Abstract
Objective
The biological mechanisms linking obesity to insulin resistance have not been fully elucidated. We have shown that insulin resistance/glucose intolerance in diet-induced obese mice is related to a shift in the ratio of pro- and anti-inflammatory T cells in adipose tissue. We sought to test the hypothesis that the balance of T-cell phenotypes would be similarly related to insulin resistance in human obesity.
Approach and Results
Healthy overweight/obese human subjects underwent adipose-tissue biopsies and quantification of insulin-mediated-glucose disposal by the modified insulin-suppression test. T-cell subsets were quantitated by flow cytometry in visceral (VAT) and subcutaneous adipose tissue (SAT). Results showed that CD4 and CD8 T-cells infiltrate both depots, with pro-inflammatory T-helper (Th)-1, Th17 and CD8 T-cells significantly more frequent in VAT as compared with SAT. T-cell profiles in SAT and VAT correlated significantly with one another and with peripheral blood. Th1 frequency in SAT and VAT correlated directly, whereas Th2 frequency in VAT correlated inversely with plasma hsCRP concentrations. Th1 in SAT correlated with plasma interleukin-6. Th2 in both depots and peripheral blood was inversely associated with systemic insulin resistance. Relative expression of associated cytokines, measured by rtPCR, reflected flow cytometry results. Most notably, adipose tissue expression of interleukin-10 was inversely associated with insulin resistance.
Conclusion
CD4 and CD8 T-cells populate human adipose tissue and the relative frequency of Th1 and Th2 is highly associated with systemic inflammation and insulin resistance. These findings point to the adaptive immune system as a potential mediator between obesity and insulin resistance/inflammation. Identification of antigenic stimuli in adipose tissue may yield novel targets for treatment of obesity-associated metabolic disease.
Keywords: Insulin Resistance, Obesity, Adaptive Immunity, T cells, Inflammation, T-helper, Interleukin-10, CD4, CD8, Human Adipose Tissue
Introduction
Whereas obesity has been clearly linked to insulin resistance and type 2 diabetes, the underlying mechanisms have not been fully elucidated. Accumulating evidence implicates adipose tissue inflammation as a contributor to insulin resistance via increased systemic inflammation and direct impairment of insulin-mediated glucose uptake. Obese adipose tissue is associated with increases in serum and tissue pro-inflammatory molecules such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which can induce insulin resistance in vitro1,2. Macrophages accumulate in adipose tissue in proportion to BMI and adipose cell size3,4, and adopt a pro-inflammatory phenotype in response to diet-induced obesity5. It is thought that changes in hypertrophic adipose cells, such as hypoxia, necrosis, chemokine secretion, and fatty acids attract and activate macrophages1, but the specific stimuli and pathways by which this occurs are unclear. Also unclear is the potential role of other immune cells in adipose tissue, and their relationship to localized and systemic inflammation and insulin resistance. Studies in mice indicate that with diet-induced obesity, pro-inflammatory T-helper 1 (Th1) and CD8 lymphocytes infiltrate visceral adipose tissue (VAT), where they overcome the anti-inflammatory effects of Th2 and T-regulatory (Treg) cells, promoting classical activation of macrophages and systemic insulin resistance/glucose intolerance6–13. T cells have been identified in human adipose tissue14,15 but there is scant data on T-cell phenotypes or their relationship to systemic inflammation and/or insulin resistance. To test the hypothesis that numerical dominance of proinflammatory CD8 and Th1 over anti-inflammatory Th2 and T-reg cells would be related to systemic inflammation and insulin resistance in humans, we used flow cytometry to quantitate the relative frequency of these T-cell subsets in SAT and VAT of healthy overweight/obese subjects who underwent quantitative insulin-sensitivity testing.
Materials and Methods
Detailed Materials and Methods are available in the online-only Data Supplement. In brief, subjects included healthy nondiabetic women and men from the San Francisco Bay Area who were 35–65 years of age and overweight or obese. Insulin-mediated glucose uptake was quantified by the modified insulin-suppression test to yield steady-state plasma glucose (SSPG) concentrations, for which higher values indicate relative inability to dispose of a glucose load under experimentally-fixed insulin concentrations. Adipose tissue biopsy samples from subcutaneous and/or visceral depots were flash frozen for real-time polymerase chain reaction (rtPCR), and collagenase digested for flow cytometry of isolated stromal-vascular cells. Same-day blood samples were obtained for flow cytometry and measurement of IL-6 and high-sensitivity c-reactive protein (hsCRP).
Results
Forty-seven prescreened, nondiabetic, healthy subjects were studied: 20 Roux-en-Y gastric bypass patients with sampling of both SAT and VAT for flow cytometric analysis of T-cell subsets (n=10) and/or rtPCR of T-cell associated cytokines (n=10), and 27 nonsurgical overweight/moderately-obese subjects with sampling of SAT alone for similar analyses (Supplementary Table 1). Thirteen nonsurgical subjects underwent collagenase digestion of fresh adipose tissue for flow cytometric analysis of T-cell subsets: six of these subjects plus an additional 14 frozen tissue specimens from subjects meeting study criteria and harvested in an identical manner were included in the rtPCR analyses of T-cell associated cytokine expression. Same-day peripheral blood for flow cytometry was obtained from all subjects who underwent flow-cytometric analysis of adipose tissue. Using the staining and gating strategies depicted in Supplementary Figure 1, we determined relative frequencies of T-cell subsets in SAT, VAT and peripheral blood. Primary analyses were of two types: 1) comparison of T-cell subsets and relevant cytokines in SAT vs VAT depots from gastric bypass patients who provided tissue from both depots (Tables 1 and 2), including relationships between fat depots and peripheral blood T cells (Figure 2) and plasma hsCRP, a marker of systemic inflammation, (Figure 3); 2) measurement of the association between T-cell subsets/cytokines and systemic insulin resistance, which was quantified in all nonsurgical subjects but only approximately half of the bariatric surgery subjects, who were reluctant to undergo time-intensive metabolic testing prior to their surgery (Figure 4).
Table 1.
Flow cytometric analysis of T-cell subsets in peripheral blood mononuclear cells (PBMC), subcutaneous (SAT), and visceral (VAT) adipose tissue in bariatric surgery subjects
| T-cell subset | PBMC (n=10) | SAT (n=10) | VAT (n=10) | p-value* SAT vs VAT | p-value*,a,b,c PBMC vs SAT and/or VAT |
|---|---|---|---|---|---|
| CD4 T cells (%total T) | 61.9 ± 15 | 59.6 ± 14.1 | 53.6 ± 12.2 | 0.008 | c |
| Th1 (%CD4 T) | 18.4 ± 13 | 47 ± 19 | 63.0 ± 5 | 0.008 | c |
| Th2 (%CD4 T) | 0.63 ± 0.6 | 1.9 ± 1.1 | 1.4 ± 0.8 | 0.15 | a |
| Th17 (%CD4 T) | 0.53 ± 0.5 | 3.5 ± 1.9 | 5.6 ± 3.2 | 0.013 | c |
| Treg (%CD4 T) | 1.4 ± 1.0 | 2.2 ± 2.7 | 3.8 ± 4.3 | 0.42 | NS |
| CD8 T cells (%total T) | 32.9 ± 13.8 | 30.7 ± 9.6 | 38.4 ± 6.8 | 0.87 | NS |
| IFNγ+ CD8 cells (%CD8 T) | 30.9 ± 19.3 | 69.2 ± 15.6 | 81.3 ± 7.0 | 0.04 | c |
Paired Student’s t-test
p<0.05 for PBMC vs SAT
p<0.05 for PBMC vs VAT
p<0.05 for PBMC vs both SAT and VAT
Table 2.
Relative expression of T-cell associated cytokines quantified by real-time polymerase chain reaction in subcutaneous (SAT) and visceral (VAT) adipose tissue from bariatric surgery subjects
| Cytokine | SAT (n=10) | VAT (n=10) | p-value* SAT vs VAT |
|---|---|---|---|
| IL-6 | 1.3 ± 1.2 | 2.7 ± 1.6 | 0.02 |
| IL-10 | 1.3 ± 0.9 | 0.32 ± 0.32 | 0.08 |
| IL-17 | 1.09 ± 0.54 | 3.63 ± 3.62 | 0.04 |
| IFN-γ | 1.65 ± 1.9 | 2.2 ± 1.6 | 0.57 |
Paired Student’s t-test
Figure 2.
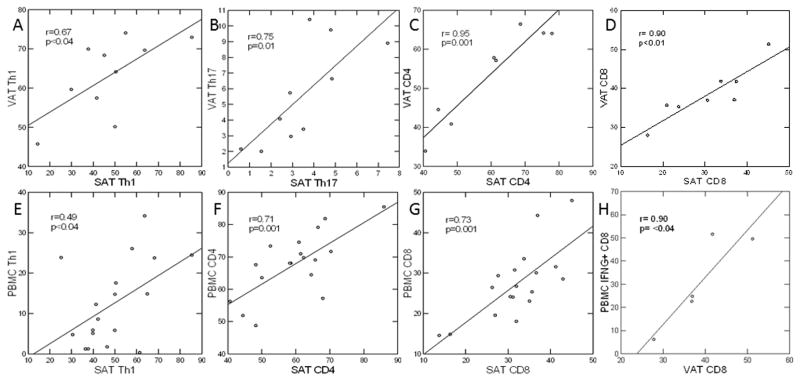
BMI-adjusted correlations between Th subsets, expressed as percentage of CD4 cells, in SAT and VAT (A–D) and between Th subsets in adipose tissue and PBMC (E–H). Due to limited cell quantity not all surface markers could be measured on every sample.
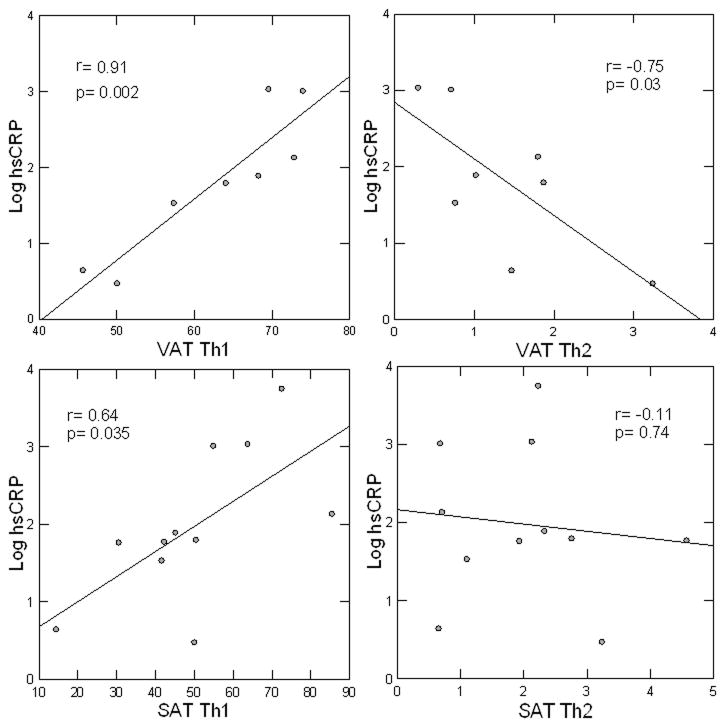
Figure 3.
BMI-adjusted correlations between log plasma hsCRP (mg/dL) and Th subsets, expressed as percentage of CD4 cells in SAT and VAT. Due to limited cell quantity not all surface markers could be measured on every sample.
Figure 4.
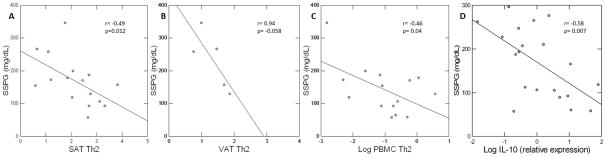
BMI-adjusted correlations between Th2, expressed as percentage of CD4 cells, in SAT (A) and VAT (B) and insulin resistance (SSPG), and between SAT IL-10 expression and SSPG (C). Due to limited cell quantity not all surface markers could be measured on every sample.
Differences in T cell frequency according to regional fat depot
Pro-inflammatory Th1, Th17 and IFNγ+ CD8 cells, expressed as % of total CD4 or CD8 T-cells, were markedly increased in VAT relative to SAT, and in both adipose compartments relative to PBMC (Table 1). This finding can also be seen in Figure 1, depicting intense staining for both CD4 and CD8 in the VAT of an IR subject as compared to SAT. Interestingly, CD4 and CD8 density in a comparable VAT sample of an IS control with similar BMI was much less dense. Although there was no significant difference in anti-inflammatory %Treg between SAT, VAT and PBMC, the relative frequency of anti-inflammatory Th2 cells was significantly higher in SAT, but not VAT, as compared to PBMC. T-cell profiles in SAT and VAT were highly correlated with each other with respect to Th1, Th17, CD4, and CD8 cell frequency (Figure 2A–D), but not Th2 (r=0.46, p=0.19) or Treg (r=−0.13, p=0.76). Of note, the frequency of Th1 in both adipose tissue depots was 10–20-fold greater than that of Th2, Treg, and Th17. Given the robust correlations between T cell subsets in SAT and VAT, we considered the possibility that T cell profiles might be reflected in peripheral blood. In support of this idea, strong correlations were seen between PBMC and SAT for Th1, CD4, and CD8 T-cell frequency, and despite the small number, between PBMC and VAT for CD4 (Figure 2E–H) and CD8 frequency (r=0.82, p=0.09, not shown). Neither Th2, Treg, nor Th17 in PBMC were correlated with frequency in SAT or VAT, confirming that observed correlations were not an artifact from blood contamination of tissue. To assess expression of Th cytokine genes in adipose tissue, we performed rtPCR on VAT and SAT from 10 healthy bariatric subjects (Table 2). Expression of IL-6, a marker of Th1 and M1 macrophage activation, was 2-fold greater in VAT than SAT (p=0.02), and IL-17, a marker of Th17 activation (also pro-inflammatory), was 3-fold greater in VAT than SAT (p=0.04). IL-10, a marker of Th2 and M2 activation and potential mediator of insulin sensitivity, was 4-fold lower in VAT versus SAT (p=0.08).
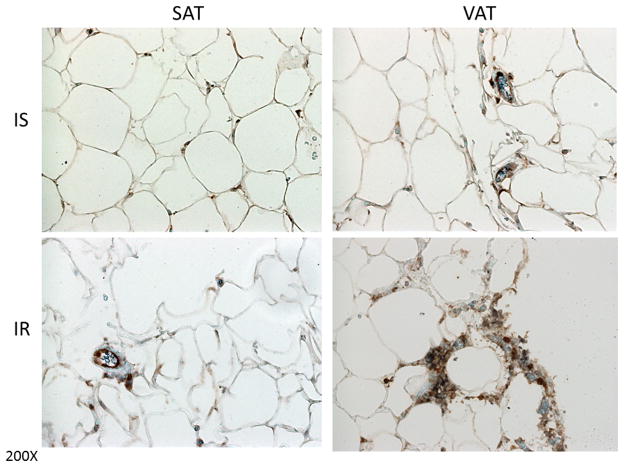
Figure 1.
Representative histology of CD4 and CD8 T cells in human subcutaneous (SAT) and visceral (VAT) adipose tissue in insulin-sensitive (IS) and insulin-resistant (IR) subjects. Brown indicates CD4-positive cells whereas blue indicates CD8-positive cells. Images are shown at 200× magnification. As indicated in the right panel, VAT from the IR subject has more CD4 and CD8 cells compared to the IS subject. IS subject is a 60 yr Caucasian female, BMI 41 kg/m2, SSPG 129 mg/dl, plasma IL6 8.8 pg/ml; IR subject is a 56 yr Caucasian female, BMI 47.3 kg/m2, SSPG 346 mg/dl, and plasma IL6 28.3 pg/ml.
T-cell phenotype: relationship to circulating inflammatory markers
To ascertain whether Th profiles in adipose tissue were associated with systemic inflammation, correlations between Th1 and Th2 in SAT and VAT were performed with respect to both plasma hsCRP and IL-6 concentrations in our cohort. Th1 in VAT was robustly associated with plasma hsCRP (r=0.91, p=0.002), whereas Th2 was inversely associated with plasma hsCRP (r= −0.75, p=0.03) (Figure 3). Th1, but not Th2, in SAT was also strongly associated with hsCRP (r= 0.64, p=0.035). Frequencies of the other CD4 and CD8 cells were not associated with hsCRP. Results were unchanged after adjustment for sex. These results point to VAT as having a particularly strong association with systemic inflammation, with strong bidirectional relationships between Th1 and Th2 and plasma hsCRP. Th1, but not Th2, frequency in SAT was also correlated with IL-6 concentrations in peripheral blood (Supplementary Figure 2) (r=0.65, p=0.03, n=15). A similar trend was also demonstrated for Th1 in VAT although sample size was too limited to draw firm conclusions (r=0.86, p=0.38, n=4).
T-cell subsets: relationship to insulin resistance
To ascertain the relationship between Th profiles and systemic insulin resistance, we quantified insulin-mediated glucose uptake using the modified insulin suppression test (see online data supplement for detailed method), denoted by the steady-state plasma glucose (SSPG), in subjects who were willing. While studies in obese mice have shown that pro-inflammatory Th1 and CD8 cells play important roles in the adipose tissue inflammation that underlies insulin resistance6,8, we found no correlation between insulin resistance and the percentages of Th1, CD8, or Th17 cells in SAT, VAT or PBMC. Also in contrast to mice studies13, anti-inflammatory Treg frequency in SAT and VAT was not significantly associated with SSPG (data not shown). On the other hand, despite lower overall frequency in both SAT and VAT than pro-inflammatory Th1, the percent of anti-inflammatory Th2 was inversely correlated with insulin resistance (Figure 4A–B). Th2 in PBMC was also significantly inversely associated with SSPG (Figure 4C). Thus, the results indicate that Th2 bias is independently inversely associated with insulin resistance.
To further explore the relationship between Th2 and insulin resistance, we measured the relative expression of IL-10, a Th2-secreted cytokine that potently inhibits proinflammatory cytokines and has also been shown to enhance whole-body insulin sensitivity in mice11. Relative expression of this cytokine in the SAT of 20 moderately-obese, BMI-matched subjects was significantly inversely associated with SSPG (p=0.007) (Figure 4D), supporting the importance of an anti-inflammatory bias in adipose tissue with regard to protection from systemic insulin resistance. IL-10 is also secreted by cells other than Th2, and the stimulus to and source of IL-10 in adipose tissue are worthy of further investigation.
Discussion
This study shows, for the first time, that there exists an abundant infiltrate of Th1 and Th2 CD4 cells, as well as IFN-γ+CD8 T cells, in the subcutaneous and visceral adipose tissue of healthy overweight and obese individuals, and confirms prior studies demonstrating Th17 cells are present in human fat. Importantly, results demonstrate that subpopulations of T cells differ according to regional fat depot and are associated with both systemic inflammation and insulin resistance. In our healthy overweight/obese humans, pro-inflammatory Th1, Th17 and IFNγ+ CD8 cells were markedly increased in VAT relative to SAT and peripheral blood. Despite these differences, proinflammatory T-cell frequencies in VAT correlated highly with SAT, and with peripheral blood, indicating that triggers to the adaptive immune response occur in multiple adipose tissue depots, and may even be systemic. Th1 and Th2 cells in VAT bore robust bidirectional relationships to plasma hsCRP, a systemic inflammatory marker that indicates increased risk for both cardiovascular disease and type 2 diabetes. Further, Th1 frequency in SAT was associated with both plasma hsCRP and IL-6 concentrations. Finally, and perhaps most importantly, Th2 frequency in adipose tissue and peripheral blood correlated inversely with insulin resistance, indicating a protective effect. Adipose tissue expression of IL-10, a Th2-related cytokine was also highly inversely associated with insulin resistance. These relationships were independent of obesity per se, and thus the relative dominance of Th1 versus Th2 responses in adipose tissue may account in part for the metabolic heterogeneity that is now well described in overweight/obese humans18.
The current findings represent an important extension to currently published data on the potential role of inflammatory cells as mediators of insulin resistance. While the majority of studies investigating inflammation and insulin resistance have focused on macrophages, three recent studies in mice6,8,13 demonstrated an important role for CD4 and CD8 lymphocytes in the development of insulin resistance. We previously demonstrated that CD4 T cells in adipose tissue promote insulin resistance in diet-induced obese mice via dramatic increases in pro-inflammatory Th1 over static numbers of anti-inflammatory Treg and Th2. CD4+ T cell transfer, predominantly through Th2 cells, reversed insulin resistance, supporting a causal role. In addition, treatment of obese WT and ob/ob (leptin-deficient) mice with CD3-specific antibody or its F(ab′)2 fragment reduced the predominance of Th1 cells over Treg cells, reversing insulin resistance for months6. Two other studies in mice have shown that Treg and CD8 T cells play opposing roles in adipose tissue inflammation and insulin resistance13,8, and another showed that obesity was associated with selective expansion of the pro-inflammatory Th17 sublineage14. Importantly, two of these studies6,13 demonstrated very little heterogeneity of the T-cell receptor repertoire in VAT, implying localized antigenic stimulation and expansion of antigen-specific T cells. This is plausible, as it is known that hypertrophic or hypoxic adipose cells become necrotic, releasing cellular contents and/or secreted molecules into the extracellular space which serve as antigens to T cells2,4.
Despite the impressive findings in rodent studies, human data on the role of T cells in adipose tissue is scant and there are no studies addressing their potential relationship to insulin resistance. To our knowledge, there are only three published studies demonstrating CD4 T cell subsets in human adipose tissue using flow cytometry. The first, which examined SAT only, demonstrated increased prevalence of Th17 and Th22 cells in metabolically-abnormal versus metabolically-normal obese individuals15. In this study, CD4 T cells staining for IL13 (Th2) and IFN-γ (Th1) were not associated with metabolic profile. It is important to note that the BMI of the metabolically-abnormal versus metabolically-normal obese subjects in this study was 43.8 versus 34.9 kg/m2, which does not rule out the possibility that obesity per se contributed to the findings. Furthermore, T cells were expanded in culture for two weeks prior to flow cytometric analysis, which leaves open the possibility that T-cell phenotypes were altered by the culture process. The second study,17 which compared SAT and VAT of morbidly-obese diabetic and nondiabetic patients with SAT of non-obese controls, also showed increased frequency of IL17 and IL22-secreting T cells in obese subjects with or without diabetes as compared to the nonobese controls. Furthermore, exposure of CD4 T cells to macrophage-derived or recombinant IL1B increased production of IL17 and IL22, suggesting cross-talk between immune cells in adipose tissue. The third study demonstrated increased Th17 in VAT of overweight/obese as compared with lean women18. Like the first study, these results cannot exclude the possibility that alterations in T-cell phenotypes were due to obesity per se, since the comparisons were made between largely different BMI groups. Beyond these reports, there is one published study documenting the presence of CD4 cells in human fat via immunohistochemistry19, and one showing a relative increase in gene expression of FOXP3, a marker for anti-inflammatory Treg cells, in SAT as compared with VAT13.
Indirect support for our finding that adipose tissue Th subsets are associated with human insulin resistance is found in one published study demonstrating the presence of both classically-activated pro-inflammatory macrophages and alternatively-activated anti-inflammatory macrophages in human adipose tissue, with associations between the ratio of pro-to-anti-inflammatory macrophages and insulin resistance as measured by the homeostasis model assessment of insulin resistance (HOMA-IR)20. While the signals responsible for macrophage activation in adipose tissue have not been clearly elucidated, we have shown in mice that pro-inflammatory Th1 cells stimulate macrophage activation via secretion of IFN-γ6. Thus, it is conceivable that localized T-cell activation in adipose tissue is a primary event, which results in macrophage recruitment and activation. This is consistent with findings in mice fed a high-fat diet, in which T-cell infiltration into VAT and insulin resistance were observed at 5 weeks but macrophage recruitment was not observed until 10 weeks of feeding19. T cell secreted cytokines may also directly impair insulin action in target tissues. Inflammatory cytokines (eg TNF-α) elaborated by activated T-cells directly impair insulin-mediated glucose uptake via stimulation of IKKB and JNK1. Other cytokines, such as IL-10, secreted by anti-inflammatory T cells, are associated with enhanced insulin sensitivity and protection from inflammation in mice5. Our results support a protective role for IL-10, and are the first to demonstrate an association between expression of this cytokine in human adipose tissue and systemic insulin sensitivity.
In the context of accumulating data implicating inflammation as causal in the development of atherosclerotic vascular disease and type 2 diabetes, the potential role of T cells in determining systemic inflammation is of great interest. The current results show that T-cells in VAT, both CD8 and CD4, adopt a proinflammatory phenotype as compared to SAT. Furthermore, the frequencies of Th1 and Th2 in VAT are bidirectionally and robustly associated with circulating hsCRP, an inflammatory marker predictive of both type 2 diabetes and atherosclerotic heart disease. hsCRP is secreted by the liver in response to stimulation by IL-6, a classic proinflammatory cytokine. Given the proximity of VAT to the liver, which is the main source of hsCRP, it is plausible that inflammatory immune cells in VAT provide a particularly potent stimulus to hsCRP secretion, via direct cytokine release into the portal vein. In the current study, expression of IL-6, secreted by Th1 and pro-inflammatory macrophages, was significatntly higher in VAT than in SAT, supporting the hypothesis that proinflammatory immune cells in VAT play a particularly important role in determining hsCRP release by the liver. Whether inflammation in VAT is a stronger determinant of systemic inflammation than is SAT is an important question, and whereas prior clinical studies show that abdominal and visceral fat mass bear modestly stronger independent associations with plasma hsCRP than total adiposity21,22, this is the first study to characterize immune cell phenotypes in VAT with respect to systemic inflammation. Additional data supporting a role of T-cells in determining systemic inflammation are found in an increasing number of studies showing that the balance of pro- to anti-inflammatory T-cells is associated with development of atherosclerotic plaque23.
Our study is limited by the relatively small number of pre-surgical subjects willing to undergo comprehensive insulin-resistance testing (SSPG) and the inability to perform comprehensive flow cytometric analysis for all T cell subsets on every tissue sample. Furthermore, as in most human studies, it is difficult to prove causality. In particular, proving that Th2 cells protect humans from insulin resistance would require selective manipulation of Th2 cells, in vivo, which is not feasible. Considering the current findings together with those from our prior DIO mice studies, in which transfer of Th2 cells protected against the development of insulin resistance6, the current results are highly suggestive of a similar relationship in humans.
In summary, the current findings extend previous studies in mice, and add to the small but growing body of literature in humans implicating T lymphocytes in obesity-related insulin resistance and type 2 diabetes. Not only are Th1 and Th2 CD4 cells, as well as IFN-γ+CD8 T cells, abundantly present in the VAT and SAT of healthy overweight and obese humans, but the balance of pro- to anti-inflammatory subtypes is associated with systemic inflammation and insulin resistance. The high frequencies of Th1, Th2 and Th17 cells in two separate adipose tissue depots is consistent with antigenic stimulation in these tissues, and indicates a widespread effect. Thus, these results provide compelling evidence for a role of the adaptive immune system in systemic inflammation and insulin resistance in overweight/obese humans. Identification of antigens and other key components of this process may ultimately prove useful in the prevention and treatment of obesity-associated metabolic diseases.
Supplementary Material
Significance.
The biological mechanisms linking obesity to insulin resistance are not fully elucidated. It has been suggested that inflammation in adipose tissue may play a role: pro-inflammatory cytokines and macrophages have been identified in human adipose tissue, and overfeeding mice shifts macrophage phenotype in adipose tissue from anti- to pro-inflammatory. Other types of immune cells may also potentiate localized inflammation and insulin resistance. In mice, the ratio of pro- to anti-inflammatory T-helper (Th) cells in adipose tissue increases with weight gain, and blocking their activity prevents obesity-induced insulin resistance. Here, we show for the first time that Th1(pro-inflammatory) and Th2 (anti-inflammatory) cell frequency in human subcutaneous and visceral adipose tissue correlate with systemic inflammation and insulin resistance. These results extend findings in mice to humans, and provide strong support for a role of the adaptive immune system in mediating obesity-induced inflammation and insulin resistance.
Acknowledgments
All authors contributed to the manuscript.
Sources of Funding
Grant support for this study was provided by National Institutes of Health/National Institute of Digestive Diseases and Diabetes, R01DK080436, R01DK071309, DK096038. Clinical Trials Identifier: NCT00285844.
Abbreviations
- BMI
body mass index
- hsCRP
high sensitivity c-reactive protein
- IL
interleukin
- PBMC
peripheral blood mononuclear cells
- rtPCR
real-time polymerase chain reaction
- SAT
subcutaneous adipose tissue
- SSPG
steady-state plasma glucose
- Th
T-helper
- Treg
T-regulatory
- VAT
visceral adipose tissue
Footnotes
Disclosures
There are no conflicts of interest to disclose.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzshneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg S, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg A, Obin M. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winer S, Chan Y, Paltsr G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nature Med. 2009;15:1–10. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc. 2011;70:408–417. doi: 10.1017/S0029665111000565. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 9.Odegaard JI, et al. Macrophage-specific PPARg controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiemesen MM, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong EG, Ko HJ, Cho YR, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park-Min KH, Antoniv TT, Ivashkiv LB. Regulation of macrophage phenotype by long-term exposure to IL-10. Immunobiology. 2005;210:77–86. doi: 10.1016/j.imbio.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Shosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T-cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–38. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 15.Fabbrini E, Cella M, McCartney SA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–374. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in prevalence of risk factors for cardiovascular disease and type 2 diabetes in obese individuals: impact of differences in insulin sensitivity. Archives Int Med. 2007;167:642–8. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 17.Dalmas E, Venteclef E, Caer C, Poitou C, Cremer I, Aron-Winewsky J, Lacroix-Desmazes, Bayry J, Kaveri SV, Clement K, Andre S, Guerre-Millo M. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014 doi: 10.2337/db13-1511. published ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Bertola A, Ciucci T, Rousseau D, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kintscher, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue. Artereoscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 20.Wentworth JM, Naselli G, Brown WA, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pou KM, Massaro JM, Hoffmann U, Vasan RS, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 22.Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol. 2010;106:56–61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–58. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.