Abstract
An epizootic of beak abnormalities (avian keratin disorder) was recently detected among wild birds in Alaska. Here we describe the gross, histologic, and ultrastructural features of the disease in 30 affected adult black-capped chickadees (Poecile atricapillus). Grossly, there was elongation of the rhamphotheca, with varying degrees of lateral deviation, crossing, and gapping between the upper and lower beak. Not uncommonly, the claws were overgrown and there was alopecia, scaling, and crusting of the skin. The most prominent histopathologic features in the beak included epidermal hyperplasia, hyperkeratosis, and core-like intrusions of necrotic debris. In affected birds, particularly those with moderate to severe beak overgrowth, there was remodeling of premaxillary and mandibular bones and various dermal lesions. Lesions analogous to those found in beaks were present in affected claws, indicating that this disorder may target both of these similar tissues. Mild to moderate hyperkeratosis occurred in other keratinized tissues, including skin, feather follicles, and, occasionally, sinus epithelium, but typically only in the presence of microbes. We did not find consistent evidence of a bacterial, fungal, or viral etiology for the beak lesions. The changes observed in affected birds did not correspond with any known avian diseases, suggesting a potentially novel hyperkeratotic disorder in wild birds.
Keywords: avian keratin disorder, black-capped chickadee, beak, claw, deformity, hyperkeratosis, integument, wild birds
An epizootic of beak abnormalities, termed “avian keratin disorder,” was recently documented in black-capped chickadees (Poecile atricapillus) and northwestern crows (Corvus caurinus) in Alaska.8,31 The estimated prevalence of the disorder in affected populations of these two species (6.5% and 16.9%, respectively) exceeds all published rates of gross deformities in wild birds.8 In addition, as many as 28 other, mostly nonmigratory, species in the Pacific Northwest region of North America may be affected.8 New reports suggest the emergence of a similar disorder in the United Kingdom.11 The high rate of occurrence in multiple avian species across a growing geographic area raises concern about possible causes, including infectious agents, toxins, or metabolic disorders. However, the etiology and pathogenesis of this emerging disease are not yet known.
Various forms of beak deformities, including elongation, crossing, and lateral deviation, which impair feeding, preening, and other normal behaviors, are present in affected birds.8,31 Study of this disease in Alaska has highlighted important fitness consequences.4,8 Evidence of high mortality rates and reduced reproductive success8,9,33 suggests the potential for deleterious population-level impacts. Beak deformities in other species have been attributed to a variety of toxic, nutritional, infectious, and traumatic etiologies, although precise mechanisms are often difficult to determine.10,30 Underlying pathologic changes associated with beak deformities in black-capped chickadees have not yet been described and it is unknown whether this disease affects multiple keratinized tissues or is primarily restricted to the beak.
Here we document the gross, microscopic, and ultrastructural features of avian keratin disorder in affected black-capped chickadees. Our objectives were to characterize the suite of lesions associated with this disease, screen for potential pathogens, and establish the basis for future investigations.
Materials and Methods
Study Animals and Gross Pathology
In spring and fall 2008 and spring 2011, we used funnel traps and mist nets to capture adult black-capped chickadees from several locations in south-central and interior Alaska.8 We examined beaks, claws, skin, and feathers for gross abnormalities. Standard beak measurements included nares-to-tip (anterior end of right nare to tip of the upper beak), gonys (base of the gonys to the tip of the lower beak), overbite (chord of upper beak beyond the lower beak), underbite (chord of the lower beak beyond the upper beak), and gapping (maximum distance between the lateral margins of the upper and the lower beak). We used the case definition presented by Handel et al.8 to identify gross beak deformities. Briefly, a beak was considered abnormal if it met one or more of the following criteria: nares-to-tip >8.5 mm, overbite or underbite >1.0 mm, or lateral deviation of the upper and lower beak.8 We then assigned three categories of severity based on nares-to-tip and/or gonyl measurements: mild (≤9.0 mm), moderate (9.1–14.9 mm), and severe (≥15.0 mm).
A total of 30 birds with gross beak deformities was included for examination. Twenty-two birds with normal beaks were selected as controls and provided baseline information on normal microanatomy and ultrastructure of the chickadee beak and claw.32 A subset (n = 16 affected, n = 14 controls) of the birds used in this study was held in captivity for up to five months at the University of Alaska Fairbanks (UAF) Biological Research and Diagnostics Facility as part of a separate investigation prior to post-mortem examination.33 All birds that died while in captivity (n = 9 affected) were necropsied immediately by UAF Veterinary Services personnel. All other individuals were euthanized with isoflurane using the open-drop method upon capture or at the termination of the captive study and kept cool (~4° C) until necropsy.
This study was completed under guidance of the UAF and the USGS Alaska Science Center Institutional Animal Care and Use Committees (Assurance #07-49, 08-57). Measurements are reported as means ± standard deviation.
Radiography
High-magnification radiography (Dage XD7500NT, Nordson Corporation, Westlake, OH) was used to examine the underlying bone structure of the beak from a subset of affected birds (n = 18) and controls (n = 14).32 Images of the head and beak were collected at 20–100× magnification (40kV, 2.8W) using frame averaging (n = 256) with Dage XiDAT software (Nordson Corporation).
Histopathology
All major tissues and organ systems (brain, spinal cord, eyes, heart, lung, kidneys, liver, pancreas, adrenal gland, axial and appendicular bone, skeletal muscle, proventriculus, ventriculus, and intestine) from five affected birds and three controls were examined using light microscopy. To rule out hepatic disease, which has been proposed as a cause of beak hyperkeratosis in other species,10 liver tissues from an additional 10 affected animals and 10 controls were examined. For detailed histopathology of keratinized tissues, beak, claw, skin, feather follicles, and epithelial lining of nasal sinuses were sampled from 25 affected animals and 19 controls (keratinized tissues from the other 5 affected birds and 3 controls were processed separately for electron microscopy). Tissues were fixed in 10% neutral buffered formalin, processed using an automated tissue processor (Shandon Citadel 2000; GMI Inc., Ramsey, MN), and embedded in paraffin blocks (Reichert-Jung Model 8040 Tissue Embedding Center with vacuum; Reichert Technologies, Buffalo, NY). We used methods described in Van Hemert et al.32 to decalcify and soften beaks and claws. Beak tissues were trimmed for sectioning along either the mid-sagittal (n = 20 affected, n = 14 controls) or transverse plane (n = 5 affected, n = 5 controls). For transverse sections, the upper and lower beaks in apposition were cut at four reference locations based on the upper beak: immediately proximal to and 1, 3, and 5 mm from the distal edge of the external nares. Skin, feather follicles, and sinuses were included with head and beak sections. The hallux was embedded whole and the block was faced by serial sectioning to the mid-sagittal plane. Blocks were cut into 5-µm sections using an American Optical 820 Spencer microtome (American Optical, Buffalo, NY), mounted onto charged glass slides (VWR Superfrost Plus Micros Slide; VWR International, Radnor, PA), and stained with hematoxylin and eosin using standard procedures.20 Select sections were stained with Masson’s trichrome stain for keratin, collagen, and bone (American Master Tech Scientific, Lodi, CA), Periodic acid Schiff stain for carbohydrates (PAS; American Master Tech Scientific), phosphotungstic acid hematoxylin stain for fibrin, collagen, and elastin (American Master Tech Scientific), Gram stain for bacteria (Newcomer Supply, Middleton, WI), Grocott Methenamine Silver stain for fungi (GMS; Ventana Medical Systems, Tucson, AZ), Ziehl-Neelsen acid-fast bacilli stain (Newcomer Supply), and Steiner stain for spiral bacteria (Ventana Medical Systems), according to each manufacturer’s specifications. External laboratory controls and positive staining within slides were used to confirm the reliability of these stains.
Transmission Electron Microscopy
Transmission electron microscopy (TEM) was used to screen for pathogens and to resolve uncertainties in the characterization of lesions that were identified with histopathology in five affected and three unaffected chickadees. Pathogen screening was conducted by negative and positive staining techniques applied to sections from multiple tissues. Detailed ultrastructural tissue examination of keratinized tissues targeted lesions identified with light microscopy, including areas of hyperkeratosis and the junction between the inner and outer cornified plates. All assessments were made relative to controls.
Fresh feather, skin, liver, kidney, lungs, and intestinal tissues were sampled for virus screening with negative staining. Tissues were placed in 1 ml of double-distilled water, then homogenized to produce an opalescent suspension and centrifuged at 4000 rpm (Eppendorf, Hamburg, Germany). Supernatant was transferred to airfuge tubes (Belkman Coulter, Brea, CA) and centrifuged at 30 psi using an airfuge (Belkman Coulter) for 10 min. Supernatant was then removed and the pellet was reconstituted with 10 µl of double-distilled water. We transferred 5 µl of the sample to parafilm and then placed formvar-coated copper grids (Electron Microscopy Sciences, Hatfield, PA) on top of the drop for 10 min. Excess liquid was wicked and the grids were stained with 1% phosphotungstic acid (Electron Microscopy Sciences) for 1 min.
For examination of tissue ultrastructure, beak, nail, and skin were fixed in 10% buffered formalin immediately after euthanasia. Beak and nail tissues were then decalcified in EDTA for a maximum of 8 weeks. Transverse sections of preselected areas of the beak were collected at two locations: approximately 2 mm and 5 mm distal of the nares (at the tip of the premaxillary and mandibular bones). Thereafter, samples were post-fixed in 0.166 M cacodylate-buffered, 3% glutaraldehyde with 1% tannic acid solution (Electron Microscopy Sciences), followed by a second postfixation treatment in 1% osmium tetroxide (Electron Microscopy Sciences). Using a graded series of ethyl alcohol, 1.0-mm3 tissue blocks were dehydrated and then embedded in Embed (Electron Microscopy Sciences). Embedded samples were trimmed and sectioned on a Leica UC6 Ultramicrotome (Leica Microsystems). Thin sections (60–90 nm) were collected on 100-mesh copper grids (Electron Microscopy Sciences), then grids were stained with 5% uranyl acetate for 20 min and Satos’ lead citrate for 6 min.
We examined samples with a JEOL 1200 EX II transmission electron microscope (JEOL LTD, Tokyo, Japan) and obtained images using a Veleta 2K × 2K camera with iTEM software (Olympus SIS, Munster, Germany).
Results
Gross Pathology
In affected birds the rhamphotheca was elongated, compared to that of controls, with varying degrees of lateral deviation, crossing, and gapping between the upper and lower beak. Deformities ranged from mild overgrowth to severe crossing and elongation (Figs. 1a, 2a, 3a). At necropsy, beak measurements of affected birds (n = 30) averaged 12.7 ± 5.2 mm (6.3–25.0 mm) from nares to tip and 9.4 ± 3.3 mm (6.3–18.9) mm along the gonys. For control beaks (n = 22), the mean nares-to-tip length was 7.3 ± 0.3 mm (6.0–7.8 mm) and mean gonys length was 6.5 ± 0.4 mm (6.0–7.9 mm). In affected birds, the surface of the beak was typically rough and irregular, with raised lateral ridges and discoloration (pale or white compared to normal black pigmentation). The superficial layers of the hard-cornified beak epidermis were also sometimes thickened or exhibited flaking or peeling. Upon histologic sectioning, hard-cornified beak tissues of affected birds were noticeably more brittle and more easily fractured than those of unaffected birds.
Fig. 1.
a. Black-capped chickadee with mild overgrowth of the upper beak.
b. Radiograph of head and beak; black-capped chickadee with mild overgrowth of the upper beak. Note slight beak elongation relative to underlying bone core.
c. Radiograph of beak; black-capped chickadee with mild overgrowth of the upper beak. Note lack of bone remodeling at the tips of the premaxillary and mandibular bones (arrows).
Fig. 2.
a. Black-capped chickadee with moderate overgrowth of the upper beak.
b. Radiograph of head and beak; black-capped chickadee with moderate overgrowth of the upper beak. Note beak elongation relative to underlying bone core.
c. Radiograph of beak; black-capped chickadee with moderate overgrowth of the upper beak. There is minor bone remodeling at the tips of the premaxillary and mandibular bones (arrows).
Fig. 3.
a. Black-capped chickadee with severe overgrowth of the upper and lower beak.
b. Radiograph of head and beak; black-capped chickadee with severe overgrowth of the upper and lower beak. Note severe beak elongation relative to underlying bone core.
c. Radiograph of beak; black-capped chickadee with severe overgrowth of the upper and lower beak. There is significant bone remodeling at the tips of the premaxillary and mandibular bones (arrows), resulting in recurved and decurved appearance.
Among 30 birds with beak deformities, 5 (17%) had one or more obviously elongated claws and 3 (10%) had other claw abnormalities, including thickening, raised lateral ridges, and peeling of superficial layers. Macroscopic claw lesions were also identified in 2 of 25 birds with normal beaks. One of these (5%) had a slightly elongated hallux and the other (5%) showed lateral ridges in several claws. Both of these birds had grossly normal claws at capture and developed lesions while in captivity; the appearance of the beak remained normal and thus they were assessed as controls.
Mild to severe alopecia, xerosis, scaling, and/or exudation were present on the head, face, lores, or abdomen in 25 of 30 (83%) affected birds; lesions were most severe in birds previously housed in captivity. Of the 22 control birds, 3 (14%) that had been previously housed in captivity had mild skin lesions.
Radiography
The underlying bones of the beak were of approximately normal length (Figs. 1b, 2b, 3b), but bone deformation at the tip of the premaxilla and/or mandible was evident in 15 of the 18 (83%) affected birds that were examined by high-magnification radiography. Deviation from the frontal plane toward superficial aspects of the beak resulted in recurved and decurved appearance of the premaxilla and mandible, respectively (Figs. 2c, 3c). In most of these cases, the margins of the distal tip of the bone were indistinct or irregular, which contrasted with the clearly demarcated, consistent margins observed in birds with normal beaks. Severity of bone lesions was typically proportional to the amount of beak overgrowth (Figs. 1–3).
Histopathology
Beaks and claws
Hyperkeratosis occurred in the cornified plate of nearly all abnormal beaks (Table 1). Severity of hyperkeratosis varied widely, ranging from slight thickening of the cornified epidermis to thickening and elongation to more than triple the beak’s normal size (Figs. 4a, 5a, 6a). The presence and severity of other abnormal features, including hyperplasia and dermal and osseous lesions, typically correlated with the relative amount of beak overgrowth (Figs. 4b, 5b, 6b).
Table 1.
Severity of histopathologic lesions observed in the beak of black-capped chickadees with mild, moderate, or severe beak overgrowth. Columns show number of animals with lesions and average severitya of lesion for each group. Similar lesions were not observed in controls
| Lesion | Mild (n = 7) |
Moderate (n = 8) |
Severe (n = 10) |
|||
|---|---|---|---|---|---|---|
| Cornified plate | ||||||
| Hyperkeratosis | 7 | + | 8 | ++ | 10 | +++ |
| Focal retention of nucleus | 5 | + | 8 | ++ | 10 | ++ |
| Debris in junctional line | 2 | + | 8 | ++ | 10 | +++ |
| Laminar disorganization | 5 | + | 8 | ++ | 10 | +++ |
| Increased exfoliation/fracturing | 4 | + | 8 | ++ | 10 | ++ |
| Germinative layer | ||||||
| Hyperplasia | 5 | + | 8 | + | 10 | ++ |
| Dermis | ||||||
| Edema/fibrosis/increased cellularity | 4 | + | 8 | + | 10 | +++ |
| Neovascularization | 4 | + | 8 | ++ | 10 | ++ |
| Hyperpigmentation | 4 | + | 7 | + | 10 | ++ |
| Bone | ||||||
| Remodeling | 4 | + | 8 | ++ | 10 | +++ |
| Myelofibrosis | 2 | + | 8 | ++ | 10 | +++ |
Severity defined as mild (+), moderate (++), or severe (+++).
Fig. 4.
a. Beak; black-capped chickadee with mild beak overgrowth. Note mild hyperkeratosis (arrow). HE.
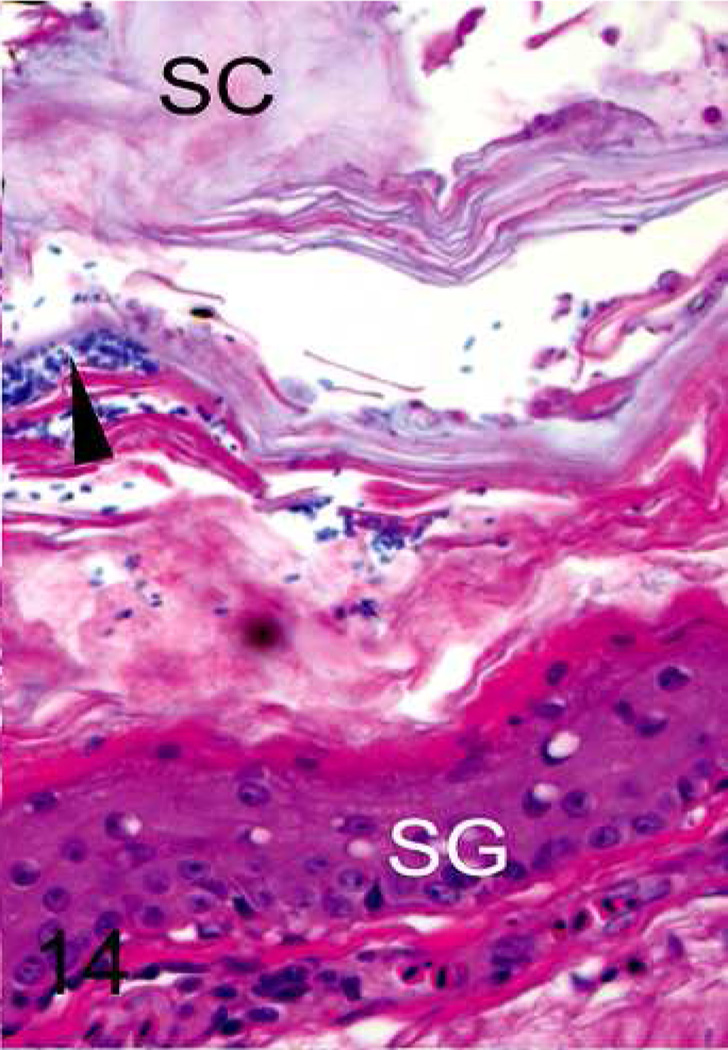
b. Upper beak; black-capped chickadee with mild beak overgrowth. There is mild hyperkeratosis of the stratum corneum (SC), mild hyperplasia in the stratum germinativum (SG), and a slightly irregular margin at the tip of the premaxilla (arrowhead). HE.
Fig. 5.
a. Beak; black-capped chickadee with moderate beak overgrowth. Note obvious hyperkeratosis (arrow). HE.
b. Upper beak; black-capped chickadee with moderate beak overgrowth. There is moderate hyperkeratosis of the stratum corneum (SC), mild hyperplasia in the stratum germinativum (SG), preexisting and neovascularization (asterisk), myelofibrosis (m), and slight remodeling at the tip of the premaxilla (arrowhead). HE.
Fig. 6.
a. Beak; black-capped chickadee beak with severe beak overgrowth. Note severe hyperkeratosis (arrow), extensive eosinophilic lines that represent sequestrations of incompletely differentiated corneocytes, and core-like intrusions along the junctional line of the inner and outer cornified plates. HE.
b. Upper beak; black-capped chickadee with severe beak overgrowth. There is severe hyperkeratosis of the stratum corneum (SC), moderate hyperplasia in the stratum germinativum (SG), neovascularization (asterisk), fibrosis, and severe remodeling of the premaxillary bone (arrowhead). Clusters of eosinophilic cells with altered morphology and retention of nuclear material are evident in the stratum corneum (arrow). HE.
Examination of the stratum corneum in normal beaks with polarized light microscopy revealed a typical linear arrangement of cells (Fig. 7). By comparison, the stratum corneum in affected beaks appeared disorganized, as indicated by attenuation and irregular, swirling, and scalloped patterns of birefringence (Fig. 8).32 Individual corneocytes were disarrayed along the juncture between the external and buccal plates in birds with moderate to severe beak overgrowth. There was increased exfoliation of the cornified laminae along external surfaces of the beak in affected birds. Microorganims were occasionally observed in these fissures; two (8.0%; n = 25) specimens had isolated pockets of Gram-positive cocci and three (12%; n = 25) other specimens had PAS-positive fungi in the superficial layers of the upper or lower beak.
Fig. 7.
a. Polarized light micrograph of upper beak; black-capped chickadee with normal beak. Dorsal (DP) and buccal (BP) plates cover the premaxillary bones (Mx) of the upper beak. Note the linear organization of the stratum corneum and the proportional thickness of the dorsal plate (double-headed arrow). HE.
b. Polarized light micrograph of lower beak; black-capped chickadee with normal beak. Buccal (BP) and ventral (VP) plates cover the mandibular bones (Md) of the lower beak. Note the linear organization of the stratum corneum. HE.
Fig. 8.
a. Polarized light micrograph of upper beak; black-capped chickadee with moderate beak overgrowth. Note disorganized appearance of the stratum corneum, indicated by attenuation and irregular patterns of birefringence and disarray and separation (asterisk), between the dorsal (DP) and buccal (BP) plates that cover the maxillary (Mx) bones. The proportional thickness (double-headed arrow) of the dorsal plate is significantly increased relative to the normal appearance shown in Fig. 7a. HE.
b. Polarized light micrograph of upper beak; black-capped chickadee with moderate beak overgrowth. Note disorganized appearance of the stratum corneum, indicated by attenuation and irregular, swirling, and scalloped patterns of birefringence (arrowheads) and disarray and separation (asterisk), between the buccal (BP) and ventral (VP) plates that cover the mandibular bones (Md). HE.
Cells that resembled those from the stratum transitivum, characterized by retention of the nucleus, increased eosinophilia, and polyhedral rather than flattened morphology (Fig. 9), occurred frequently in the stratum corneum of affected beaks. These transitional cells were often concentrated near the distal tip of the dermis, along the margins of the juncture between the inner and outer cornified plates, and, in some cases, sequestered in linear formations parallel to the germinal epithelium (Fig. 6). Globular, lacuna-like lesions containing amorphous, eosinophilic material were also common in the stratum corneum of abnormal beaks (Fig. 10). These lacunae were diffusely distributed along the cornified laminae and occupied spaces varying from the size of a single corneocyte (~10 µm) to more than 150 µm in length.
Fig. 9.
Lower beak; black-capped chickadee with severe beak overgrowth. Insets on the right correspond to layers shown in boxes, from top to bottom: a region of the stratum corneum (SC) showing corneocytes with normal appearance; a region of the stratum corneum with incompletely differentiated and eosinophilic corneocytes; the stratum germinativum (SG); and the interface between the dermis (De) and mandibular bone (Md). Note increased eosinophilia, polyhedral (rather than flattened) morphology, and incomplete differentiation of corneocytes (asterisk) in focal areas of the stratum corneum. Hyperplasia and disorganization of basal cells (black arrow) are evident in the stratum germinativum. Osseous changes include increased cellularity and active osteocytes (white arrowhead), and production of woven bone. Hyperpigmentation is evident in the dermis and marrow (black arrowheads). HE.
Fig. 10.
Lower beak; black-capped chickadee with severe beak overgrowth. Hyperkeratosis of the stratum corneum (SC), hyperplasia of the stratum germinativum (SG), and significant remodeling of the mandibular bone (Md) are evident. The buccal plate (BP) is thickened (double-headed arrow) relative to normal appearance. Insets above highlight two specific changes associated with beak hyperkeratosis: lacuna-like lesions in the stratum corneum (upper left box) and a core-like intrusion between the buccal (BP) and ventral (VP) plates (lower right box). Left inset shows eosinophilic globules (arrow) that consist of keratin debris in the stratum corneum. Right inset shows highly nucleated material consisting of red and white blood cells and necrotic corneocytes in the core-like intrusion. HE.
A core-like intrusion that originated near the distal tip of the dermis and continued along the junctional line of the external and buccal plates of the stratum corneum, often persisting to the tip of the beak, was common in affected birds (Fig. 10), especially in those with moderate to severe beak overgrowth. This characteristic lesion consisted of a columnar aggregate of tightly clustered nuclear remnants of red and white blood cells and amorphous, eosinophilic debris, sometimes interspersed with eosinophilic globules (Fig. 10). No organisms were detected with PAS, Gram, Ziehl-Neelsen acid-fast, GMS, and Steiner staining of this material and no other evidence of microbes was detected in these areas. Similar lesions were not observed in controls.
Mild to moderate hyperplasia and, occasionally, mild focal dysplasia were present in the germinal epithelium of the beak in affected animals (Fig. 9). In some focal areas, typically near the distal apex of the dermis, the basal layer increased from a single band of keratinocytes to a disorganized stratum several cells thick. In many cases, these basal cells were also hypertrophic and exhibited irregular, prismatic morphology compared to the normal cuboidal shapes observed in controls. Seven of 25 (28%) affected animals and no controls presented with intranuclear, eosinophilic inclusions (Fig. 11), sometimes accompanied by intracytoplasmic, eosinophilic inclusions, in the germinative layer of the beak. These inclusions were not widespread and were difficult to locate. Material recovered from paraffin blocks was not suitable for electron microscopy and additional study will be required to determine the nature of the inclusions and if viral particles are present.
Fig. 11.
Epidermis of upper beak; black-capped chickadee with severe beak overgrowth. Arrows show examples of intranuclear, eosinophilic inclusions that were observed in the germinative layer of the beak of some affected birds. HE.
Lesions consistently observed in the dermis of affected beaks included neovascularization, edema, fibrosis, and hyperpigmentation (Figs. 5b, 6b, 9). In some cases mild inflammation, perineuritis, and axonal degeneration and demyelination were also present. Lesions typically occurred near the distal tip of the dermis and were most notable in severely affected birds. There were no dermal lesions in birds with normal beaks.
Osseous lesions, including focal bone lysis, new bone formation, osteomyelitis, and myelofibrosis, occurred in the underlying bones of the beak in most affected birds (Figs. 5b, 6b). These lesions typically were found near the tip of the premaxilla or the mandible. Affected beaks had increased scalloping along the external margins of lamellar bone, disconnected fragments of highly remodeled tissue, osteoclastic resorption, hypercellularity, and localized proliferation of woven bone (Figs. 6b, 9). These features contrasted with the more quiescent, continuous lamellar bone structure typical of controls.32 In affected beaks, marrow spaces often contained necrotic debris, fibrosis, and inflammatory cells. The most prominent bone lesions occurred in animals with severe beak overgrowth (Table 1).
Among affected birds (n = 25), there often was mild to moderate hyperkeratosis (40%) and occasionally mild hyperplasia (16%) in the epithelial lining of the nasal sinuses, typically in association with bacteria or fungi (Fig. 12). These features were more common among birds previously housed in captivity (n = 8) but also occurred in some wild-caught birds (n = 2). In 2 cases (1 captive, 1 wild), mild lymphoplasmatic infiltration was also present. Four birds with normal beaks (21%; n = 19), all of which had been previously housed in captivity, exhibited bacterial infection with mild hyperkeratosis in the sinuses.
Fig. 12.
Nasal sinus; black-capped chickadee with mild beak overgrowth. There is severe hyperkeratosis and bacterial infection (asterisk). HE.
Mild hyperkeratosis occurred in claws of most affected birds (68%; n = 25) and in claws of several controls (21%; n = 19). Core-like intrusions of nuclear remnants and amorphous debris, similar to those described in beaks, were observed in claws of a high proportion of birds with abnormal beaks (64%; n = 25) and more than one third of controls (37%; n = 19) (Fig. 13). These lesions originated near the dermal apex and typically continued along the juncture of the dorsal and ventral plates of the claw, often extending to its distal tip. No microorganisms were detected in claw tissues, but intranuclear, eosinophilic inclusions, similar to those found in the beak, occurred in the germinative layer of the claw in several affected birds. The staining and refractive properties of lesions in the stratum corneum of the claw under polarized light were nearly identical to those of lesions in the beak. However, claws were more difficult to process for histology than beaks and the quality of these slides was not adequate to consistently evaluate the germinative layer, dermis, or bone.
Fig. 13.
Claw; black-capped chickadee with moderate beak overgrowth. There is mild hyperkeratosis of the dorsal plate (double-headed arrow) with clusters of eosinophilic corneocytes (arrowhead) in the stratum corneum. Inset corresponds to the box in the main figure and shows a core-like intrusion of highly nucleated material along the juncture between the dorsal and ventral plates (asterisk). This material is composed of red and white blood cells and degenerating corneocytes. HE.
Skin and feathers
Lesions in skin and feather follicles of the head were observed in all affected birds (n = 25) and in several controls (32%; n = 19). These lesions were characterized by hyperkeratosis, occasional hyperplasia, and the presence of microbes (Figs. 14, 15). Similar to macroscopic observations, microscopic lesions were most remarkable in birds with severe beak overgrowth, particularly those that had been previously housed in captivity. Mild and moderate cases were restricted to the superficial layers of the epidermis without a visible dermal response. Severe cases included inflammation of the dermis, with lymphoplasmatic infiltration and mild to moderate edema. Lesions in birds with normal beaks were mild and occurred only among the subset of birds previously housed in captivity. Lesions in affected free-ranging birds were mild or moderate, whereas lesions in affected birds previously housed in captivity ranged from mild to severe. Skin samples from two captive birds with severe lesions were submitted to the Washington Animal Disease Diagnostic Laboratory for microbial analysis; both samples had mixed bacterial growth that was dominated by coagulase-negative Staphyloccocus species but also included Penicillium species.
Fig. 14.
Skin of the head; black-capped chickadee with severe beak overgrowth. Hyperkeratosis in the stratum corneum (SC) and hyperplasia in the stratum germinativum (SG) are associated with staphylococcal bacteria (arrowhead). HE.
Fig. 15.
Skin of the head; black-capped chickadee with moderate beak overgrowth. Hyperkeratosis (arrows) of the skin and feather follicles (FF) is associated with yeast-like microorganisms (arrowhead; inset). HE.
Other tissues
Lesions were observed in several other tissues but these occurred inconsistently. One affected specimen exhibited a focal, moderate area of necrosis with histiocytic infiltration in skeletal muscle of the breast and mild, multifocal lymphocytic portal hepatitis. One affected and one unaffected specimen each had multifocal, moderate areas of necrosis with histiocytic infiltration in the myocardium and a small area of coagulative necrosis of the mucosa in the proventriculus. A lipoma removed from the subcutis of the furcular hollow of one severely affected bird had well-differentiated adipocytes encapsulated within multiple lobules. All other tissues were unremarkable.
Transmission Electron Microscopy
Electron microscopy did not reveal consistent evidence of viruses in affected animals. Although viral particles were detected in several birds, these differed among individuals in both location and appearance. In thin sections, intracytoplasmic virus-like particles 80–100 nm in diameter were present in mesenchymal cells of the dermis of the upper beak of one moderately affected bird and virus-like particles ~100 nm in diameter were free in the intercellular space in the germinal epithelium of the lower beak of one severely affected bird. Negative staining revealed 90–100 nm non-enveloped icosahedral particles of the Family Adenoviridae in the liver of one mildly affected bird and in the kidney of another mildly affected bird. Viral particles were not detected in any other tissues of affected birds or controls.
Examination of selected areas of affected beaks with TEM showed focal and generally mild morphological changes relative to controls. In affected beaks, corneocytes located in the hyperkeratotic stratum corneum were often swollen, had lightly indented cell membranes, and retained more chromatin and nuclear debris than normal corneocytes (Figs. 16, 17). However, similar to results from light microscopy, lesions were detected with TEM in only some areas of the stratum corneum in abnormal beaks. In other regions of the stratum corneum, particularly near the base of the beak, the ultrastructure of the corneocytes was indistinguishable between affected animals and controls. The appearance and amount of s-keratin bundles and melanin granules contained within intact corneocytes did not differ between affected beaks and controls upon qualitative examination. In addition, organelles and intercellular attachments of corneocytes from affected animals were not obviously abnormal. Cells from the hyperplastic germinative layer in affected beaks were normal in appearance in most cases (Fig. 18). Occasionally, intranuclear cytoplasmic invaginations composed of dense packets of fine filaments were observed in the cells of the stratum germinativum of abnormal beaks. Additional study is required to determine if these features correspond with the eosinophilic, intranuclear inclusions that were visible with light microscopy. Ultrastructural changes observed in the dermis and bone were similar to histologic lesions and there was evidence of active bone remodeling and edema (Fig. 19).
Fig. 16.
Electron micrograph of the stratum corneum of the beak epidermis; black-capped chickadee with severe beak overgrowth. Picture corresponds to the superior inset in Fig. 9 that shows corneocytes using light microscopy. Note indentation of plasma membranes along the margins of the cell (arrowheads) and retention of nuclear material (arrow). Uranyl acetate-lead citrate.
Fig. 17.
Electron micrograph of the stratum corneum of the beak epidermis; black-capped chickadee with severe beak overgrowth. Picture corresponds to the second inset from the top in Fig. 9 that shows corneocytes with increased cell eosinophilia and polyhedral morphology. These incompletely differentiated corneocytes have abundant, densely packed bundles of β-keratin (arrowheads show cell margins), and retain nuclear material (arrows). Uranyl acetate-lead citrate.
Fig. 18.
Electron micrograph of the stratum germinativum of the beak epidermis; black-capped chickadee with severe beak overgrowth. Picture corresponds to the third inset from the top in Fig. 9. Note relatively unaltered appearance of the cells of the hyperplastic germinative layer. Arrows show margins of a columnar basal cell with intact nucleus (white asterisk) in the stratum basale (SB). A maturing keratinocyte (black asterisk) in the stratum intermedium (SI) has normal ultrastructure. Uranyl acetate-lead citrate.
Fig. 19.
Electron micrograph of the interface between the dermis (De) and reactive bone tissue in the premaxillary bone of the beak; black-capped chickadee with severe beak overgrowth. Picture corresponds to the bottom inset in Fig. 9. Note osteocyte (arrow) surrounded by newly formed extracellular matrix (Ex) and an active osteoblast (asterisk) at the margin of the bone. Melanin granules are visible in the processes of pigmented cells (arrowheads) within an edematous dermis. Uranyl acetate-lead citrate.
The characteristic core-like intrusions that occurred along the juncture between the external and buccal plates of the stratum corneum consisted of free-floating erythrocytes, inflammatory cells, keratinocytes, and corneocyte debris (Fig. 20). These cells and associated debris were not confined within an endothelium and were in direct contact with adjacent corneocytes of the stratum corneum. Increased degeneration of corneocytes was also evident along the margins of this juncture in affected beaks. Ultrastructural examination did not reveal any evidence of microorganisms in these areas, confirming results from special stains and light microscopy. The lacuna-like lesions observed along laminae of the stratum corneum were consistent with degenerated or necrotic corneocytes (Fig. 21). When examined with electron microscopy, degenerated corneocytes showed a more electron-dense cytoplasm with disorganized, variably dense packets of keratin filaments. In addition, these sometimes formed small to large granular and finely filamentous aggregations that corresponded with the eosinophilic globules visible with light microscopy.
Fig. 20.
a. Stratum corneum of the beak epidermis; black-capped chickadee with severe beak overgrowth. There is a core-like intrusion of red and white blood cells and necrotic corneocytes between the dorsal and buccal plates of the upper beak. Box highlights region examined with electron microcopy in Fig. 20b. Toluidine blue.
b. Electron micrograph of core-like intrusion in the stratum corneum of the beak epidermis; black-capped chickadee with severe beak overgrowth. Arrowheads show cell margin of a degenerated corneocyte that contains amorphous globules of keratin debris (asterisks). Uranyl acetate-lead citrate.
Fig. 21.
a. Lacuna-like lesions (asterisk) in the stratum corneum of the beak epidermis; black-capped chickadee with severe beak overgrowth. Asterisk corresponds to area examined with electron microscopy in Fig. 21b. Toluidine blue.
b. Electron micrograph of a lacuna-like lesion in the stratum corneum of the beak epidermis; black-capped chickadee with severe beak overgrowth. There is a degenerating corneocyte (asterisk) visible in the stratum corneum. Note dense and retracted cytoplasm with tubular and filamentous arrays and melanin granules (arrow). Uranyl acetate-lead citrate.
Discussion
This study was prompted by a recent outbreak of beak deformities in thousands of black-capped chickadees and other wild birds in Alaska. Reports of beak abnormalities are not uncommon in the published literature, but these are typically limited to a single gross observation or clinical case without corresponding in-depth pathologic examination and description. Detailed study of the beak and other keratinized tissues has been conducted in association with certain toxicoses,23 nutritional disorders,22,36 and viral diseases,5,26,28 which typically exhibit distinctive features. The gross, microscopic, and ultrastructural lesions that we observed in affected black-capped chickadees do not closely resemble characteristics of these or other known avian diseases but instead suggest a novel hyperkeratotic disorder in wild birds.
The microscopic changes that we observed in affected chickadees are consistent with rapid production of keratin resulting in overgrowth of the rhamphotheca. The most characteristic features of this disorder occurred in the beak epidermis and included hyperplasia and hyperkeratosis. Also there were discrete, multifocal clusters of corneocytes with altered morphology and increased retention of chromatin. These cells looked similar to those from the stratum transitivum and may have been the product of rapid differentiation associated with accelerated epidermal growth. This explanation concurs with results from a previous study in which we documented increased rates of beak growth in chickadees with beak overgrowth and deformity.33 The location of these less mature cells, typically near the tip of the dermis and in distal regions of the stratum corneum, also corresponds to the dominant longitudinal growth of the beak epidermis.21,29,33
Other lesions in affected beaks were suggestive of disruption of the integrity of the cornified plate of the beak. We consistently encountered increased brittleness and fracturing during processing and observed disorganization of the cornified laminae with polarized light. Microscopically, affected beaks showed degeneration of corneocytes, resulting in lacuna-like lesions throughout the stratum corneum and altered structure along the margins of the juncture between the inner and outer plates of the beak. However, intracellular components of intact corneocytes, including s-keratin bundles, did not differ ultrastructurally between normal and affected beaks. Additional analysis of beak tissue is necessary to identify what mechanisms are responsible for alterations to the cornified epidermis.
The core-like intrusions that were common in birds with beak deformities consisted of red and white blood cells and corneocyte debris. These lesions occurred in a restricted region of the stratum corneum where inner and outer horn layers merge. In a normal beak, columns of transitional cells form a structurally significant junction at this site,18,32 which presents a break in the otherwise continuous barrier formed by laminae of the hard-cornified epidermis. Thus, this junction may provide a natural channel through which free-floating cells and necrotic debris can be routed. Neovascularization at the tip of the dermis was a common finding in affected birds and it is possible that hemorrhage due to trauma or chronic strain associated with overgrowth of the beak could partially explain the presence of erythrocytes in these areas. Chronic strain to the beak tip may also contribute to the infiltration of inflammatory cells that we observed in the dermis and along the junctional line of the beak. In moderately to severely affected birds, the core-like intrusions of necrotic material sometimes continued all the way to the distal tip of the beak. Despite this potential breach of the beak integument, we did not detect any microorganisms within these areas.
In addition to abnormalities in the epidermis, there was significant bone remodeling as well as various osseous and dermal lesions, particularly in birds with moderate to severe beak overgrowth. This pattern could be explained by one of two competing hypotheses: (1) osseous and dermal lesions are associated with the primary pathogenesis of beak deformities and may subsequently induce abnormal growth of the beak epidermis or, (2) osseous and dermal lesions are a secondary consequence of trauma associated with a grossly elongated beak. Histologic and radiographic examination of cases across a wide range of severity and with known duration for some individuals allowed us to evaluate these possibilities. Due to the identification of this disorder via beak overgrowth, which typically requires at least several weeks to be detectable macroscopically,33 we considered all lesions in affected birds to be chronic. However, several birds with slight deformities had no dermal or osseous lesions, which suggested that epidermal lesions may precede changes in other tissues of the beak. Similarly, birds known to have had a relatively recent onset of deformities (<6 months) had no or only mild lesions in the bone and dermis. Generally, the most severe osseous and dermal lesions occurred in birds with the most pronounced beak overgrowth. In addition, the recurved and decurved appearance of the premaxilla and mandible, respectively, correlates with the direction of torque that would be exerted on rhamphothecal tips in grossly elongated beaks. It is therefore plausible that bone remodeling may occur in response to increased mechanical strain associated with such forces. Based on these observations, we suspect that osseous and vascular changes are secondary consequences rather than primary lesions of the disorder. It is also likely that lesions in all beak tissues (epidermis, dermis, bone) may be further exacerbated by amplification of the inflammatory response associated with repeated, chronic stress to the elongated beak. Although logistically challenging due to the difficulty of early detection in wild birds, repeated radiographic examination from the point of onset to development of severe lesions, combined with targeted histopathology to determine the nature of the lesion, would be useful for evaluating this pathologic sequela.
There was some evidence that multiple keratinized tissues were affected by this disorder. Claws, which share many biochemical and structural properties with beaks,2,6,13 had similar pathology. Although gross overgrowth of the claw was observed in only a small number of affected individuals, there was frequent occurrence of mild hyperkeratosis and core-like intrusions of red and white blood cells and corneocyte debris similar to those found in the beak upon microscopic examination. The occurrence of nearly identical lesions in the claw suggests that both of these tissues are affected similarly, despite the more conspicuous presentation in the beak. Claw length was not explicitly measured so overgrowth may have occurred more frequently than was detected macroscopically. In addition, because claws are thinner and more fragile than beaks, claws may be more likely to break when they become elongated, potentially masking the effects of hyperkeratosis. Surprisingly, there was a relatively high rate of core-like intrusions in claws of birds with normal beaks. In most of these cases, there were no macroscopic lesions observed, although this may have been due in part to the difficulty of detecting overgrowth in claws as described above. The presence of these lesions in claws of birds with normal beaks suggests that the disorder may manifest first in either the beak or the claw, and may not immediately result in changes that are grossly detectable. Given the resemblance between the lesions in beaks and claws, this explanation seems likely. However, the core-like lesions observed in the claw may not be unique to this disorder and could be explained by other etiologies. For example, if hemorrhage is an important factor for producing such lesions, breakage of claws due to normal wear and tear could result in similar presentation.
Hyperkeratosis and hyperplasia in skin, feather follicles, and sinus epithelium occurred more frequently and more severely in birds with beak deformities than in those with normal beaks. However, we observed bacteria and/or fungi in nearly all of these cases, suggesting that epidermal lesions are likely to be secondary consequences of opportunistic infection. Bacterial and fungal infections in skin, feather follicles, and sinuses of birds with beak deformities could be related to proximate causes such as compromised preening ability, poor nutritional status, or reduced immune function,3 which may be directly or indirectly related to this disorder. Although not explicitly assessed in this study, the integrity of skin integument may also be compromised, thus reducing the skin’s ability to serve as a natural barrier. The α-keratins in skin, feathers, and sinus epithelium are of a different structure than the β-keratins of the beak and claws,13 but processes of keratinization are similar and may be subject to some of the same controls.12 In general, epidermal lesions were more severe in affected birds housed in captivity than birds sampled directly from the wild. Captive conditions, including warmer ambient temperatures, may have promoted microbial growth, and may partially explain the observed differences in severity. However, the presence of similar lesions in both groups suggests that these are not merely a product of captivity and may be important secondary consequences of this disorder. Given the harsh environmental conditions of winter in Alaska, epidermal infection could significantly compromise a bird’s ability to thermoregulate and may contribute to mortalities, thus reducing the likelihood that we would encounter free-ranging birds with severe lesions.
There was no consistent evidence of bacterial, fungal, or viral pathogens that could explain the etiology of this disorder. These results correspond with previous diagnostic testing of affected chickadees.8 However, further research is needed to evaluate whether intranuclear inclusions identified in the germinative layer of the beak and claw in some affected birds could be due to the presence of a virus or, alternatively, may be non-pathogenic cytoplasmic invaginations. Some viral diseases, including psittacine beak and feather disease and avian pox, have been reported to cause hyperkeratosis of the beak and other keratinized tissues, but these are typically accompanied by characteristic lesions in other tissues.5,25,28 The gross and microscopic presentation of non-keratinized tissues in chickadees was generally unremarkable and no viruses were identified in negative staining of organs known to host circoviruses, pox viruses, and polyoma viruses in other species.5 The inclusions apparent with light microscopy differed in appearance from viral inclusions that are typical of circovirus or avian pox virus, but more closely resembled those of polyoma viruses, herpes viruses, and adenoviruses.5
The results of our examinations are not consistent with any known avian diseases and eliminate most differential diagnoses. Overgrowth of the rhamphotheca is sometimes reported in captive birds, but such lesions are usually attributed to lack of wear,1 rather than to accelerated growth, which occurs in association with beak deformities in black-capped chickadees.33 Metabolic disorders, such as those related to deficiencies of vitamin A, zinc, or biotin, may also be responsible for beak overgrowth and abnormalities in other keratinized tissues,15,17 but chickadees with beak deformities did not display lesions typical of these or other reported nutritional problems. In addition, previous micronutrient analyses did not reveal differences between affected and unaffected birds.9 Beak abnormalities previously attributed to toxic etiologies in other avian species, including exposure to elevated levels of selenium or organochlorines,7,19,24 exhibit very different gross and microscopic lesions than what we observed in this study. There was no evidence of lesions in the liver or other organs commonly affected by some toxicants27 and preliminary toxicological analyses did not identify abnormally high levels of metals or organochlorines in affected birds.9 However, dioxins and dioxin-like compounds have been associated with keratin disorders in other taxa14,16,34,35 and additional study is required to rule out environmental contaminants as etiological agents of this disorder. Other naturally-produced toxins, including mycotoxins, have not yet been investigated and may warrant consideration.
A suite of macroscopic features, including beak overgrowth, has been observed in keratinized tissues of birds affected by “avian keratin disorder.”8,31 Microscopic and ultrastructural examination of tissues from affected birds confirmed and characterized lesions in beak, claw, skin, feather, and sinuses, but were not sufficient to determine whether or not the lesions corresponded with a primary keratin disorder. Additional study is needed to resolve this uncertainty and to clarify the associated terminology. In particular, biochemical analysis and immunohistochemistry of beak and claw tissues would provide insights about keratin expression and pathogenesis. Such efforts would also help to establish a basis for targeted investigation of specific keratins that may be disrupted. Other diagnostic testing, including further research on the nature of inclusions observed in this study, is also necessary to identify likely etiological agents. In addition, epidemiological analyses may reveal whether an infectious mode of transmission is likely. Recent patterns of emergence11,31 suggest rapid spread across a large geographic area and highlight the potential for this disease to have significant effects on wild bird populations.
Acknowledgements
We thank L. Pajot and J. Terenzi for field assistance. R. Swor, D. Ariyakumar, and D. Muldoon assisted in the laboratory. J. Blake, C. Terzi, J. Jack, and C. Willetto provided animal care and veterinary oversight. CV received funding from an NSF Graduate Research Fellowship and an Angus Gavin Memorial Bird Research Grant. Other sources of financial support were provided by the United States Geological Survey and the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH; Grant Number 5P20RR016466 to TMO). D. Mulcahy, A. Wunschmann, and two anonymous reviewers offered helpful comments that improved this manuscript. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Footnotes
Contact for reprints: Caroline Van Hemert, USGS Alaska Science Center, 4210 University Dr., Anchorage, AK 99508, USA; cvanhemert@usgs.gov
Contributor Information
C. Van Hemert, US Geological Survey, Alaska Science Center, 4210 University Drive, Anchorage, AK 99508, USA Department of Biology and Wildlife, Institute of Arctic Biology, University of Alaska Fairbanks, PO Box 757000, Fairbanks, AK 99775, USA.
A. G. Armién, Department of Veterinary Population Medicine, VDL, College of Veterinary Medicine, University of Minnesota, 1333 Gortner Avenue, St. Paul, MN 55108-1098, USA
J. E. Blake, UAF Animal Resources Center, University of Alaska Fairbanks, PO Box 756980, Fairbanks, Alaska 99775, USA
C. M. Handel, US Geological Survey, Alaska Science Center, 4210 University Drive, Anchorage, AK 99508, USA
T. M. O’Hara, Department of Biology and Wildlife, Institute of Arctic Biology, University of Alaska Fairbanks, PO Box 757000, Fairbanks, AK 99775, USA
References
- 1.Arnall L. Conditions of the beak and claw in the budgerigar. J Small Anim Pract. 1965;6:135–144. [Google Scholar]
- 2.Brush AH. Patterns in the amino acid compositions of avian epidermal proteins. Auk. 1980;97:742–753. [Google Scholar]
- 3.Cooper JE, Harrison GJ. Dermatology. In: Ritchie BW, Harrison GJ, Harrison LR, editors. Avian Medicine: Principles and Application. Lake Worth, FL: Wingers Publishing; 1994. pp. 607–639. [Google Scholar]
- 4.D'Alba L, Van Hemert C, Handel CM, Shawkey MD. A natural experiment on the condition-dependence of achromatic plumage reflectance in black-capped chickadees. PLoS ONE. 2011;6:e25877. doi: 10.1371/journal.pone.0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher OJ, Abdul-Aziz T. Avian Histopathology. 3rd ed. Jacksonville, FL: American Association of Avian Pathologists; 2008. [Google Scholar]
- 6.Frenkel MJ, Gillespie JM. The proteins of the keratin component of bird's beaks. Aust J Biol Sci. 1976;29:467–479. doi: 10.1071/bi9760467. [DOI] [PubMed] [Google Scholar]
- 7.Gilbertson M, Kubiak T, Ludwig J, Fox G. Great Lakes embryo mortality, edema, and deformities syndrome (GLEMEDS) in colonial fish-eating birds: similarity to chick-edema disease. J Toxicol Env Health. 1991;33:455–520. doi: 10.1080/15287399109531538. [DOI] [PubMed] [Google Scholar]
- 8.Handel CM, Pajot LM, Matsuoka SM, et al. Epizootic of beak deformities among wild birds in Alaska: an emerging disease in North America? Auk. 2010;127:882–898. [Google Scholar]
- 9.Handel CM, Pajot LM, Matsuoka SM, et al. Anchorage, AK: USGS Alaska Science Center; 2006. [Accessed July 2012]. Potential role of environmental contaminants in the pathology of beak deformities among black-capped chickadees in south-central Alaska. Final report. http://alaska.usgs.gov/science/biology/landbirds/beak_deformity/pdfs/Handel_et_al_2006_final_report_sm.pdf. [Google Scholar]
- 10.Harrison GJ. Disorders of the integument. In: Harrison GJ, Harrison LR, editors. Clinical Avian Medicine and Surgery. Philadelphia, PA: WB Saunders Company; 1986. pp. 397–407. [Google Scholar]
- 11.Harrison T. Beak deformities of garden birds. British Birds. 2011;104:538–541. [Google Scholar]
- 12.Homberger DG. The case of the cockatoo bill, horse hoof, rhinoceros horn, whale baleen, and turkey beard: the integument as a model system to explore the concepts of homology and non-homology. In: Dutta HM, Datta Munshi JS, editors. Vertebrate Functional Morphology: Horizon of Research in the 21st Century. Enfield, NJ: Science Publishers; 2001. pp. 317–343. [Google Scholar]
- 13.Homberger DG, Brush AH. Functional-morphological and biochemical correlations of the keratinized structures in the African grey parrot. Psittacus erithacus (Aves). Zoomorph. 1986;106:103–114. [Google Scholar]
- 14.Jackson T, Halbert F. A toxic syndrome associated with the feeding of polybrominated biphenyl-contaminated protein concentrate to dairy cattle. J Am Vet Med Assoc. 1974;165:437–439. [PubMed] [Google Scholar]
- 15.Keymer IF, Samour J. Disorders of the digestive system. In: Samour J, editor. Avian Medicine. 2nd ed. New York, NY: Elsevier; 2008. pp. 281–302. [Google Scholar]
- 16.Kimbrough RD. Skin lesions in animals and humans: a brief overview. Banb Report. 1984;18:357–363. [Google Scholar]
- 17.Klasing KC. Comparative Avian Nutrition. New York, NY: Cabi International; 1998. [Google Scholar]
- 18.Lucas AM, Stettenheim PR. Avian Anatomy: Integument. Washington, DC: US Department of Agriculture; 1972. Agriculture Handbook 362. [Google Scholar]
- 19.Ludwig JP, Kurita Matsuba H, Auman HJ, et al. Deformities, PCBs, and TCDD-equivalents in double-crested cormorants (Phalacrocorax auritus) and Caspian terns (Hydroprogne caspia) of the upper Great Lakes 1986–1991: testing a cause-effect hypothesis. J Great Lakes Res. 1996;22:172–197. [Google Scholar]
- 20.Luna G. AFIP Manual of Histologic Staining Techniques. 3rd ed. New York, NY: McGraw-Hill Publications; 1968. [Google Scholar]
- 21.Menzel R, Lüdicke M. Funktionell-anatomische und autoradiographische untersuchungen am schnabelhorn von papageien (Psittaci) Zool Jahrb Abt Anat Ontog Tiere. 1974;93:175–218. [Google Scholar]
- 22.Monroe AD, Latimer KS, Pesti GM, Bakalli RI. Pathology and histology of dietary tryptophan deficiency in broiler chicks. Avian Dis. 2003;47:1393–1398. doi: 10.1637/7039. [DOI] [PubMed] [Google Scholar]
- 23.O'Toole D, Raisbeck MF. Experimentally induced selenosis of adult mallard ducks: clinical signs, lesions, and toxicology. Vet Path. 1997;34:330–340. doi: 10.1177/030098589703400409. [DOI] [PubMed] [Google Scholar]
- 24.Ohlendorf HM, Hoffman DJ, Saiki MK, Aldrich TW. Embryonic mortality and abnormalities of aquatic birds: apparent impacts of selenium from irrigation drainwater. Sci Tot Env. 1986;52:49–63. [Google Scholar]
- 25.Olsen GH. Oral biology and beak disorders of birds. Vet Clin of North Am Ex Anim Pract. 2003;6:505–521. doi: 10.1016/s1094-9194(03)00030-6. [DOI] [PubMed] [Google Scholar]
- 26.Pass DA, Perry RA. The pathology of psittacine beak and feather disease. Aust Vet J. 1984;61:69–74. doi: 10.1111/j.1751-0813.1984.tb15520.x. [DOI] [PubMed] [Google Scholar]
- 27.Poppenga RH. Avian toxicology. In: Gupta RC, editor. Veterinary Toxicology: Basic and Clinical Principles. New York, NY: Elsevier; 2007. pp. 663–688. [Google Scholar]
- 28.Schmidt RE, Reavill DR, Phalen DN. Pathology of Pet and Aviary Birds. Ames, IA: Iowa State Press; 2003. [Google Scholar]
- 29.Seki Y, Bodde SG, Meyers MA. Toucan and hornbill beaks: a comparative study. Acta Biom. 2010;6:331–343. doi: 10.1016/j.actbio.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Tully TN, Jr, Lawton MPC, Dorrestein GM. Handbook of Avian Medicine. 2nd ed. New York, NY: Butterworth-Heinemann, Elsevier Science Limited; 2000. [Google Scholar]
- 31.Van Hemert C, Handel CM. Beak deformities in northwestern crows: evidence of a multispecies epizootic. Auk. 2010;127:746–751. [Google Scholar]
- 32.Van Hemert C, Handel CM, Blake JE, Swor RM, O'Hara TM. Microanatomy of passerine hard-cornified tissues: beak and claw structure of the black-capped chickadee (Poecile atricapillus) J Morph. 2012;273:226–240. doi: 10.1002/jmor.11023. [DOI] [PubMed] [Google Scholar]
- 33.Van Hemert C, Handel CM, O'Hara TM. Evidence of accelerated beak growth associated with avian keratin disorder in black-capped chickadees (Poecile atricapillus) J Wildl Dis. 2012;48:686–694. doi: 10.7589/0090-3558-48.3.686. [DOI] [PubMed] [Google Scholar]
- 34.Vos JG, Beems RB. Dermal toxicity studies of technical polychlorinated biphenyls and fractions thereof in rabbits. Toxicol Appl Pharm. 1971;19:617–633. doi: 10.1016/0041-008x(71)90294-8. [DOI] [PubMed] [Google Scholar]
- 35.White SS, Birnbaum LS. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Heal C. 2009;27:197–211. doi: 10.1080/10590500903310047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wight PAL, Dewar WA. The histopathology of zinc deficiency in ducks. J Path. 1976;120:183–191. doi: 10.1002/path.1711200308. [DOI] [PubMed] [Google Scholar]