Abstract
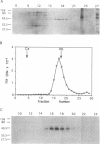
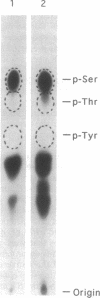
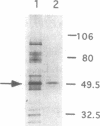
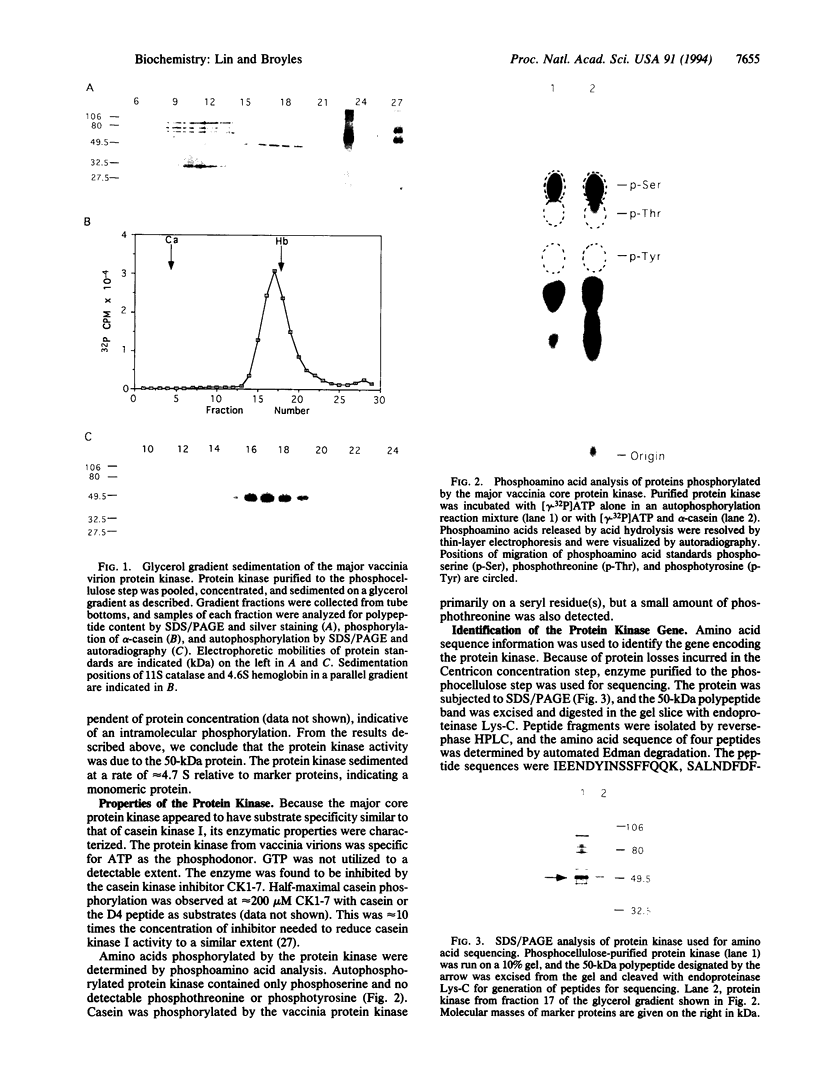
The major protein kinase activity from vaccinia virus core particles was purified to near homogeneity. The protein kinase is a 50-kDa polypeptide that is shown here to phosphorylate primarily seryl residues in alpha-casein, a casein kinase I-specific peptide substrate, and itself through autophosphorylation. The sequence of four peptides derived from the protein kinase demonstrated that it is encoded by the vaccinia virus F10L gene. Expression of the F10L gene product in bacteria as a fusion with glutathione S-transferase confirmed that the vaccinia F10L gene encodes the protein kinase. We have termed this enzyme vaccinia protein kinase 2 (VPK2) to distinguish it from the protein kinase encoded by the vaccinia B1R gene. Targeted disruption of the VPK2 gene with a positive selectable marker demonstrated that all viruses with a disrupted gene also possessed a wild-type gene, suggesting that VPK2 is essential for viability. The discovery of a second essential protein kinase encoded by vaccinia virus, in addition to a protein phosphatase, underscores the importance of protein phosphorylation in poxvirus biogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banham A. H., Smith G. L. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology. 1992 Dec;191(2):803–812. doi: 10.1016/0042-6822(92)90256-o. [DOI] [PubMed] [Google Scholar]
- Baylis S. A., Banham A. H., Vydelingum S., Dixon L. K., Smith G. L. African swine fever virus encodes a serine protein kinase which is packaged into virions. J Virol. 1993 Aug;67(8):4549–4556. doi: 10.1128/jvi.67.8.4549-4556.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Yuen L., Shuman S., Moss B. Purification of a factor required for transcription of vaccinia virus early genes. J Biol Chem. 1988 Aug 5;263(22):10754–10760. [PubMed] [Google Scholar]
- Bunnell B. A., Heath L. S., Adams D. E., Lahti J. M., Kidd V. J. Increased expression of a 58-kDa protein kinase leads to changes in the CHO cell cycle. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7467–7471. doi: 10.1073/pnas.87.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T., Hagiwara M., Hidaka H. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989 Mar 25;264(9):4924–4927. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Davis R. E., Mathews C. K. Acidic C terminus of vaccinia virus DNA-binding protein interacts with ribonucleotide reductase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):745–749. doi: 10.1073/pnas.90.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Flotow H., Roach P. J. Role of acidic residues as substrate determinants for casein kinase I. J Biol Chem. 1991 Feb 25;266(6):3724–3727. [PubMed] [Google Scholar]
- Frame M. C., Purves F. C., McGeoch D. J., Marsden H. S., Leader D. P. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J Gen Virol. 1987 Oct;68(Pt 10):2699–2704. doi: 10.1099/0022-1317-68-10-2699. [DOI] [PubMed] [Google Scholar]
- Franke C. A., Rice C. M., Strauss J. H., Hruby D. E. Neomycin resistance as a dominant selectable marker for selection and isolation of vaccinia virus recombinants. Mol Cell Biol. 1985 Aug;5(8):1918–1924. doi: 10.1128/mcb.5.8.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Broyles S. S., Dixon J. E. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature. 1991 Mar 28;350(6316):359–362. doi: 10.1038/350359a0. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Howard S. T., Smith G. L. Two early vaccinia virus genes encode polypeptides related to protein kinases. J Gen Virol. 1989 Dec;70(Pt 12):3187–3201. doi: 10.1099/0022-1317-70-12-3187. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Kao S. Y., Bauer W. R. Biosynthesis and phosphorylation of vaccinia virus structural protein VP11. Virology. 1987 Aug;159(2):399–407. doi: 10.1016/0042-6822(87)90479-x. [DOI] [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Characterization of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2430–2437. [PubMed] [Google Scholar]
- Kleiman J. H., Moss B. Purification of a protein kinase and two phosphate acceptor proteins from vaccinia virions. J Biol Chem. 1975 Apr 10;250(7):2420–2429. [PubMed] [Google Scholar]
- Lin S., Chen W., Broyles S. S. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J Virol. 1992 May;66(5):2717–2723. doi: 10.1128/jvi.66.5.2717-2723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel R. E., Anderson M. K., Evans E., Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990 Feb;64(2):574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel R. E., Traktman P. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J Virol. 1992 Jul;66(7):4413–4426. doi: 10.1128/jvi.66.7.4413-4426.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemond H., Moss B. Phosphoprotein component of vaccinia virions. J Virol. 1973 Jun;11(6):961–970. doi: 10.1128/jvi.11.6.961-970.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Golder M., Moss B. Insertional mutagenesis of the vaccinia virus gene encoding a type I DNA topoisomerase: evidence that the gene is essential for virus growth. Virology. 1989 May;170(1):302–306. doi: 10.1016/0042-6822(89)90384-x. [DOI] [PubMed] [Google Scholar]
- Shuman S., Surks M., Furneaux H., Hurwitz J. Purification and characterization of a GTP-pyrophosphate exchange activity from vaccinia virions. Association of the GTP-pyrophosphate exchange activity with vaccinia mRNA guanylyltransferase . RNA (guanine-7-)methyltransferase complex (capping enzyme). J Biol Chem. 1980 Dec 10;255(23):11588–11598. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]