Induced pluripotent stem cells (iPSCs) from patients’ somatic tissues provide a viable source to create autologous cardiomyocytes (CMs) for potential cardiac-related cell therapies. However, a gap between the generation of iPSC-derived cardiomyocytes (iPSC-CMs) and the successful intra-cardiac engraftment of the cells to restore heart function remains to be bridged. Clinical data reporting engraftment of cells within human heart tissue has not been without its challenges, with significant cell loss from the site of delivery due to the physical stress of the cardiac cycle and the hostile inflammatory response within the infarct zone.[1–3] Hydrogels have been proven to support the survival of multiple cell types and have served as a platform for cell transplantation.[4, 5] Yet the use of tissue-adhesive, temperature-sensitive hydrogels to deliver iPSC-derived cardiomyocytes to infarcted heart remains to be explored. Therefore, we developed a polymer hydrogel to encapsulate, deliver, and integrate iPSC-CMs into infarcted myocardium to restore heart function.
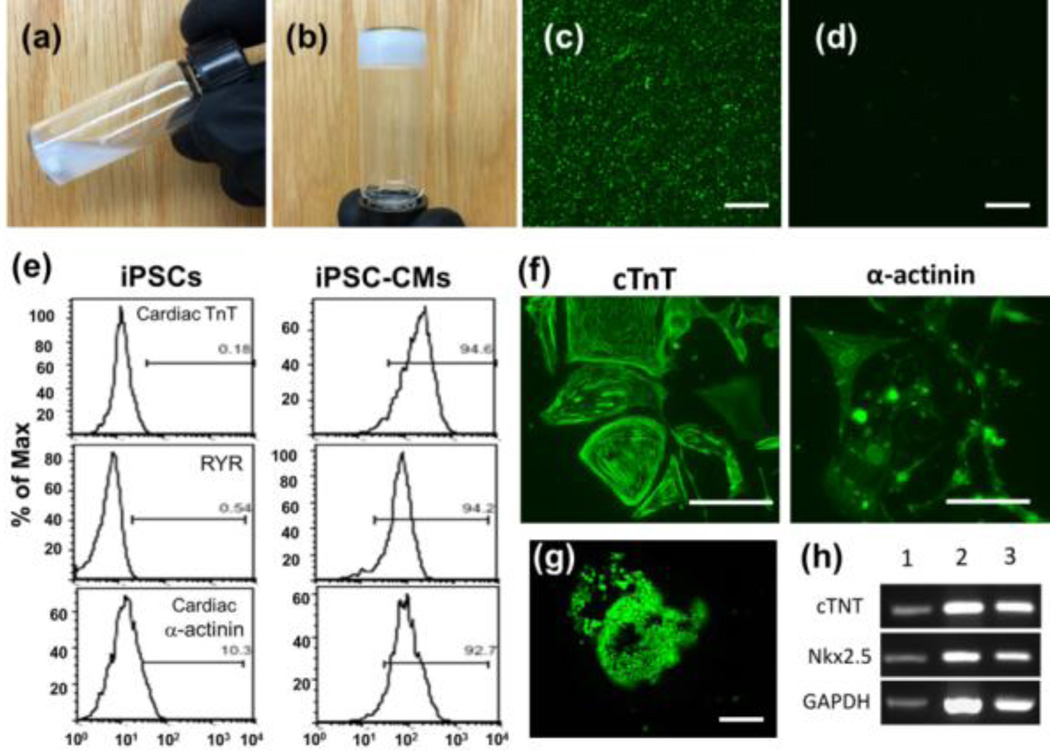
A temperature-sensitive biodegradable copolymer (polyethylene glycol-co-poly-ε-caprolactone (PEG-PCL)) was synthesized [6] and conjugated with a collagen-binding peptide (SYIRIADTNIT). [7] The polymer was soluble in aqueous solutions at room temperature and underwent solution-to-gel transition at 37°C (Fig. 1 a,b).[6] As the peptide-modified polymer had a strong affinity to collagen I in vitro (Fig. 1c), it was expected that it would significantly increase the binding of the hydrogel to an infarcted heart, thus immobilizing iPSC-CMs within the damaged myocardium. Functional, beating cardiomyocytes were derived from patient fibroblast-derived iPSCs using a “Matrigel sandwich” method.[8] Cardiac differentiation was confirmed by fluorescence-activated cell sorting (FACS) and by immunostaining with known cardiac markers cardiac troponin T (cTNT), ryanodine receptor 2 (RYR) and α-actinin (Fig. 1f). The iPSC-CMs encapsulated in the polymer hydrogel at 37°C for two weeks maintained their viability (Fig. 1g) and cardiac phenotype, as evidenced by strong expression of troponin T and Nkx2.5 (Fig. 1h); thus, PEG-PCL does not appear to have obvious toxic effects on iPSC-CMs.
Fig. 1.
PEG-PCL copolymer was (a) dissolved in PBS at RT and (b) gelled at 37°C within 5 min (Sol-gel transition). Collagen-binding study was performed by adding peptide-modified polymer to collagen-coated surface at 37°C, and unbound polymer was washed away. Dylight488-conjugated antibodies targeting PEG was used to recognize (c) the adhered modified polymer (green fluoresence) (d) unmodified polymer (no fluorescence). (e) FACS analysis of induced cardiomyocytes using cardiac makers cTnT, RYR2, and α-actinin. (f) The iPSC-CMs were further verified by immunostaining of cTnT and α-actinin. The encapsulation study was performed by mixing cells with polymer solution and gelled at 37 °C. After two weeks, live cells were stained by calcein AM (green fluorescence) (g). Cardiac marker expression was assessed by PCR (h). Column 1: cardiomyocyte positive control; 2: iPSC-CMs encapsulated in hydrogel for 2 weeks; 3: iPSC-CMs grown atop polymer hydrogels in tissue culture plates for 2 weeks. Scale bars represent 100 µm.
Engraftment of iPSC-CMs into infarcted myocardium using the peptide-modified hydrogel and its impact on infarcted heart function and structure were assessed with a rat myocardial infarction (MI) model. All animal surgery and animal care were approved by the Institutional Animal Care and Use Committee (IACUC) at Vanderbilt University (protocol: M/12/074). The left anterior descending (LAD) coronary artery of nude rats was ligated to induce MI. At 30 minutes post-MI, iPSC-CMs alone, or modified copolymer solution with or without iPSC-CMs (2–4 million/rat) were injected around the infarct border zone. A negative control group received the LAD ligation and phosphate-buffered saline (PBS) injection without cells or copolymer.
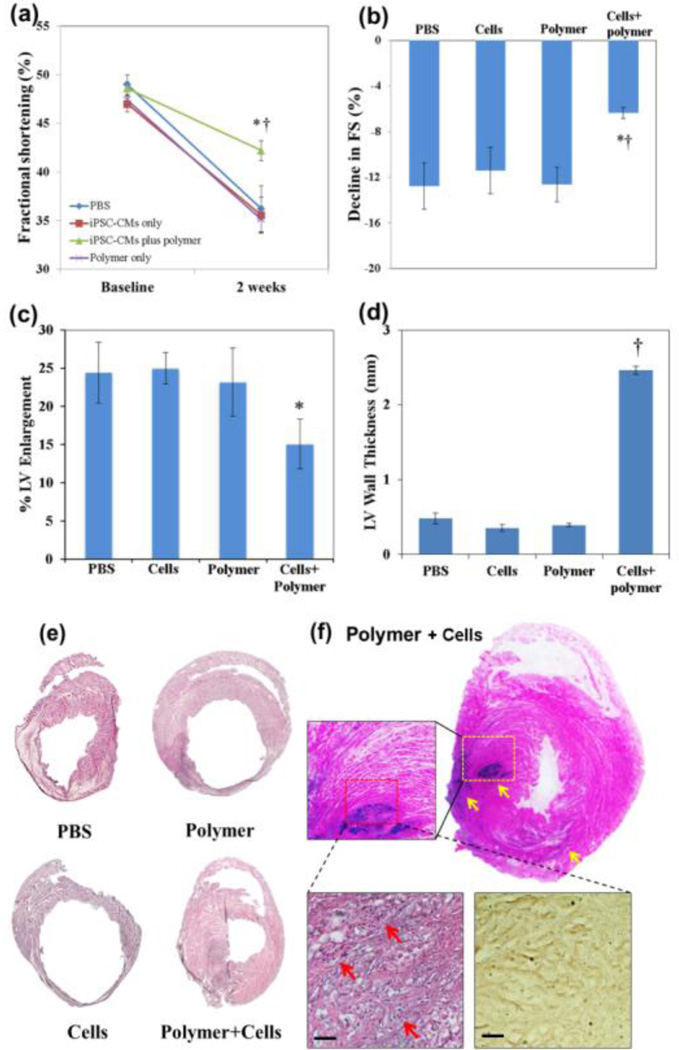
At two weeks post-injection, heart dimensions and functional output were assessed by echocardiography. All groups had ventricular dilation and reduced fractional shortening (FS) (Fig. 2). However, rats treated with iPSC-CM-encapsulated copolymer demonstrated significantly less decline in FS (Δ= −6.37±0.49%) compared to other groups (Δ= −12.77±2.04%, −11.44±2.04% and −12.65±1.53% for PBS control, iPSC-CM only, and polymer only groups, respectively (Fig. 2a). p=0.016 vs. PBS, p=0.021 vs. iPSC-CM only, and p=0.005 vs. polymer only). Overall, the iPSC-CM plus polymer group demonstrated 50.1%, 28.2% and 49.6% improvement in LV systolic function over PBS, iPSC-CM only and polymer only groups (Fig. 2b). Moreover, rats treated with iPSC-CM plus polymer demonstrated a trend toward less LV enlargement (Δ= 15.07±3.24%) compared to other groups (Δ=24.44±3.99%, 25.02±2.03% and 23.17±4.51% for PBS control, iPSC-CM only, and polymer only groups, respectively; Fig. 2c) although this reached statistical significance only in comparison to the iPSC-CM group (p=0.032). Overall, iPSC-CM plus polymer group demonstrated 38.3%, 39.8% and 35.0% less LV enlargement over PBS, iPSC-CM only and polymer only groups, suggesting that iPSC-CM encapsulated in polymer curtailed adverse ventricular remodeling better than other treatment modalities.
Fig. 2.
The effects of hydrogel-encapsulated iPSC-CMs on left ventricle function and remodeling. (a–c) Echocardiography was performed before the ligation of rat LAD coronary arteries (baseline) and again 2 weeks post-delivery (n=5 rats per group). (a, b) The decline in left ventricular (LV) fractional shortening (FS) was significantly less in iPSC-CMs plus polymer (cells+polymer) group compared to other groups (one-way ANOVA, * p<0.05 vs. PBS and cells only groups, † p<0.01 vs. polymer only group). (c) There was a trend toward less LV enlargement in cells plus polymer group compared to other groups, reaching *p<0.05 vs. cell only group. (d) LV wall thickness of the cells plus polymer group was significantly higher than all other groups based on histology staining (one-way ANOVA, † p<0.001 vs. sham, cells only and polymer only groups; n=3 per group). (e) H&E staining of rat hearts. (f) Staining for human nuclei around the infarct area in rat myocardum two weeks after MI/transplantation demonstrated the presence of human nuclei in the heart injected with human iPSC-CMs within polymer hydrogel (arrows). Implanted cells were cardiac α-actinin positive at 2-weeks post-delivery (brownish, bottom right). Scale bars: 50 µm.
Histological examination of the hearts was performed, and the LV anterior free wall thickness was measured using ImageJ and averaged from 3 randomly selected regions in each rat heart. Result demonstrated that, in addition to LV chamber enlargement, the LAD ligation resulted in dramatic thinning and significant fibrosis of the LV anterior free wall at two weeks in control groups (Fig. 2e). In contrast, heart injected with polymer-encapsulated iPSC-CMs had smaller LV chamber, thicker LV free wall and less fibrosis (Fig. 2e). Overall, hearts in the iPSC-CM plus polymer group were significantly thicker than all other groups; the average LV anterior wall thickness of cell plus polymer group was 2.46±0.06mm, compared with 0.48±0.07, 0.35±0.05 and 0.39±0.02mm in PBS, cell only and polymer only groups, respectively (Fig. 2d, p<0.001). In summary, implantation of iPSC-CMs encapsulated in polymer hydrogel was much more effective at limiting adverse LV remodeling and preserving cardiac function after MI than other treatment modalities. Importantly, as implantation of iPSC-CMs or polymer alone did not elicit as favorable outcomes as the iPSC-CMs plus polymer group, we attribute the latter group’s synergistic effects to enhanced survival of transplanted iPSC-CMs in vivo. Consistent with this notion, staining for human nuclei confirmed the presence of iPSC-CMs, delivered with the polymer hydrogel, in the peri-infarct region of the host rat myocardium at 2 weeks (Fig. 2f); moreover, the implanted cells maintained their cardiac phenotype, as demonstrated by positive staining of cardiac α-actinin (Fig. 2f). By contrast, no human nuclei were detected in hearts of control groups at 2 weeks.
In conclusion, we describe a temperature-sensitive, collagen-binding hydrogel based system to deliver human iPSC-derived cardiomyocytes to improve cardiac structure and function in infarcted rat heart. Moreover, our studies indicate that the beneficial effects of encapsulating iPSC-CMs in hydrogel are mediated through enhanced survival of transplanted iPSC-CMs in vivo. While future studies are needed to demonstrate long-term functional engraftment of transplanted cells, our study illustrates a promising biomaterial-based approach to overcome a commonly recognized obstacle to the potentially revolutionary cell-based approaches to repair failing hearts: survival of donor cells in the infarcted heart.
Acknowledgements
This study was supported by NIH HL091465 and NSF DMR 1006558 to HJS, and NIH HL104040 and VA Merit BX000771 to CCH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 2.Schierling W, Kunz-Schughart LA, Muders F, Riegger GA, Griese DP. Fates of genetically engineered haematopoietic and mesenchymal stem cell grafts in normal and injured rat hearts. J Tissue Eng Regen Med. 2008;2:354–364. doi: 10.1002/term.104. [DOI] [PubMed] [Google Scholar]
- 3.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 4.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–I173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 5.Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, et al. Tissue-engineered injectable collagenbased matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114:I138–I144. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- 6.Zachman AL, Wang X, Tucker-Schwartz JM, Fitzpatrick ST, Lee SH, Guelcher SA, et al. Uncoupling angiogenesis and inflammation in peripheral artery disease with therapeutic peptide-loaded microgels. Biomaterials. 2014;35:9635–9648. doi: 10.1016/j.biomaterials.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalamajski S, Aspberg A, Oldberg A. The decorin sequence SYIRIADTNIT binds collagen type I. Journal of Biological Chemistry. 2007;282:16062–16067. doi: 10.1074/jbc.M700073200. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]