Abstract
Motor neurons send out axons to peripheral muscles while their cell bodies remain in the ventral spinal cord. The unique configuration of motor neurons spanning the border between the CNS and PNS has been explained by structural barriers such as boundary cap (BC) cells, basal lamina and radial glia. However, mechanisms in motor neurons that retain their position have not been addressed yet. Here we demonstrate that the Islet1 (Isl1) and Islet2 (Isl2) transcription factors, which are essential for acquisition of motor neuron identity, also contribute to restrict motor neurons within the neural tube. In mice that lack both Isl1 and Isl2, large numbers of motor neurons exited the neural tube, even prior to the appearance of BC cells at the ventral exit points. Transcriptional profiling of motor neurons derived from Isl1 null embryonic stem cells revealed that transcripts of major genes involved in repulsive mechanisms were misregulated. Particularly, expression of Neuropilin1 (Npr1) and Slit2 mRNA was diminished in Islet mutant mice, and these could be target genes of the Islet proteins. Consistent with this mechanism, Robo and Slit mutations in mice and knockdown of Npr1 and Slit2 in chick embryos caused motor neurons to migrate to the periphery. Together, our study suggests that Islet genes engage Robo-Slit and Neuropilin-Semaphorin signaling in motor neurons to retain motor somata within the CNS.
Keywords: Islet1, Islet2, Neuropilin1, Slit2, motor neuron somata
Introduction
Motor neurons that control locomotion are a unique population of the CNS whose cell bodies lie in the neural tube but whose axons exit the neural tube and project toward muscles in the periphery. Numerous studies have investigated the initial acquisition of motor neuron identity and axon pathfinding. However, the key mechanisms that direct axons toward the motor exit points (MEPs) located in the ventral spinal cord while retaining the motor somata within the CNS are still unclear. Around embryonic day 9 (E9.0) in mice, motor neurons are born in the pMN domain of the ventral spinal cord (Nornes and Carry, 1978). Subsequently, they migrate laterally to occupy ventro-lateral positions, and their axons converge and pass through MEPs. Although several structural barriers such as neural crest-derived boundary cap (BC) cells, radial glia and basal lamina have been proposed to prevent motor neuron somata from escaping the neural tube, motor neurons are frequently found outside the neural tube when the specification of motor neurons is defective (Lee and Song, 2013, Niederlander and Lumsden, 1996, Thaler, et al., 2004, Vermeren, et al., 2003). Thus, mechanisms that retain cell bodies within the neural tube appear to include factors located within the motor neurons themselves.
Members of the LIM homeodomain (LIM-HD) transcription factor family play a role in numerous aspects of motor neuron development, including the initial acquisition of motor neuron identity and the diversification of motor columns (Ericson, et al., 1992, Kania and Jessell, 2003, Song, et al., 2009, Thaler, et al., 2004, Tsuchida, et al., 1994). The LIM-HD proteins Isl1 and Isl2 are very similar with 72% protein identity (98% in HD domain and 82% in the LIM domains). Isl1 first appears in all motor neurons when motor neurons exit the cell cycle, and the expression of Isl2 follows (Pfaff, et al., 1996, Thaler, et al., 2004). Later, expression of Isl1 and Isl2 becomes restricted to some of the motor columns, and this is important for assigning motor columnar identity. Consistent with an important function in motor neuron identity, conditional elimination of Isl1 in the CNS results in a loss of motor neurons and the formation of ectopic V2a interneurons (Song, et al., 2009). In Isl2 null mice, however, only visceral motor neurons are affected and mis-positioned on the dorsal side of the spinal cord (Thaler, et al., 2004). Thus, Isl1 and Isl2 may serve only partially overlapping functions in motor neuron development.
In this study, we identify a new function of Isl1 and Isl2, which allows axons but not cell bodies to penetrate MEP. In Islet mutant mice, many motor neurons exit from the neural tube regardless of their subtype, and this exit begins even before BC cells appear at the MEP. We find that the emigration of motor neurons in these animals is accompanied by downregulation of Neuropilin1 and Slit2 transcripts, raising the possibility that the latter genes may be targets of Islet proteins and that they may be responsible for preventing neuronal migration. Consistent with this hypothesis, we demonstrated that Robo and Slit mutant mice, as well as chick embryos with depleted Nrp1 and Slit2 transcripts, have motor neuron cell bodies that emigrate out of the neural tube. Our results suggest that repulsive activity in motor neurons controlled by the Islet proteins is a key mechanism maintaining the boundary between CNS and PNS.
Material and Methods
Mice
Isl1 hypo, Isl2 null mice and Hb9::GFP mice were described previously (Lee, et al., 2004, Song, et al., 2009, Sun, et al., 2008, Thaler, et al., 2004). The Robo and Slit mutant strains were gifts of Dr. Marc Tessier-Lavigne, Rockefeller University (Long, et al., 2004). Wildtype C56BL/6 and CD-1 mice (6–8 weeks old) were purchased from Damul Science and Charles River Laboratories, respectively. Robo and Slit PCR genotyping was performed as previously described (Grieshammer, et al., 2004, Long, et al., 2004, Plump, et al., 2002). All experiments used protocols approved by the Animal Care and Ethics Committees of the Gwangju Institute of Science and Technology (GIST), or by the University of Nevada, Reno Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The day when a vaginal plug was detected was designated embryonic day 0.5 (E0.5).
Immunohistochemistry, immunocytochemistry and in situ hybridization
Embryos were obtained and processed for immunohistochemistry or in situ hybridization as described previously (Song, et al., 2009). The following antibodies were used: rabbit and guinea pig anti-Hb9 (Thaler, et al., 1999), guinea pig anti-Lhx3 (Sharma, et al., 1998), guinea pig anti-Chx10 (Thaler, et al., 2002), rabbit anti-Foxp1 (Abcam), rabbit anti-Krox20 (Covance), rabbit and guinea pig anti-Isl1/2 (Ericson, et al., 1992), mouse anti-Neurofilament (DSHB), mouse anti-GFP (Sigma), rabbit anti-β-III-tubulin (Covance), rabbit anti-β-galactosidase (Cappel) antibodies. For immunocytochemistry, dissociated cultured cells were fixed and immunostained with antibodies including rabbit anti-Robo1 and Robo2 (kind gift of Dr. Elke Stein, Yale) and mouse anti-Isl1 (DSHB). Previous characterization of the Robo1 and Robo2 antisera confirmed that specific labeling was lost in homozygous mutants. For in situ hybridization, embryonic cDNA at E10.5 or E12.5 was used to generate riboprobes using an Advantage cDNA PCR kit (Clonetech).
Chick in ovo electroporation
Nrp1 siRNA/scrambled siRNA (Bron, et al., 2004), Slit2 morpholino/control morpholino (Giovannone, et al., 2012) and siRNAs/scrambled siRNA against Semaphorin ligands (see Supplementary Methods) were electroporated with GFP into the chick spinal cord at Hamburger and Hamilton (HH) stages 10 to 12 and harvested at HH stages 20 to 25. Electroporation was carried out using a square wave electroporator (BTX) with 5 pulses of 25 V, 50 ms at 1 s intervals.
Microarray analysis
Embryonic stem (ES) cells were derived from littermate blastocysts of Isl1 heterozygous intercrosses (also containing Hb9::gfp transgenes) and cultured in standard ES cell conditions as described (Macfarlan, et al., 2011). To induce motor neuron differentiation, ES cells were adapted to gelatinized dishes for two passages, and 106 trypsinized cells were seeded in mDiff medium (1:1 Knockout DMEM:DMEM/F12 (Invitrogen), 5% Knockout™ Serum Replacement (Invitrogen), 1X NEAA (Mediatech), 2 mM L-glutamine, 14.3 mM 2-mercaptoethanol) in bacterial grade 10 cm2 dishes, and the medium was changed every two days. RA (1 μM) and smoothened agonist (1 μM, Calbiochem) were added to induce motor neuron differentiation after 2 days of EB formation. Hb9::gfp positive cells were then collected on day 6 by FACS. RNA was prepared from mES-derived MNs using an RNEasy kit (Qiagen) with on-column DNAse digestion. dscDNA was generated from 100 ng-1 μg of total RNA using a GeneChip3′ IVT Express Kit (Affymetrix), fragmented, and hybridized to Affymetrix Mouse Genome 430 2.0 expression arrays. Differentially expressed genes in four wild type ES cell lines (heterozygous and homozygous) and three knockout lines (independent replicates from a single ES cell line) were identified using Vampire and the default settings (http://genome.ucsd.edu/microarray/).
Quantification and statistics
At least 6 sections from 3 embryos were analyzed for each genotype and axial level. Limb levels were determined by Raldh2 expression in adjacent sections. To measure % of sections with ectopic motor neurons in the chick spinal cord, the proportion of sections that contained ectopic motor neurons on either side of the neural tube was calculated. Statistical significance was analyzed by unpaired Student’s t-test and the Kruskal-Wallis test for multiple comparisons.
Results
Soma emigration of motor neurons occurs when the level of Islet protein expression is low
Since Isl1 and Isl2 co-exist in spinal motor neurons, they may play redundant roles that might be revealed only when both gene products were eliminated (Hutchinson and Eisen, 2006, Thaler, et al., 2004). To test this idea, we investigated motor neuron formation in Isl1 hypomorphic (Isl1 hypo) mice and Isl2 null (Isl2 KO) mice (Sun, et al., 2008, Thaler, et al., 2004). Isl1 hypo mice make low level of Isl1 due to the insertion of a neo cassette that reduces the splicing efficiency of Isl1 transcripts (Sun, et al., 2008). We examined expression of Isl1 and Isl2 in these mice by immunohistochemistry using antibodies against Isl1 at subsaturating concentrations (Sup. Fig. 1A-D, H). Isl1 protein was reduced to as little as 19.8% of normal in Isl1 hypo mice and to 6.2% in Isl1 hypo; Isl2 KO mice. In Isl1 hypo mice, Isl2 expression was reduced and it was almost undetectable in Isl2 KO and Isl1 hypo; Isl2 KO mice (Sup. Fig. 1E-G, I). Thus, the level of total Islet proteins decreased in the order Isl2 KO, Isl1 hypo and Isl1 hypo; Isl2 KO.
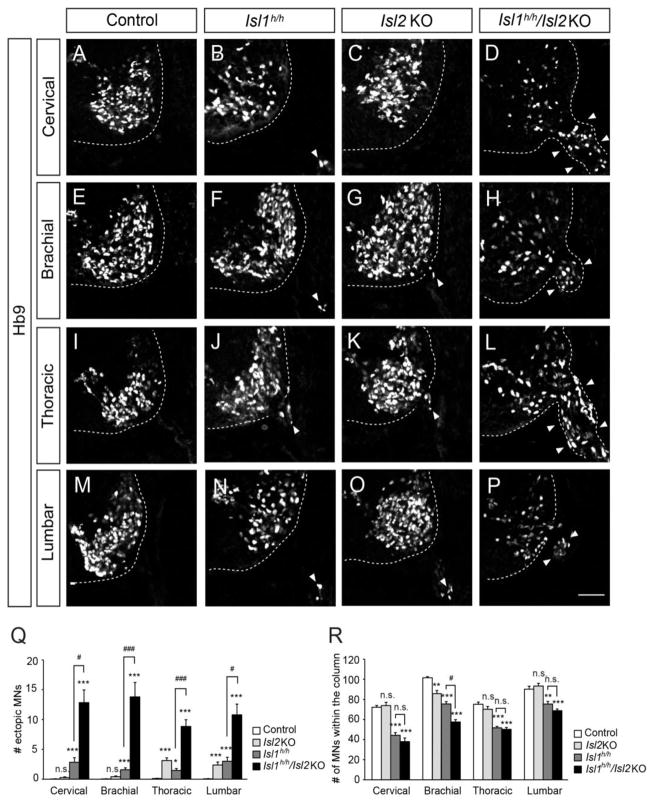
When we examined the motor columns in the spinal cords of E11.5 Islet mutant mice, we found significant numbers of motor neurons labeled by Hb9, a transcription factor present in postmitotic motor neurons, outside the neural tube (Fig. 1). A few ectopic motor neurons were detected at the cervical level in Isl2 KO and Isl1 hypo mice (about 1 cell per quadrant of the spinal cord in Isl2 KO and 4 cells in Isl1 hypo mice). This is in stark contrast to littermate controls in which virtually no cells escaped from the neural tube. Remarkably, more than 15 ectopic motor neurons were found in Isl1 hypo; Isl2 KO mice, indicating that Isl1 and Isl2 cooperate to confine motor neurons. We also examined motor neurons in Isl1 conditional Nestin-Cre knockout mice that selectively remove Isl1 expression in the CNS (Song, et al., 2009). Isl1 conditional Nestin-Cre knockout mice had lower numbers of ectopic motor neurons (2 cells per section), indicating that elimination of Isl1 is not sufficient to fully emigrate motor neurons (Sup. Fig. 2). Similar results were obtained at other segmental levels of the spinal cord (Fig. 1Q). Somatal emigration was accompanied by corresponding reductions in motor neurons within the neural tube (Fig. 1R). Taken together, these observations indicate that motor neurons escape the neural tube when the level of Islet proteins is low.
Figure 1. Ectopic motor neurons in the ventral roots of Isl1 hypo (Isl1h/h) and Isl1 hypo; Isl2 (Isl1h/h; Isl2 KO) null mice.
(A–P) Motor neurons labeled with Hb9 are present in the neural tube and the periphery (arrowheads, B, D, F–H, J–L, N–P) Control, Isl1 hypo, Isl2 KO and Isl1 hypo; Isl2 KO mice (n >10 sections in 5 embryos). (Q, R) The number of ectopic Hb9+ cells in the periphery (Q) and Hb9+ motor neurons in the column (R). Error bars represent SEM. **p < 0.01, ***p < 0.001 vs. control; #p < 0.05, ###p < 0.001 vs. Isl1 hypo; Kruskal-Wallis test. Scale bar: 50 μm.
Distribution and specification of motor columns are altered in Islet mutant spinal cords
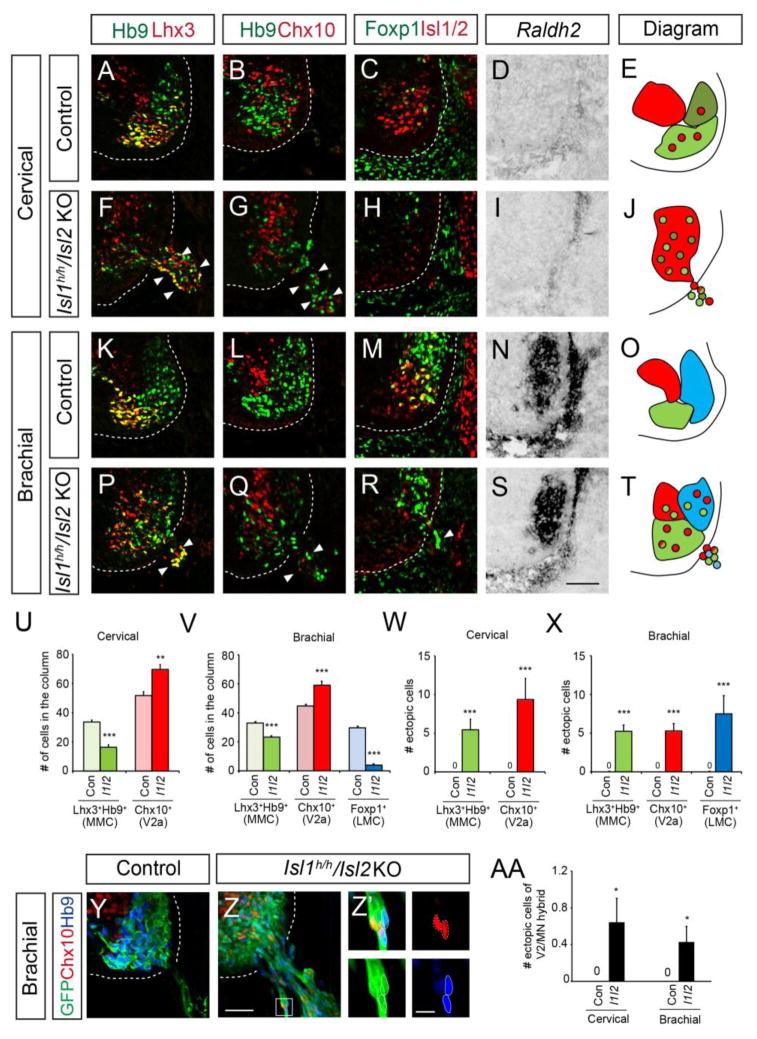
We previously showed that motor neuron identity is compromised in the absence of Isl1 such that some motor neurons display V2 interneuronal traits (Song, et al., 2009). To see whether this change was related to the emigration of motor neurons, we examined motor columnar identity in Islet mutant mice. The medial and lateral motor columns are determined by the combinatorial expression of Lhx3, Hb9, Isl1/2, Foxp1 and Raldh2. We also examined V2a interneurons marked by Chx10 since Isl1 mutant cells often display ectopic Chx10 expression (Song, et al., 2009, Thaler, et al., 2004). At cervical levels, the number of median motor column (MMC) neurons that expressed Hb9 and Lhx3 decreased in Isl1 hypo; Isl2 KO mice (33.7 cells in control; 16.3 cells in Isl1 hypo; Isl2 KO mice) (Fig. 2A, E, F, J, U), whereas the number of Chx10-expressing cells increased (51.8 cells in control; 69.6 cells in Isl1 hypo; Isl2 KO mice), an increase that is roughly the same as the number of missing MMC neurons (Fig. 2B, E, G, J, U). Furthermore, the extra V2a interneurons were mostly found in the territory of the motor columns and they could be labeled by an Hb9-gfp reporter, which selectively marks motor axons. This indicates that the fates of putative motor neurons, initially labeled by Hb9-gfp reporter, were compromised in the absence of Islet proteins and the cells became switched to V2a interneuronal type (Fig. 2G, Q, Z). Similar increases in the numbers of MMC neurons and V2a interneurons occurred in Isl1 hypo; Isl2 KO mice at brachial levels (Fig. 2K, L, O, Q, T, V), while the number of lateral motor column (LMC) neurons identifiable by the presence of Foxp1 and Raldh2 was reduced by 70.3% (Fig. 2M–O, R–T, V). Thus, the number of motor neurons decreases and the number of V2a interneurons increases when Islet expression is low, which could be due to altered specification as well as emigration of motor neurons.
Figure 2. Columnar identity is compromised in Islet mutant spinal cords.
(A–D, F–I, K–N, P–S) Expression of Hb9, Lhx3, Chx10, Foxp1, Isl1/2 and Raldh2 at cervical and brachial levels in E11.5 mouse spinal cords of Isl1 hypo and Isl1 hypo; Isl2 KO (I1/I2) mice (n > 6 sections in 3 embryos). Ectopic cells outside the neural tube are marked with arrowheads (F, G, P, Q, R). (E, J, O, T) Diagrams of motor columns and ectopic motor neurons of each genotype; MMC (light green), HMC (olive), LMC (blue) and V2a (red). (U, V) Quantification of motor columns and V2a interneurons in the neural tube, as indicated. (W, X) Quantification of ectopic cells with the indicated markers. (Y-AA) Immunolabeling and quantification of extraspinal cells in Isl1 hypo; Isl2 KO; Hb9::GFP mice. Images are z-stack images (Y, Z) or single plane images Z′ (high power images in Z; Scale bar: 10 μm). Dotted lines mark Chx10+GFP+ cells and solid lines mark Hb9+GFP+ cells. Error bars represent SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control; unpaired Student’s t-test; n.s., not significant. Scale bar: 50 μm.
Next, we examined the identity of the ectopic motor neurons to see whether those motor neurons that erroneously express the V2a interneuronal marker are prone to escape the neural tube. We did not find any sign of apoptosis in these ectopic cells at E11.5 (Sup. Fig. 3). At cervical levels, 5.4 ectopic cells on average were MMC neurons (Lhx3+Hb9+), and 9.4 cells were V2a interneurons (Fig. 2W). Almost all the Chx10-expressing ectopic cells were labeled by Hb9-gfp, indicating that they were originally motor neurons and their fates had undergone conversion in the absence of Islet (Fig. 2Z-Z′). At brachial levels, MMC, LMC and V2a interneurons were all found outside the neural tube, with more than 5 cells of each subtype (Fig. 2P–T, X). Only a few cells were Hb9+Chx10+ hybrids (0.6 cells at cervical levels and 0.4 cells at brachial levels) (Fig. 2AA). We observed similar changes in the number of cells in motor columns and ectopic cells at thoracic and lumbar levels (Sup. Fig. 4). Since not all the ectopic motor neurons were hybrid or Chx10-expressing cells, mis-specification may not be the major cause of their positioning defect. We noted that the organization of motor columns in Isl2 KO mice was normal, with few ectopic cells, unlike the situation in the Isl1 hypo; Isl2 KO mice. The Isl1 hypo mice had mild phenotypes compared to the Isl1 hypo; Isl2 KO mice (Sup. Fig. 5). Together, all types of motor neurons escape the spinal cord regardless of their columnar identity when both Islet genes are downregulated.
The peripheral structural barrier is formed normally but is disrupted by emigrating motor neurons
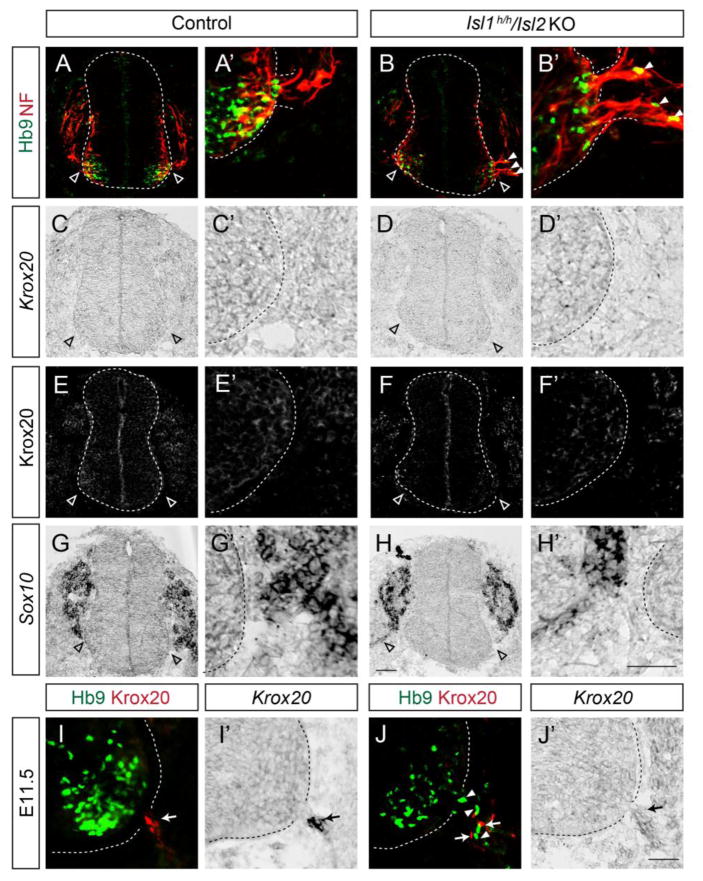
The presence of extraspinal motor neurons has been reported previously when peripheral structures such as the basal lamina, boundary cap (BC) cells and radial glia were compromised (Halfter, et al., 2002, Lee and Song, 2013, Vermeren, et al., 2003). Although there is no report that BC cells express Isl1 or Isl2, we examined whether peripheral barrier formation is normal in the absence of Islet proteins. BC cells originate from neural crest cells that produce Sox10 along their migratory route, and Krox20 once they arrive at MEPs (Vermeren, et al., 2003). At E9.75 when neural crest cells were still migrating, a stream of cells expressing Sox10 transcripts were detected near MEPs but it was uncertain whether it contained BC cells or not. Nevertheless, none of Krox20 protein and mRNA was detected at MEPs, implying that BC cells have not established yet (Fig. 3C–H′). Surprisingly, motor axons labeled by Neurofilament proteins exited the MEPs at this stage and several ectopic motor somata appeared in Isl1 hypo; Isl2 KO mice (Fig. 3A–B′). This suggests that motor cell bodies escape before the time when BC cells function as a barrier. At E11.5, the Krox20-expressing BC cells in Isl1 hypo; Isl2 KO mice were scattered and intermingled with motor somata unlike those in littermate controls, in which the BC cells were clustered at the MEPs (Fig. 3I–J′). Nevertheless, a few scattered BC cells in the mutants imply that BC cells were recruited but they failed to group properly. Perhaps BC cells are mechanically blocked by emigrating motor neurons (Fig. 3J). We also found that the structures of the basal lamina, radial glia and peripheral glia, were intact in the Isl1 hypo; Isl2 KO mice (Sup. Fig. 6). Together, these findings suggest that the initial exodus of motor neurons may inhibit BC cell formation, and that this may later aggravate the defect of the CNS/PNS border in Islet mutant mice.
Figure 3. Motor somata exit the neural tube before the settlement of boundary cap (BC) cells in Islet mutant spinal cords.
(A–B′) Motor neurons (Hb9, green) are ectopically located in the ventral roots (Neurofilament, red) of Isl1 hypo; Isl2 KO mice at E9.75. (C–H′) BC cells labeled by Krox20 protein or mRNA as well as migrating neural crest cells labeled by Sox10 mRNA are absent in the dorsal and ventral exit points in adjacent sections of A and B. Empty arrowheads indicate MEPs (A–H′). (I–J′) At E11.5, BC cell clusters are present at MEPs in control mice (arrows, I, I′), while scattered BC cells (arrows, J, J′) intermingled with ectopic motor neurons (arrowheads, J) are seen in Isl1 hypo; Isl2 KO mice. Expression of Krox20 was assessed by immunohistochemistry and in situ hybridization in adjacent sections. Scale bars: 50 μm.
Reduced expression of Neuropilin1 and Slit2 in the absence of Isl1 and Isl2
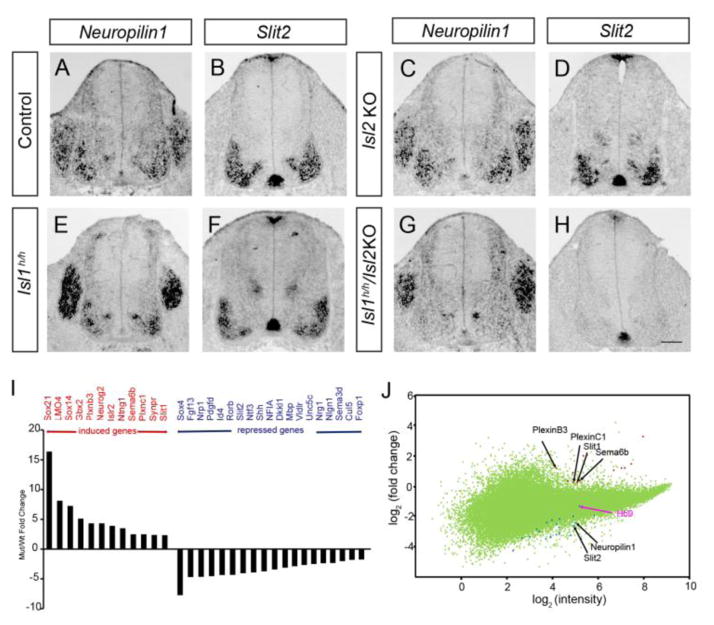
Since the Islet proteins are transcription factors, they are likely to control a set of genes required for migrating cell bodies to be retained in the neural tube. Expression of these genes is expected to be downregulated in the absence of Islet since Islet proteins mainly act as activators (Pfaff, et al., 1996, Zhang, et al., 2009). To identify the gene products responsible for the occurrence of ectopic motor neurons, we performed microarray screens using Isl1 knockout embryonic stem cells differentiated into motor neurons with retinoic acid and smoothened agonist (Wichterle, et al., 2002). About 275 transcripts were significantly induced and 182 transcripts downregulated in Isl1 null cells (> 0.5 fold change compared to the control). The expression of several genes related to axon guidance and cell migration was altered in the Islet null cells (Fig. 4I, J) (Supplementary Table 1). In particular, Neuropilin1 and Slit2 transcripts were absent from the spinal cord motor neurons of Isl1 hypo; Isl2 KO mice but not from those of Isl2 KO or Isl1 hypo mice (Fig. 4). It is important to note that Slit2 expression was maintained in the floor plate (Fig 4H), so that the Islet mutant embryos have a motor neuron-specific loss of Slit expression. Expression of Neuropilin2, PlexinA1, Slit1, Slit3, Robo1, Robo2 was unchanged in the Islet mutants (Sup. Fig. 7).
Figure 4. Reduced expression of Neuropilin1 and Slit2 in the absence of Isl1 and Isl2.
(A–H) Neuropilin1 and Slit2 transcripts are almost undetectable at motor columns of Isl1 hypo; Isl2 KO mice. (I) List of genes altered in Isl1 null ES cells. (J) Scatter plot of the gene expression profiles comparing Isl1 KO ES with control ES cells by cDNA microarrays. Scale bar: 100 μm.
Reduction of Semaphorin and Slit-Robo signaling triggers ectopic motor neurons outside the neural tube
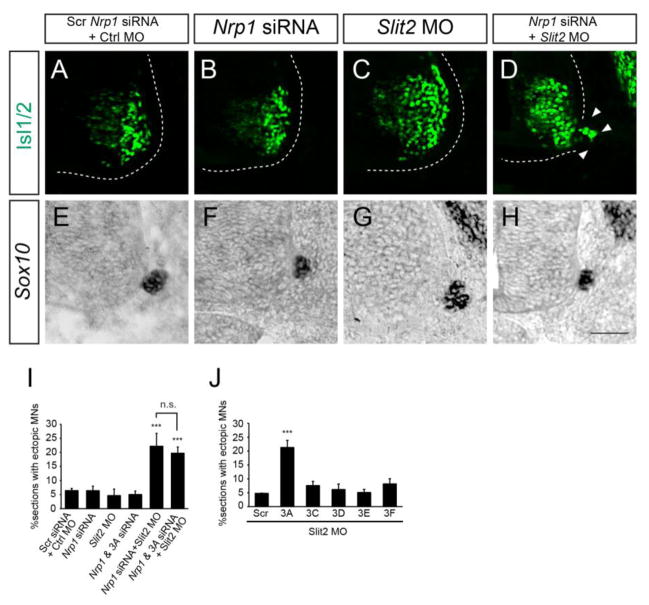
The downregulation of Nrp1 and Slit2 in the absence of Isl1 and Isl2 led us to think that the products of these genes might be responsible for preventing the emigration of motor neurons in wild type organisms. To test this, we electroporated Nrp1 siRNA and/or Slit2 morpholino (MO) into chick neural tube, downregulated expression of the corresponding transcripts (Bron, et al., 2004, Giovannone, et al., 2012). The introduction of Nrp1 siRNA or Slit2 MO separately did not cause motor neuron emigration (Fig. 5A–C, I). However, downregulation of both transcripts resulted in the escape of more motor neurons (> 2.8-fold) than in the control (Fig. 5D, I). Importantly, BC structures were intact in all the knockdown conditions (Fig. 5E–H).
Figure 5. Repression of Semaphorin and Slit-Robo signaling results in extraspinal motor neurons.
(A–D) Knockdown of Nrp1 siRNA and Slit2 MO but not Nrp1 siRNA or Slit2 MO alone causes ectopic migration of motor neurons in the chick neural tube (arrowheads, D) (n > 80 sections in 6 embryos). (E–H) Sox10+ BC cells are intact in embryos in which Nrp1 and Slit2 are downregulated. (I, J) Quantification of % sections with ectopic motor neurons treated with Nrp1 siRNA, Sema3A (3A) siRNA and Slit2 MO (I), and semaphorin siRNAs and Slit2 MO (J). Error bars represent SEM. ***p < 0.001 vs. control; one-way ANOVA test, post hoc Tukey’s multiple comparison test (I); unpaired Student’s t-test (J); n.s., not significant. Scale bar: 50 μm
Nrp1 interacts with Sema3 family members, which are present in the developing spinal cord (He and Tessier-Lavigne, 1997, Kitsukawa, et al., 1997, Kolodkin, et al., 1997). Previous studies and our in situ hybridization showed that Sema3A, 3C, 3D, 3E and 3F were present in motor neurons but none of them were found in BC cells (Bron, et al., 2007) (Sup. Fig. 8). Sema3B and 3G are present in BC cells but elimination of them does not affect position of motor neurons (Bron, et al., 2007). Knockdown of individual Semaphorin ligands or knockdown of both Sema3A siRNA and Nrp1 siRNA was not sufficient to trigger motor neuron emigration (Fig. 5I, data not shown). However, when co-electroporated with Slit2 MO, Sema3A siRNA increased the escape of motor neurons by 4-fold, similar to the effect of Nrp1 siRNA (Fig. 5J). Introducing Sema3A siRNA, Nrp1 siRNA and Slit2 MO did not further enhance the induction of ectopic motor neurons (Fig. 5I).
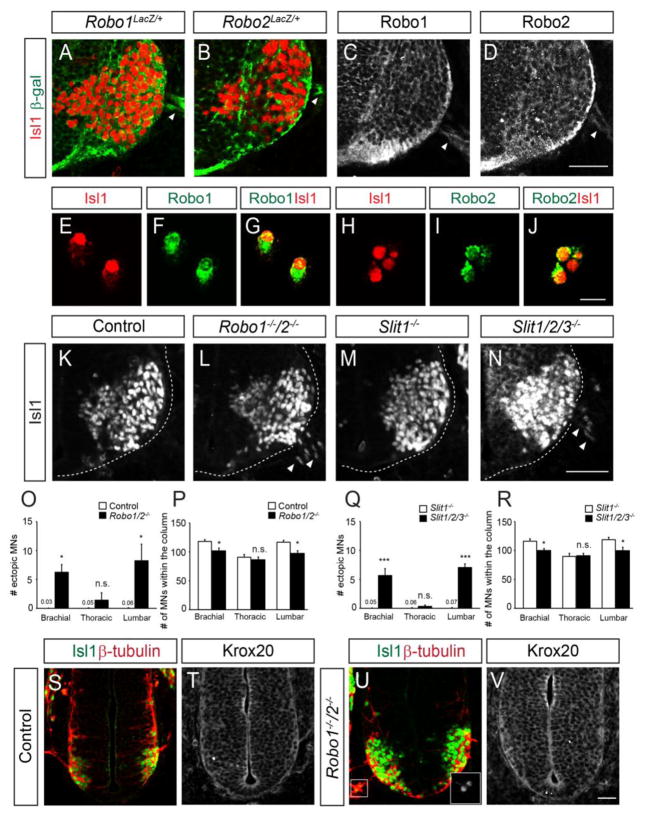
The induction of extraspinal motor neurons in the absence of Nrp1 could be simply explained by a failure of motor neurons to respond to BC cells that transmit repellent agents such as semaphorins (Bron, et al., 2007, Mauti, et al., 2007). However, we found that BC cells did not express Robo or Slit (see Fig. 4, 6 and Sup. Fig. 7). Hence Slit-Robo signaling may occur between motor neurons (Brose, et al., 1999, Jaworski and Tessier-Lavigne, 2012). To investigate the presence of Robo receptors in motor neurons, we examined the distribution of β-galactosidase in spinal cord sections of heterozygous Robo1+/− and Robo2+/− mice carrying Robo1 and Robo2 mutant alleles in which β-geo and LacZ-tau, respectively, had been inserted (Long, et al., 2004). The spinal motor neuron cell bodies and axons of both heterozygotes produced β-galactosidase (Fig. 6A–B). In addition, immunostaining of spinal cord sections and dissociated spinal motor neurons with anti-Robo1 and anti-Robo2 antibodies showed that both Robo1 and Robo2 receptors were present on the cell membranes and in the cytoplasm of motor neuron cell bodies (Fig. 6C–J). The presence of Robo expression on motor neurons and not on BC cells suggests a cell-autonomous function in motor neurons.
Figure 6. Disruption of Slit-Robo signaling results in ectopic motor neurons.
(A, B) β-galactosidase and Isl1 labeling of cryosections of Robo1lacZ/+ (n=3 embryos, n=6 sections) and Robo2lacZ/+ (n=3 embryos, n=6 sections) embryos. (C–J) Anti-Robo1 and anti-Robo2 antibody labeling of cryosections (C, D; n=3 embryos, n=6 sections) and dissociated cells (E–J, n=3 embryos) from ventral spinal cords. Robo receptors are present on Isl1+ cell bodies. (K–N) Isl1 antibody labeling of spinal cord sections at brachial levels. Robo1−/−;2−/− and Slit1−/−;2−/−;3−/− embryos have numerous Isl1+ cell bodies outside the neural tube at the lumbar level. (O–R) Graphs show numbers of ectopic motor neurons at different levels of the spinal cord, and the numbers of motor neurons within the spinal column of Robo and Slit mutant embryos. (S–V) Isl1 and β–tubulin immunostaining of sections of E9.75 Robo1−/−;2−/− embryos. Adjacent sections were immunostained for Krox20. Inset shows high power image of Isl1+ motor neurons in the periphery (U). Error bars represent SEM. *p < 0.05, ***p < 0.001 vs. control; unpaired Student’s t-test; n.s., not significant. Scale bars: A–D, K–N, 50 μm; E–J, 20 μm; S–V, 50 μm.
Next, we investigated if Slit/Robo signals were involved in keeping ventral motor neurons inside the neural tube, using Robo and Slit knockout embryos (Fig. 6K–R). In Robo1−/−;2−/− or Slit1−/−;2−/−;3−/− embryos, significant numbers of spinal motor neurons were positioned outside the spinal cord at the brachial and lumbar levels (Fig. 6K–O, Q). No significant number of ectopic motor neurons was detected at the thoracic level, suggesting that Slit-Robo signaling is region-specific (Fig. 6O, Q). Extraspinal motor neurons in Robo mutants were also found at E9.75 when Krox20-expressing BC cells were not found at the MEPs (Fig. 6S–V). Hence, Slit-Robo signaling prevents motor neuron emigration prior to BC cells. Lastly, the motor axons at the MEPs in Robo, Slit mutants and Isl1 hypo; Isl2 KO were severely defasciculated at the MEPs, forming wider and disorganized motor nerves, which may affect MEP structures (Sup. Fig. 9). Together, the similarity of motor neuron emigration in Robo, Slit, and Islet mutants is consistent with a mechanism in which Islet transcription factors are responsible for retaining motor cell bodies within the neural tube by acting through Slit/Robo signaling.
Discussion
Overlapping and distinct roles of Islet proteins in motor neuron development
Isl1 and Isl2 share a high level of homology, especially in their DNA binding region, and their expression overlaps in most postmitotic motor neurons, raising the possibility that they play redundant roles in motor neuron development. However Isl1 appears to be more crucial since it regulates the expression of Isl2, and elimination of Isl1 leads to more severe phenotype (Hutchinson and Eisen, 2006, Pfaff, et al., 1996, Song, et al., 2009, Thaler, et al., 2004). Although the Islet proteins have been mainly implicated in motor neuron specification, genetic elimination of Isl1 or Isl2 alone in mice and zebrafish also results in errors in axon pathfinding and in the retention of motor somata within columns (Hutchinson and Eisen, 2006, Thaler, et al., 2004).
Several lines of evidence show that Isl1 and Isl2 control the transcription of similar sets of genes. Their DNA binding-homeodomains (HD) in multiple species differ at most by one amino acid (Gadd, et al., 2011). The introduction of Isl2 mRNA into Isl1-knockdown mice rescued the defective production of motor neurons (Hutchinson and Eisen, 2006). Likewise, when co-electroporated with Lhx3 into the chick neural tube, Isl2 induced extra motor neurons, like Isl1 (Song, et al., 2009). However, some of the functions of Islet proteins may involve distinct aspects of motor neuron development. In zebrafish, the Islet proteins have separate functions although they co-exist in the same neurons: Isl1 is required for motor neuron formation and Isl2 for axon projection (Hutchinson and Eisen, 2006). In mice, the absence of Isl2 results in mis-specification of visceral neurons although Isl1 expression is normal (Thaler, et al., 2004). In this study, we demonstrated that massive emigration of motor neurons occurs when both Islet proteins are downregulated. This is not simply due to errors in cell identity because both motor neurons and ectopically induced V2a interneurons emigrate with similar extent. Removal of Isl1 or Isl2 individually resulted in few if any extraspinal motor neurons. Since Isl2 transcript are significantly reduced in Isl1 cKO, both Isl1 and Isl2 may be involved in positioning motor neurons (Song, et al., 2009). At least two genes, Slit2 and Npr1, appear to be involved in motor neuron positioning. The expression of neither of these genes is dependent on Isl1 only (or on Isl2 only), since expression of Slit2 and Npr1 was normal when Isl1 or Isl2 was individually downregulated. Although a genome-wide study is needed to determine whether they control identical sets of genes, we suggest that both Isl1 and Isl2 activate the transcription of major genes whose products have repulsive activity, and they thus serve partially redundant roles in positioning motor somata.
Central and peripheral mechanisms that dictate motor neuron positioning
Motor neurons comprise motor columns with distinct locations in the CNS and thus the mechanisms that set the position of motor somata within the neural tube are closely associated with motor neuron specification. For instance, LIM-HD transcription factors such as Isl2 and Lhx1 are critical for the location of visceral and limb-innervating motor neurons, respectively, and ETS factors are implicated in motor pool formation in later periods (Livet, et al., 2002, Palmesino, et al., 2010, Thaler, et al., 2002). Clustering of motor somata relied on multiple mechanisms: cell-to cell adhesion mediated by cadherins, repellent activity of Slit from the floor plate and reelin signaling that determine the mediolateral axis (Demireva, et al., 2011, Kim, et al., 2014, Palmesino, et al., 2010).
In addition to central mechanisms that determine the three-dimensional position of motor neurons within the neural tube, peripheral mechanisms include BC cells, perineurial glia and Schwann cells, which set the barrier between the CNS and PNS and prevent the emigration of motor neurons (Kucenas, et al., 2008, Vermeren, et al., 2003). Recently, CNS-derived radial glia and MEP glia were also implicated in this process, indicating that multiple cell types involved (Kucenas, et al., 2008, Lee and Song, 2013). BC cells comprise major cellular structure that sets the boundary between the CNS and PNS (Kucenas, et al., 2008, Vermeren, et al., 2003). BC cells migrate from the dorsal roof plate and arrive at the MEPs around the time when motor axons exit the neural tube (Niederlander and Lumsden, 1996). The exact timing of BC cell arrival is still uncertain, but recent EM examination shows that motor axons leave the CNS before BC cells arrive, implying that BC cells may not be the unique barrier to motor neuron emigration (Altman and Bayer, 1982, Altman and Bayer, 1984, Bravo-Ambrosio and Kaprielian, 2011, Fraher, et al., 2007, Fraher and Rossiter, 1983). This is consistent with our observation that motor somata of Islet mutant exited the neural tube before Krox20-expressing BC cells appear at the MEPs. BC cells were recruited to the MEPs in Islet mutant mice, although their clustering appeared to be hindered by already emigrating motor neurons. It is unlikely that Isl1 or Isl2 play any role in BC cells since we did not observe any expression of Isl1 or Isl2 in BC cells (see Sup. Fig. 1). Nevertheless, disrupted BC clusters caused by initially exiting motor neurons may contribute or aggravate the later breakout of motor neurons, raising the possible involvement of BC cells in Islet mutant mice. Thus, Islet factors may link both central (BC cell-independent) and peripheral (BC cell-dependent) mechanisms to control cell body positioning.
Slit-Robo and Semaphorin-Neuropilin signaling maintains the position of motor somata
The involvement of Semaphorin signaling in motor somata has been suggested in several studies mainly between motor neurons and BC cells: Sema6A is present in BC cell clusters, and repel motor somata that express Npr2 or Plexins (Bron, et al., 2007, Mauti, et al., 2007). Knockdown or genetic deletion of Npr2, Sema6A and PlexinA induces ectopic motor neurons in BC-cell dependent manner (Bron, et al., 2007, Mauti, et al., 2007). In this study, we demonstrated that a class 3 family member Sema3A and its receptor Npr1 is also involved in this mechanism. In Islet mutants, Nrp1 was downregulated in motor neurons and knockdown of Sema3A emigrated motor neurons. Thus, BC cells may utilize multiple Semaphorin pathways to secure motor cell bodies.
Several studies have implicated Slit-Robo signals in controlling motor axons and somata. The Slit-Robo signals prevent midline entry by motor neurons and guides motor axons towards the MEPs, as demonstrated by studies of Robo1 and Robo2-deficient mice in which many motor axons cross the midline or display projection errors (Bai, et al., 2011, Bravo-Ambrosio, et al., 2012, Hammond, et al., 2005). In Drosophila, Robo2 and Robo3 whose expression is controlled by motor neuron-specific transcription factor Hb9 determine the medio-lateral position of motor axons (Santiago, et al., 2014). Significantly, the cell bodies of hindbrain neurons are found on the floor plate in Robo1/2 null mice, indicating that the Slit-Robo signal dictates the position of the cell bodies in addition to axons (Kim, et al., 2011, 2015). Although the role of Slit-Robo in motor neurons seems obvious, the location and the mode of interaction between Robos and Slits are still uncertain. Studies by us and others show that Robos and Slits are not expressed in other relevant nearby structures including BC cells and peripheral glia but are present in motor neurons, especially in motor axons and cell bodies, as well as Slit expression in the floor plate (Brose, et al., 1999, Jaworski and Tessier-Lavigne, 2012). Early motor somata in Robo mutants escaped the neural tube when BC cells have not established yet, implying this could be BC cell-independent.
The fact that both the receptor Robo and the ligand Slit are co-expressed in motor neurons raises the possibility that Slit-Robo signals are transmitted between motor neurons. Indeed, an autocrine/juxtacrine function of Slit was implicated in the phrenic motor nerve in which elimination of Slit2 ligand was found to defasciculate motor axons in vivo, while increase of exogenous Slit2 could increase the fasciculation of motor axons growing out in culture (Jaworski and Tessier-Lavigne, 2012). Interestingly, Purkinje cells in the cerebellum also use autocrine regulation of Slit as a self-avoidance mechanism that permits complex dendritic arborization (Gibson, et al., 2014). In the present study, we observed that the reduction of Slit expression in motor neurons caused an increase in extraspinal motor somata and defasciculated axons. How Slit-Robo signaling stalls motor somata and promotes motor nerve fasciculation remains unknown. One possibility is that Slit2 proteins secreted from motor neurons accumulate in the base membrane with the help of dystroglycan near the MEP and repel motor somata from the MEP (Wright, et al., 2012). If this is the case, motor neurons develop a dual barrier that retains their cell bodies: Slit2 inside the MEP and Sema6A in BC cells outside the MEP. Alternative possibility is that bundling of axons affects the intactness of MEP. When Slit level is low, the resulting defasciculated motor axons could exit independently over a wider area, widening the motor exit points, and, as a result, allowing motor somata to escape. After the normally compact exit point is formed, the arrival of BC cells would then be important to maintain or enforce the bounds of the exit point. Neuropilin present in motor axons may then interact with Semaphorin in BC cells to conduct BC cell-dependent barrier mechanisms at the CNS/PNS border.
It is important that both Npr1 and Slit2 are jointly controlled by Islet factors and participate in positioning motor somata. Our microarray analysis as well as other ChIP-related analyses point to Npr1 and Slit2 as potential targets of Isl1 protein (Lee, et al., 2012, Mazzoni, et al., 2013). Neither of them may fully compensate each other since the segmental levels that each signal influences are slightly different. Ectopic motor neurons in the Robo and Slit mutants were only present at the limb level but not at thoracic level, whereas emigration of motor neurons was more severe at the lumbar level when Semaphorin signaling was perturbed (Bron, et al., 2007, Mauti, et al., 2007). In addition, thoracic motor neurons are more likely to emigrate when reelin signaling is missing (Lee and Song, 2013). Such variations in different axis cannot be simply explained by distribution of relevant ligands and receptors because they are broadly expressed along the rostocaudal axis of the spinal cord (data not shown). Recently, several studies suggested the potential cross-talk between Slit and Semaphorin signaling by sharing their receptors; Robo1 interacts with Nrp1, and Slit2 binds to PlexinA1 (Delloye-Bourgeois, et al., 2015, Hernandez-Miranda, et al., 2011). Thus, more complicated and dramatic outcome may arise when both pathways are compromised. Nevertheless, in Islet mutants, motor neurons emigrated in all segmental levels and the number is far greater, when compared to other mutants, indicating that additional genes may be controlled by Islet proteins more or less (Bron, et al., 2007, Lee and Song, 2013, Mauti, et al., 2007). In our microarray analysis, we observed that expression level of more than 400 genes altered in the absence of Isl1. Although we focused on our analysis in genes downregulated and identified two major targets of Islet, other genes may also involve in motor neuron positioning, i.e., unknown target genes whose level was moderately altered, or genes whose expression levels were indirectly changed in the absence of Isl1. Thus, together with Slit2 and Nrp1, multiple genes under the influence of Islet may contribute to constraining motor somata within the neural tube in total.
Supplementary Material
Highlights.
Isl1 and Isl2 retain motor somata within the neural tube
Slit2 and Nrp1 are target genes of Islet factors in motor neurons
Downregulation of Isl1 and Isl2 results in emigration of motor neurons
Acknowledgments
We thank the BioImaging Research Center and Systems Biology Research Center at GIST for use of the confocal imaging facility. Support for M-RS and this research was provided by grants from NRF (2013R1A1A2058548), the Cell Dynamics Research Center, NRF (2007-0056157), Intergrative Aging Research Center of Gwangju Institute of Science and Technology and KHIDI HI14C3484. Support for GSM and MK include NIH RO1 NS054740 and R21 NS077169 to GSM, and use of tissue culture and imaging core facilities was supported by P20 RR-016464, P20 GM103440, P20 GM103554, and P20 GM103650.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Advances in Anatomy, Embryology and Cell Biology. 1982 doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- 2.Altman J, Bayer SA. The development of the rat spinal cord. Advances in Anatomy, Embryology, and Cell Biology. 1984;85:1. doi: 10.1007/978-3-642-69537-7. [DOI] [PubMed] [Google Scholar]
- 3.Bai G, Chivatakarn O, Bonanomi D, Lettieri K, Franco L, Xia C, Stein E, Ma L, Lewcock JW, Pfaff SL. Presenilin-dependent receptor processing is required for axon guidance. Cell. 2011;144:106–118. doi: 10.1016/j.cell.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravo-Ambrosio A, Kaprielian Z. Crossing the border: molecular control of motor axon exit. International journal of molecular sciences. 2011;12:8539–8561. doi: 10.3390/ijms12128539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo-Ambrosio A, Mastick G, Kaprielian Z. Motor axon exit from the mammalian spinal cord is controlled by the homeodomain protein Nkx2. 9 via Robo-Slit signaling. Development. 2012;139:1435–1446. doi: 10.1242/dev.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bron R, Eickholt BJ, Vermeren M, Fragale N, Cohen J. Functional knockdown of neuropilin-1 in the developing chick nervous system by siRNA hairpins phenocopies genetic ablation in the mouse. Developmental dynamics. 2004;230:299–308. doi: 10.1002/dvdy.20043. [DOI] [PubMed] [Google Scholar]
- 7.Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev. 2007;2:21. doi: 10.1186/1749-8104-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 9.Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nature neuroscience. 2015 doi: 10.1038/nn.3893. [DOI] [PubMed] [Google Scholar]
- 10.Demireva EY, Shapiro LS, Jessell TM, Zampieri N. Motor neuron position and topographic order imposed by β-and γ-catenin activities. Cell. 2011;147:641–652. doi: 10.1016/j.cell.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 12.Fraher JP, Dockery P, O’Donoghue O, Riedewald B, O’Leary D. Initial motor axon outgrowth from the developing central nervous system. Journal of anatomy. 2007;211:600–611. doi: 10.1111/j.1469-7580.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraher JP, Rossiter JP. Cell clusters on rat ventral roots: postnatal development. Journal of anatomy. 1983;137:555. [PMC free article] [PubMed] [Google Scholar]
- 14.Gadd MS, Bhati M, Jeffries CM, Langley DB, Trewhella J, Guss JM, Matthews JM. Structural basis for partial redundancy in a class of transcription factors, the LIM homeodomain proteins, in neural cell type specification. Journal of Biological Chemistry. 2011;286:42971–42980. doi: 10.1074/jbc.M111.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson DA, Tymanskyj S, Yuan RC, Leung HC, Lefebvre JL, Sanes JR, Chédotal A, Ma L. Dendrite Self-Avoidance Requires Cell-Autonomous Slit/Robo Signaling in Cerebellar Purkinje Cells. Neuron. 2014;81:1040–1056. doi: 10.1016/j.neuron.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannone D, Reyes M, Reyes R, Correa L, Martinez D, Ra H, Gomez G, Kaiser J, Ma L, Stein MP. Slits affect the timely migration of neural crest cells via robo receptor. Developmental dynamics. 2012;241:1274–1288. doi: 10.1002/dvdy.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grieshammer U, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Developmental cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- 18.Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. The Journal of neuroscience. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond R, Vivancos V, Naeem A, Chilton J, Mambitisaeva E, Andrews W, Sundaresan V, Guthrie S. Slit-mediated repulsion is a key regulator of motor axon pathfinding in the hindbrain. Development. 2005;132:4483–4495. doi: 10.1242/dev.02038. [DOI] [PubMed] [Google Scholar]
- 20.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Miranda LR, Cariboni A, Faux C, Ruhrberg C, Cho JH, Cloutier JF, Eickholt BJ, Parnavelas JG, Andrews WD. Robo1 regulates semaphorin signaling to guide the migration of cortical interneurons through the ventral forebrain. The Journal of neuroscience. 2011;31:6174–6187. doi: 10.1523/JNEUROSCI.5464-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchinson SA, Eisen JS. Islet1 and Islet2 have equivalent abilities to promote motoneuron formation and to specify motoneuron subtype identity. Development. 2006;133:2137–2147. doi: 10.1242/dev.02355. [DOI] [PubMed] [Google Scholar]
- 23.Jaworski A, Tessier-Lavigne M. Autocrine/juxtaparacrine regulation of axon fasciculation by Slit-Robo signaling. Nature neuroscience. 2012;15:367–369. doi: 10.1038/nn.3037. [DOI] [PubMed] [Google Scholar]
- 24.Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A: EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim M, Fontelonga T, Roesener AP, Lee H, Gurung S, Mendonca PRF, Mastick GS. Motor neuron cell bodies are actively positioned by slit/Robo repulsion and Netrin/DCC attraction. Developmental biology. 2014 doi: 10.1016/j.ydbio.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M, Roesener AP, Mendonca PRF, Mastick GS. Robo1 and Robo2 have distinct roles in pioneer longitudinal axon guidance. Developmental biology. 2011;358:181–188. doi: 10.1016/j.ydbio.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-Semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 28.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 29.Kucenas S, Takada N, Park HC, Woodruff E, Broadie K, Appel B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nature neuroscience. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H, Song MR. The structural role of radial glial endfeet in confining spinal motor neuron somata is controlled by the Reelin and Notch pathways. Experimental neurology. 2013;249:83–94. doi: 10.1016/j.expneurol.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Lee SK, Jurata LW, Funahashi J, Ruiz EC, Pfaff SL. Analysis of embryonic motoneuron gene regulation: derepression of general activators function in concert with enhancer factors. Development. 2004;131:3295–3306. doi: 10.1242/dev.01179. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Cuvillier JM, Lee B, Shen R, Lee JW, Lee SK. Fusion protein Isl1 Lhx3 specifies motor neuron fate by inducing motor neuron genes and concomitantly suppressing the interneuron programs. Proceedings of the National Academy of Sciences. 2012;109:3383–3388. doi: 10.1073/pnas.1114515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, Jessell TM, Arber S. ETS Gene Pea3 Controls the Central Position and Terminal Arborization of Specific Motor Neuron Pools. Neuron. 2002;35:877–892. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 34.Long H, Sabatier C, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- 35.Macfarlan TS, Gifford WD, Agarwal S, Driscoll S, Lettieri K, Wang J, Andrews SE, Franco L, Rosenfeld MG, Ren B. Endogenous retroviruses and neighboring genes are coordinately repressed by LSD1/KDM1A. Genes & development. 2011;25:594–607. doi: 10.1101/gad.2008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev. 2007;2:28. doi: 10.1186/1749-8104-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzoni EO, Mahony S, Closser M, Morrison CA, Nedelec S, Williams DJ, An D, Gifford DK, Wichterle H. Synergistic binding of transcription factors to cell-specific enhancers programs motor neuron identity. Nature neuroscience. 2013;16:1219–1227. doi: 10.1038/nn.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niederlander C, Lumsden A. Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development. 1996;122:2367–2374. doi: 10.1242/dev.122.8.2367. [DOI] [PubMed] [Google Scholar]
- 39.Nornes HO, Carry M. Neurogenesis in spinal cord of mouse: an autoradiographic analysis. Brain research. 1978;159:1–16. doi: 10.1016/0006-8993(78)90105-1. [DOI] [PubMed] [Google Scholar]
- 40.Palmesino E, Rousso DL, Kao TJ, Klar A, Laufer E, Uemura O, Okamoto H, Novitch BG, Kania A. Foxp1 and lhx1 coordinate motor neuron migration with axon trajectory choice by gating Reelin signalling. PLoS biology. 2010;8:e1000446. doi: 10.1371/journal.pbio.1000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM Homeobox Gene Isl1 in Motor Neuron Generation Reveals a Motor Neuron Dependent Step in Interneuron Differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 42.Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 43.Santiago C, Labrador JP, Bashaw GJ. The Homeodomain Transcription Factor Hb9 Controls Axon Guidance in Drosophila through the Regulation of Robo Receptors. Cell reports. 2014;7:153–165. doi: 10.1016/j.celrep.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- 45.Song MR, Sun Y, Bryson A, Gill GN, Evans SM, Pfaff SL. Islet-to-LMO stoichiometries control the function of transcription complexes that specify motor neuron and V2a interneuron identity. Development. 2009;136:2923–2932. doi: 10.1242/dev.037986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, Dykes IM, Liang X, Eng SR, Evans SM, Turner EE. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nature neuroscience. 2008;11:1283–1293. doi: 10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- 48.Thaler JP, Koo SJ, Kania A, Lettieri K, Andrews S, Cox C, Jessell TM, Pfaff SL. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41:337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- 49.Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 50.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 51.Vermeren M, Maro GS, Bron R, McGonnell IM, Charnay P, Topilko P, Cohen J. Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron. 2003;37:403–415. doi: 10.1016/s0896-6273(02)01188-1. [DOI] [PubMed] [Google Scholar]
- 52.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 53.Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD. Dystroglycan organizes axon guidance cue localization and axonal pathfinding. Neuron. 2012;76:931–944. doi: 10.1016/j.neuron.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Wang WP, Guo T, Yang JC, Chen P, Ma KT, Guan YF, Zhou CY. The LIM-homeodomain protein ISL1 activates insulin gene promoter directly through synergy with BETA2. Journal of molecular biology. 2009;392:566–577. doi: 10.1016/j.jmb.2009.07.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.