Abstract
Context
Eye contact is a fundamental component of human social behavior. Individuals with fragile X syndrome (fraX), particularly male subjects, avoid eye contact and display other social deficits. To date (to our knowledge), this behavior in fraX has been studied only in female subjects, who show lesser degrees of gaze aversion.
Objective
To determine the neural correlates of the perception of direct eye gaze in adolescent boys with fraX using functional magnetic resonance imaging.
Design
Cross-sectional study.
Setting
Academic medical center.
Participants
Thirteen adolescent boys with fraX, 10 boys with developmental delay, and 13 typically developing control subjects.
Main Outcome Measures
Behavioral performance and brain activation during functional magnetic resonance imaging were evaluated during the presentation of faces with eye gaze directed to or averted away from subjects and during successive presentations of stimuli with eye gaze directed toward the subject. Whole-brain and region of interest analyses and regression analyses with task performance were performed.
Results
Significantly greater activation was observed in prefrontal cortices in controls compared with boys having fraX, who (in contrast) demonstrated elevated left insula activation to direct eye gaze stimuli. Furthermore, compared with controls, boys with fraX showed greater sensitization in the left amygdala with successive exposure to direct gaze.
Conclusions
Compared with controls, boys with fraX display distinct patterns of brain activation in response to direct eye gaze. These results suggest that aberrant neural processing of direct eye gaze in subjects with fraX may be related to the associated avoidant response.
The maintenance and regulation of eye gaze during social interactions represent a fundamental nonverbal cue that we use to express intimacy, acknowledge hierarchy, regulate interactions, exercise social control, and facilitate communication to obtain services or achieve goals.1,2 A disruption in the ability to maintain, regulate, and perceive eye gaze has a profound influence on social development.3
A striking characteristic of individuals with fragile X syndrome (fraX) is their propensity to avoid direct eye gaze during social interactions. Fragile X syndrome, the most common known form of inherited neurodevelopmental disorder, affects approximately 1 in 4000 male subjects and 1 in 8000 female subjects and is caused by a mutation to a single gene at Xq27.3.4,5 The mutation reduces production of the fragile X mental retardation protein (FMRP), which has been shown to be involved in the regulation of synaptic plasticity and function.6 Although the disorder affects both sexes, male subjects with fraX usually have more serious manifestations of the syndrome because the mutation occurs on their single X chromosome. Behavioral investigations of individuals with fraX have shown that eye gaze aversion occurs approximately 80% of the time in male subjects and 60% of the time in female subjects during social interactions,7 and male subjects with fraX demonstrate a distinct greeting behavior of a turned upper body and direct gaze avoidance.3,8–10
Neuroimaging studies of eye gaze processing in individuals with fraX may allow investigators to identify links among gene, brain, and social behavior. Although gaze aversion and the unusual greeting behavior are characteristic behavioral features of male subjects with fraX, the brain basis of gaze perception has not been studied in this group of individuals, to our knowledge. A study10 conducted in our laboratory investigated the neural correlates of gaze processing in female subjects with fraX and found decreased activation in the superotemporal gyrus (STG) when viewing faces with direct vs averted gaze compared with age-matched control subjects. Another study11 examined male subjects with autism, a population also noted for eye gaze avoidance, and found positive correlations between time spent fixating on the eyes and activation in the fusiform gyrus and amygdala.
Structural neuroimaging studies12–14 indicate that individuals with fraX have aberrant morphologic structure of the fusiform gyrus, amygdala, and STG. Altered structure (and consequently function) of these brain areas has potential neurobiologic relevance for understanding gaze aversion in fraX. In particular, the fusiform gyrus, amygdala, and superotemporal sulcus comprise important elements of the neural circuitry underlying social cognition as related to processing of faces, emotion, and gaze, respectively. Other regions implicated in the neural basis of anxiety such as the insula, as well as frontal areas involved in emotion regulation such as the dorsolateral prefrontal cortex (DLPFC), are of particular interest to this study.4,15–25
In the present study, adolescent boys with fraX were compared with 2 control groups, typically developing (TD) adolescent boys and adolescent boys having developmental delay (DD) without fraX matched for age, IQ, and performance on a gaze perception task. The critical and novel comparison with the developmentally delayed control group provides insight as to whether aberrant neural activation observed in fraX is simply a function of general DD rather than specific to fraX. Based on converging data from behavioral and imaging studies of fraX, as well as emerging research on animal models, we predicted that participants with fraX in the present study would show abnormal activation profiles in brain systems related to gaze processing, arousal, and anxiety, as well as regions that modulate emotional responses and saccade activity, relative to the 2 control groups.
METHODS
SUBJECTS
Thirty-six boys participated in this study, including 13 with fraX (mean [SD] age, 15.5 [2.4] years), 10 with DD matched for age and IQ (16.1 [3.3] years), and 13 TD individuals matched for age (15.0 [2.5] years). Detailed demographic information is given in Table 1. Multivariate analysis of variance (MANOVA) and Bonferroni post hoc tests corrected for multiple comparisons were performed to examine any significant effects of diagnosis on the dependent variables of age and IQ. All groups were matched for chronologic age, and the fraX and DD groups were also matched for IQ. Written informed consent and assent were obtained from parents and participants, respectively. This study was approved by the Stanford University Administrative Panel on Human Subjects in Medical Research.
Table 1.
Demographics and Behavioral Results
| Variable | Mean (SD)
|
||
|---|---|---|---|
| TD Group (n=13) | fraX Group (n=13) | DD Group (n=10) | |
| Age, mean (SD) y | 15.0 (2.5) | 15.5 (2.4) | 16.1 (3.3) |
| WISC full-scale IQ, mean (SD) | 116.8 (11.6) | 61.0 (14.8) | 62.4 (9.4) |
| Accuracy, % | |||
| DirectGz | 93.1 (8.1) | 68.5 (18.9) | 72.2 (18.5) |
| AvertedGz | 95.6 (5.7) | 58.0 (25.3) | 65.9 (35.7) |
| RT, ms | |||
| DirectGz | 863.6 (157.9) | 929.1 (174.6) | 1028.9 (122.4) |
| AvertedGz | 931.1 (189.7) | 935.9 (246.5) | 1041.4 (112.3) |
| Accuracy, % | |||
| DirectGz-1 trial | 92.7 (9.0) | 67.7 (20.6) | 71.5 (18.9) |
| DirectGz-2 trials | 94.2 (10.0) | 75.4 (17.1) | 72.5 (26.0) |
| RT, ms | |||
| DirectGz-1 trial | 863.0 (148.2) | 924.6 (153.0) | 1023.2 (112.3) |
| DirectGz-2 trials | 860.3 (171.3) | 868.3 (156.8) | 975.8 (135.0) |
Abbreviations: AvertedGz, all conditions with averted gaze (face forward with averted gaze and averted face with averted gaze combined); DD, developmental delay; DirectGz, all conditions with direct gaze (forward face with direct gaze and averted face with direct gaze combined); fraX, fragile X syndrome; RT, response time; TD, typically developing; WISC, Wechsler Intelligence Scale for Children.
BEHAVIORAL ASSESSMENT AND GROUP CRITERIA
Intellectual functioning was assessed using the Wechsler Intelligence Scale for Children, Third Edition,26 for participants younger than 17 years and the Wechsler Adult Intelligence Scale, Third Edition,27 for participants 17 years and older. All diagnoses of fraX were confirmed by Southern blot analysis,28,29 and levels of FMRP were calculated based on methods described by Willemsen et al.30 Criteria for inclusion of individuals with DD were a negative diagnosis of fraX (based on DNA testing), a full-scale IQ score of less than 85, and a score of less than 15 on the Autism Screening Questionnaire.31 Participants with TD were included if they had a full-scale IQ score of between 90 and 130 and were free of significant psychiatric or behavioral problems based on a T score within 1 SD of the mean of a normative standardized sample on the Child Behavior Checklist for ages 4 to 18 years32 and on the Symptom Checklist 90–Revised for ages 16 years and older.33 Participants with TD were also selected to age match those in the fraX group. All subjects were noted to be right-handed except 1 individual with fraX who was left-handed and 3 participants with DD who did not complete a handedness assessment. Subjects with TD were excluded from the study if they took psychiatric medications. All psychiatric medications taken by participants having diagnoses of DD and fraX were preapproved for the study by a psychiatrist (A.L.R.) and included selective serotonin reuptake inhibitors (5 subjects with fraX and 1 subject with DD), norepinephrine and dopamine reuptake inhibitors (2 subjects with fraX and 1 subject with DD), methylphenidate hydrochloride (2 subjects with fraX and 3 subjects with DD), risperidone (1 subject with fraX), and divalproex (1 subject with fraX). Subjects were asked to discontinue stimulant medications 24 hours before imaging; however, 1 participant with DD was unable to comply for safety reasons.
TASK AND STIMULI
The functional magnetic resonance (fMR) imaging task was an event-related jittered design with 4 experimental conditions and a resting baseline. The 4 conditions were a combination of face and gaze directed toward or away from the subject as follows: (1) forward face with direct gaze (FD), (2) forward face with averted gaze (FA), (3) averted face with direct gaze (AD), and (4) averted face with averted gaze (AA). Stimuli consisted of color face photographs of 120 college-aged models with neutral facial expression against a common solid-color background taken at a distance of about 2 m (male to female ratio, 6.6:5.4; white to nonwhite race/ethnicity ratio, 9.3:2.7). Averted faces and gaze were turned approximately 45° away. The sex and race/ethnicity of the models were distributed similarly across the 4 conditions. There was a total of 30 trials per condition.
Each stimulus was presented for 1750 milliseconds, followed by a 250-millisecond duration fixation cross. The mean intertrial interval was 1570 milliseconds (range, 250–4250 milliseconds). For each trial, subjects were instructed to use the right index finger to press a button if the person in the photograph was looking at them and to use the right middle finger to press an adjacent button if the person was looking away from them, irrespective of face direction. Correct and incorrect responses and response times (RTs) were recorded if they occurred between 150 and 2000 milliseconds after the stimulus presentation. There were 2 runs, with each run lasting 4 minutes and 32 seconds. Behavioral measures for 1 participant with TD and for 2 participants with DD were unavailable because of a computer malfunction.
Task performance was assessed by percentage correct and RT for DirectGz (all conditions with direct gaze [FD and AD combined]) and for AvertedGz (all conditions with averted gaze [FA and AA combined]). Furthermore, to examine the effect of successively viewing stimuli with direct or averted gaze (relevant to our fMR imaging analysis of adaptation and sensitization, described in the “fMR Imaging Data Analysis” subsection of the “Methods” section), we calculated accuracy and RT for DirectGz stimuli when trials were not repeated (DirectGz-1 trial) or were repeated (DirectGz-2 trials). MANOVA and Bonferroni post hoc tests, to correct for multiple comparisons, were performed.
IMAGE ACQUISITION
All subjects participated in mock imaging to familiarize them with the imaging procedures. Images were acquired on a 1.5-T whole-body MR system (General Electric Signa; GE Medical Systems, Milwaukee, Wisconsin) located at the Lucas Center for Magnetic Resonance Spectroscopy and Imaging (Stanford University). The entire supratentorial brain was imaged at each of 272 time points. Eighteen axial-oblique sections (6 mm thick, 1 mm skip) were prescribed parallel to the anterior-posterior intercommissural line using a T2-weighted gradient-echo spiral-pulse sequence sensitive to blood oxygen level–dependent contrast34 with the following acquisition parameters: repetition time, 2000 milliseconds; echo time, 30 milliseconds; flip angle, 89°; field of view (FOV), 24×24 cm; acquisition matrix, 64×64 pixels; and voxel size, 3.75×3.75×6 mm.
fMR IMAGING DATA ANALYSIS
The fMR image processing and statistical analysis were performed using Statistical Parametric Mapping software (SPM2; Wellcome Department of Cognitive Neurology, London, England). Images were reconstructed and realigned to the reference functional volume. Images were normalized using the mean functional volume resampled to 2×2×2-mm voxels in Montreal Neurological Institute stereotaxic space and smoothed using a gaussian kernel of 4-mm full-width at half maximum.
Statistical analyses were performed using a general linear model, adapting gaussian random fields theory. Individual subjects’ data were high pass filtered at 120 seconds, globally scaled, and analyzed using a fixed-effects model to determine areas with significantly greater activation for the contrast DirectGz>AvertedGz.
Individual contrast images were then combined to perform group statistical analysis using a random-effects model. Within-group activation maps were created using 1-sample t tests. Between-group comparisons were performed using 1-way ANOVA for the primary contrast, DirectGz>AvertedGz. When there was a significant effect, post hoc analyses were performed by extracting the appropriate contrast estimates (linear combination of beta parameters) and by examining the direction of the effects using a Bonferroni correction for between-group comparisons and 1-sample t tests within each diagnostic group. This approach was performed to examine whether each group shows significantly greater activation for DirectGz compared with AvertedGz or vice versa, which cannot be discerned from the ANOVA.
To investigate the effects of successive presentations (DirectGz-1 trial and DirectGz-2 trials), we examined brain regions that demonstrated decreased (which we define as adaptation) or increased (which we define as sensitization) activation with repetition of DirectGz stimuli. Although the underlying neural mechanism is not completely understood, evidence suggests that this technique may be useful in elucidating dynamic changes in regional activation associated with specific perceptual and cognitive processes (eg, using this technique, others have shown that limbic regions fail to adapt to specific repeated emotion-related stimuli, while the fusiform gyrus, on the other hand, adapts to repeated face stimuli).35,36 Therefore, we performed adaptation and sensitization analyses to determine if individuals with fraX show abnormal repetition effects for directed, but not averted, gaze stimuli within brain regions associated with arousal, anxiety, or gaze perception. For each subject, we modeled the number of repetitions of DirectGz stimuli as stick functions (http://www.poldracklab.org/teaching/psych254/psych254_lecture9.pdf); DirectGz-1 trials were assigned a value of 1, DirectGz-2 trials a value of 2, and intertrial intervals and non-onset images a value of 0. This series of numbers was then convolved using the hemodynamic response function to create general linear models. In this study, one-third of all trials were repeated (one-sixth of trials for each gaze direction).
Region of interest (ROI) analyses, focusing on the STG and amygdala as a priori hypothesized regions, were performed for the ANOVA for DirectGz>AvertedGz and for repetition to DirectGz. The ROIs were created using Automatic Anatomical Labeling software.37 Contrast estimates were extracted from regions that showed significant effects in the whole-brain analysis and in STG and amygdala ROIs to interrogate the nature of the effect. Post hoc tests for these ROI analyses were performed using the same method as that described for the whole-brain DirectGz>AvertedGz contrast. Regression analyses (Pearson product moment correlation r) were performed within each group to examine the associations between activation and task performance for DirectGz>AvertedGz and for the repetition effects to DirectGz.
To investigate whether the effect of repetition was specific to direct gaze, control analyses were performed similar to those suggested by Grill-Spector et al.35 Brain activation associated with repetition of other conditions, namely, AvertedGz (FA and AA) and DirectFc (FD and FA), was compared with that of the DirectGz condition.
Statistical thresholds of P<.001 uncorrected with an extent threshold of 10, as well as P<.05 small-volume correction, were used for whole-brain and ROI analyses, respectively. A threshold of P<.05 was used for other analyses based on extracted values from ROIs derived from whole-brain or a priori defined regions (ie, amygdala and STG). Coordinates of activation were converted from Montreal Neurological Institute to Talairach space using the mni2tal function (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml). The x, y, z coordinates were then located using Talairach Daemon38,39 and were confirmed using the Talairach Atlas.40
RESULTS
DEMOGRAPHIC AND BEHAVIORAL DATA
Demographics
Table 1 gives detailed demographic and behavioral results. There was no significant difference in age among the 3 groups (F2,32 = 1.04, P = .37). Subject IQ showed a main effect of group (F2,32=71.43, P <.001), which was driven by significant differences between the TD group and other groups (TD>DD and TD>fraX, P <.001 for both) but not between the DD and fraX groups (P >.99). The fraX group displayed a range of FMRP levels from 6.5% to 77%, with a mean (SD) of 23% (26.3%). The range of CGG repeats was 193 to 963. Eight of these subjects demonstrated the FMR1 full mutation (allele size >200 repeats), 4 had mosaicism for full mutation and premutation (<200 repeats) alleles, and 1 demonstrated FMR1 allele size at the boundary between full and premutation status (193 repeats).
Task Performance
Examination of task accuracy using a 3-group (TD, DD, and fraX) MANOVA, corrected for multiple comparisons, revealed a main effect of group for DirectGz trials (F2,33=8.49, P =.001) and for AvertedGz trials (F2,33=8.06, P=.002). In addition, post hoc tests showed significant differences in task accuracy between the TD group and other groups (P=.02 and P=.03 vs DD; P=.001 vs fraX) but not between the DD and fraX groups (P>.99 for all). Measures of RT for correct trials showed no significant group effects for DirectGz (F2,33=2.64, P =.09) or for AvertedGz (F2,33=0.87, P =.43).
We performed a 3-group (TD, DD, and fraX) MANOVA, corrected for multiple comparisons, to examine task accuracy as a function of the number of repetitions of directed gaze stimuli. This analysis indicated a main effect of group for DirectGz-1 trial (F2,33=7.61, P=.002) and for DirectGz-2 trials (F2,33=4.90, P =.01). Post hoc tests showed that the TD group had significantly higher accuracy compared with the other groups for DirectGz-1 trial (P =.03 for DD and P =.003 for fraX) and for DirectGz-2 trials (P =.04 for DD and for fraX), but no significant differences were observed between the fraX group and the DD group based on the number of repetitions (P >.99 for DirectGz-1 trial and DirectGz-2 trials). Measures of RT for the DirectGz-1 trial (F2,33=3.02, P =.06) and DirectGz-2 trials (F2,33=1.52, P =.24) showed no significant main effects of group.
NEUROIMAGING DATA
Brain Activation Related to the Direction of Gaze
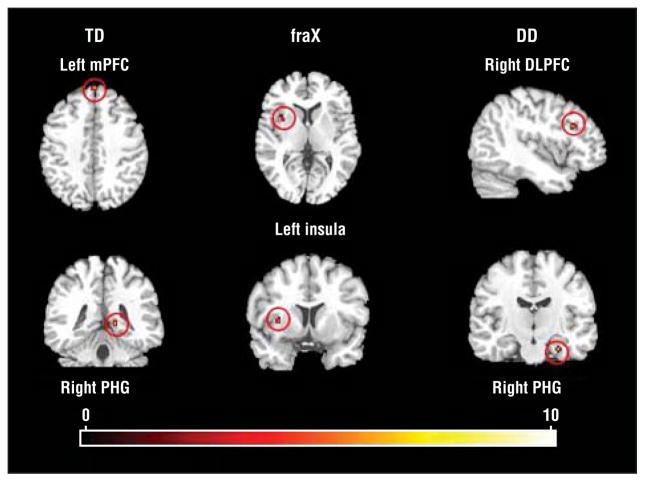
Within-group comparisons of the contrast DirectGz>AvertedGz revealed significant activation in distinct regions in the fraX, TD, and DD groups (Figure 1 and Table 2). The fraX group showed significant activation in the left anterior insula, the TD group in the right parahippocampal gyrus and the left medial frontal gyrus, and the DD group in the right midfrontal and parahippocampal gyri.
Figure 1.
Within-group results for DirectGz>AvertedGz. DirectGz is all conditions with direct gaze (forward face with direct gaze and averted face with direct gaze combined). AvertedGz is all conditions with averted gaze (forward face with averted gaze and averted face with averted gaze combined). All regions significant at P<.001 and size of 80 mm3 or greater. DD indicates developmental delay; DLPFC, dorsolateral prefrontal cortex; fraX, fragile X syndrome; mPFC, medial prefrontal cortex; PHG, parahippocampal gyrus; TD, typically developing; and red dots, increased neural activation. Color gradations indicate t scores.
Table 2.
Within-Group Whole-Brain Results for DirectGz>AvertedGz
| Variable | Brodmann Area | Talairach Coordinates
|
T Score | Volume, mm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| fraX group left insula and claustrum | Not applicable | −34 | 8 | 3 | 5.47 | 168 |
| TD group | ||||||
| Right parahippocampal gyrus | 30 | 14 | −39 | 6 | 6.51 | 88 |
| Left medifrontal gyrus | 9 | −4 | 56 | 36 | 6.67 | 88 |
| DD group | ||||||
| Right midfrontal gyrus | 9 | 40 | 23 | 25 | 9.64 | 112 |
| Right midfrontal gyrus | 9 | 34 | 30 | 24 | 5.07 | 80 |
| Right parahippocampal gyrus | 35 | 26 | −15 | −21 | 8.99 | 88 |
Abbreviations: DD, developmental delay; fraX, fragile X syndrome; TD, typically developing.
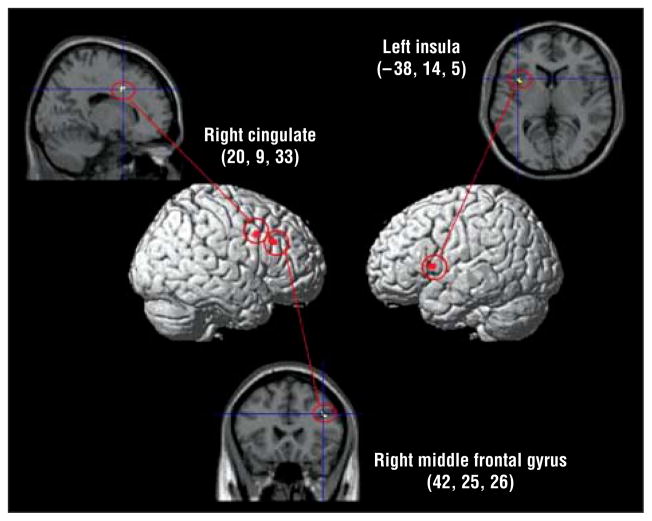
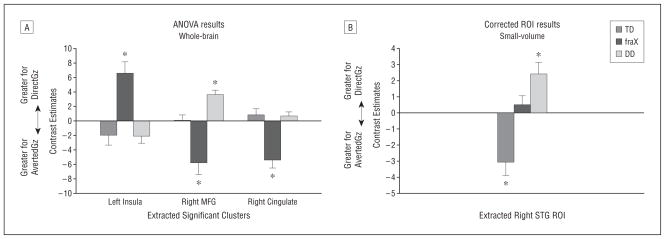
Whole-brain ANOVA for the DirectGz>AvertedGz contrast revealed a main effect of group in the right cingulate gyrus (F32 = 14.42), right midfrontal gyrus (F32=11.80), and left insula (F32=12.12) (P <.001 for all) (Figure 2 and Table 3). These 3 regions were extracted and analyzed for the direction of the effect within each region (ie, whether each region showed significantly greater activation for DirectGz, AvertedGz, or neither for each group) (Figure 3A). These results showed that the TD and DD groups had significantly greater activation than fraX group in the right midfrontal gyrus (P=.004 for TD>fraX and P<.001 for DD>fraX) and cingulate (P <.001 for both) DirectGz>AvertedGz for the contrast; however, this was due to the fraX group showing a significantly greater response to the opposite contrast, AvertedGz>DirectGz, in those regions. The fraX group demonstrated “negative” activation for the contrast DirectGz>AvertedGz (ie, greater response to AvertedGz than DirectGz) in the right midfrontal gyrus (t12=−3.54, P =.004) and cingulate (t12=−4.83, P <.001). The TD and DD groups failed to show significant differences between these 2 conditions in the cingulate (P=.34 for TD and P =.29 for DD). However, the fraX group had a significantly greater response in the left insula than the TD group (P <.001) and the DD group (P = .001) to DirectGz>AvertedGz. Activation in the left insula negatively correlated with performance on DirectGz trials for the fraX group only (r12=−0.59, P =.03 for fraX; r13=0.03, P =.93 for TD; and r9=0.13, P =.74 for DD).
Figure 2.
Whole-brain analysis of variance showing the main effects of group for DirectGz>AvertedGz. DirectGz is all conditions with direct gaze (forward face with direct gaze and averted face with direct gaze combined). AvertedGz is all conditions with averted gaze (forward face with averted gaze and averted face with averted gaze combined). All regions significant at P <.001 and size of 80 mm3 or greater. Medial regions are projected onto the lateral surface for display purposes. The numbers represent the Talairach coordinates.
Table 3.
Between-Group Results Showing the Main Effect of Group for DirectGz>AvertedGz
| Region | Brodmann Area | Talairach Coordinates
|
F Score | Volume, mm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right cingulate gyrus | 32 | 20 | 9 | 33 | 14.42 | 144 |
| Right midfrontal gyrus | 9 | 42 | 25 | 26 | 11.80 | 112 |
| Left insula | 13 | −38 | 14 | 5 | 12.12 | 104 |
Abbreviations: See Table 1 for DirectGz and AvertedGz.
Figure 3.
Contrast estimates for DirectGz>AvertedGz. DirectGz is all conditions with direct gaze (forward face with direct gaze and averted face with direct gaze combined). AvertedGz is all conditions with averted gaze (forward face with averted gaze and averted face with averted gaze combined). A, Results from analysis of variance (ANOVA) between groups at P <.001. B, Significant small-volume correction regions of interest (ROIs) at P <.05. *Within-group significance by a 1-sample t test at P <.05. Peak activation Talairach coordinates for the right superotemporal gyrus (STG) were 65, −26, and 22. DD indicates developmental delay; fraX, fragile X syndrome; MFG, midfrontal gyrus; TD, typically developing.
Among the a priori hypothesized regions (bilateral STG and amygdala), only the STG showed significant effects of group using ROI analyses (P = .05 corrected, extracted values shown in Figure 3B). Bonferroni post hoc tests showed that the TD group differed significantly from the other groups (P = .003 vs fraX and P <.001 vs DD).
Change in Activation With Successive Presentation of Direct Gaze Stimuli
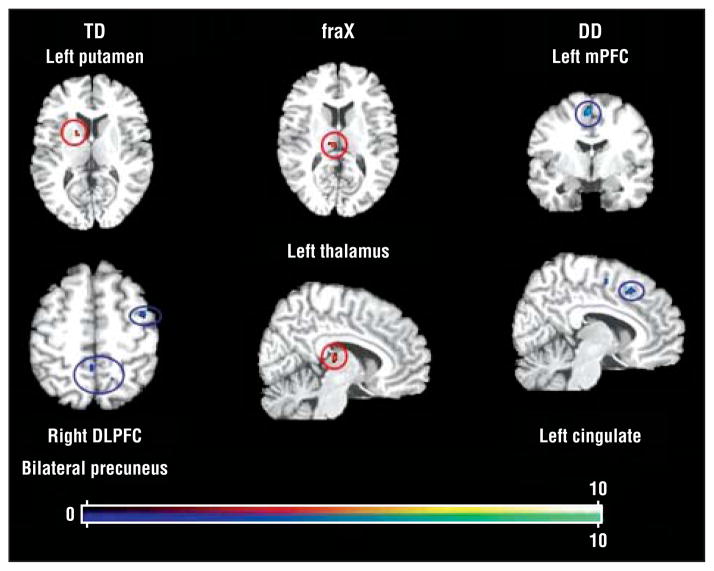
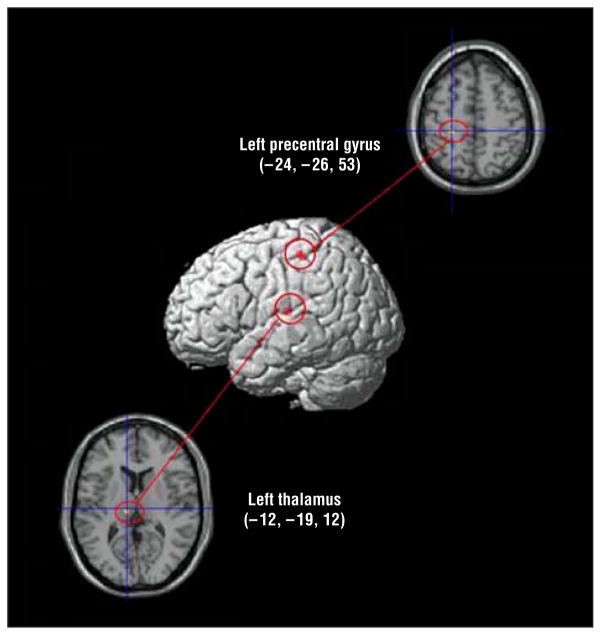
Regions that showed decreased (adaptation) and increased (sensitization) activation with successive DirectGz stimuli are summarized for each group (Table 4 and Figure 4). We found a significant main effect of group in the left precentral gyrus (F32=18.87) and in the left dorsomedial thalamus (F32=14.80) (P <.001 for both) (Table 5 and Figure 5).
Table 4.
Within-Group Whole-Brain Results for Adaptation to DirectGz and for Sensitization to DirectGz
| Variable | Brodmann Area | Talairach Coordinates
|
T Score | Volume, mm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Adaptation | ||||||
| TD group | ||||||
| Right midfrontal gyrus | 6 | 38 | 4 | 47 | 4.64 | 80 |
| Right precuneus | 7 | 15 | −52 | 49 | 5.5 | 104 |
| Left precuneus | 5 | −4 | −42 | 50 | 6.43 | 328 |
| DD group | ||||||
| Left medial frontal gyrus | 6 | −4 | −46 | 51 | 10.96 | 144 |
| Left cingulate | 32 | −10 | 20 | 41 | 6.16 | 104 |
|
| ||||||
| Sensitization | ||||||
| TD group right lentiform nucleus | Putamen | 16 | 4 | 7 | 7.36 | 96 |
| fraX group left thalamus | Dorsomedial nucleus | −10 | −19 | 12 | 5.94 | 160 |
Abbreviations: DD, developmental delay; DirectGz, all conditions with direct gaze (forward face with direct gaze and averted face with direct gaze combined); fraX, fragile X syndrome; TD, typically developing.
Figure 4.
Within-group results for repetition to DirectGz (all conditions with direct gaze [forward face with direct gaze and averted face with direct gaze combined]). All regions significant at P<.001 and size of 80 mm3 or greater. DD indicates developmental delay; DLPFC, dorsolateral prefrontal cortex; fraX, fragile X syndrome; mPFC, medial prefrontal cortex; TD, typically developing; red dots, sensitization; and blue dots, adaptation. Color gradations indicate t scores.
Table 5.
Between-Group Results Showing the Main Effect of Group for Successive DirectGz Trials
| Region | Brodmann Area | Talairach Coordinates
|
F Score | Volume, mm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left precentral gyrus | 4 | −24 | −26 | 53 | 18.87 | 112 |
| Left thalamus | Dorsomedial nucleus | −12 | −19 | 12 | 14.80 | 136 |
Figure 5.
Whole-brain analysis of variance for the main effect of group for repetition to DirectGz (all conditions with direct gaze [forward face with direct gaze and averted face with direct gaze combined]). All regions significant at P <.001 and size of 80 mm3 or greater. Medial regions are projected onto the lateral surface for display purposes. The numbers represent the Talairach coordinates.
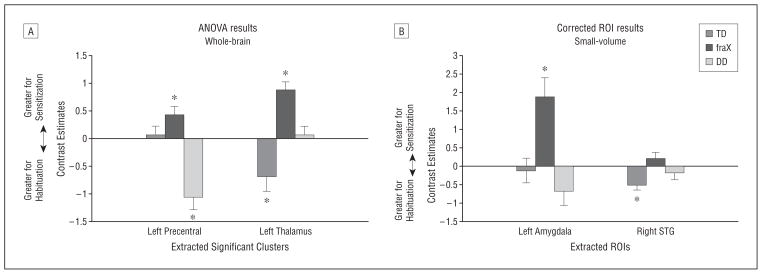
Post hoc analyses of the contrast estimates extracted from significant clusters (Figure 6A) showed the fraX group to have significantly greater sensitization in the left dorsal thalamus with successive DirectGz stimuli than the TD group (P <.001) and the DD group (P =.03). The DD group showed significantly greater adaptation than the other groups with successive DirectGz stimuli in the left precentral gyrus (P <.001 for all). When assessed using 1-sample (ie, within group) t tests, only the subjects with fraX showed significant responses (sensitization) in both regions (t12=5.75, P <.001 for thalamus and t12=2.77, P =.02 for precentral gyrus), while the TD and DD groups showed significant responses (adaptation) in 1 region each (t12 = 2.65, P = .02 for TD thalamus and t9=4.42, P =.002 for DD precentral gyrus).
Figure 6.
Contrast estimates for repetition to DirectGz (all conditions with direct gaze [forward face with direct gaze and averted face with direct gaze combined]). A, Results from between-group analysis of variance (ANOVA) at P<.001. B, Small-volume–corrected regions of interest (ROIs) at P<.05. *Within group significance by a 1-sample t test at P<.05. Peak activation Talairach coordinates for ROIs are −16, −1, and −12 for the left amygdala and 57, −42, and 13 for the right superotemporal gyrus (STG). DD indicates developmental delay; fraX, fragile X syndrome; TD, typically developing.
We then performed ROI analyses in our a priori hypothesized regions and found main effects of group in the left amygdala and in the right STG (extracted values plotted in Figure 6B). Bonferroni post hoc tests showed the fraX group to have significant sensitization in the left amygdala (P = .004). The fraX group showed a significant difference from both control groups only in the left amygdala, with greater sensitization in the fraX group than in the TD group (P =.006) or the DD group (P =.001). This region also showed significant negative correlation with performance on successive trials in the fraX group (r12=−0.56, P =.04 for DirectGz-2 trials), a trend toward a significant positive correlation in the TD group (r12=0.57, P =.05 for DirectGz-2 trials), and no relationship in the DD group (r8=0.006, P =.99).
Specificity of Adaptation and Sensitization Effects During Presentation of Direct Gaze Stimuli
Whole-brain ANOVA of AvertedGz and DirectFc conditions showed no significant adaptation or sensitization effects in the same brain regions that adapted or sensitized to DirectGz stimuli. This indicated an effect in those regions specific to DirectGz repetition.
COMMENT
This study demonstrates unique brain activation profiles in adolescent boys with fraX compared with age-matched and age and IQ–matched male control subjects when viewing photographs of individuals with direct or averted gaze and when judging gaze direction. We found that individuals with fraX showed distinct activation and sensitization patterns for processing eye gaze relative to both control groups. Individuals with fraX demonstrated greater activation to direct eye gaze in regions associated with arousal and anxiety such as the insula and amygdala. In response to averted eye gaze, the fraX group showed greater activation in prefrontal regions known to be involved in executive processes, including saccade inhibition and decision making. Examination of the effects of successive direct gaze exposure revealed sensitization in brain regions related to arousal and perception in participants with fraX, including the amygdala, dorsal thalamus, and precentral gyrus. The fraX group did not show significant adaptation in any of the brain regions we analyzed. Previous neuroimaging findings among healthy volunteers indicate that a typical response to successive exposure is to decrease activation,35,36 which was the case in our study for the participants with TD in all regions except the left lentiform nucleus. These results suggest that adolescent boys with fraX have aberrant processing of direct gaze stimuli, including sensitization, in brain regions associated with anxiety and visceral arousal.
The hyperarousal of the insula to direct gaze, along with the sensitization of the amygdala during successive exposure, indicates an aberrant emotional response to direct gaze in subjects with fraX. However, because we did not collect measures of physiologic arousal such as heart rate or skin conductance, we are unable to make a more definite statement about emotional arousal to direct gaze stimuli in fraX. Activation of the insula has been shown to be involved in anxiety responses, visceral arousal, and attention.18,21,23 The negative correlation between performance on DirectGz and insula activation indicates a relationship between anxiety and task performance in the fraX group. However, because a similar relationship is not seen in the DD group, with equally low performance scores, this association is not likely to be related to anxiety about poor performance but is more likely to be related to anxiety about direct gaze stimuli. Greater activation in a region noted for having a role in anxiety is consistent with the behavioral phenotype of male subjects with fraX when asked to make eye contact.3,7
When exposed to successive direct gaze stimuli, the fraX group demonstrated sensitization in the left dorsal thalamus, a region not only implicated in specific phobias but also thought to promote activation in other brain regions associated with visceral arousal such as the basal ganglia, insula, and amygdala. This suggests that the fraX group responded to successive direct gaze stimuli with increased rather than habituated anxiety.41,42 The fraX group also showed a significant sensitization effect in the left amygdala. The amygdala has been reported to be involved in gaze processing20 and fear responses.16 In addition, Strauss et al43 showed that sensitization of the amygdala was associated with successive presentations of aversive stimuli (angry facial expressions) in healthy adults. Sensitization in a region known for fear responses provides additional evidence to suggest that the fraX group was not modulating their fear or anxiety but rather was intensifying such a response. We found a significant negative correlation between accuracy on successive trials and sensitization in the left amygdala in the fraX group. This result indicates that increased anxiety in the fraX group had a negative effect on task performance, similar to the performance correlation found in the insula. Much like our sensitization results, recent data in subjects with autism suggest heightened amygdala activation associated with time spent fixating on faces and eyes.11 It is unknown whether subjects with autism also show sensitization of amygdala activation with successive eye gaze stimulation. Future studies are needed to differentiate fraX from (non-fraX) autism with respect to sensitization of the amygdala to eye gaze.
Other significant differences between the fraX and control groups were in regions noted for their role in executive function and social cognition. These were the activation patterns associated with the right midfrontal gyrus of the DLPFC and the right cingulate. The DLPFC is a region implicated for executive control, including saccade inhibition, prediction, working memory,44,45 perceptual decision making,42 and modulation of emotional response.17,46 The DD group demonstrated greater activation in the DLPFC to direct eye gaze, while the fraX group showed a greater response to averted eye gaze. These results suggest that similar cognitive processes in this region may have been used in the DD and fraX groups, although for different conditions. In addition, the fraX group showed greater activation to averted gaze in the rostral portion of the right midcingulate gyrus, while both control groups showed no significant effect in this region. Greater activation seen in the DLPFC and cingulate to averted gaze is important in analyzing the gaze avoidance pattern in fraX, as affected individuals have been reported to selectively avoid mutual eye contact but not indirect social gaze.47 Recent research has described cortical control networks that involve the DLPFC, cingulate, and insula in tasks of inhibition and social cognition.48,49 Similar networks have been shown to have aberrant connectivity in individuals with high-functioning autism, another population demonstrating direct gaze avoidance.50,51 Future studies using functional connectivity methods would help explicate these networks in fraX.
Similar to a previous study10 among female subjects with fraX, we found aberrant activation to direct gaze (greater averted gaze) in the right STG in the fraX group compared with the control groups. However, the present study found greater activation to averted gaze compared with direct gaze in the TD control group. This is opposite to the result in the DD group and to the result found in the previous study. The existing literature has not definitively determined if activation in the STG is greater for direct gaze or for averted gaze in healthy adults.25,52,53
Limitations of this study include a small sample size, the absence of an age- and IQ-matched control group with autism, and a lack of correlated behavioral indexes of regional activation. The distinction between gaze avoidance features associated with fraX compared with autism is unclear because of the high prevalence of autism among the fraX population (approximately 25% among male subjects).54 Future studies, including eye tracking and psychophysiologic monitoring, might help to clarify the association between brain activation, anxiety, and eye gaze behaviors in fraX.
In summary, our findings indicate aberrant neural processing during gaze perception and lack of neural adaptation to successive gaze stimuli in adolescent boys with fraX. This study shows that there are functional brain correlates to the striking gaze aversion behavior seen in this population, as well as a decreased ability of these individuals to adapt to direct eye gaze stimuli. It remains unclear whether the differences seen between the fraX group and the control groups are due to learned behavior or if their behavior stems from altered development of neural networks as a direct result of the fraX mutation. The most probable case is that gaze avoidance behavior develops as a consequence of genetic and neurobiologic influences interacting with “common” environmental conditions such as the contingent removal of social demands.7 We believe that the challenge of understanding the multifaceted process of social cognition can be partially overcome by studying specific behavioral phenotypes (such as gaze processing) in more pathogenetically homogeneous populations such as those with fraX.
Acknowledgments
Funding/Support: This study was funded by grant MH50047 from the National Institutes of Health and by the Lynda and Scott Canel Fund for Fragile X Research (Dr Reiss).
Footnotes
Author Contributions: Ms Watson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Additional Contributions: Shelli Kesler, PhD, provided advice throughout the writing process, and Heidi Baumgartner, BA, proofread the manuscript. We thank the families who participated in the study.
References
- 1.Kleinke CL. Gaze and eye contact: a research review. Psychol Bull. 1986;100(1):78–100. [PubMed] [Google Scholar]
- 2.Patterson ML. A sequential functional model of nonverbal exchange. Psychol Rev. 1982;89(3):231–249. [Google Scholar]
- 3.Wolff PH, Gardner J, Paccla J, Lappen J. The greeting behavior of fragile X males. Am J Ment Retard. 1989;93(4):406–411. [PubMed] [Google Scholar]
- 4.Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: analyzing gene-brain-behavior relationships in child developmental psychopathologies. Dev Psychopathol. 2003;15(4):927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- 5.Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64(1):196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci U S A. 2001;98(13):7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall S, DeBernardis M, Reiss A. Social escape behaviors in children with fragile X syndrome. J Autism Dev Disord. 2006;36(7):935–947. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- 8.Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, Jenkins EC, Brown WT. Social gaze, social avoidance, and repetitive behavior in fragile X males: a controlled study. Am J Ment Retard. 1988;92(5):436–446. [PubMed] [Google Scholar]
- 9.Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Effects of age and communication level on eye contact in fragile X males and non–fragile X autistic males. Am J Med Genet. 1991;38(2–3):498–502. doi: 10.1002/ajmg.1320380271. [DOI] [PubMed] [Google Scholar]
- 10.Garrett AS, Menon V, MacKenzie K, Reiss AL. Here’s looking at you, kid: neural systems underlying face and gaze processing in fragile X syndrome. Arch Gen Psychiatry. 2004;61(3):281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- 11.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnea-Goraly N, Eliez S, Hedeus M, Menon V, White CD, Moseley M, Reiss AL. White matter tract alterations in fragile X syndrome: preliminary evidence from diffusion tensor imaging. Am J Med Genet B Neuropsychiatr Genet. 2003;118B (1):81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- 13.Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10(1):17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- 14.Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, Erba HW, Ringel J, Hayashi KM, Patnaik S, Golianu B, Kraemer HC, Thompson PM, Piven J, Reiss AL. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63(1):40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37(4):8–25. [PubMed] [Google Scholar]
- 16.Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9(3):382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 17.Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28 (5):409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosbras MH, Laird AR, Paus T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp. 2005;25(1):140–154. doi: 10.1002/hbm.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooker CI, Paller KA, Gitelman DR, Parrish TB, Mesulam MM, Reber PJ. Brain networks for analyzing eye gaze. Brain Res Cogn Brain Res. 2003;17(2):406–418. doi: 10.1016/s0926-6410(03)00143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Ito K, Fukuda H, Kojima S, Nakamura K. The human amygdala plays an important role in gaze monitoring: a PET study. Brain. 1999;122(pt 4):779–783. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- 21.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 22.Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18(6):2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60(4):402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 25.Wicker B, Michel F, Henaff MA, Decety J. Brain regions involved in the perception of gaze: a PET study. Neuroimage. 1998;8(2):221–227. doi: 10.1006/nimg.1998.0357. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Intelligence Scale for Children–Third Edition: Administration and Scoring Manual. San Antonio, TX: Psychological Corp; 1991. [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale–Third Edition: Administration and Scoring Manual. San Antonio, TX: Psychological Corp; 1997. [Google Scholar]
- 28.Oostra BA, Jacky PB, Brown WT, Rousseau F National Fragile X Foundation. Guidelines for the diagnosis of fragile X syndrome. J Med Genet. 1993;30(5):410–413. doi: 10.1136/jmg.30.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rousseau F, Heitz D, Biancalana V, Blumenfeld S, Kretz C, Boué J, Tommerup N, Van Der Hagen C, DeLozier-Blanchet C, Croquette MF, Gilgenkrantz S, Jalbert P, Voelckel MA, Oberlé I, Mandel JL. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- 30.Willemsen R, Smits A, Mohkamsing S, van Beerendonk H, de Haan A, de Vries B, van den Ouweland A, Sistermans E, Galjaard H, Oostra BA. Rapid antibody test for diagnosing fragile X syndrome: a validation of the technique. Hum Genet. 1997;99(3):308–311. doi: 10.1007/s004390050363. [DOI] [PubMed] [Google Scholar]
- 31.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism Screening Questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 32.Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington: Dept of Psychiatry, University of Vermont; 1991. [Google Scholar]
- 33.Derogatis LR. SCL-90: Administration, Scoring & Procedures Manual–I for the Revised Version and Other Instruments of the Psychopathology Rating Scale Series. Baltimore, MD: Johns Hopkins University Press; 1977. [Google Scholar]
- 34.Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39(3):361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 35.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci U S A. 2004;101(26):9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzourio-Mazoyer N, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer BJM. Automated Anatomical Labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 38.Lancaster JLSJ, Rainey L, Freitas CS, Fox PT. The Talairach Daemon: a database server for Talairach Atlas labels. Neuroimage. 1997;5(4 pt 2):S633. [Google Scholar]
- 39.Lancaster JLWM, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme-Spratton Inc; 1988. [Google Scholar]
- 41.McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22(18):8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus: anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39(2–3):107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 43.Strauss MM, Makris N, Aharon I, Vangel MG, Goodman J, Kennedy DN, Gasic GP, Breiter HC. fMRI of sensitization to angry faces. Neuroimage. 2005;26(2):389–413. doi: 10.1016/j.neuroimage.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 44.Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126(pt 6):1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- 45.Pierrot-Deseilligny C, Ploner CJ, Muri RM, Gaymard B, Rivaud-Pechoux S. Effects of cortical lesions on saccadic: eye movements in humans. Ann N Y Acad Sci. 2002;956:216–229. doi: 10.1111/j.1749-6632.2002.tb02821.x. [DOI] [PubMed] [Google Scholar]
- 46.Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc Natl Acad Sci U S A. 2006;103(26):10023–10028. doi: 10.1073/pnas.0603949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Parent-child dyadic gaze patterns in fragile X males and in non–fragile X males with autistic disorder. J Child Psychol Psychiatry. 1989;30(6):845–856. doi: 10.1111/j.1469-7610.1989.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 48.Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 49.Satpute AB, Lieberman MD. Integrating automatic and controlled processes into neurocognitive models of social cognition. Brain Res. 2006;1079(1):86–97. doi: 10.1016/j.brainres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62(3):198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizuno A, Villalobos ME, Davies MM, Dahl BC, Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104(1):160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 52.Calder AJ, Lawrence AD, Keane J, Scott SK, Owen AM, Christoffels I, Young AW. Reading the mind from eye gaze. Neuropsychologia. 2002;40(8):1129–1138. doi: 10.1016/s0028-3932(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3 (1):80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- 54.Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]