Abstract
To dissect the genetic architecture of sexual dimorphism in obesity-related traits, we evaluated the sex–genotype interaction, sex-specific heritability and genome-wide linkages for seven measurements related to obesity. A total of 1,365 non-diabetic Chinese subjects from the family study of the Stanford Asia–Pacific Program of Hypertension and Insulin Resistance were used to search for quantitative trait loci (QTLs) responsible for the obesity-related traits. Pleiotropy and co-incidence effects from the QTLs were also examined using the bivariate linkage approach. We found that sex-specific differences in heritability and the genotype–sex interaction effects were substantially significant for most of these traits. Several QTLs with strong linkage evidence were identified after incorporating genotype by sex (G × S) interactions into the linkage mapping, including one QTL for hip circumference [maximum LOD score (MLS) = 4.22, empirical p = 0.000033] and two QTLs: for BMI on chromosome 12q with MLS 3.37 (empirical p = 0.0043) and 3.10 (empirical p = 0.0054). Sex-specific analyses demonstrated that these linkage signals all resulted from females rather than males. Most of these QTLs for obesity-related traits replicated the findings in other ethnic groups. Bivariate linkage analyses showed several obesity traits were influenced by a common set of QTLs. All regions with linkage signals were observed in one gender, but not in the whole sample, suggesting the genetic architecture of obesity-related traits does differ by gender. These findings are useful for further identification of the liability genes for these phenotypes through candidate genes or genome-wide association analysis.
Introduction
Obesity resulting from an excess of body fat is a known risk factor for chronic diseases, such as heart disease, diabetes, hypertension, stroke, dyslipidemia, osteoarthritis, and some cancers (Burton et al. 1985). Its prevalence has increased dramatically since 1960 and become a serious public health problem. As a complex trait, obesity arises from the interactions of multiple genes and environmental factors. Studies have shown that heredity plays a major role in the development of body size and obesity (Price and Lee 2001; Price et al. 2000). Recent reports of genome scans on obesity traits have also found linkage to regions on several chromosomes in diverse populations (Rankinen et al. 2006). In a recent meta-analysis of the 15 genome-wide association analysis (GWA), studies for BMI not only replicated common variants of fat mass and obesity associated (FTO) and melanocortin 4 receptor (MC4R) but also identified 6 additional loci: transmembrane protein 18 (TMEM18), potassium channel tetramerisation domain containing 15 (KCTD15), glucosamine-6-phosphate deaminase 2 (GNPDA2), SH2B adaptor protein 1 (SH2B1), mitochondrial carrier homolog 2 (MTCH2), and neuronal growth regulator 1 (NEGR1) (Willer et al. 2009). Although their functional importance awaits further validation studies, several of the candidate genes are considered to act on the central nervous system to cause obesity.
Sexual dimorphism in body fat content and its distribution has long been recognized in both humans and animals. Generally, males are taller, weigh more, and tend to have an accumulation of central fat, while females are smaller, have greater total body fat, and have a smaller accumulation of central fat (Lemieux et al. 1993; Pietrobelli et al. 2002). Sex differences in occurrence of metabolic diseases might be partly mediated via differences in body fat distribution; and the expression of sexual dimorphism in body fat may involve a substantial underlying genetic component. Hence, studying sex-specific genetic architecture may help in detecting susceptibility loci for body-fat-related traits. So far, several studies have shown genotype by sex interactions (Comuzzie and Allison 1998; Comuzzie et al. 1993; Diego et al. 2006; Martin et al. 2002; Towne et al. 1999; Voruganti et al. 2006) or sex-specific QTLs in obesity-related traits (Lewis et al. 2005) in African-Americans, white Americans, and Mexican Americans. The study of genotype-by-sex (G × S) interactions or sex-specific QTLs on fat-related traits, however, has lacked an examination of a Chinese population. In the present study, we applied the variance component method (Almasy and Blangero 1998) to assess the significance of genotype-by-sex interactions on obesity-related traits and, further, to identify sex-specific susceptible QTLs responsible for these traits. In addition, we performed bivariate linkage analyses to examine the pleiotropy and coincident effects of the sex-specific QTLs for these traits to understand the genetic architecture of sexual dimorphism in shape and size. Linkage findings have helped in improving statistical power in whole-genome association studies through, for example, a weighting procedure, as some regions of the genome may be favored because of prior investigations or knowledge of the biological function of particular genes (Roeder et al. 2006). These findings are useful for further identification of the liability genes for complex phenotypes through candidate genes or GWA by taking gender into consideration.
Subjects and methods
The study used data collected from the Stanford Asia–Pacific Program of Hypertension and Insulin Resistance (SAPPHIRe) study, which was designed to investigate susceptibility genes for hypertension and insulin resistance in selected Chinese and Japanese populations (Ranade et al. 2001, 2003; Wu et al. 2002; Yang et al. 2003). The subjects of this study were concordant siblings (all siblings with hypertension) and discordant siblings (at least one hypertensive sibling) recruited through probands. Probands were ascertained as those with age at onset of 35–60 years, or those over 60 who had available documentation of their hypertension status prior to age 60. A total of 2,525 subjects of Japanese or Chinese descent were recruited from centers in San Francisco, Hawaii, and Taiwan. All subjects underwent a clinical and fasting laboratory examination, with written informed consent obtained prior to examination. Diabetic individuals defined by the WHO criteria uncovered as a result of SAPPHIRe laboratory work or previously diagnosed were excluded in our analyses, as diabetic individuals usually have abnormal traits measures. The details of recruitment, exclusion criteria, phenotyping, and genotyping have been described elsewhere (Ranade et al. 2003).
As it is helpful in studying a genetically homogeneous population, we focused on the Chinese population in the previous and present studies, because the number of Japanese is very limited (352 in total) in this study. We used “sib_kin,” a module of ASPEX software (http://www.aspex.sourceforge.net/) to identify non-paternities. When a non-paternity was identified that individual was excluded from the analysis (Ranade et al. 2003). It turned out that 1,365 non-diabetic Chinese subjects (118 parents and 1,247 siblings) with genotyping data from 411 nuclear families were included in this study. The number of families with 2–8 siblings was 138, 122, 63, 37, 16, 5, and 1, respectively. The study was approved by the Institutional Review Board of all the Institutes involved.
Genotyping
Whole blood was obtained from all consenting family members for DNA extraction. DNA was prepared using commercial kits (Puregene, Gentra Systems, Minneapolis, MN). Genotyping was performed at the Marshfield Medical Research Foundation (Marshfield, WI) using the Weber screening set 9 (Research Genetics, Inc., Huntsville, AL) (Chuang et al. 2004). The genotyping procedure used 376 autosomal markers representing short tandem repeat polymorphisms and yielded an average map density of 9.16 cM. Genotyping quality was monitored by typing 30 samples in duplicate. An error rate of ~1% was estimated based on these duplicated samples. A published sex-averaged genetic linkage map available from the Marshfield website (http://www.research.marshfieldclinic.org/genetics/GeneticResearch/compMaps.asp) was used for linkage analysis, with the marker order confirmed based on a release from that site. Data for three markers, which had significant Mendelian inconsistencies, were excluded from linkage analysis. ASPEX software was used to examine Mendelian inconsistencies. In the situations where an error was found, the genotype data for all the members of the family at that marker were converted to missing (see Ranade et al. for additional details; Ranade et al. 2003).
Phenotyping
The phenotypes in this study included BMI, height, hip circumference (hip), weight, waist–hip ratio (WHR), and waist circumference (waist). To directly assess the genetic effect of body fat, we also studied percent body fat (PBF), defined by PBF = 1.2 × (BMI) + 0.23 × (age) − 10.8 × (sex: male 1, female 0) − 5.4 (Deurenberg et al. 1991). Before conducting linkage analyses, empirical normal quartile transformation (Diego et al. 2007; Peng et al. 2007) was applied to normalize the scaled rank of trait values using an inverse normal transformation.
Covariates
The covariates adjusted for in the linkage analyses in the whole sample included age, gender, smoking, physical activity, alcohol consumption, and field center. The detailed definitions of these covariates are described in Chiu et al. (2005). These covariates were considered to be possible confounders, and were all included in these analyses regardless of their significances.
Genome-wide multipoint linkage analyses
The variance-components model partitions, the variability of a trait into components for a quantitative trait loci (QTL), the residuals polygenic component, and the random environmental component (Almasy and Blangero 1998); namely,
where the phenotypic variance is the sum of the genetic variance and the environmental variance .
To model G × S interaction, additional parameters are needed. Assuming the probability of an individual having a specific genotype is independent of sex, the expected additive genetic covariance between a pair of male and female relatives [COV(GM, GF)] may be defined as.
where subscripts M and F refer to male and female, respectively; ϕ is the coefficient of kinship between the two individuals; and ρG(M,F) is the genetic correlation between the expressions of the trait in the two sexes. The across-sex QTL correlation coefficient is constrained to one.
The variance component method implemented in SOLAR was used to perform genome screens under standard linkage and G × S interaction models across all 22 autosomes.
For the standard linkage case, the likelihood ratio statistic; namely, twice the logarithm of the likelihood ratio, is asymptotically distributed as a 1/2:1/2 mixtures of a and a point mass at zero, denoted by . Log of odds (LOD) scores were calculated as logarithm to base 10 of the likelihood ratios. For the linkage version of the G × S interaction models, the QTL correlation coefficient was constrained to one, and because of this the likelihood ratio statistic is asymptotically distributed as 1/4 . To make the MLS scores from this statistic comparable to the standard model under univariate analysis, we provided the corrected MLS score denoted by MLS[1] on the basis of the appropriate distribution (Diego et al. 2007).
In the bivariate model, the additive genetic (ρg) and environmental (ρe) correlations between the two traits represent the effects of shared genes, or pleiotropy, and of shared environmental factors, respectively, on the phenotypic variance in a trait. We conducted a series of bivariate quantitative genetic analyses for all pair-wise combinations of obesity-related traits using the bivariate approach implemented in SOLAR 2.0.
To test pleiotropy and coincident linkage, likelihoods for linkage model in which ρq was estimated were compared with models in which ρq was constrained to 0 (no shared major gene effects in the region, i.e., co-incident linkage) and constrained to 1 or −1 (complete pleiotropy). In the case of ρq constrained to 0, the difference between these likelihoods is distributed as a χ2 with 1 degree of freedom. When ρq is constrained to 1 or −1, a boundary, the difference in likelihoods is distributed as a 1/2:1/2 mixture of and a point mass at 0 (Amos et al. 2001). The hypothesis of ρq = 1 or −1 (complete pleiotropy) or the hypothesis of ρq = 0 (coincidence linkage) was rejected when p < 0.05.
Pointwise statistical significance was assessed through computer simulations to avoid inflated type I error rate. A fully informative marker was simulated under the null hypothesis of no linkage, with the observed family structures and phenotypes. We generated 10,000 (or at least 100,000 for regions with MLS ≥ 3) replicates for each interested region, and the resulting distribution was used to derive empirical p values. This procedure was implemented in the SOLAR program.
Results
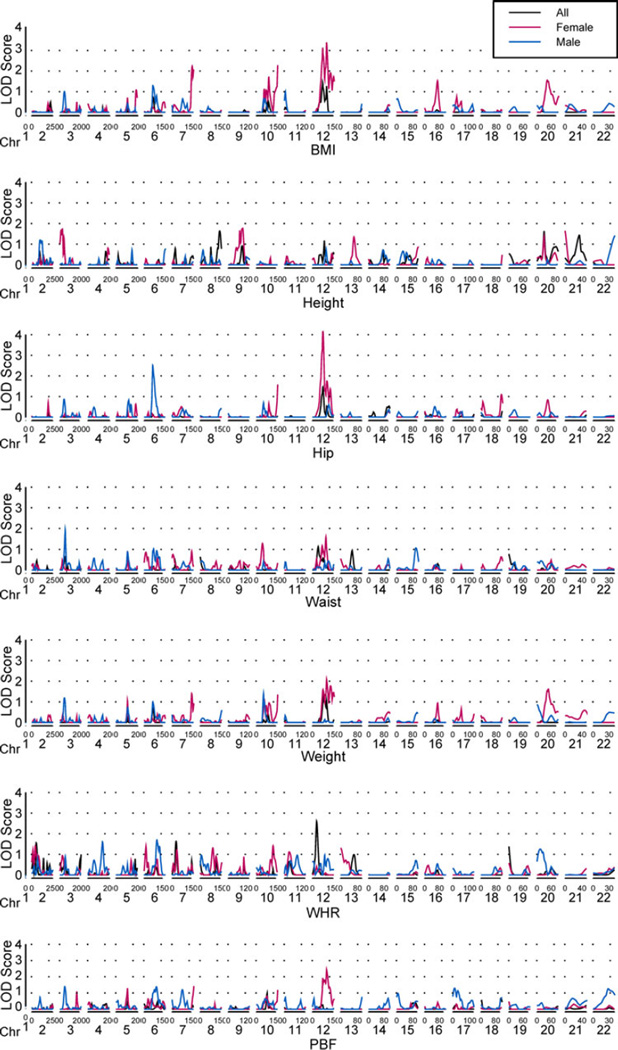
Phenotypically, the fatness-related traits all significantly differed by gender (Table 1). Almost all of the measures were lower in females than in males, except for PBF, which was significantly higher in females. The gender effects remained highly significant (p < 0.0001) after adjusting for age, field center and other environmental factors (data not shown). The overall heritability estimates of these fatness-related traits range from 0.35 to 0.82 (Table 2). To study the gender effect, we calculated the heritability estimates of various phenotypes stratified by sex. The heritability estimates are mostly higher in females than in males, except that for height (Fig. 1; Table 3). For example, the heritability estimates of BMI, waist, and WHR were as high as 0.61, 0.83, and 0.89, respectively, in females; and reduced to 0.29, 0.14, and 0.39, respectively, in males (Table 3). All heritability estimates were significantly different from zero (Fig. 1). In addition, the differences in heritability for BMI, waist, WHR, and PBF between males and females were statistically significant (p = 0.0019, 2.96 × 10−6, 0.00017, and 0.019, respectively).
Table 1.
Gender difference in fat-related phenotypes
| Gender | Male | Female |
|---|---|---|
| Subjects (n) | 608 | 757 |
| Age (years) | 49.99 ± 10.5 (608)a | 51.28 ± 11.25 (757) |
| BMI (kg/m2) | 25.70 ± 3.20 (485)b | 24.52 ± 3.46 (573) |
| Height (cm) | 167.33 ± 5.85 (485)b | 156.03 ± 5.07 (573) |
| Weight (kg) | 72.05 ± 10.22 (484)b | 59.73 ± 8.97 (573) |
| Waist (cm) | 88.45 ± 8.48 (485)b | 80.1 ± 10.28 (572) |
| Hip (cm) | 97 ± 6.09 (485)a | 95.44 ± 7.31 (572) |
| WHR | 0.91 ± 0.06 (485)b | 0.84 ± 0.08 (572) |
| PBF | 25.85 ± 4.34 (485)b | 35.29 ± 4.84 (573) |
Mean ± SD (n)
p < 0.05 (male vs. female)
p < 0.0001 (male vs. female)
Table 2.
Regions with maximum LOD score (MLS) ≥ 1.87 in the univariate genome-wide multipoint linkage analysis
| Trait | Heritability | Without G × S | With G × S | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosomes | cM | MLS | Empirical p value |
Chromosomes | cM | MLS | Empirical p value |
ρG | p value for testing H0:|ρG| = 1 |
||
| BMI | 0.39 | 12q14.1 | 74 | 1.45 | 0.0081 | 10q26.3 | 166 | 2.40 | 0.0069 | 0.52 | 0.088 |
| 12q14.1 | 75 | 3.10 | 0.0054 | ||||||||
| 12q23.1 | 103 | 3.37 | 0.0043 | ||||||||
| Height | 0.82 | 8q24.22 | 150 | 1.64 | 0.0067 | 9q21.31 | 78 | 2.04 | 0.0069 | −0.0087 | 1.2E-15 |
| 18q23 | 113 | 1.94 | 0.0089 | ||||||||
| 21q21.1 | 0 | 1.88 | 0.010 | ||||||||
| Hip | 0.36 | 12q15 | 76 | 1.47 | 0.0049 | 6p12.3 | 72 | 2.39 | 0.0018 | 0.47 | 0.038 |
| 12q15 | 76 | 4.22 | 0.000033 | ||||||||
| Waist | 0.41 | 12p12.1 | 42 | 1.14 | 0.017 | 12q23.1 | 98 | 1.61 | 0.0146 | −0.998 | 0.5 |
| Weight | 0.35 | 12q21.2 | 84 | 1.38 | 0.012 | 12q23.1 | 103 | 1.72 | 0.024 | −0.31 | 0.0002 |
| WHR | 0.49 | 12p13.1 | 30 | 2.59 | 0.0008 | 7p15.3 | 38 | 2.55 | 0.0030 | 0.078 | 0.0021 |
| 12p13.1 | 30 | 2.04 | 0.0071 | ||||||||
| PBF | 0.60 | 10q21.3 | 84 | 0.99 | 0.0179 | 5q15 | 105 | 2.09 | 0.0030 | −0.15 | 1.4E-10 |
| 10q21.3 | 81 | 1.98 | 0.0043 | ||||||||
| 12q14.1 | 74 | 2.08 | 0.0033 | ||||||||
| 15q26.2 | 101 | 2.70 | 0.0005 | ||||||||
Fig. 1.
Autosome-wide multipoint linkage analyses for seven body fat traits, black lines for the combined sample, pink lines for females and blue lines for males. The chromosomal markers are represented on the x axis, and the LOD scores are shown on the y axis. Multipoint LOD scores are depicted by solid lines where age, sex and other environmental factors were adjusted
Table 3.
Regions with MLS ≥ 1.87 in the gender-specific univariate genome-wide multipoint linkage analysis
| Trait | Female | Male | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosomes | cM | MLS | Empirical p value |
Heritability | Chromosomes | cM | MLS | Empirical p value |
Heritability |
p value for testing H0: |
|
| BMI | 7q36.1 | 160 | 2.23 | 0.0032 | 0.61 | 6p12.3 | 72 | 1.29 | 0.0083 | 0.29 | 0.0019 |
| 7q36.2 | 174 | 2.06 | 0.0042 | ||||||||
| 10q26.3 | 166 | 2.29 | 0.0029 | ||||||||
| 12q14.1 | 75 | 3.13 | 0.0007 | ||||||||
| 12q23.1 | 103 | 3.39 | 0.00055 | ||||||||
| 12q24.22 | 131 | 1.91 | 0.0060 | ||||||||
| Height | 9q22.31 | 99 | 1.76 | 0.0092 | 0.86 | 22q12.3 | 41 | 1.41 | 0.012 | 0.99 | 0.31 |
| Hip | 12q14.3 | 76 | 4.23 | 6.7E-6 | 0.48 | 6p12.3 | 71 | 2.52 | 0.0003 | 0.39 | 0.28 |
| Waist | 12q22 | 98 | 1.57 | 0.0074 | 0.83 | 3p24.1 | 56 | 1.93 | 0.0015 | 0.14 | 2.96E-06 |
| Weight | 12q23.1 | 103 | 2.08 | 0.0023 | 0.50 | 10p11.22 | 56 | 1.39 | 0.0059 | 0.44 | 0.70 |
| WHR | 10q25.2 | 130 | 1.41 | 0.0086 | 0.89 | 6q16.2 | 106 | 1.69 | 0.0012 | 0.39 | 0.00017 |
| PBF | 12q14.1 | 75 | 1.93 | 0.0047 | 0.73 | 3p24.1 | 51 | 1.39 | 0.0023 | 0.56 | 0.019 |
| 12q23.1 | 103 | 2.43 | 0.0019 | ||||||||
There was no significant peak in our original autosomal-wide scan for these fatness-related traits. The power of detecting linkage increased dramatically after incorporating G × S interactions into the linkage analysis. The maximum MLS scores (MLS) ranged from 0.99 to 2.59 before incorporating the interaction term; but after G × S interactions were incorporated, the range of MLS increased to 1.61–4.22 (Table 2). The G × S interactions characterized by the across-gender genetic correlation coefficients (ρG) were significantly different from 1 for height (p = 1.2 × 10−15), hip (p = 0.038), weight (p = 0.0002), WHR (p = 0.0021), and PBF (p = 1.4 × 10−10) after adjusting for environmental factors in the linkage analyses (Table 2). It is worth noting that the genetic effects on weight and PBF were in opposite directions in two genders, as they were negatively correlated.
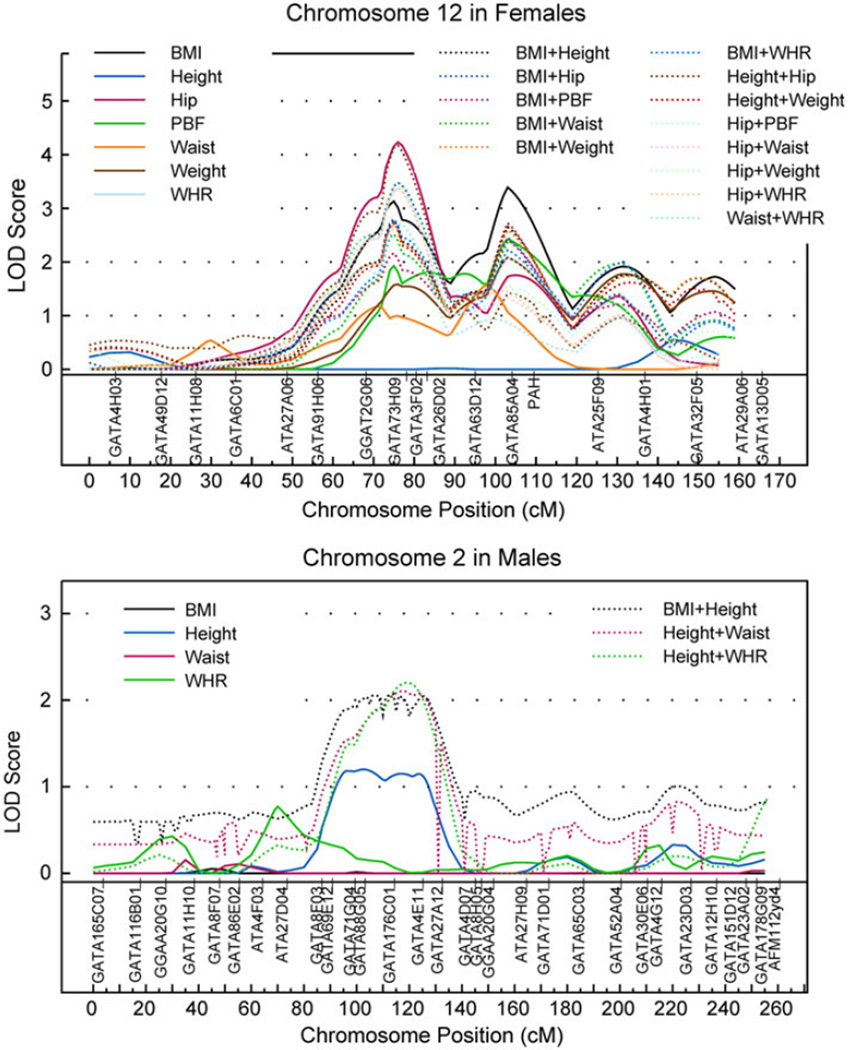
After observing the strong interaction effects on several fatness-related traits, we searched for QTLs controlling these traits by males and females separately (Fig. 2). As illustrated in Table 3, some QTLs influencing BMI were detected only in women on chromosomes 7q (MLS = 2.23 or 2.06, the corresponding empirical p = 0.0032 or 0.0042), 10q (MLS = 2.29, empirical p = 0.0029), and 12q14.1 (MLS = 3.13, empirical p = 7.0 × 10−4); the QTL nearby on 12q14.3 also influenced hip (MLS = 4.23, empirical p = 6.7 × 10−6) and PBF (MLS = 1.93, empirical p = 0.0047). The other QTLs for BMI were on 12q23.1 (MLS = 3.39, empirical p = 5.5 × 10−4) and 12q24.2 (MLS = 1.91, empirical p = 0.0060). These QTLs also controlled weight (MLS = 2.08, empirical p = 0.0023) and PBF (MLS = 2.43, empirical p = 0.0019). Because several traits were found to be controlled by common loci, we performed bivariate linkage analyses for pair-wise traits to investigate whether the common loci have a pleiotropy effect on the pair-wise traits. Most of the QTLs showed pleiotropic effects for various trait pairs, other than a few that showed evidence of coincidence linkage (Table 4). In females, the QTLs on 12q for BMI have a pleiotropic effect on all traits except for height and WHR, where the BMI and height showed evidence of coincident linkage. A QTL influencing hip on 6p12.3 (MLS = 2.52, empirical p = 0.0003) and one QTL influencing waist on chromosomes 3p24.1 (MLS = 1.93, empirical p = 0.0015) were identified only in men (Table 3). For females, most of the QTLs on chromosome 12q showed pleiotropic effects on pair-wise traits (the highest MLS[1] = 3.48, empirical p = 0.00095), only some of them showed coincidence linkage on chromosomes 5q, 10q, and 12q. None of the susceptible QTLs responsible for height and WHR were identified in women or men in the univariate analyses; yet the combinations of BMI–height, height–hip, height–weight, and WHR–hip pairs showed coincidence linkage on chromosome 12q (the highest MLS[1] = 4.17, empirical p < 0.000001) in females. In males, the pleiotropic effects were observed for height–waist, height–WHR pairs on chromosome 2q and for hip–waist pair on chromosome 6p. Several new QTLs not identified in the univariate linkage analyses were found in the bivariate analyses in either females or males for various pair-wise traits. For example, the additional QTLs on chromosomes 1p35, 3p26, 5q31, 9q21, 10q22, 12q13, and 21q11 were identified for several trait pairs in females; and the QTLs on chromosomes 2p11, 2q12, 2q14, 8q24, 10p11, 11q23, 12q23, and 22q12 were identified for various trait pairs in the bivariate analyses only for males.
Fig. 2.
Comparisons of LOD scores from univariate (solid lines) versus those from bivariate linkage analyses (dotted lines). LOD scores from the bivariate approach were converted to the LOD scores with equivalent p values in the univariate approach. Chromosome 12 in females and chromosome 2 in males are presented
Table 4.
Regions with peak LOD scores C 2.87 in gender-specific genome-wide bivariate multipoint linkage analysis
| Females | Males | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait 1 | Trait 2 | Chromosomes | cM | MLS[1] | MLS | Empirical p value |
Complete coincidence p value |
Complete pleiotropy p value |
Chromosomes | cM | MLS[1] | MLS | Empirical p value |
Complete coincidence p value |
Complete pleiotropy p value |
||
| BMI | Height | 12 | 75 | 2.78 | 3.75 | 0.0011 | 0.28 | 0.00074b | 2 | 104 | 2.04 | 2.91 | 0.015 | 0.58 | 0.074 | ||
| 12 | 103 | 2.65 | 3.60 | 0.0016 | 0.70 | 0.091 | 2 | 107 | 2.05 | 2.92 | 0.015 | 0.42 | 0.90 | ||||
| 2 | 114 | 2.07 | 2.94 | 0.014 | 0.30 | 1 | |||||||||||
| 2 | 116 | 2.05 | 2.92 | 0.015 | 0.32 | 1 | |||||||||||
| 2 | 125 | 2.04 | 2.91 | 0.015 | 0.35 | 1 | |||||||||||
| 10 | 55 | 2.09 | 2.97 | 0.014 | 0.084 | 1 | |||||||||||
| 22 | 41 | 2.12 | 3.00 | 0.013 | 0.82 | 0.21 | |||||||||||
| BMI | Hip | 1 | 63 | 2.06 | 2.93 | 0.012 | 0.81 | 0.66 | |||||||||
| 10 | 97 | 3.24 | 4.25 | 0.00025 | 0.062 | 0.0017b | |||||||||||
| 12 | 70 | 2.50 | 3.44 | 0.00495 | 0.00028 | 0.25a | |||||||||||
| 12 | 76 | 3.48 | 4.52 | 0.00095 | 1.3E-05 | 0.48a | |||||||||||
| 12 | 103 | 2.45 | 3.38 | 0.0053 | 0.00014 | 0.5a | |||||||||||
| BMI | PBF | 12 | 75 | 2.17 | 3.06 | 0.0027 | 0.00028 | 0.5a | 11 | 111 | 2.91 | 3.89 | 0.0002 | 0.059 | 1 | ||
| 12 | 103 | 2.41 | 3.33 | 0.0015 | 0.00014 | 0.37a | |||||||||||
| BMI | Waist | 7 | 173 | 2.09 | 2.97 | 0.0045 | 0.052 | 0.5 | |||||||||
| 12 | 75 | 2.52 | 3.46 | 0.0022 | 0.0027 | 0.29a | |||||||||||
| 12 | 103 | 2.37 | 3.29 | 0.0026 | 0.00077 | 0.28a | |||||||||||
| BMI | Weight | 3 | 28 | 2.08 | 2.96 | 0.0043 | 0.046 | 1 | |||||||||
| 12 | 75 | 2.71 | 3.67 | 0.0014 | 7.9E-05 | 0.5a | |||||||||||
| 12 | 103 | 2.59 | 3.54 | 0.0017 | 6.4E-05 | 0.5a | |||||||||||
| 21 | 0 | 2.05 | 2.93 | 0.0047 | 0.51 | 0.20 | |||||||||||
| BMI | WHR | 10 | 166 | 2.28 | 3.19 | 0.0043 | 0.77 | 0.25 | |||||||||
| 12 | 75 | 2.81 | 3.77 | 0.0012 | 0.95 | 0.50 | |||||||||||
| 12 | 103 | 2.23 | 3.12 | 0.0049 | 0.11 | 1 | |||||||||||
| Height | Hip | 12 | 70 | 2.94 | 3.93 | 0.00035 | 0.78 | 0.73 | |||||||||
| 12 | 76 | 4.17 | 5.28 | 5.0E-06 | 0.77 | 0.00041b | |||||||||||
| Height | Waist | 2 | 117 | 2.10 | 2.98 | 0.004 | 0.011 | 1a | |||||||||
| 2 | 124 | 2.08 | 2.95 | 0.0044 | 0.011 | 1a | |||||||||||
| 3 | 56 | 2.07 | 2.95 | 0.0044 | 0.80 | 1 | |||||||||||
| Height | Weight | 12 | 75 | 2.73 | 3.69 | 0.0013 | 0.29 | 0.00084b | |||||||||
| 12 | 103 | 2.72 | 3.68 | 0.0013 | 0.67 | 0.00068b | |||||||||||
| Height | WHR | 3 | 28 | 2.30 | 3.21 | 0.0022 | 0.18 | 0.097 | 2 | 119 | 2.20 | 3.10 | 0.0005 | 0.0058 | 1 | ||
| Hip | PBF | 12 | 69 | 2.44 | 3.37 | 0.0015 | 0.004 | 0.37a | 12 | 119 | 2.30 | 3.21 | 0.0003 | 0.70 | 1 | ||
| 12 | 76 | 3.41 | 4.44 | 0.0003 | 0.00012 | 0.40a | |||||||||||
| 12 | 103 | 2.08 | 2.96 | 0.0034 | 0.00026 | 0.5a | |||||||||||
| Hip | Waist | 5 | 139 | 2.10 | 2.98 | 0.0022 | 0.084 | 0.028b | 6 | 70 | 2.31 | 3.22 | 0.0013 | 0.011 | 0.33a | ||
| 12 | 70 | 2.42 | 3.35 | 0.001 | 0.0039 | 0.20a | 6 | 72 | 2.28 | 3.22 | 0.0013 | 0.0092 | 0.37a | ||||
| 12 | 76 | 3.36 | 4.39 | 0.0002 | 0.0012 | 0.28a | 8 | 155 | 2.41 | 3.22 | 0.0009 | 0.46 | 0.0097b | ||||
| 8 | 157 | 2.46 | 3.22 | 0.0006 | 0.49 | 1 | |||||||||||
| Hip | WHR | 12 | 70 | 2.43 | 3.36 | 0.0008 | 0.95 | 0.44 | |||||||||
| 12 | 76 | 3.37 | 4.40 | 0.00005 | 0.89 | 0.0043b | |||||||||||
| Hip | Weight | 12 | 69 | 2.52 | 3.46 | 0.0019 | 0.00016 | 0.5a | 10 | 57 | 2.34 | 3.25 | 0.0008 | 0.018 | 0.025 | ||
| 12 | 76 | 3.50 | 4.54 | 0.00013 | 1.1E-05 | 0.5a | 10 | 61 | 2.26 | 3.16 | 0.001 | 0.040 | 0.039 | ||||
| PBF | Waist | 9 | 78 | 2.04 | 2.91 | 0.0005 | 0.29 | 0.24 | |||||||||
| PBF | Weight | 3 | 15 | 2.22 | 3.11 | 0.0016 | 0.71 | 0.71 | |||||||||
| 3 | 28 | 3.11 | 4.11 | 0.0002 | 0.89 | 1 | |||||||||||
| Waist | WHR | 12 | 76 | 2.72 | 3.68 | 0.0005 | 0.91 | 0.56 | |||||||||
MLS[1] the bivariate LOD score converted to LOD score with an equivalent p value in univariate linkage analysis MLS maximum LOD score in bivariate linkage analysis
Pleiotropy linkage
Coincident linkage
We examined the genes located within the 1-LOD support intervals for the signals over a LOD score of 3 from the linkage analyses. Depending on the regions, the number of candidate genes within a support interval varies from 56 to 411. Among them, the gene high mobility group AT-hook 2 (HMGA2) in 12q14.3 was identified for BMI, hip and for the trait pairs of BMI–hip, hip–PBF, hip–waist, hip–WHR, hip–weight and PBF–weight. This gene was also identified in European ancestry for height with a p of 5.9 × 10−09 (Weedon et al. 2008). In addition, the genes in the region of 12q23.1, including insulin-like growth factor 1 (IGF1), phenylalanine hydroxylase (PAH), pro-melanin-concentrating hormone (PMCH), nucleoporin 37 kDa (NUP37), were also identified in Caucasian, Korean, European-American, Hispanic and African-American (Chen et al. 2004; Kim et al. 2010; Li et al. 2004; Norris et al. 2005; Perusse et al. 2001; Sun et al. 1999) for some obesity-related traits. The ghrelin/obestatin prepropeptide (GHRL) gene was found to be associated with BMI in tall obese children and with obesity in Swedish obese females (Barlow et al. 1991; Korbonits et al. 2002; Ukkola et al. 2001). Several genes in these regions are novel and are potentially associated with obesity-related metabolic mechanisms. These potential candidate genes warrant further studies.
Discussion
In the present study, we examined seven quantitative fatness-related traits and all of them were sexually dimorphic in trait values and showed gender difference in linkage. BMI, waist, WHR, and PBF showed evidence of differences in heritability between genders, while height, hip, WHR, and PBF showed evidence of significant G × S interactions. BMI and waist are sexually dimorphic phenotypically, but did not show significant G × S interaction. Height, hip, and weight have significant G × S interaction, but have no significant sex differences in heritability. On the other hand, WHR and PBF showed significant gender difference in sex-specific heritability and in G × S interaction effects. These findings suggest that many genes or the genetic mechanism within them and may function differently in the genders.
In the gender-specific univariate linkage analyses, we identified a few QTLs on chromosome 12q in females. Interestingly, the region 12q23–24 showed evidence of linkage (MLS[1] = 2.08–2.72) for the hip–PBF trait pair for both genders and also showed evidence of pleiotropy from the QTLs on this region for pairwise combinations of BMI, hip, PBF, waist, and weight in the bivariate analysis. These results suggest that some of the genes in this region play important roles in various fatness-related traits. Thus far, this region has also been identified in various studies, such as for PBF in non-Hispanic whites and African-American women (Lewis et al. 2005); for PBF, BMI, and waist circumference in European-Americans (Li et al. 2004); for abdominal fat in European-American families from Quebec (Perusse et al. 2001); for BMI, total AUC of BMI, and incremental AUC (calculated as total AUC-baseline AUC) of BMI in white sibships (Chen et al. 2004); for stature in the Framingham Heart Study (Geller et al. 2003); and for total lean body mass in British females (Livshits et al. 2007). Of note, this region harbors several positional candidates, including IGF1, PAH, PMCH, NUP37, and chromosome 12 open-reading frame 48 (C12orf48). Among them, IGF1 is of particular interest in its potential gender-specific biological effects via the action of IGF1 and its receptor IGF1 receptor (IGF1R). Although this biological system is not similar to those described for insulin-like growth factor 2 (IGF2) and IGF2 receptor (IGF2R) where genomic imprinting mechanism was described nicely for paternal- or maternal allele of the gene(s) through imprinting mechanism (Barlow et al. 1991; Kalscheuer et al. 1993; Xu et al. 1993), there is increasing evidence showing genetic imprinting of other molecules which controls the upstream or downstream of IFG1–IGF1R signaling (Drake et al. 2009; Miyoshi et al. 1998). Whether and how IGF1 influences various fatness-related traits in females await further investigation. In our preliminary analyses, we found that certain SNPs of the IGF1 gene showed highly significant association of various fatness-related traits (Wang et al., submitted) and the association varies by gender (unpublished data). In addition, the trait pairs with high genetic correction coefficients (0.52–0.82), including BMI–hip, BMI–PBF, BMI–waist, BMI–weight, hip–PBF, hip–waist, and hip–weight, all showed evidence of a gender-specific pleiotropy effect in this region (Table 4). These results indicate that these traits may be regulated by a set of common genes.
The QTL for BMI on 7q36 in females was also identified in Old Order Amish for leptin (Hsueh et al. 2001); and for PBF in non-Hispanic whites and African-American females (Lewis et al. 2005). For males, the QTL on chromosome 6p12.3 (MLS 2.52, p = 0.0003) was previously reported to be linked to plasma leptin concentration in Pima Indians (Walder et al. 2000). We confirmed several other QTLs reported previously, including QTLs on 3p24.1 (MLS 1.93, empirical p = 0.0015) for waist in males (Dong et al. 2005; Gorlova et al. 2003), and 7q (MLS 2.23 or 2.06, corresponding empirical p = 0.00032 or 0.0042) and 10q (MLS 2.29, empirical p = 0.0029) for BMI in females (Butler et al. 1998; Le Stunff et al. 2000; Li et al. 1999). In males, the pleiotropic effects were observed from the QTLs on chromosome 6p for hip–waist pair and on chromosome 2q for height–waist pair. These findings are consistent with our notion that the fatness-related traits may be controlled by a set of common genes.
Human height is another clear example of sexual dimorphism with high heritability (0.86 for females and 0.99 for males in this study). Genome-wide linkage scans have suggested quite a few regions linked to body height (Liu et al. 2006). Although there was no significant linkage peak for height in our univariate analysis, when G × S interaction was taken into consideration for linkage analyses we observed the peaks on chromosomes 9, 18, and 21 (MLS 2.04, 1.94, and 1.88, respectively), which replicated the QTL reported on chromosome 9q22.31 for height in Caucasians (Liu et al. 2006); Nevertheless, this QTL was observed only in females (LOD 1.76, empirical p = 0.0092). In the bivariate analysis, the QTLs for height have only coincident effects with BMI, hip, or weight in females; yet have only pleiotropic effects with waist or WHR in males. Note that it is more appropriate to use sex-specific map for the sex-specific linkage analysis. The impact of using the sex-specific maps warrants further investigation.
There is a concern that different ethnic groups may have different recombination rates as a result of genomic variations, which would generate misspecification of the genetic map and reduce the power of linkage analyses (Ju et al. 2008). Ju et al. (2008) showed that Mongolians have about 1.9 fewer recombination per meiosis when compared with Caucasians. As a result, genetic lengths of the whole genome and chromosomes of the Mongolian map are shorter than those of Caucasian map. Jorgenson et al. (2005) studied the ethnic differences in genetic map, including Caucasians and Chinese and found that although the maps for the different groups are general similar, regional and genome-wide differences across ethnic groups do exist. They showed that the regions with significant differences in interval length between Caucasians and Chinese include the regions of 6p24.1–21.1, 8p23.1 and 12q24.31–24.32. In the present study, we did not identify significant loci in these regions, which eliminate the likelihood of having false-positive findings in these regions. However, Daw et al. (2000) found that map misspecification may also cause negative bias in regions with actual linkage, although in most cases, bias is modest. They also found that in the absence of linkage, map misspecification can cause positive or negative bias. Indeed, linkage analyses require appropriate genetic maps for identifying correct loci. The impact of using the Marshfield map on our linkage analysis warrants further investigation.
We demonstrated that the QTLs controlling fatness-related traits vary in genders. The sexual dimorphism phenomenon in these fatness-related traits suggests some of the gender-specific autosomal effects, we observed might be caused by interaction with sex-linked genes, and also that many might result from hormonal influences on gene expression and regulation or other environmental factors that are related to gender (Weiss et al. 2006). The power of identifying QTLs for the traits increased substantially by incorporating genders into the linkage mapping and by studying trait pairs simultaneously. Most of the findings replicated reports from other studies in different ethnic groups. These results are informative for association mapping and warrant further investigation to elucidate the gender-specific underlying genetic mechanism of the QTLs or positional candidates responsible for these traits.
Acknowledgments
We thank patients and their families for participating in this study. We also thank the members of the SAPPHIRe project for their help. We thank Dr. David Hinds and Ms. Guan-Yi Hung for assistance with data. We thank the reviewers for their constructive and insightful comments, which have greatly improved the quality of this manuscript. This work was supported in part by the Grant from the National Science Council (NSC 96-3112-B002-002 to L.M.C) and by the Grants BS-097-PP-03 (C.A.H) and BS-097-PP-04 (Y.F.C) from the National Health Research Institutes.
Footnotes
Conflict of interest None.
Contributor Information
Y.-F. Chiu, Division of Biostatistics and Bioinformatics, Institute of Population Health Sciences, National Health Research Institutes, 35 Keyan Rd., Zhunan, Miaoli 350, Taiwan, ROC
L.-M. Chuang, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan Graduate Institute of Clinical Medicine, National Taiwan University, Taipei, Taiwan.
H.-Y. Kao, Division of Biostatistics and Bioinformatics, Institute of Population Health Sciences, National Health Research Institutes, 35 Keyan Rd., Zhunan, Miaoli 350, Taiwan, ROC
K.-C. Shih, Division of Endocrinology and Metabolism, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
M.-W. Lin, Institute of Public Health, National Yang-Ming University, Taipei, Taiwan Department of Medical Research and Education, Taipei Veterans General Hospital, Taipei, Taiwan.
W.-J. Lee, Department of the Education and Research, Taichung Veterans General Hospital, Taichung, Taiwan
T. Quertermous, Division of Cardiovascular Medicine, Falk Cardiovascular Research Center, Stanford University, Palo Alto, CA, USA
J. D. Curb, Pacific Health Research Institute, Honolulu, HI, USA
I. Chen, Cedars-Sinai Medical Center, Los Angeles, CA, USA
B. L. Rodriguez, Pacific Health Research Institute, Honolulu, HI, USA
C. A. Hsiung, Email: hsiung@nhri.org.tw, Division of Biostatistics and Bioinformatics, Institute of Population Health Sciences, National Health Research Institutes, 35 Keyan Rd., Zhunan, Miaoli 350, Taiwan, ROC.
References
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos C, de Andrade M, Zhu D. Comparison of multivariate tests for genetic linkage. Hum Hered. 2001;51:133–144. doi: 10.1159/000053334. [DOI] [PubMed] [Google Scholar]
- Barlow DP, Stoger R, Herrmann BG, Saito K, Schweifer N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature. 1991;349:84–87. doi: 10.1038/349084a0. [DOI] [PubMed] [Google Scholar]
- Burton BT, Foster WR, Hirsch J, Van Itallie TB. Health implications of obesity: an NIH consensus development conference. Int J Obes. 1985;9:155–170. [PubMed] [Google Scholar]
- Butler MG, Hedges L, Hovis CL, Feurer ID. Genetic variants of the human obesity (OB) gene in subjects with and without Prader–Willi syndrome: comparison with body mass index and weight. Clin Genet. 1998;54:385–393. doi: 10.1111/j.1399-0004.1998.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Li S, Cook NR, Rosner BA, Srinivasan SR, Boerwinkle E, Berenson GS. An autosomal genome scan for loci influencing longitudinal burden of body mass index from childhood to young adulthood in white sibships: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:462–469. doi: 10.1038/sj.ijo.0802610. [DOI] [PubMed] [Google Scholar]
- Chiu YF, Chuang LM, Hsiao CF, Hung YJ, Lin MW, Chen YT, Grove J, Jorgenson E, Quertermous T, Risch N, Hsiung CA. An autosomal genome-wide scan for loci linked to prediabetic phenotypes in nondiabetic Chinese subjects from the Stanford Asia-Pacific Program of Hypertension and Insulin Resistance Family Study. Diabetes. 2005;54:1200–1206. doi: 10.2337/diabetes.54.4.1200. [DOI] [PubMed] [Google Scholar]
- Chuang LM, Chiu YF, Sheu WH, Hung YJ, Ho LT, Grove J, Rodriguez B, Quertermous T, Chen YD, Hsiung CA, Tai TY. Biethnic comparisons of autosomal genomic scan for loci linked to plasma adiponectin in populations of Chinese and Japanese origin. J Clin Endocrinol Metab. 2004;89:5772–5778. doi: 10.1210/jc.2004-0640. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Allison DB. The search for human obesity genes. Science. 1998;280:1374–1377. doi: 10.1126/science.280.5368.1374. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Blangero J, Mahaney MC, Mitchell BD, Stern MP, MacCluer JW. Quantitative genetics of sexual dimorphism in body fat measurements. Am J Hum Biol. 1993;5:725–734. doi: 10.1002/ajhb.1310050616. [DOI] [PubMed] [Google Scholar]
- Daw EW, Thompson EA, Wijsman EM. Bias in multipoint linkage analysis arising from map misspecification. Genet Epidemiol. 2000;19:366–380. doi: 10.1002/1098-2272(200012)19:4<366::AID-GEPI8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- Diego VP, Voruganti VS, Dyer TD, Cole SA, Almasy L, Rainwater DL, Mahaney MC, MacCluer JW, Blangero J, Comuzzie AG. Genotype × sex interaction analyses identify a region on chromosome 20 contributing to anthropometric measures of obesity in the San Antonio Family Heart Study. Suppl Obes. 2006;14:A267. [Google Scholar]
- Diego VP, Rainwater DL, Wang XL, Cole SA, Curran JE, Johnson MP, Jowett JB, Dyer TD, Williams JT, Moses EK, Comuzzie AG, Maccluer JW, Mahaney MC, Blangero J. Genotype 9 adiposity interaction linkage analyses reveal a locus on chromosome 1 for lipoprotein-associated phospholipase A2, a marker of inflammation and oxidative stress. Am J Hum Genet. 2007;80:168–177. doi: 10.1086/510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Li WD, Geller F, Lei L, Li D, Gorlova OY, Hebebrand J, Amos CI, Nicholls RD, Price RA. Possible genomic imprinting of three human obesity-related genetic loci. Am J Hum Genet. 2005;76:427–437. doi: 10.1086/428438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake NM, Park YJ, Shirali AS, Cleland TA, Soloway PD. Imprint switch mutations at Rasgrf1 support conflict hypothesis of imprinting and define a growth control mechanism upstream of IGF1. Mamm Genome. 2009;20:654–663. doi: 10.1007/s00335-009-9192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller F, Dempfle A, Gorg T. Genome scan for body mass index and height in the Framingham Heart Study. BMC Genet. 2003;4(Suppl 1):S91. doi: 10.1186/1471-2156-4-S1-S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlova OY, Amos CI, Wang NW, Shete S, Turner ST, Boerwinkle E. Genetic linkage and imprinting effects on body mass index in children and young adults. Eur J Hum Genet. 2003;11:425–432. doi: 10.1038/sj.ejhg.5200979. [DOI] [PubMed] [Google Scholar]
- Hsueh WC, Mitchell BD, Schneider JL, St Jean PL, Pollin TI, Ehm MG, Wagner MJ, Burns DK, Sakul H, Bell CJ, Shuldiner AR. Genome-wide scan of obesity in the Old Order Amish. J Clin Endocrinol Metab. 2001;86:1199–1205. doi: 10.1210/jcem.86.3.7358. [DOI] [PubMed] [Google Scholar]
- Jorgenson E, Tang H, Gadde M, Province M, Leppert M, Kardia S, Schork N, Cooper R, Rao DC, Boerwinkle E, Risch N. Ethnicity and human genetic linkage maps. Am J Hum Genet. 2005;76:276–290. doi: 10.1086/427926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Park H, Lee MK, Kim JI, Sung J, Cho SI, Seo JS. A genome-wide Asian genetic map and ethnic comparison: the GENDISCAN study. BMC Genomics. 2008;9:554. doi: 10.1186/1471-2164-9-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalscheuer VM, Mariman EC, Schepens MT, Rehder H, Ropers HH. The insulin-like growth factor type-2 receptor gene is imprinted in the mouse but not in humans. Nat Genet. 1993;5:74–78. doi: 10.1038/ng0993-74. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HI, Park T, Kim K, Lee JE, Cho NH, Shin C, Cho YS, Lee JY, Han BG, Yoo HW, Lee JK. Identification of 15 loci influencing height in a Korean population. J Hum Genet. 2010;55:27–31. doi: 10.1038/jhg.2009.116. [DOI] [PubMed] [Google Scholar]
- Korbonits M, Gueorguiev M, O’Grady E, Lecoeur C, Swan DC, Mein CA, Weill J, Grossman AB, Froguel P. A variation in the ghrelin gene increases weight and decreases insulin secretion in tall, obese children. J Clin Endocrinol Metab. 2002;87:4005–4008. doi: 10.1210/jcem.87.8.8881. [DOI] [PubMed] [Google Scholar]
- Le Stunff C, Le Bihan C, Schork NJ, Bougneres P. A common promoter variant of the leptin gene is associated with changes in the relationship between serum leptin and fat mass in obese girls. Diabetes. 2000;49:2196–2200. doi: 10.2337/diabetes.49.12.2196. [DOI] [PubMed] [Google Scholar]
- Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–467. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- Lewis CE, North KE, Arnett D, Borecki IB, Coon H, Ellison RC, Hunt SC, Oberman A, Rich SS, Province MA, Miller MB. Sex-specific findings from a genome-wide linkage analysis of human fatness in non-Hispanic whites and African Americans: the HyperGEN study. Int J Obes (Lond) 2005;29:639–649. doi: 10.1038/sj.ijo.0802916. [DOI] [PubMed] [Google Scholar]
- Li WD, Reed DR, Lee JH, Xu W, Kilker RL, Sodam BR, Price RA. Sequence variants in the 50 flanking region of the leptin gene are associated with obesity in women. Ann Hum Genet. 1999;63:227–234. doi: 10.1046/j.1469-1809.1999.6330227.x. [DOI] [PubMed] [Google Scholar]
- Li WD, Dong C, Li D, Zhao H, Price RA. An obesity-related locus in chromosome region 12q23–24. Diabetes. 2004;53:812–820. doi: 10.2337/diabetes.53.3.812. [DOI] [PubMed] [Google Scholar]
- Liu YZ, Xiao P, Guo YF, Xiong DH, Zhao LJ, Shen H, Liu YJ, Dvornyk V, Long JR, Deng HY, Li JL, Recker RR, Deng HW. Genetic linkage of human height is confirmed to 9q22 and Xq24. Hum Genet. 2006;119:295–304. doi: 10.1007/s00439-006-0136-y. [DOI] [PubMed] [Google Scholar]
- Livshits G, Kato BS, Wilson SG, Spector TD. Linkage of genes to total lean body mass in normal women. J Clin Endocrinol Metab. 2007;92:3171–3176. doi: 10.1210/jc.2007-0418. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Mahaney MC, Almasy L, MacCluer JW, Blangero J, Jaquish CE, Comuzzie AG. Leptin’s sexual dimorphism results from genotype by sex interactions mediated by testosterone. Obes Res. 2002;10:14–21. doi: 10.1038/oby.2002.3. [DOI] [PubMed] [Google Scholar]
- Miyoshi N, Kuroiwa Y, Kohda T, Shitara H, Yonekawa H, Kawabe T, Hasegawa H, Barton SC, Surani MA, Kaneko-Ishino T, Ishino F. Identification of the Meg1/Grb10 imprinted gene on mouse proximal chromosome 11, a candidate for the Silver–Russell syndrome gene. Proc Natl Acad Sci USA. 1998;95:1102–1107. doi: 10.1073/pnas.95.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris JM, Langefeld CD, Scherzinger AL, Rich SS, Bookman E, Beck SR, Saad MF, Haffner SM, Bergman RN, Bowden DW, Wagenknecht LE. Quantitative trait loci for abdominal fat and BMI in Hispanic-Americans and African-Americans: the IRAS Family study. Int J Obes (Lond) 2005;29:67–77. doi: 10.1038/sj.ijo.0802793. [DOI] [PubMed] [Google Scholar]
- Peng B, Yu R, Dehoff K, Amos C. Normalizing a large number of quantitative traits using empirical normal quantile transformation. BMC Proc. 2007;1(Suppl 1):S156. doi: 10.1186/1753-6561-1-s1-s156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusse L, Rice T, Chagnon YC, Despres JP, Lemieux S, Roy S, Lacaille M, Ho-Kim MA, Chagnon M, Province MA, Rao DC, Bouchard C. A genome-wide scan for abdominal fat assessed by computed tomography in the Quebec Family Study. Diabetes. 2001;50:614–621. doi: 10.2337/diabetes.50.3.614. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Allison DB, Heshka S, Heo M, Wang ZM, Bertkau A, Laferrere B, Rosenbaum M, Aloia JF, Pi-Sunyer FX, Heymsfield SB. Sexual dimorphism in the energy content of weight change. Int J Obes Relat Metab Disord. 2002;26:1339–1348. doi: 10.1038/sj.ijo.0802065. [DOI] [PubMed] [Google Scholar]
- Price RA, Lee JH. Risk ratios for obesity in families of obese African-American and Caucasian women. Hum Hered. 2001;51:35–40. doi: 10.1159/000022957. [DOI] [PubMed] [Google Scholar]
- Price RA, Reed DR, Guido NJ. Resemblance for body mass index in families of obese African American and European American women. Obes Res. 2000;8:360–366. doi: 10.1038/oby.2000.43. [DOI] [PubMed] [Google Scholar]
- Ranade K, Wu KD, Risch N, Olivier M, Pei D, Hsiao CF, Chuang LM, Ho LT, Jorgenson E, Pesich R, Chen YD, Dzau V, Lin A, Olshen RA, Curb D, Cox DR, Botstein D. Genetic variation in aldosterone synthase predicts plasma glucose levels. Proc Natl Acad Sci USA. 2001;98:13219–13224. doi: 10.1073/pnas.221467098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade K, Hinds D, Hsiung CA, Chuang LM, Chang MS, Chen YT, Pesich R, Hebert J, Chen YD, Dzau V, Olshen R, Curb D, Botstein D, Cox DR, Risch N. A genome scan for hypertension susceptibility loci in populations of Chinese and Japanese origins. Am J Hypertens. 2003;16:158–162. doi: 10.1016/s0895-7061(02)03245-4. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Perusse L, Bouchard C. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Roeder K, Bacanu SA, Wasserman L, Devlin B. Using linkage genome scans to improve power of association in genome scans. Am J Hum Genet. 2006;78:243–252. doi: 10.1086/500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Gagnon J, Chagnon YC, Perusse L, Despres JP, Leon AS, Wilmore JH, Skinner JS, Borecki I, Rao DC, Bouchard C. Association and linkage between an insulin-like growth factor-1 gene polymorphism and fat free mass in the HERITAGE Family Study. Int J Obes Relat Metab Disord. 1999;23:929–935. doi: 10.1038/sj.ijo.0801021. [DOI] [PubMed] [Google Scholar]
- Towne B, Almasy L, Siervogel RM, Blangero J. Effects of genotype × sex interaction on linkage analysis of visual event-related evoked potentials. Genet Epidemiol. 1999;17(Suppl 1):S355–S360. doi: 10.1002/gepi.1370170760. [DOI] [PubMed] [Google Scholar]
- Ukkola O, Ravussin E, Jacobson P, Snyder EE, Chagnon M, Sjostrom L, Bouchard C. Mutations in the preproghrelin/ghrelin gene associated with obesity in humans. J Clin Endocrinol Metab. 2001;86:3996–3999. doi: 10.1210/jcem.86.8.7914. [DOI] [PubMed] [Google Scholar]
- Voruganti VS, Diego VP, Blangero J, Haack K, Cole SA, Ebbesson S, Devereux R, Fabsitz RR, Howard BV, Comuzzie AG, MacCluer JW. A QTL for genotype by sex specific interaction for anthropometric measurements in Alaskan Eskimos on chromosome 19q12–13: the GOCADAN Study. Suppl Obes. 2006;14:A8. doi: 10.1038/oby.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder K, Hanson RL, Kobes S, Knowler WC, Ravussin E. An autosomal genomic scan for loci linked to plasma leptin concentration in Pima Indians. Int J Obes Relat Metab Disord. 2000;24:559–565. doi: 10.1038/sj.ijo.0801197. [DOI] [PubMed] [Google Scholar]
- Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, Freathy RM, Perry JR, Stevens S, Hall AS, Samani NJ, Shields B, Prokopenko I, Farrall M, Dominiczak A, Johnson T, Bergmann S, Beckmann JS, Vollenweider P, Waterworth DM, Mooser V, Palmer CN, Morris AD, Ouwehand WH, Zhao JH, Li S, Loos RJ, Barroso I, Deloukas P, Sandhu MS, Wheeler E, Soranzo N, Inouye M, Wareham NJ, Caulfield M, Munroe PB, Hattersley AT, McCarthy MI, Frayling TM. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nat Genet. 2006;38:218–222. doi: 10.1038/ng1726. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O’Rahilly S, Purmann C, Rees MG, Ridderstrale M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KD, Hsiao CF, Ho LT, Sheu WH, Pei D, Chuang LM, Curb D, Chen YD, Tsai HJ, Dzau VJ, Cox D, Tai TY. Clustering and heritability of insulin resistance in Chinese and Japanese hypertensive families: a Stanford-Asian Pacific Program in Hypertension and Insulin Resistance sibling study. Hypertens Res. 2002;25:529–536. doi: 10.1291/hypres.25.529. [DOI] [PubMed] [Google Scholar]
- Xu Y, Goodyer CG, Deal C, Polychronakos C. Functional polymorphism in the parental imprinting of the human IGF2R gene. Biochem Biophys Res Commun. 1993;197:747–754. doi: 10.1006/bbrc.1993.2542. [DOI] [PubMed] [Google Scholar]
- Yang WS, Hsiung CA, Ho LT, Chen YT, He CT, Curb JD, Grove J, Quertermous T, Chen YD, Kuo SS, Chuang LM. Genetic epistasis of adiponectin and PPARgamma2 genotypes in modulation of insulin sensitivity: a family-based association study. Diabetologia. 2003;46:977–983. doi: 10.1007/s00125-003-1136-2. [DOI] [PubMed] [Google Scholar]