, toxicity, or other EV properties has not been rigorously evaluated.
Enhancing EV Yield and Scale-Up
Manufacturing therapeutic vesicle preparations necessitates large-scale production as well as isolation and purification under controlled conditions. Vesicles produced by extrusion or filtration of cells may be more easily scaled up to achieve high yields. For secreted vesicles, scale-up is more difficult but mirrors challenges faced in the field of recombinant protein therapeutics. Multiple strategies exist to increase vesicle release from cells. For example, raising intracellular calcium increases vesicle production, so ionophore treatment (e.g., with A23187 or ionomycin) or surface receptor stimulation (e.g., the P2X7 purinergic receptor) (130, 131) can increase vesicle production. In addition, serum starvation or endotoxin increases EV production (132). Different treatments (and physical methods) may alter the composition and functionality of vesicles. Creation of immortalized cells from primary cells is another strategy to scale up vesicle production. For example, EV release from embryonic stem cell–derived MSCs was increased by overexpressing an oncogene, c-myc. EVs from these cells retained their protective function in a mouse model of myocardial ischemia/reperfusion injury, although transfer of c-myc DNA/RNA into normal cells may be oncogenic.
Quantifying Potency of EV-Based Products
For therapeutic applications, rigorously quantifying EV product potency, efficacy, and dose is crucial, which is challenging for such complex particles. Evaluating specific loading (cargo molecules per vesicle) would require vesicles counts, which can be determined using nanoparticle-tracking analysis (vesicles <800 nm) or flow cytometry (vesicles >1 μm), although the optimality and limitations of such methods are under debate (133, 134). Vesicle components can also be quantitated, e.g., by measuring total protein, lipid, or RNA content, but none of these is considered a gold standard. Importantly, vesicle dose determination may be confounded by the presence of vesicle aggregates or stable, nonvesicular, extracellular RNA complexes. Vesicle integrity and stability impacts dose efficacy, and freeze-thawing or sonication of vesicles may reduce their biological activities; there is currently no general quality control assay for the intactness of vesicles (54, 135).
EV-Inspired Bioengineered Artificial Vesicles
A strategy for combining ease of manufacturing and desirable EV functionalities is to engineer artificial lipid vesicles that incorporate EV features or components. For example, the high PS content of EV membranes results in a rigid vesicle that is resistant to lipolytic and proteolytic degradation in the circulation (28). Mimicking this rigid structure could enhance the stability of artificial lipid vesicles in vivo and increase their probability of reaching targets prior to being cleared or degraded. The incorporation of EV lipids into artificial vesicles could also enhance delivery of vesicle contents to recipient cells. PS is important in EV uptake, and its incorporation into liposomes could enhance their uptake by DCs and other phagocytes. EV lipids may also enhance other mechanisms of vesicle uptake, such as plasma membrane fusion. The addition of cholesterol to PS vesicles increases the rate of intervesicle fusion, serving as a proxy for vesicle fusion with lipid rafts in cell membranes (136). Thus, liposomes that incorporate PS and cholesterol may be well suited for cytoplasmic delivery of EV cargo. Furthermore, the use of EV lipids to enhance liposome delivery is effective in vivo. The addition of the glycosphingolipid N-octanoylglucosylceramide to liposomes composed of hydrogenated soy phosphatidylcholine, cholesterol, and distearylphosphatidylethanolamine-PEG2000 increased delivery of doxorubicin to B16 mouse melanoma cells in culture and in vivo (137), supporting the combined use of artificial and natural lipids.
EV lipids may also be selected to modulate interactions between liposomes and the immune system. The inclusion of PS in antigen-delivering liposomes induced Th1-type cell-mediated immunity in a dose-dependent manner (138). In contrast, PS liposomes, but not phosphatidylcholine liposomes, inhibited DC maturation in response to LPS stimulation (139), indicating that PS may play varying roles in different immune contexts. Mixed EV lipids can also modulate the immune response. Intestine-derived EV-like nanoparticles (IDENs) induced NKT cell anergy (140) via a process that required signaling between prostaglandin E2 (PGE2) displayed on IDENs and PGE2 receptors on NKT cells. Liposomes formed using lipids purified from these intestinal EVs induced the same reduction in NKT cell cytokine secretion as did IDENs. Thus, reconstitution of EV-derived lipids into liposomes is a viable method for developing well-defined nanoparticles that replicate the immune-modifying properties of the native vesicles. The effects of EV-derived lipids are multifactorial, impacting liposome structure, circulation time, uptake by target cells, and immune responses.
Mimicking the protein composition of EVs could also enable the development of immune modulatory liposomes. Liposomes containing MHC-cytomegalovirus (CMV) peptide complexes and Fab fragments to activate LFA-1, CD27, CD28, 4-1BB, and CD40L were able to trigger the expansion of CD8+ effector memory cells and the secretion of IFN-γ when incubated with peripheral blood mononuclear cells from CMV-positive individuals (141). Thus, incorporating even a limited number of proteins into a liposome may achieve EV-like properties such as antigen presentation and stimulation of an immune response.
Open Questions
Which vesicle population is the most suitable for drug delivery? Given the heterogeneity of vesicles, it is not clear which vesicle population will prove the best for carrying appropriate amounts of different therapeutic cargoes, which will have the highest bioavailability, and whether such optimal vesicle subsets will overlap. For a given therapeutic indication, different vesicle populations should be tested in parallel. For example, in mammalian vesicles, the total RNA content of small vesicles appears higher than that of larger vesicles (142), but different types of RNAs may be differentially distributed. Liposomes <100–150 nm in diameter are superior in bioavailability, transcapillary passage, and biological activity compared to larger particles (143). Thus, among EVs, small-size vesicles (the so-called exosome fraction) are the most likely to be useful for drug delivery. For naturally secreted vesicles, the desired size range could be achieved by selection of an appropriate isolation and purification protocol. In the case of artificial vesicles, vesicle size can be manipulated by tuning the process characteristics (e.g., geometry of the microchannels during extrusion or pore sizes during filtration) (129).
How can clinical-grade EVs be produced? Clinical Good Manufacturing Practice (cGMP)-grade standards for clinical use require uniformity, consistency, and reproducibility in the quantity and quality of a given therapeutic agent. It is particularly challenging to establish cGMP-grade EV preparations, as EVs are highly complex and their composition and production are highly variable. The factors contributing to this diversity are still under investigation. Furthermore, EV stability also differs by vesicle source and preparation method, and a reliable quality control test for membrane integrity is lacking.
How can natural EV and liposome components be combined to maximize stability, targeting, and therapeutic delivery? Because manufacturing practices for liposomes and nanoparticles are already well developed and enable efficient cargo molecule loading, an attractive strategy may be to enhance the performance of these synthetic delivery vehicles by incorporating specific lipids or proteins of EVs known to increase stability, targeting, and uptake. This enhancement could use purified molecules (e.g., synthetic lipids or recombinant proteins), or these elements could be extracted from EVs, although extraction could lead to contamination with other components. Importantly, such enhanced liposome formulations also provide a test bed in which to study the effects of EV components in isolation and to identify the components that best enhance the therapeutic properties of the EV-inspired artificial vesicles.
CLINICAL TRIALS AND COMMERCIALIZATION
Clinical Trials
Building upon a growing body of promising preclinical evidence, EV-based therapies are making their way into the clinic (Table 1). EV-based cancer vaccines have already been tested in Phase I clinical trials evaluating safety (144). In one EV clinical trial, DCs were isolated from patients with advanced metastatic melanoma and incubated with melanoma peptide antigens to induce presentation of the antigen on the cell surface in association with MHC. EVs were then isolated from the DC-conditioned medium and reintroduced into patients to promote an immune response against melanoma. In some patients, minor inflammatory responses at the site of EV injection (mild swelling, redness, and DTH responses) and low-grade fever were observed after EV administration. However, patients tolerated administration of EVs for up to 21 months (145). In a similar trial, non-small-cell lung cancer patients injected with EVs weekly for 4 weeks manifested low-level inflammatory responses (146). Finally, a clinical trial in which tumor ascites–derived EVs were isolated and reintroduced along with granulocyte-macrophage colony-stimulating factor demonstrated that the only adverse response to EV vaccination was mild inflammatory responses at the site of vaccination (147). Thus, repeated administration of autologous EVs is well tolerated. The therapeutic benefit of EV vaccination has not yet been validated; however, disease progression was halted in some patients in the Phase I clinical trials after EV vaccination (146, 147). Based on these promising results, DC EVs for non-small-cell lung cancer treatment will soon be tested in a Phase II clinical trial (http://clinicaltrials.gov/show/NCT01159288). EVs are also being tested as potential therapeutic delivery vehicles in a Phase I clinical trial of plant-derived EVs delivering curcumin for treatment of colon cancer (http://clinicaltrials.gov/show/NCT01294072).
Table 1.
Clinical and preclinical investigation of extracellular vesicle–based therapies
| Vesicle type and source | Administrative route |
Recipient | Therapeutic effect | Reference |
|---|---|---|---|---|
| Preclinical studies: mouse | ||||
|
| ||||
| Liposomes enriched with N-octanoyl-glucosylceramide |
i.v. | Tumor-bearing nude mice | Enhanced Dox delivery to tumors via EV versus via liposomes |
137 |
|
| ||||
| EVs from mouse MSCs | i.v. | Hypoxia-treated FVB/N mice |
Decreased lung inflammation and hypoxia-induced hypertension |
42 |
|
| ||||
| EVs from the human ESC-derived MSC line HuES9.E1 |
i.v. | C57BL6/J mice after myocardial infarction before reperfusion |
Reduced myocardial infarct size and inflammation |
43 |
|
| ||||
| EVs derived from mouse bone marrow MSCs |
s.c. with 4T1 cells |
BALB/c mice | Reduced growth and vascularization of tumors |
46 |
|
| ||||
| EVs derived from spleen and lymph node cells of TNP-tolerized mice |
i.p. | TNP-sensitized C57BL/6 mice |
Inhibited contact sensitivity | 51 |
|
| ||||
| EVs derived from mouse BMDC overexpressing IL-10, IL-4, or FasL |
Footpad | C57BL/6 mice prior to DTH induction |
Suppressed DTH | 53 |
|
| ||||
| EVs derived from mouse BMDC overexpressing IL-10, IL-4, or FasL |
i.v. | Collagen-immunized DBA/1 mice |
Delayed collagen-induced arthritis |
53 |
|
| ||||
|
Bacteroides fragilis OMVs with polysaccharide A |
Oral | BALB/c mice prior to colitis induction |
Decreased colitis-induced weight loss, colon shrinkage, and colitis |
55 |
|
| ||||
| EVs derived from grape juice | Oral | C57BL/6 mice concurrent with colitis induction |
Decreased colitis-induced colon shrinkage and mortality |
12 |
|
| ||||
| EVs from RAW264.7 cell line pulsed with Mycobacterium tuberculosis proteins |
i.n. vaccine | C57BL/6 mice | Decreased growth of M. tuberculosis in the lung |
61 |
|
| ||||
| EVs from a mouse splenic DC cell line, pulsed with Toxoplasma gondii antigens |
s.c. | Female CBA/J mice prior to mating and T. gondii exposure |
Fewer T. gondii cysts in mother and pup brains, increased pup survival |
63 |
|
| ||||
| OMVs derived from Bordetella pertussis |
i.v. | BALB/c mice prior to B. pertussis challenge |
Decreased B. pertussis counts and increased type 1 antibody production compared to animals given Tdap vaccine before challenge |
64 |
|
| ||||
| OMVs derived from Escherichia coli | i.p. | C57BL/6 and BALB/c mice prior to E. coli challenge |
100% survival postchallenge, versus 20% for unvaccinated mice |
92 |
|
| ||||
| EVs from BMDCs cultured with OVA peptide and α glucosylceramide |
i.v. | Tumor-bearing C57BL/6 mice |
Decreased tumor growth and prolonged survival |
67 |
|
| ||||
| EVs from HEK293 expressing let-7a and RGD-targeting peptide |
i.v. | Tumor-bearing RAG−/− mice |
Decreased tumor growth | 73 |
|
| ||||
| EVs from HEK293T expressing CD and UPRT |
Intratumoral injection |
Tumor-bearing nude mice |
Decreased tumor growth; elimination of tumor in two-thirds of treated mice |
72 |
|
| ||||
| Dox-loaded EVs from DCs expressing iRGD |
i.v. | Tumor-bearing nude mice |
Decreased tumor growth compared to free Dox or untargeted EVs |
74 |
|
| ||||
| Nanovesicles extruded from RAW264.7 cells in presence of Dox |
i.v. | Tumor-bearing BALB/c mice |
Decreased tumor growth compared to Dox liposomes |
93 |
|
| ||||
| Curcumin-loaded EVs from EL-4 cells |
i.p. with LPS | C57BL/6 mice | Increased survival of LPS-induced septic shock |
71 |
|
| ||||
| Curcumin-loaded EVs from EL-4 cells |
Intranasal | C57BL/6 mice | Decreased EAE clinical score compared to free curcumin |
75 |
|
| ||||
| EVs derived from EL-4 cells loaded with Stat 3 inhibitor |
Intranasal | Tumor-bearing C57BL/6 mice |
Decreased tumor size and prolonged survival compared to free inhibitor |
75 |
|
| ||||
| Phase I studies | ||||
|
| ||||
| EVs from autologous DCs pulsed with melanoma peptide antigens |
Intradermal and s.c. |
15 patients | Minor inflammation, no major toxicity. One patient: MART-1–T cell response and tumor shrinkage; one patient: minor response (loss of a spinal cord lesion); two patients: stabilization |
145 |
|
| ||||
| EVs from autologous DCs pulsed with MAGE peptides |
Intradermal and s.c. |
9 patients | No major toxicity. One-third of patients: MAGE-specific T cell responses; two patients: increased NK cell lysis |
146 |
|
| ||||
| EVs from autologous ascites fluid of colorectal cancer patients |
s.c. | 37 patients; 13 also received GM-CSF |
No major toxicity. Patients receiving EVs and GM-CSF: 80% showed cytotoxic T cell responses to colon cancer peptide CAP-1, with one stabilization and one minor response |
147 |
|
| ||||
| OMVs from Neisseria meningitidis |
i.m. injection | Human patients | MenBVac and MeNZB vaccines are safe and effective |
65 |
Abbreviations: BMDC, bone marrow–derived DC; CAP-1, carcinoembryonic antigen peptide-1; CD, cluster of differentiation; DC, dendritic cell; Dox, doxorubicin; DTH, delayed type hypersensitivity; EAE, experimental autoimmune encephalomyelitis; ESC, embryonic stem cell; EV, extracellular vesicle; FasL, Fas ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; i.m., intramuscular; i.n., intranasal; i.p., intraperitoneal; iRGD, internalizing arginine-glycine-asparagine; i.v., intravenous; LPS, lipopolysaccharide; MAGE, Melanoma Antigen GEne; MART-1, melanoma antigen recognized by T cells-1; MSC, mesenchymal stem cell; NK, natural killer; OMV, outer membrane vesicle; OVA, ovalbumin; RGD, arginine-glycine-asparagine; s.c., subcutaneous; Tdap, tetanus-diphtheria-acellular pertussis; TNP, trinitrophenyl; UPRT, uracil phosphoribosyltransferase.
Commercialization
Biotechnology companies are actively involved in commercializing EV-based diagnostics, and activity is moving toward therapeutic applications as well. Strategies for EV isolation and purification are being commercialized by many companies (e.g., System Biosciences, Life Technologies, Qiagen, HansaBioMed, Cell Guidance Systems, and Exosome Diagnostics). EV-based diagnostic platforms are being developed and marketed for cancer (Exosome Diagnostics, Exosomics Siena, Exosome Sciences), viral infection (Exosome Sciences), and siRNA therapy (Alnylam). In addition, several large pharmaceutical companies are investigating the potential of vesicle-based biomarkers. Finally, Aethlon Medical is investigating the therapeutic value of EV depletion in cancer. Clinical evaluation of EV therapeutics is still at an early stage but is rapidly expanding, and it is likely to drive commercial investment if clinical evaluations can recapitulate promising preclinical data.
Open Questions
What regulatory mechanisms are required for clinical EV applications? Although the US Food and Drug Administration regulates final approval for clinical applications, the National Institutes of Health DNA Recombinant Advisory Committee may also be involved in evaluating EVs derived from genetically modified cells or EV-mediated delivery of genetic materials. Notably, the EV-associated concerns discussed above, such as the oncogenic potential of EVs produced by transformed cells and the presence of retrovirus-like particles and possibly other viruses in EV preparations, are safety concerns for which substantial precedence and experience exist in the context of other biological therapeutics (148–150).
What clinical applications will be realized first? EVs have already made their debut as vaccination vehicles with great promise to promote immune responses, and this trend will likely continue with respect to infectious agents and potentially cancer. The immunosuppressive potential of EVs may also prove to be a promising therapeutic avenue for managing at-risk pregnancies and treating autoimmune diseases. The ability of EVs to act as stealth vehicles that mediate delivery while avoiding immune rejection may also promote their use for gene therapy, including vector-mediated gene replacement and genome editing, as well as a more efficient means to delivery antisense oligonucleotides and siRNAs.
CONCLUSION
The therapeutic potential of EVs presents exciting new avenues for intervention in many diseases. EVs span a broad range of vesicle types, cells and species of origin, processes of biogenesis, and modes of cellular uptake and fate. Promising aspects include the ability of EVs to transport genetic information, as well as drugs and proteins, to target specific cell types and to increase the stability of therapeutic cargoes in vivo. This new technology fits well into current knowledge and use of gene therapy vectors, oligonucleotides, liposomes, and nanoparticles, and great potential exists for engineering new vehicles that combine advantageous elements. Two overall approaches are emerging: (a) harnessing the natural therapeutic potential of cellular-derived vesicles and enhancing this capacity through the introduction of molecules that facilitate targeting, uptake, and loading EVs with specific protein or RNA cargoes; and (b) incorporating specific EV-mimetic features or molecules into manufactured liposomes or nanoparticles to pair these novel features with platforms for which manufacturing ease and clinical experience is currently superior. Realizing the potential of EV-based therapies will require investigation of the open questions posed here to drive EV research from promising phenomenological observations to quantitative evaluation of translational investigations.
ACKNOWLEDGMENTS
We thank Ms. Suzanne McDavitt for skilled editorial assistance and Ms. Emily Mills at Millstone Design for preparation of figures. This work was supported by the National Science Foundation’s Graduate Research Fellowship Program (NSF GRFP) award DGE-0824162 (to M.E.H.); U19 CA179563, which is supported by the National Institutes of Health (NIH) Common Fund through the Office of Strategic Coordination/Office of the NIH Director; NIH award CA069246; Voices Against Brain Cancer; the Richard Floor Biorepository Fund (to X.O.B.); the Northwestern University Prostate Cancer Specialized Program of Research Excellence (SPORE) through NIH award P50 CA090386; and a 3M Non-Tenured Faculty Award (to J.N.L.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Lee Y, El Andaloussi S, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012;21:R125–34. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 2.El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Nat. Rev. Drug Discov. 2013;12:347–57. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 3.Marcus ME, Leonard JN. FedExosomes: engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals. 2013;6:659–80. doi: 10.3390/ph6050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagiwara K, Ochiya T, Kosaka N. A paradigm shift for extracellular vesicles as small RNA carriers: from cellular waste elimination to therapeutic applications. Drug Deliv. Transl. Res. 2014;4:31–37. doi: 10.1007/s13346-013-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kittel A, Falus A, Buzas E. Microencapsulation technology by nature: cell derived extracellular vesicles with therapeutic potential. Eur. J. Microbiol. Immunol. 2013;3:91–96. doi: 10.1556/EuJMI.3.2013.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning AJ, Kuehn MJ. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J. Mol. Microbiol. Biotechnol. 2013;23:131–41. doi: 10.1159/000346548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues ML, Nakayasu ES, Almeida IC, Nimrichter L. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J. Proteomics. 2014;97:177–86. doi: 10.1016/j.jprot.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJ, et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLOS ONE. 2010;5:e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantel PY, Marti M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell Microbiol. 2014;16:344–54. doi: 10.1111/cmi.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013;4:1867. doi: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju S, Mu J, Dokland T, Zhuang X, Wang Q, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013;21:1345–57. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, et al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr. Biol. 2014;24:519–25. doi: 10.1016/j.cub.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koles K, Budnik V. Exosomes go with the Wnt. Cell. Logist. 2012;2:169–73. doi: 10.4161/cl.21981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houseley J, LaCava J, Tollervey D. RNA-quality control by the exosome. Nat. Rev. Mol. Cell Biol. 2006;7:529–39. doi: 10.1038/nrm1964. [DOI] [PubMed] [Google Scholar]
- 16.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 17.Morello M, Minciacchi VR, de Candia P, Yang J, Posadas E, et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle. 2013;12:3526–36. doi: 10.4161/cc.26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contreras-Galindo R, Kaplan MH, Contreras-Galindo AC, Gonzalez-Hernandez MJ, Ferlenghi I, et al. Characterization of human endogenous retroviral elements in the blood of HIV-1-infected individuals. J. Virol. 2012;86:262–76. doi: 10.1128/JVI.00602-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLOS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prada I, Furlan R, Matteoli M, Verderio C. Classical and unconventional pathways of vesicular release in microglia. Glia. 2013;61:1003–17. doi: 10.1002/glia.22497. [DOI] [PubMed] [Google Scholar]
- 21.Pallet N, Sirois I, Bell C, Hanafi LA, Hamelin K, et al. A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics. 2013;13:1108–20. doi: 10.1002/pmic.201200531. [DOI] [PubMed] [Google Scholar]
- 22.Wurdinger T, Gatson NA, Balaj L, Kaur B, Breakefield XO, Pegtel DM. Extracellular vesicles and their convergence with viral pathways. Adv. Virol. 2012;2012:767694. doi: 10.1155/2012/767694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA. 2003;100:10592–97. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J. Cell Biol. 2008;183:1187–91. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Curr. Biol. 2013;23:906–11. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llorente AS, Sylvänne T, Kauhanen D, Róg T, Orłowski A, et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta. 2013;1831:1302–9. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 28.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochemistry. 2004;380:161–71. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal M, Sainte-Marie J, Philippot JR, Bienvenue A. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for “aminophospholipid translocase.”. J. Cell. Physiol. 1989;140:455–62. doi: 10.1002/jcp.1041400308. [DOI] [PubMed] [Google Scholar]
- 30.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–71. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 31.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteomics. 2009;6:267–83. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 32.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–44. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Curtis I, Meldolesi J. Cell surface dynamics – how Rho GTPases orchestrate the interplay between the plasma membrane and the cortical cytoskeleton. J. Cell Sci. 2012;125:4435–44. doi: 10.1242/jcs.108266. [DOI] [PubMed] [Google Scholar]
- 34.Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 2010;13:328–35. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J. Cell. Physiol. 2013;228:1487–95. doi: 10.1002/jcp.24304. [DOI] [PubMed] [Google Scholar]
- 36.Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta. 2013;24:1843–1612. doi: 10.1016/j.bbamcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keyel PA, Heid ME, Watkins SC, Salter RD. Visualization of bacterial toxin induced responses using live cell fluorescence microscopy. J. Vis. Exp. 2012;68:e4227. doi: 10.3791/4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 2000;6:769–75. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 39.van der Vlist EJ, Arkesteijn GJA, van de Lest CHA, Stoorvogel W, Nolte-’t Hoen ENM, Wauben MHM. CD4+ T cell activation promotes the differential release of distinct populations of nanosized vesicles. J. Extracell. Vesicles. 2012;1:18364. doi: 10.3402/jev.v1i0.18364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 41.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans E, et al. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA. 2010;107:6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–11. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–12. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–64. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pusic AD, Kraig RP. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia. 2014;62:284–99. doi: 10.1002/glia.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLOS ONE. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianco NR, Kim SH, Morelli AE, Robbins PD. Modulation of the immune response using dendritic cell–derived exosomes. Methods Mol. Biol. 2007;380:443–55. doi: 10.1007/978-1-59745-395-0_28. [DOI] [PubMed] [Google Scholar]
- 48.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J. Immunol. 2006;176:1534–42. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 50.Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 2013;191:5515–23. doi: 10.4049/jimmunol.1301885. [DOI] [PubMed] [Google Scholar]
- 51.Bryniarski K, Ptak W, Jayakumar A, Pullmann K, Caplan MJ, et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J. Allergy Clin. Immunol. 2013;132:170–81. doi: 10.1016/j.jaci.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim HD, Maxwell JA, Kong FK, Tang DCC, Fukuchi K. Induction of anti-inflammatory immune response by an adenovirus vector encoding 11 tandem repeats of Aβ1–6: toward safer and effective vaccines against Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2005;336:84–92. doi: 10.1016/j.bbrc.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 53.Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, et al. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflamma-tory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J. Immunol. 2007;179:2242–49. doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- 55.Shen Y, Torchia MLG, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–20. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. USA. 2013;110:12048–53. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Liu K, Liu Y, Xu Y, Zhang F, et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 58.Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, et al. Dendritic cell-derived exosomes for cancer immunotherapy: What’s next? Cancer Res. 2010;70:1281. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 59.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 2007;179:1913–25. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 60.Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J. Immunol. 2006;177:3757–62. doi: 10.4049/jimmunol.177.6.3757. [DOI] [PubMed] [Google Scholar]
- 61.Cheng Y, Schorey JS. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur. J. Immunol. 2013;43:3279–90. doi: 10.1002/eji.201343727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, et al. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine. 2011;29:3818–25. doi: 10.1016/j.vaccine.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 63.Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine. 2009;27:1750–57. doi: 10.1016/j.vaccine.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 64.Gaillard ME, Bottero D, Errea A, Ormazabal M, Zurita ME, et al. Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine. 2014;32:931–37. doi: 10.1016/j.vaccine.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 65.Sandbu S, Feiring B, Oster P, Helland OS, Bakke HS, et al. Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines. Clin. Vaccine Immunol. 2007;14:1062–69. doi: 10.1128/CVI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine. 2011;29:1413–20. doi: 10.1016/j.vaccine.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 67.Gehrmann U, Hiltbrunner S, Georgoudaki AM, Karlsson MC, Näslund TI, Gabrielsson S. Synergistic induction of adaptive antitumor immunity by codelivery of antigen with α-galactosylceramide on exosomes. Cancer Res. 2013;73:3865–76. doi: 10.1158/0008-5472.CAN-12-3918. [DOI] [PubMed] [Google Scholar]
- 68.Lee EY, Park KS, Yoon YJ, Lee J, Moon HG, et al. Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis. PLOS ONE. 2012;7:e33330. doi: 10.1371/journal.pone.0033330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 2003;33:522–31. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 70.Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012;11:839–49. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 71.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18:1606–14. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol. Ther. 2013;21:101–8. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21:185–91. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian Y, Li S, Song J, Ji T, Zhu M, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 75.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011;19:1769–79. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–45. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 77.Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat. Immunol. 2002;3:1156–62. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 78.Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J. Immunol. 2008;180:3081–90. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 79.Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc. Natl. Acad. Sci. USA. 2014;111:711–16. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bijnsdorp IV, Geldof AA, Lavaei M, Piersma SR, van Moorselaar RJA, Jimenez CR. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J. Extracell. Vesicles. 2013;2:22097. doi: 10.3402/jev.v2i0.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–56. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 82.Shin SJ, Smith JA, Rezniczek GA, Pan S, Chen R, et al. Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2013;110:19414–19. doi: 10.1073/pnas.1309720110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 2013;288:34343–51. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA. 2013;110:7312–17. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–56. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 86.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 2008;180:7249–58. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 87.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–66. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 88.Balaj L, Lessard R, Dai L, Cho Y-J, Pomeroy SL, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rak J, Guha A. Extracellular vesicles–vehicles that spread cancer genes. Bioessays. 2012;34:489–97. doi: 10.1002/bies.201100169. [DOI] [PubMed] [Google Scholar]
- 90.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu S, Liu C, Su K, Wang J, Liu Y, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J. Immunol. 2007;178:6867–75. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 92.Kim OY, Hong BS, Park KS, Yoon YJ, Choi SJ, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J. Immunol. 2013;190:4092–102. doi: 10.4049/jimmunol.1200742. [DOI] [PubMed] [Google Scholar]
- 93.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 94.Wahlgren J, Karlson TDL, Brisslert M, Vaziri Sani F, Telemo E, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release. 2013;172:229–38. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 96.Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 2014;448:41–49. doi: 10.1016/j.ab.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–52. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 98.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–85. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 99.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA. 2012;109:4146–51. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J. Biol. Chem. 2011;286:14383–95. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLOS ONE. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 2009;11:1143–49. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y, Liu D, Chen X, Li J, Li L, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell. 2010;39:133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. Analysis of microRNA and protein transfer by exosomes during an immune synapse. Methods Mol. Biol. 2013;1024:41–51. doi: 10.1007/978-1-62703-453-1_4. [DOI] [PubMed] [Google Scholar]
- 105.Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Ströbel T, et al. miR-1289 and “zipcode”-like sequence enrich mRNAs in microvesicles. Mol. Ther. Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koppers-Lalic D, Hackenberg M, van Eijndhoven ME, Sabogal Pineros Y, Sie D, et al. Comprehensive deep-sequencing analysis reveals non-random small RNA incorporation into tumour exosomes and biomarker potential. Proc. Int. Soc. Extracell. Vesicles, 2nd, Boston, Apr. 17–20. 2013 [Google Scholar]
- 107.Rechavi O, Erlich Y, Amram H, Flomenblit L, Karginov FV, et al. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev. 2009;23:1971–79. doi: 10.1101/gad.1789609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kosaka N, Iguchi H, Yoshioka Y, Hagiwara K, Takeshita F, Ochiya T. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J. Biol. Chem. 2012;287:1397–405. doi: 10.1074/jbc.M111.288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, et al. Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol. Ther. 2011;19:395–99. doi: 10.1038/mt.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012;14:249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 111.Hartman ZC, Wei J, Glass OK, Guo H, Lei G, et al. Increasing vaccine potency through exosome antigen targeting. Vaccine. 2011;29:9361–67. doi: 10.1016/j.vaccine.2011.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68:1228–35. doi: 10.1158/0008-5472.CAN-07-3163. [DOI] [PubMed] [Google Scholar]
- 113.Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–94. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maguire CA, Balaj L, Sivaraman S, Crommentuijn M, Ericsson M, et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012;20:960–71. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–71. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lai CP, Tannous BA, Breakefield XO. Noninvasive in vivo monitoring of extracellular vesicles. Methods Mol. Biol. 2014;1098:249–58. doi: 10.1007/978-1-62703-718-1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–16. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., II Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–96. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 119.Nolte-’t Hoen ENM, Buschow SI, Anderton SM, Stoorvogel W, Wauben MHM. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–81. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 120.Vallhov H, Gutzeit C, Johansson SM, Nagy N, Paul M, et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J. Immunol. 2011;186:73–82. doi: 10.4049/jimmunol.1001145. [DOI] [PubMed] [Google Scholar]
- 121.Ruiss R, Jochum S, Mocikat R, Hammerschmidt W, Zeidler R. EBV-gp350 confers B-cell tropism to tailored exosomes and is a neo-antigen in normal and malignant B cells–a new option for the treatment of B-CLL. PLOS ONE. 2011;6:e25294. doi: 10.1371/journal.pone.0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Atai NA, Balaj L, van Veen H, Breakefield XO, Jarzyna PA, et al. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J. Neuro-Oncology. 2013;115:343–51. doi: 10.1007/s11060-013-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA. 2013;110:17380–85. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 2011;81:1171–82. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 125.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284:34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 2000;74:2777–85. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Temchura VV, Tenbusch M, Nchinda G, Nabi G, Tippler B, et al. Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus. Vaccine. 2008;26:3662–72. doi: 10.1016/j.vaccine.2008.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jo W, Jeong D, Kim J, Cho S, Jang SC, et al. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14:1261–69. doi: 10.1039/c3lc50993a. [DOI] [PubMed] [Google Scholar]
- 130.Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, et al. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J. Immunol. 2009;182:5052–62. doi: 10.4049/jimmunol.0802968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Constantinescu P, Wang B, Kovacevic K, Jalilian I, Bosman GJCGM, et al. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim. Biophys. Acta. 2010;1798:1797–804. doi: 10.1016/j.bbamem.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 132.Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb. Haemost. 2008;100:878–85. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- 133.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 134.György B, Pálóczi K, Kovács A, Barabás E, Bekő G, et al. Improved circulating microparticle analysis in acid-citrate dextrose (ACD) anticoagulant tube. Thromb. Res. 2014;133:285–92. doi: 10.1016/j.thromres.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 135.Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, et al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 2005;174:6440–48. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 136.Shavnin SA, Pedroso de Lima MC, Fedor J, Wood P, Bentz J, Düzgüneş N. Cholesterol affects divalent cation-induced fusion and isothermal phase transitions of phospholipid membranes. Biochim. Biophys. Acta. 1988;946:405–16. doi: 10.1016/0005-2736(88)90416-6. [DOI] [PubMed] [Google Scholar]
- 137.van Lummel M, van Blitterswijk WJ, Vink SR, Veldman RJ, van der Valk MA, et al. Enriching lipid nanovesicles with short-chain glucosylceramide improves doxorubicin delivery and efficacy in solid tumors. FASEB J. 2011;25:280–89. doi: 10.1096/fj.10-163709. [DOI] [PubMed] [Google Scholar]
- 138.Yotsumoto S, Kakiuchi T, Aramaki Y. Enhancement of IFN-γ production for Th1-cell therapy using negatively charged liposomes containing phosphatidylserine. Vaccine. 2007;25:5256–62. doi: 10.1016/j.vaccine.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 139.Chen X, Doffek K, Sugg SL, Shilyansky J. Phosphatidylserine regulates the maturation of human dendritic cells. J. Immunol. 2004;173:2985–94. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- 140.Deng ZB, Zhuang X, Ju S, Xiang X, Mu J, et al. Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. J. Immunol. 2013;190:3579–89. doi: 10.4049/jimmunol.1203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De La Peña H, Madrigal JA, Rusakiewicz S, Bencsik M, Cave GWV, et al. Artificial exosomes as tools for basic and clinical immunology. J. Immunol. Methods. 2009;344:121–32. doi: 10.1016/j.jim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 142.Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles. 2013;2:20677. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hwang KJ, Padki MM, Chow DD, Essien HE, Lai JY, Beaumier PL. Uptake of small liposomes by non-reticuloendothelial tissues. Biochim. Biophys. Acta. 1987;901:88–96. doi: 10.1016/0005-2736(87)90259-8. [DOI] [PubMed] [Google Scholar]
- 144.Mignot G, Roux S, Thery C, Ségura E, Zitvogel L. Prospects for exosomes in immunotherapy of cancer. J. Cell Mol. Med. 2006;10:376–88. doi: 10.1111/j.1582-4934.2006.tb00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl. Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morse MA, Garst J, Osada T, Khan S, Hobeika A, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dai S, Wei D, Wu Z, Zhou X, Wei X, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008;16:782–90. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nienhuis AW. Development of gene therapy for blood disorders: an update. Blood. 2013;122:1556–64. doi: 10.1182/blood-2013-04-453209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller JL, Petteway SR, Jr, Lee DC. Ensuring the pathogen safety of intravenous immunoglobulin and other human plasma-derived therapeutic proteins. J. Allergy Clin. Immunol. 2001;108:S91–94. doi: 10.1067/mai.2001.117823. [DOI] [PubMed] [Google Scholar]
- 150.Farshid M, Taffs RE, Scott D, Asher DM, Brorson K. The clearance of viruses and transmissible spongiform encephalopathy agents from biologicals. Curr. Opin. Biotechnol. 2005;16:561–67. doi: 10.1016/j.copbio.2005.07.006. [DOI] [PubMed] [Google Scholar]