Abstract
Compelling evidence indicates that type 2 diabetes mellitus (T2D), insulin resistance (IR), and metabolic syndrome are often accompanied by cognitive impairment. However, the mechanistic link between these metabolic abnormalities and CNS dysfunction requires further investigations. Here, we evaluated whether adipose tissue (AT) IR and related metabolic alterations resulted in CNS changes by studying synapse lipid composition and function in the adipocyte-specific ecto-nucleotide pyrophosphate phosphodiesterase overexpressing transgenic (AtENPP1-Tg) mouse, a model characterized by white adipocyte IR, systemic IR, and ectopic fat deposition. When fed a high-fat diet (HFD), AtENPP1-Tg mice recapitulate essential features of the human metabolic syndrome, making them an ideal model to characterize peripherally induced CNS deficits. Using a combination of gas chromatography and western blot analysis, we found evidence of altered lipid composition, including decreased phospholipids and increased triglycerides (TG) and fatty acid (FFA) in hippocampal synaptosomes isolated from HFD-fed AtENPP1-Tg mice. These changes were associated with impaired basal synaptic transmission at the Schaffer collaterals to hippocampal cornu ammonis 1 (CA1) synapses, decreased phosphorylation of the GluN1 glutamate receptor subunit, down-regulation of insulin receptor expression and up-regulation of the FFA receptor 1.
Keywords: Cognitive dysfunction, ENPP1, Glutamate receptors, Insulin Resistance, Lipids, synaptic transmission
INTRODUCTION
A growing body of evidence indicates that cognitive impairment is associated with obesity, metabolic syndrome, and type 2 diabetes (T2D) (Calvo-Ochoa and Arias 2014; Elias et al. 2003; Hassing et al. 2004), suggesting common yet undefined pathogenic mechanisms among the conditions. However, the cellular and molecular mechanisms driving these peripherally induced CNS deficits remain elusive in this emerging area of interest. This critical knowledge gap precludes the development of therapeutic interventions to prevent and/or treat cognitive impairment in the growing population affected by the metabolic complications of obesity.
Altered production of adipokines and lipid metabolism regulation in adipose tissue (AT) (i.e., AT dysfunction) affect the plasma concentrations of circulating adipokines and free fatty acids (FFA). This aberrant endocrine signaling triggers numerous functional changes throughout the body that are part of the complex cluster of metabolic abnormalities that increase the risks for T2D and cardiovascular diseases in obese patients.
We have developed an adipocyte-specific ecto-nucleotide pyrophosphate phosphodiesterase overexpressing transgenic (AtENPP1-Tg) animal model of metabolic syndrome and systemic IR, which is highly suitable for evaluating peripherally driven changes in central synapses triggered by adipocyte IR. The AtENPP1-Tg mouse is characterized by adipocyte and systemic IR, increased circulating FFA, and TG deposition in the liver, all of which are induced by the adipocyte-specific overexpression of ecto-nucleotide pyrophosphate phosphodiesterase (ENPP1), a negative modulator of the insulin receptor (Pan et al. 2011). We have shown translational validity of our model by identifying association between higher AT ENPP1 expression and systemic abnormalities of glucose and lipid metabolism in humans (Chandalia et al. 2012). In the present study, we used the AtENPP1-Tg mouse model to investigate the effects of AT-induced metabolic alterations of systemic lipid and glucose metabolism on lipid composition and functional activity of hippocampal synapses, and to explore involved candidate molecular mechanisms.
MATERIALS AND METHODS
Animals
Eighty-one adult male AtENPP1-Tg (transgenic, Tg) and C57Bl/6J (wild type, WT) mice were randomized to receive dietary intervention. Animals were generated through a breeding program at UTMB and were individually housed in their filter-top cages in a temperature-controlled environment at 22°C, humidity 40%, and a 12:12-h light-dark cycle. Understanding the 3 R’s of Russell and Birch (Replacement, Refinement and Reduction), the number of mice required for each experiment was calculated based on the minimal number of animal required to detect a significant difference based on power analysis. The study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch (UTMB, Galveston, TX, USA) and was performed according to the guidelines of the National Institutes of Health Guidelines on the use of laboratory animals.
Experimental Procedure
Following weaning at 8 weeks of age, a random group of Tg and WT siblings were pair-fed with high-fat chow (60% calories from fat - 37.1% saturated; Research Diets D12492, New Brunswick, NJ, USA). Another random group of Tg and WT siblings were fed a regular chow (RC) diet (4% calories from fat; Teklad 7001; Teklad, Madison, WI). Animal weights were recorded weekly. In the morning following 12 weeks of the respective diets, the animals were brought into the lab. and were either intracardially perfused and decapitated for electrophysiological studies, under general anesthesia (see below), or were immediately sacrificed by anesthetic overdose (5% isoflurane inhalation confirmed by chest opening) and their brains collected, flash-frozen in liquid nitrogen, and stored at −80°C for future analyses.
Synaptosome isolation
Hippocampi were dissected and homogenized from frozen brains. Synaptosomes were isolated using a sucrose gradient and ultracentrifugation, as previously described (Bjorklund et al. 2012). We employed samples with synaptosome yields ≥25 mg for lipid measurements.
Lipid composition in hippocampal synaptosomes
Synaptosome lipids were extracted with the chloroform:methanol (2:1) Folch extraction (n=4-12 per group; a total of 38). TG, diacylglycerols (DAG), and phospholipids were isolated using TLC, as previously described (Pan et al. 2011). Briefly, TG, DAG, and phospholipid bands on TLC plates were identified, scraped off, and processed to separate fatty acids and glycerol. The fatty acids were derivitized and quantified with a gas chromatography-flame ionization detector (GC-FID 6890N model – Agilent Technologies, Santa Clara, CA, USA) using a Supelco SP-2330 fused silica capillary column (30 m × 0.25 mm × 0.2 μm film thickness). The concentrations of TG, DAG, and phospholipids were calculated using an internal standard method as described previously (Zhang et al. 2011).
Extracellular recording of hippocampal synaptic responses
The mice (n=4-5 per group; a total of 18) were anesthetized with 2-2-2-tribromoethanol (Sigma-Aldrich - 250 mg/Kg IP) and intracardially perfused with an ice-cold sucrose-based solution containing (in mM 56 NaCl, 100 sucrose, 2.5 KCl, 20 glucose, 5 MgCl2, 1 CaCl2, 30 NaHCO3, 1.25 NaH2PO4, and 1 kynurenic acid). Following decapitation and harvesting hippocampi, horizontal hippocampal slices (350 μm) were cut with a vibratome VT1200S (Leica, IL) in the sucrose-based solution and transferred to a recovery chamber with 95% O2 and 5% CO2 bubbled artificial CSF (ACSF) containing (in mM) 125 NaCl, 2.5 KCl, 2 MgCl2, 2.5 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose) at 31.5°C. After at least 90 min of recovery, recordings were performed in ACSF in a submerged chamber, at 30.5 ± 0.5°C. Recordings of field excitatory postsynaptic potentials (fEPSPs) were performed in the cornu ammonis 1 (CA1) subfield with a tungsten electrode connected to an A-M Model 1800 Differential AC Amplifier (A-M Systems, Carlsborg, WA, USA). Cornu ammonis 3 (CA3) Schaffer collaterals were stimulated by a bipolar tungsten electrode, with 0.1-ms pulses of constant current. The traces were digitized by a Digidata 1200 interface using Clampex 7, and the slopes of the fEPSPs were measured offline with Clampfit 9.0 (PClamp software, Molecular Devices, Union City, CA, USA).
Hippocampal receptor expression
To evaluate whether ENPP1-induced adipocyte insulin resistance associates with changes in key receptor proteins involved in cognitive function, we studied the insulin and glutamatergic neuronal transmission. To this goal, content of insulin and FFA1 receptors in hippocampal tissue and levels of glutamate receptors (namely, NMDA receptor 1 subunit (GluN1) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor 1 subunit (GluA1)) in synaptosomes were determined by western blot (n=4-5 per group; a total of 18). Tissues were homogenized in cell lysis buffer (Cell Signaling Technology Inc. Danvers, MA), and mixed 1 mM PMSF, 1× protease inhibitor cocktail, and phosphatase inhibitor cocktail (Sigma-Aldrich, Saint Louis, MO). Protein concentration of hippocampal tissue lysate was measured using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc. Hercules, CA). Protein concentrations of synaptosome preparations were determined using a bicinchoninic acid protein assay kit (Thermo Scientific, Waltham, MA, USA). SDS-PAGE was used to separate proteins which were then transferred to a nitrocellulose membrane (Bio-Rad). Primary antibodies used were anti-β-actin antibody (A1978, Sigma-Aldrich, Saint Louis, MO), anti-Insulin Receptor antibody-α (sc710 – Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-Insulin Receptor antibody-β (Cat. # 610109 - BD Biosciences, San Jose, CA), anti-total GluN1 (D65B7 - 5704 - Cell Signaling Technology Inc. Danvers, MA), anti-phosphorylated-GluN1 (ser897, 3385; Cell Signaling Technology Inc., Danvers, MA), anti-total GluA1 (G12 - SC-55509; Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-phosphorylated-GluA1 (ser831 - SC-16313; Santa Cruz Biotechnology Inc., Santa Cruz, CA), and anti-FFA1 (ab109257, Abcam, Cambridge. MA). Secondary antibodies were from Southern Biotechnology Associates (Birmingham, AL). Immunoblots were detected using the ECL Western Blotting Detection Reagents (Amersham Biosciences, Piscataway, NJ). Expression levels were evaluated by quantification of relative density of each band normalized to that of corresponding β-actin band density using the NIH ImageJ software version 1.46r (NIH, Betsesda, MD).
Brain response to insulin
In order to understand alterations of insulin responses in the brain, synaptosomes were isolated form different brain regions of RC-fed WT and AtENPP1-Tg (n=2-5 each; a total of 7), thirty minutes following the intra-peritoneal administration of insulin (0.5 U/Kg). Regional expressions of protein kinase B (Akt) and glucagon synthase kinase 3β (GSK-3β) were determined by western blot, as detailed above. All antibodies used were from Cell Signaling (anti-pAKT Cat. # 2965 – anti-AKT Cat. # 4691 – anti-pGSK-3β Cat. #9323 – anti-GSK-3β Cat. #9315).
Statistical Analysis
All data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC, USA) and are presented as means ± SEM. Two-way ANOVA was used for multiple comparisons between all four groups of animals with post-hoc Bonferroni or Scheffé test was used. Significance was set at p < 0.05.
RESULTS
To determine the effect of peripherally-induced changes in insulin and lipid handling in the CNS, we included four study groups (n = 4-12 per group). The weights of the RC-fed groups were 31.8 ± 1 and 30.7 ± 1 g for WT and AtENPP1-Tg, respectively; and the weight of high fat fed animals were 37.3±3 and 36.6±3 g (WT and AtENPP1-Tg, respectively – p>0.05 each).
Lipid composition in hippocampal synaptosomes
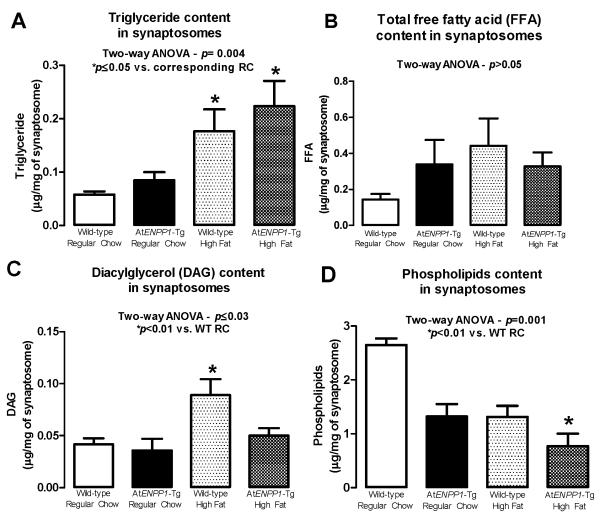
The concentrations of TG, FFA, DAG, and phospholipids in the hippocampal synaptosomes are summarized in Fig. 1. TG content (Fig. 1A) was significantly different in the four groups (p = 0.004 - ANOVA), and highest in the AtENPP1-Tg mice (*p ≤ 0.05 compared to the corresponding RC group), suggesting an additive effect of ENPP1-induced adipocyte IR and HFD on hippocampal lipid levels. Fig. 1B shows a non-significant trend towards increased synaptosomal FFA content in HFD groups. Fig. 1C shows that DAG content was increased in hippocampal synaptosomes isolated from HFD-fed WT animals, suggesting independent effects of diet and adipocyte IR on these lipid compositional changes in hippocampal synapses (p ≤ 0.03 – ANOVA; *p<0.01 vs. WT-RC). Fig. 1D shows that the synaptosomal phospholipid content was lower in AtENPP1-Tg mice and was further decreased by HFD (p = 0.001 - ANOVA; *p <0.01 vs. WT-RC).
Figure 1.
A. Neuronal triglyceride (TG) content in hippocampal synaptosomes. TG were significantly increased in response to HFD in both WT and AtENPP1-Tg mice (p = 0.004, ANOVA – *p≤0.05 vs. corresponding RC littermates). B. Neuronal FFA content in hippocampal synaptosomes. An insignificant trend toward higher FFA concentrations was detected after HFD (p > 0.05, ANOVA). C. Neuronal DAG content in hippocampal synaptosomes. DAG increased in response to a HFD in the WT mice (p ≤ 0.03 – ANOVA; *p<0.01 vs. WT-RC). D. Neuronal phospholipid content in hippocampal synaptosomes. Phospholipid content was decreased in response to ENPP1 overexpression in AtENPP1-Tg and in response to HFD. Consumption of a HFD further decreased phospholipid levels (p = 0.001 - ANOVA; *p <0.01 vs. WT-RC).
Extracellular recording of hippocampal synaptic responses
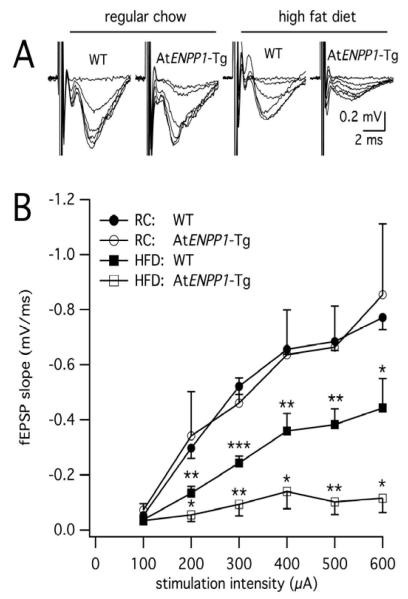
To determine whether changes in lipid synaptic content were associated with functional deficits in hippocampal circuits, we measured CA3 Schaffer collateral axons synaptic responses. Extracellular stimulation of the elicited postsynaptic responses in the CA1 (Fig. 2), but fEPSP magnitude and slope were diet and genotype dependent. For slices from mice fed a RC diet, the input-output curves of fEPSP slopes in WT mice were indistinguishable from those recorded in AtENPP1-Tg mice (n = 4-5 each, p > 0.05 - ANOVA), indicating that overexpression of the ENPP1 gene in adipose tissue was not sufficient to elicit changes in basal synaptic transmission in the CA1 hippocampal area. However, when challenged by the HFD, the AtENPP1-Tg group exhibited synaptic responses that were greatly diminished compared to HFD-fed WT littermates (n=4-5 each, p < 10−8 - ANOVA). Notably, the HFD alone had a significant effect on synapses in WT animals, with HFD-fed mice exhibiting suppressed postsynaptic responses compared to RC-fed WT mice (n=5 each, p < 10−10 - ANOVA). Indeed, HFD-fed WT animals exhibited fEPSPs with intermediate amplitudes between those from HFD-fed AtENPP1-Tg and RC-fed WT mice. This phenotype, along with the lack of effect of the AtENPP1 genotype alone on synaptic responses, indicated an interaction between genotype and diet. Two-way ANOVA analysis of fEPSPs curves across the four experimental groups confirmed that the HFD and AtENPP1 genotype additively contribute to cause the greatest degree of synaptic suppression in HFD-fed AtENPP1-Tg animals.
Figure 2. Basal synaptic transmission in the hippocampal CA1 area is greatly suppressed in HFD-fed AtENPP1-Tg animals.
A. Representative traces of extracellular field recordings in the CA1. Notice the depressed signal in the HFD-fed AtENPP1-Tg mice. B. Input/output curves of fEPSP slopes versus presynaptic stimulation intensity. fEPSP slopes were diminished in HFD-fed animals (p < 10−8 for AtENPP1-Tg HFD vs. WT HFD and p < 10−10 for WT HFD vs. WT RC, ANOVA). Asterisks signify significant decreases at a specific stimulation intensity between HFD-fed WT relative to RC-fed WT and between HFD-fed AtENPP1-Tg relative to HFD-fed WT (* p < 0.05, ** p < 0.01, *** p < 0.001).
Hippocampal receptor expression
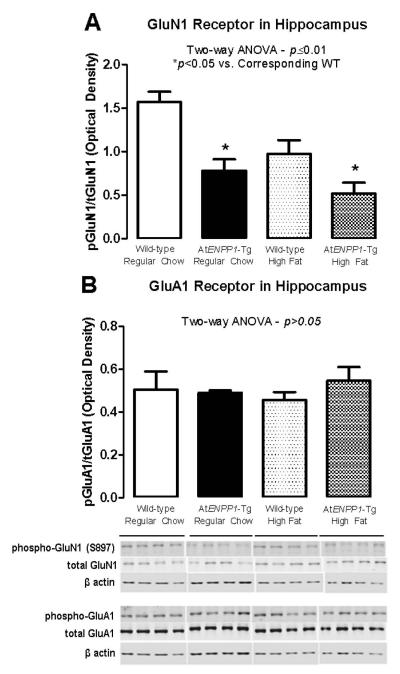
Next we addressed potential molecular mechanisms responsible for the observed suppression of synaptic transmission. As shown in Fig. 3A, there was a significant decrease in GluN1 phosphorylation in AtENPP1-Tg mice, particularly the HFD-fed group (p ≤0.01, ANOVA- *p<0.05 vs. corresponding WT). Conversely, there were no changes in the levels of phosphorylated or total GluA1 in response to adipocyte IR and/or HFD (Fig. 3B), suggesting that synaptosomal lipid changes specifically affected GluN1 phosphorylation (p >0.05, ANOVA).
Figure 3.
A. pGluN1/GluN1 ratio decreases in hippocampal synaptosomes. The ratio between phosphorylated GluN1 and total GluN1 was lower in the presence of AT-specific ENPP1 overexpression, particularly after exposure to HFD (p ≤0.01, ANOVA- *p<0.05 vs. corresponding WT). B. The pGluA1/GluA1 ratio is unchanged in hippocampal synaptosomes. The ratio between phosphorylated and total GluA1 was not affected by HFD or AT-specific ENPP1 overexpression (p >0.05, ANOVA).
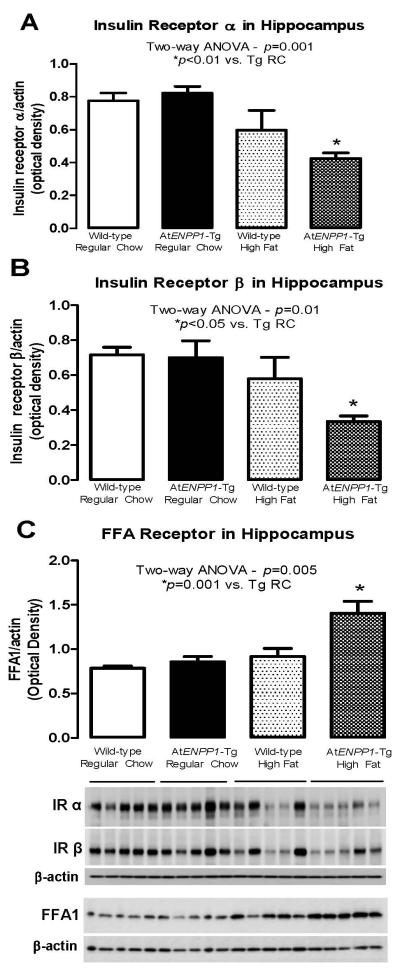
In addition, we explored the insulin and FFA1 receptor expressions in the hippocampal tissue. Only in the HFD-fed AtENPP1-Tg hippocampus, insulin receptor-α and -β subunits were down-regulated (p = 0.001 and 0.01 for α- and β-subunits, respectively, ANOVA - *p<0.01 vs. AtENPP1-Tg RC – Fig. 4A and B), while FFA1 receptors were up-regulated (p = 0.005, ANOVA – *p =0.001 vs. AtENPP1-Tg RC – Fig. 4C). These data confirm that a combination of the transgene and HFD is necessary to alter insulin receptor expression and is linked to the elevated FFA levels in this AtENPP1-Tg animal model (Pan et al. 2011).
Figure 4.
A & B Insulin receptor α and β subunits 1 levels in hippocampal tissue. Only in the HFD-fed AtENPP1-Tg hippocampus, insulin receptor-α and -β subunits were down-regulated (p = 0.001 and 0.01 for α- and β-subunits, respectively, ANOVA - *p<0.01 vs. AtENPP1-Tg RC). C. Free fatty acid receptor levels in hippocampal tissue. (p = 0.005, ANOVA – *p =0.001 vs. AtENPP1-Tg RC.
Brain response to insulin
To gain initial knowledge on post-receptor insulin signaling activation of AtENPP1Tg mice CNS, we studied the regional expression of Akt and GSK-3β in the brain of RC-fed animals in response to acute systemic insulin elevation. After 30 from intra-peritoneal insulin injection, we observed a differential regional response to insulin that was blunted in AtENPP1-Tg compared to their WT littermates (Figure 1S). It should be noted that these animals did not show a significant decrease in insulin receptor in absence of HFD (refer to Fig. 4 A and B).
DISCUSSION
Our results provide new evidence for AT IR-induced biochemical and functional changes in hippocampal synapses. We found that HFD-fed AtENPP1-Tg animals exhibited alterations in synaptic lipid composition, decreased basal synaptic transmission at the Schaffer collaterals to CA1 synapses, decreased GluN1 receptor phosphorylation, decreased insulin receptor expression and increased FFA1 receptor expression compared to WT littermates. These results extend the impact of AT dysfunction-induced lipotoxicity from peripheral organs to the brain, thus providing a novel mechanistic link between the increased risk for cognitive impairment and the metabolic complications of obesity, such as metabolic syndrome and T2D.
One innovative aspect of the present work is in the use of the AtENPP1-Tg mouse, a model of adipocyte IR and consequent manifestations of metabolic syndrome (Pan et al. 2011). Due to adipocyte-specific overexpression of ENPP1, a negative modulator of the insulin receptor, AtENPP1-Tg mice exhibit specific adipocyte IR/AT dysfunction (decreased adipocyte insulin signaling activation and defective adipocyte maturation/TG storage and adiponectin production) upon excessive caloric intake (HFD). In humans, high adipocyte lipolysis and the inability to store lipids in AT leads to FFA spillover and ectopic fat deposition in the liver and muscle, resulting in lipid and glucose metabolic alterations that recapitulate essential features of IR and metabolic syndrome (Brassard et al. 2008; Chandalia et al. 2012; Lomonaco et al. 2013; Pan et al. 2011; Ravussin and Smith 2002). Thus, AtENPP1-Tg mice are an ideal model to determine the dietary contribution of IR to the clinical manifestations of humans with metabolic syndrome and T2D.
The lipid composition of hippocampal synaptosomes from the brains of WT and AtENPP1-Tg mice was influenced by AT dysfunction. TG contents were clearly affected by the combination of diet and gene overexpression in the HFD-fed AtENPP1-Tg mouse, which are known to have high levels of AT dysfunction, circulating FFA, and IR (Pan et al. 2011). The increased plasma-derived FFA and higher glucose availability in the AtENPP1-Tg mouse brain would increase substrates for TG synthesis. Such TG excess, outside of AT, is known to cause cellular dysfunction and apoptosis (e.g., lipotoxicity), manifested as cellular IR. Indeed, we have shown evidence of elevated cellular TG content in both the liver (Pan et al. 2011) and hippocampal synapses that would potentially affect their integrity and function. A mechanistic interpretation includes the view that a recent human study showed that acute FFA elevation induced by intralipid infusion results in decreased hippocampal glucose utilization (Emmanuel et al. 2013), similar to the well-established effect of acute FFA elevation on muscle insulin-mediated glucose disposal (Boden and Chen 1995; Boden et al. 1994; Homko et al. 2003). A plausible mediator of these intracellular effects downstream of increased circulating FFA is increased levels of the lipid metabolism intermediate DAG (Yu et al. 2002; Samuel and Shulman 2012; Itani et al. 2002; Nowotny et al. 2013). Interestingly, the pattern of hippocampal synaptosome DAG content was similar to the FFA content.
Neural phospholipid content was decreased in the hippocampal synaptosomes of mice with AT dysfunction. This change was most pronounced in HFD-fed At-ENPP1-Tg mice, a combination that we previously demonstrated to lead to severe systemic IR (Pan et al. 2011). The result is of interest given the previous findings by other investigators who reported decreased phospholipids in the hippocampal region of Alzheimer’s disease patients (Prasad et al. 1998; Soderberg et al. 1991). Indeed, plasma phospholipids were recently identified as useful biomarkers for cognitive impairment (Mapstone et al. 2014). Such a decrease in phospholipids may result from hippocampal IR, similar to that reported in mice with streptozotocin-induced brain IR (Muller et al. 1998).
Because of the association between IR and cognitive impairment, next we studied whether that peripherally-induced IR could have a negative impact on synaptic transmission in the hippocampus. Extracellular field recordings revealed that the synaptic strength of the CA3 to CA1 inputs was significantly suppressed in HFD-fed AtENPP1-Tg mice. Given the role of the CA1 region and of synaptic GluN1 in cognitive function and memory formation (Morris 2013; Tsien et al. 1996), our findings might suggest reduced synaptic plasticity along with learning and memory deficit, a reduced capability of the hippocampus to decode memory engrams and store new memories; however future studies are required to directly test such hypothesis. This result is in line with the essential role of GluN1 (which is less phosphorylated and therefore less active in these mice) in hippocampal synaptic plasticity and in learning and remembering novel places in spatial navigation (Tsien et al., 1996). Furthermore, we demonstrated that the HFD was sufficient to induce synaptic deficits in the CA3-CA1 pathway, although this effect was lower in WT animals compared to AtENPP1-Tg mice.
These functional manifestations occurred concomitantly with lipid profile alterations, indicating that brain circuitry changes might explain the relationship between cognitive impairment and insulin-dependent metabolic dysfunction. While previous animal studies have reported HFD-induced memory deficits (Granholm et al. 2008; Molteni et al. 2002; Winocur and Greenwood 2005) associated with IR (McNay et al. 2010), the CNS synaptic deficits described in the present study can be clearly causally linked to peripherally induced adipocyte IR.
Next, we evaluated hippocampal expression of both α- and β- subunits of the insulin receptor and found that they were down-regulated in the HFD-fed AtENPP1-Tg mice, suggesting an interaction between gene and diet. On the other hand, FFA1 receptor expression was increased in these animals, suggesting increased neuronal FFA uptake, which may contribute to defective post-receptor CNS insulin signaling (Ruddock et al. 2008). Decreased insulin receptor expression could have a negative effect on hippocampal synaptic plasticity, resulting in memory and learning dysfunction (Calvo-Ochoa and Arias 2014), variables that remain to be evaluated in the future. The role of insulin receptor down regulation and neuronal IR in neurodegeneration is further supported by changes observed in the NIRKO (neuron-specific disruption of the IR gene) mouse (Schubert et al. 2004), in which complete knockout of brain insulin receptors mimics neuronal changes typically found in Alzheimer’s disease.
The synapse between Schaffer collaterals and CA1 pyramidal neurons is under the control of NMDA-dependent plasticity (Morris, 2013; Tsien et al., 1996). At the same time, neuronal insulin is known to control NMDA-dependent synaptic plasticity in the hippocampus, a mechanism responsible for long-term potentiation and/or depression, i.e. memory formation (Liao and Leonard 1999; Liu et al. 1995; van der Heide et al. 2005; Costello DA. 2012). For these reasons, the NMDA receptor is an excellent candidate as a molecular mechanism of the decreased synaptic transmission in AtENPP1-Tg mice. In particular, we evaluated GluN1 phosphorylation at S897, a site that has been linked to neurophatologies (Emamian et al. 2004; Hei et al. 2012). The reduction in GluN1 phosphorylation in synaptosomes isolated from AtENPP1-Tg mice supports the view that AT dysfunction affects both synaptic lipid composition and post-translational modification of a glutamate receptor subtype critical for synaptic plasticity and memory formation (Morris 2013). Our studies indicate a decrease in GluN1 phosphorylation at S897, a residue that controls NMDA receptor permeation through protein kinase A phosphorylation (Aman et al. 2014). Although the functional role of S897 phosphorylation at synapses under normal conditions is still poorly understood, severe impairment in NMDA trafficking, AMPA and NMDA-mediated synaptic transmission, and long-term potentiation along with aberrant social interaction and sensorimotor gating have been reported in GluN1 S897A knock-in phosphomutant mice (Li et al. 2009). Furthermore, evidence indicates that the S897 site is downregulated in ischemic or NMDA-induced brain damage rodent model (Hei et al. 2012) as well as in schizophrenia in humans (http://www.ncbi.nlm.nih.gov/pubmed/14973229Emamian et al. 2004). Thus, lack of phosphorylation of GluN1 at S897 is an absolute requirement for synaptic function and a causative link to neurophatologies. A reduction of the AMPA GluA1 receptor, similar to that observed in GluN1 S897A knock-in phosphomutant animals, was not detected in our current study. This observation requires further investigation, but suggests that S897 might be a point of convergence of intracellular signaling pathways, carrying specialized functions. This result provides insight into one of the possible mechanisms through which neuronal IR contributes to cognitive dysfunction by altering brain circuitry via effects on specific glutamate receptor subtypes.
CONCLUSIONS
Our results suggest that peripheral lipids and IR alter hippocampal molecular and functional integrity, which could be a pivotal mechanism responsible for the reported cognitive deficits associated with metabolic syndrome and T2D. It is therefore tempting to speculate that the mechanisms described in the present work could underscore, at least in part, the reported epidemiological link between T2D and heightened risk of developing Alzheimer’s Disease in humans. It is of interest to study the potential detrimental consequences on learning, memory, and other cognitive functions related to our current findings. Further ongoing studies in our laboratories are necessary to ultimately address this important issue.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Claudia Soto and Doaa Reda Abdelrahman, M.Sc. for providing technical assistance in the lipid composition measurements, and Lindsay Reese, PhD (SciReviser) for editing the manuscript. These studies were supported in part by NIH/NIA 1R01AG042890 to G.T; intramural funding provided by the University of Texas Medical Branch to N.A., G.T., and F.L.; and a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, NIH.
Abbreviations used
- AT
adipose tissue
- CA
cornu ammonis
- DAG
diacylglycerol
- ENPP1
ecto-nucleotide pyrophosphate phosphodiesterase 1 gene
- fEPSPs
field excitatory postsynaptic potentials
- FFA
free fatty acids
- FFA1
free fatty acid receptor 1
- GC-FID
gas chromatography-flame ionization detector
- GluA1
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor 1 subunit
- HFD
high-fat diet
- IR
insulin resistance
- GluN1
NMDA receptor 1 subunit
- RC
regular chow
- T2D
type 2 diabetes mellitus
- Tg
transgenic
- TG
triglyceride
- WT
wild type
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Aman TK, Maki BA, Ruffino TJ, Kasperek EM, Popescu GK. Separate intramolecular targets for protein kinase A control of N-methyl-D-aspartate receptor gating and Ca2+ permeability. J Biol Chem. 2014 doi: 10.1074/jbc.M113.537282. pii: jbc.M113.537282. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund NL, Reese LC, Sadagoparamanujam VM, Ghirardi V, Woltjer RL, Taglialatela G. Absence of amyloid beta oligomers at the postsynapse and regulated synaptic Zn2+ in cognitively intact aged individuals with Alzheimer’s disease neuropathology. Mol. Neurodegener. 2012;7:23. doi: 10.1186/1750-1326-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J. Clin. Invest. 1995;96:1261–1268. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J. Clin. Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassard P, Frisch F, Lavoie F, Cyr D, Bourbonnais A, Cunnane SC, Patterson BW, Drouin R, Baillargeon JP, Carpentier AC. Impaired plasma nonesterified fatty acid tolerance is an early defect in the natural history of type 2 diabetes. J. Clin. Endocrinol. Metab. 2008;93:837–844. doi: 10.1210/jc.2007-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- Calvo-Ochoa E, Arias C. Cellular and metabolic alterations in the hippocampus caused by insulin signaling dysfunction and its association with cognitive impairment during aging and Alzheimer’s disease. Animal models of study. Diabetes. Metab. Res. Rev. 2014 doi: 10.1002/dmrr.2531. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chandalia M, Davila H, Pan W, Szuszkiewicz M, Tuvdendorj D, Livingston EH, Abate N. Adipose tissue dysfunction in humans: a potential role for the transmembrane protein ENPP1. J. Clin. Endocrinol. Metab. 2012;97:4663–4672. doi: 10.1210/jc.2012-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am. J. Clin. Nutr. 2007;85:662–677. doi: 10.1093/ajcn/85.3.662. [DOI] [PubMed] [Google Scholar]
- Costello DA. Brain deletion of insulin receptor substrate 2 disrupts hippocampal synaptic plasticity and metaplasticity. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int. J. Obes. Relat. Metab. Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Karayiorgou M, Gogos JA. Decreased phosphorylation of NMDA receptor type 1 at serine 897 in brains of patients with Schizophrenia. J Neurosci. 2004;18(24(7)):1561–4. doi: 10.1523/JNEUROSCI.4650-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel Y, Cochlin LE, Tyler DJ, de Jager CA, Smith AD, Clarke K. Human hippocampal energy metabolism is impaired during cognitive activity in a lipid infusion model of insulin resistance. Brain Behav. 2013;3:134–144. doi: 10.1002/brb3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, McClearn G, Johansson B. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing. 2004;33:355–361. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- Hei MY, Tao HK, Tang Q, Yu B, Zhao LL. Decreased levels of pNR1 S897 protein in the cortex of neonatal Sprague Dawley rats with hypoxic-ischemic or NMDA-induced brain damage. Braz J Med Biol Res. 2012;45(10):962–7. doi: 10.1590/S0100-879X2012007500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 2004;28(Suppl 4):S12–21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- Homko CJ, Cheung P, Boden G. Effects of free fatty acids on glucose uptake and utilization in healthy women. Diabetes. 2003;52:487–491. doi: 10.2337/diabetes.52.2.487. [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Någren K, Solin O, Nuutila P. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–2177. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Devidze N, Barengolts D, Prostak N, Sphicas E, Apicella AJ, Malinow R, Emamian ES. NMDA receptor phosphorylation at a site affected in schizophrenia controls synaptic and behavioral plasticity. J Neurosci. 2009;23(29(38)):11965–72. doi: 10.1523/JNEUROSCI.2109-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389–1397. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, Macarthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Morris RG. NMDA receptors and memory encoding. Neuropharmacology. 2013;74:32–40. doi: 10.1016/j.neuropharm.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Muller D, Nitsch RM, Wurtman RJ, Hoyer S. Streptozotocin increases free fatty acids and decreases phospholipids in rat brain. J. Neural Transm. 1998;105:1271–1281. doi: 10.1007/s007020050130. [DOI] [PubMed] [Google Scholar]
- Nowotny B, Zahiragic L, Krog D, Nowotny PJ, Herder C, Carstensen M, Yoshimura T, Szendroedi J, Phielix E, Schadewaldt P, Schloot NC, Shulman GI, Roden M. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes. 2013;62(7):2240–8. doi: 10.2337/db12-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Ciociola E, Saraf M, Tumurbaatar B, Tuvdendorj D, Prasad S, Chandalia M, Abate N. Metabolic consequences of ENPP1 overexpression in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2011;301:E901–911. doi: 10.1152/ajpendo.00087.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau E, Beysen C, Saad N, Turner S. Dynamics of adipose tissue development by 2H2O labeling. Methods Mol. Biol. 2009;579:337–358. doi: 10.1007/978-1-60761-322-0_17. [DOI] [PubMed] [Google Scholar]
- Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 1998;23:81–88. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2002;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- Ruddock MW, Stein A, Landaker E, Park J, Cooksey RC, McClain D, Patti ME. Saturated fatty acids inhibit hepatic insulin action by modulating insulin receptor expression and post-receptor signaling. J Biochem. 2008;144(5):599–607. doi: 10.1093/jb/mvn105. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;2(148(5)):852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Küstermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Brüning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci. Biobehav. Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging. 2005;26(Suppl 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Yin W, Liao D, Kusunoki M, Xi S, Tsutsumi K, Wang Z, Lian X, Koike T, Fan J, Yang Y, Tang C. NO-1886 decreases ectopic lipid deposition and protects pancreatic beta cells in diet-induced diabetic swine. J. Endocrinol. 2004;180:399–408. doi: 10.1677/joe.0.1800399. [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;27(277(52)):50230–6. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Rodriguez NA, Wang L, Tuvdendorj D, Wu Z, Tan A, Herndon DN, Wolfe RR. Measurement of precursor enrichment for calculating intramuscular triglyceride fractional synthetic rate. J. Lipid Res. 2012;53:119–125. doi: 10.1194/jlr.M019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.