Abstract
Purpose
To evaluate postural control using a dynamic virtual reality environment and the relationship between postural metrics and history of falls in glaucoma patients.
Design
Cross-sectional study.
Participants
The study involved 42 glaucoma patients with repeatable visual field defects on standard automated perimetry (SAP) and 38 control healthy subjects.
Methods
Patients underwent evaluation of postural stability by a force platform during presentation of static and dynamic visual stimuli on stereoscopic head-mounted goggles. The dynamic visual stimuli presented rotational and translational ecologically valid peripheral background perturbations. Postural stability was also tested in a completely dark field to assess somatosensory and vestibular contributions to postural control. History of falls was evaluated by a standard questionnaire.
Main Outcome Measures
Torque moments around the center of foot pressure on the force platform were measured and the standard deviations (STD) of these torque moments were calculated as a measurement of postural stability and reported in Newton meter (Nm). The association with history of falls was investigated using Poisson regression models. Age, gender, body mass index, severity of visual field defect, best-corrected visual acuity, and STD on dark field condition were included as confounding factors.
Results
Glaucoma patients had larger overall STD than controls during both translational (5.12 ± 2.39 Nm vs. 3.85 ± 1.82 Nm, respectively; P = 0.005) as well as rotational stimuli (5.60 ± 3.82 Nm vs. 3.93 ± 2.07 Nm, respectively; P = 0.022). Postural metrics obtained during dynamic visual stimuli performed better in explaining history of falls compared to those obtained in static and dark field condition. In the multivariable model, STD values in the mediolateral direction during translational stimulus were significantly associated with history of falls in glaucoma patients (incidence-rate ratio = 1.85; 95% CI: 1.30 – 2.63; P = 0.001).
Conclusions
The study presented and validated a novel paradigm for evaluation of balance control in glaucoma patients based on the assessment of postural reactivity to dynamic visual stimuli using a virtual reality environment. The newly developed metrics were associated with history of falls and may help to provide a better understanding of balance control in glaucoma patients.
INTRODUCTION
Falls are the leading cause of injury-related death and morbidity in older adults.1 It is estimated that 1 out of 3 adults aged 65 or older falls at least once per year, and a substantial proportion of those who fall suffer moderate to severe injuries such as laceration, hip fracture, and head trauma.2, 3 Age-related changes in neural, sensory and musculoskeletal systems can lead to impaired ability to maintain postural control and to react to a sudden loss of balance, leading to an increased risk of falling.4
Maintaining postural stability requires the complex interaction between musculoskeletal and sensory systems. Several prospective studies have shown that the measurement of individual postural sway is a useful predictor of risk of falling.5, 6 The sensory components involved in postural control include vision, vestibular function and somatosensation,7 which act to inform the brain of the position and movement of the body in the three-dimensional space.8, 9 Vision plays a crucial role in detecting hazards, facilitating the adaptation of posture, and maintaining the orientation of the body upright relative to the environment.10 It has been suggested that old people may rely to a greater extent on the spatial framework provided by vision to compensate for reduced vestibular and postural sensation that occurs with aging.7, 11
Due to the important role of vision in balance control and environment navigation, it is not surprising that conditions that can lead to visual impairment such as glaucoma have been implicated as a risk factor for falls,12–14 and also is associated with greater fear of falling.15 Patients with glaucoma have over 3 times higher risk of falling compared to age-matched healthy individuals.12 The loss of retinal ganglion cells in glaucoma leads to loss of the peripheral field of vision, which has a major role in facilitating postural control because of its sensitivity to motion. However, despite this association, previous studies have shown only a weak correlation between measurements of visual field loss by standard automated perimetry (SAP) and risk of falls.16–18 This may be related to an inadequate ability of the static white-on-white stimuli of SAP to evaluate the complex demands put on vision for adequate postural control during daily activities and challenging situations.
A few previous studies have evaluated postural stability in patients with glaucoma.19–21 Although these studies have shown a significant reduction of the visual contribution to posture stabilization in glaucoma, they have not attempted to assess postural metrics that could explain history of falls in these subjects. In addition, these studies have been limited by evaluating balance control in relatively simple conditions such as with eyes closed or with eyes open and fixating on a static target. Evidence has suggested that differences in postural control may only be detectable when the inducing environment is dynamic rather than static.22–24 Therefore, stimuli consisting of dynamic information (i.e, optic flow) may be more appropriate when assessing the role of vision in postural control.24, 25
In the current study a novel paradigm for evaluation of postural control in glaucoma is presented. Postural control was evaluated using a force platform and the postural reactivity to dynamic visual information was assessed using an immersive virtual environment with head-mounted goggles. Differences in postural reactivity between glaucomatous patients and control subjects were evaluated, and the ability of the newly developed metrics in explaining history of falls was assessed in this population.
METHODS
Participants from this study were included in a prospective longitudinal study designed to evaluate functional impairment in glaucoma, the Diagnostic Innovations in Glaucoma Study (DIGS): Functional Impairment, conducted at the Visual Performance Laboratory of the University of California San Diego (UCSD). Written informed consent was obtained from all participants and the institutional review board and human subjects committee approved all methods. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and the study was conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act.
All participants underwent a comprehensive ophthalmologic examination including review of medical history, visual acuity, slit-lamp biomicroscopy, intraocular pressure measurement, gonioscopy, ophthalmoscopic examination, stereoscopic optic disc photography, and standard automated perimetry (SAP) using the Swedish Interactive Threshold Algorithm with 24-2 strategy of the Humphrey Field Analyzer II, model 750 (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Visual acuity was measured using the Early Treatment Diabetic Retinopathy Study chart and letter acuity was expressed as the logarithm of the minimum angle of resolution (logMAR). Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented any other ocular or systemic disease that could affect the optic nerve or the visual field. Optic nerve damage was assessed by masked grading of stereophotographs. Subjects were excluded if they presented with history of systemic conditions affecting the lower limbs, such as arthritis, gout, history of knee or hip replacement, or any other pathology affecting the vestibular system.
Glaucoma was defined by the presence of repeatable abnormal SAP tests (pattern standard deviation with P < 0.05 and/or a Glaucoma Hemifield Test outside normal limits) and corresponding optic nerve damage in at least one eye. Control subjects had no evidence of optic nerve damage and had normal SAP visual field tests in both eyes. Only reliable visual field tests were included (less than 33% fixation losses or false-negative errors, and less than 15% false-positive errors). Control subjects were recruited from the general population through advertisements and from the staff and employees of UCSD.
All subjects had measurements of weight and height obtained at the time of testing. These were used to calculate the body mass index (BMI) for each subject, as the quotient of mass (in kilograms) divided by the square of height (in meters). History of falls was obtained using a standard questionnaire, the Falls Screening and Referral Algorithm (EFST).26, 27 The questionnaire asked about the number of falls the patient had over the past year. A fall was considered when the individual found him/herself suddenly on the ground, without intending to get there from a sitting or standing position.
Experimental Paradigm
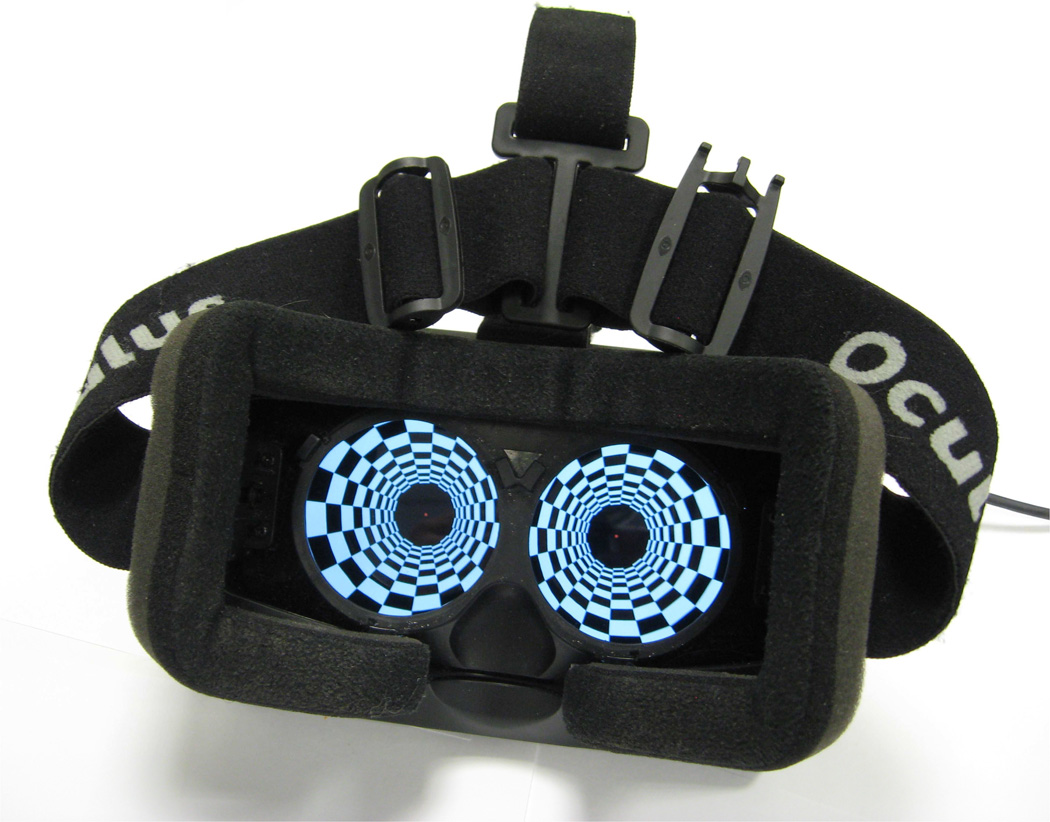
Postural reactivity to visual information was assessed using an immersive virtual environment with head-mounted stereoscopic goggles (Oculus Rift, Oculus VR, LLC, Irvine, CA) (Figure 1). The Oculus Rift presents a different projection of the virtual world onto each eye. These projections mimic the view that each individual eye would receive if the world were real. A lens is mounted in front of each eye so that the image is perceived at a wide field of view. The resulting stereoscopic 3D view presents the same information to each eye but the left eye sees an area to the left that the right eye does not see and vice versa. The binocular field of view is approximately 100 degrees diagonal. The 7-inch (18 cm) screen has a resolution of 1280 × 800 (16:10 aspect ratio) broken up into two panels of 640 pixels wide per eye. The image for each eye is presented in the panel as an inverse barrel distorted image that is then distorted by pincushion effect created by lenses in the headset, generating a correct spherical-mapped image for each eye. Postural stability was evaluated using a force platform (AMTI Optima Human Performance System, Advanced Mechanical Technology, Inc., Watertown, MA) (Figure 2). Subjects were supported by a harness system to prevent falling (Handrail and Harness Safety Structure, Bertec Corp., Columbus, OH). Subjects were required to remove their shoes and stand upright on the center of the force platform with arms by their side and feet close together.
Figure 1.
The head-mounted stereoscopic goggles used for stimulus presentation (Oculus Rift, Oculus VR, LLC, Irvine, CA).
Figure 2.
Subject performing the test while standing on the force platform and wearing the head-mounted goggles.
Subjects underwent postural assessment under four conditions:
No Oculus Rift (static condition);
Oculus Rift in a dark field, without any visual stimulation;
Oculus Rift with rotational stimulus (dynamic condition);
Oculus Rift with translational stimulus (dynamic condition).
Postural stability was initially examined without the Oculus Rift (static condition). Subjects had both eyes open and were instructed to fixate at a cross, which was displayed on the wall 100 cm away at eye level. Patients were then instructed to put on the Oculus Rift and keep their eyes always opened. Postural stability was then tested with the Oculus Rift showing a completely dark field, i.e., a black screen without any visual stimulation. As no visual input was present, this condition assessed the somatosensory and vestibular contributions to postural control.
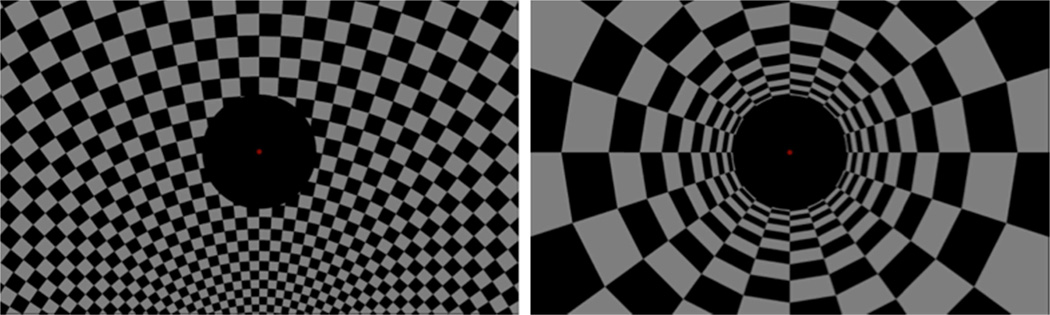
In the dynamic conditions, postural reactivity was assessed using the virtual environment in order to induce a sensation of self-motion. This was done by presenting two types of ecologically valid peripheral background perturbations through the Oculus Rift. The first was a rotational stimulus with the rotation axis at the patient’s feet (Figure 3A). The second was a peripheral translational stimulus (tunnel) with the patient’s eye point on its axis looking down the tunnel (Figure 3B). Ecological validity refers to the fact that the visual scene moved as expected when the patients moved his/her head and that the stimuli were what one would visually experience if moving through a tunnel or rotating sideways around ones feet. Both peripheral stimuli were presented only in the region outside the central 10 degrees of view, while the patient was instructed to keep fixation on a central red dot.
Figure 3.
Screenshots of the rotational (left) and translational (right) test stimuli.
The rotational and translational stimuli were comprised of a sum of four sinusoids with frequencies at 0.1167, 0.2833, 0.5167 and 0.9833 hertz. The amplitude of each sinusoid was inversely related to its frequency to prevent that the highest frequency dominates. The phases were selected to minimize the difference between the maximum magnitude of the combined signal and its root mean square (RMS) magnitude; this process is called cresting and is used to avoid salient signal characteristics caused by an excessively high signal magnitude or an excessively long period of a low signal magnitude. The resulting signal was scaled to yield an amplitude RMS of 22.5 degrees and an angular rate RMS of 28.5 degrees/second for the rotational stimulus and an amplitude RMS of 0.4m and a velocity RMS of 0.5 meters/second for the tunnel stimulus. The benefit of using sums-of-sinusoid stimuli is that the perturbation is unpredictable by the patient unlike the single stimulus. Each test consisted of 15 seconds with a stationary peripheral stimulus followed by 120 seconds of peripheral stimulation. The analysis was performed on the 120 seconds during which the peripheral stimulus was in motion.
For each one of the four test conditions described above, torque moments produced in the mediolateral and anteroposterior directions around the center of the the force plate were measured. The torque moments are generated when the patient’s center of gravity moves causing a change in the center of pressure on the force plate.28 The standard deviations of the torque moments (STD) were calculated as metrics indicative of postural stability and reported in Newton meters (Nm). Standard deviations of the mediolateral and anteroposterior torque moments were computed separately as well as the sum of them. Larger values of STD were indicative of worse postural stability.
Statistical Analysis
Descriptive statistics included mean and standard deviation of the variables. Normality assumption was assessed by inspection of histograms and using Shapiro-Wilk test. Wilcoxon signed-rank test was used for paired continuous non-normally distributed variables. Fisher’s exact test was used for group comparison for categorical variables. Student t test was used for group comparison for normally distributed variables and Wilcoxon rank-sum (Mann-Whitney) test was used for group comparison for continuous non-normally distributed variables.
The association with history of falls was investigated using Poisson regression models, where the number of falls over the previous year was used as the dependent variable and the different postural metrics as independent variables. We initially ran univariable models evaluating the association of each variable with the outcome, without considering potential confounding variables. Subsequently, multivariable models were run adjusting for age, gender, BMI, severity of visual field defect and best-corrected visual acuity. The severity of visual field defect was represented by the binocular mean sensitivity (MS) of the SAP 24-2 visual field test. Binocular MS was calculated as the average of the sensitivities of the integrated binocular visual field obtained according to the binocular summation model described by Nelson-Quigg et al.29
The multivariable model also adjusted for the STD obtained from the balance platform in the dark field condition. As described above, the STD in this condition was obtained without any visual stimulation and therefore it represents the somatosensory and vestibular contribution to balance control. As the risk of falling may also be dependent on vision-independent somatosensory and vestibular factors influencing balance control, it was important to include this variable in the multivariable model as well. Results of the Poisson regression models are given as the effect of the variables on the incidence-rate ratio (IRR).30
The Bayesian information criterion (BIC) was used to compare models with different postural metrics. The smaller the BIC, the better the fit. The difference in the BICs for two models indicates which one is preferred. A difference in the BICs greater than 5 is strong and greater than 10 is very strong in favor of the model with smaller BIC.31
All statistical analyses were performed using commercially available software Stata, version 13 (StataCorp LP, College Station, TX). The alpha level (type I error) was set at 0.05.
RESULTS
The study included 42 glaucoma patients and 38 control subjects. Table 1 presents demographic and clinical variables of the studied population. There was no significant difference in mean age in the glaucoma and control groups (69.1 ± 11.0 vs. 65.4 ± 11.8, respectively; P = 0.121). There were also no statistically significant differences in gender, race and average BMI between the two groups (Table 1).
Table 1.
Demographic and clinical characteristics of subjects included in the study
| Glaucoma (n = 42) | Control (n = 38) | P-value | |
|---|---|---|---|
| Age, years | 69.1 ± 11.0 | 65.4 ± 11.8 | 0.121a |
| Gender, n (%) female | 17 (40.5) | 22 (57.9) | 0.179b |
| Race, n (%) | |||
| Caucasian | 20 (47.6) | 21 (55.3) | 0.150b |
| African-American | 14 (33.4) | 16 (42.1) | |
| Asian | 4 (9.5) | 1 (2.6) | |
| Other | 4 (9.5) | 0 (0.0) | |
| BMI, kg/m2 | 25.8 ± 4.5 | 25.8 ± 4.5 | 0.919a |
| MD SAP 24-2 (worse eye), dB | −8.0 ± 5.7 | −1.0 ± 1.6 | <0.001a |
| MD SAP 24-2 (better eye), dB | −3.0 ± 3.5 | 0.0 ± 1.4 | <0.001a |
| Binocular MS SAP 24-2, dB | 27.8 ± 3.0 | 31.0 ± 1.8 | <0.001a |
| Binocular visual acuity, logMAR | −0.03 ± 0.11 | −0.08 ± 0.10 | 0.027c |
BMI = body mass index; kg/m2 = kilograms per square meter; MD = mean deviation; SAP = standard automated perimetry; dB = decibels; MS = mean sensitivity; logMAR = logarithm of the minimum angle of resolution.
Values are presented as mean ± standard deviation, unless otherwise noted.
Wilcoxon rank-sum test.
Fisher’s exact test.
Student t test.
Combining data from the two groups, average overall STD during dynamic translational stimulus was larger than that for static condition (4.51 ± 2.22 Nm vs. 4.09 ± 2.11 Nm; P = 0.001). Similarly, the average overall STD for dynamic rotational stimulus was significantly larger than that for the static condition (4.81 ± 3.21 Nm vs. 4.09 ± 2.11 Nm, respectively; P = 0.017). These results confirm that the dynamic stimuli were effective in inducing perturbations on postural control compared to the static condition.
Table 2 shows results according to diagnostic categories. For the dark field condition, no statistically significant differences were seen in postural stability between glaucoma and controls (4.84 ± 2.83 Nm vs. 3.94 ± 1.96 Nm, respectively; P = 0.123). For the static stimulus, glaucoma patients had significantly larger overall STD than controls (4.69 ± 2.26 Nm vs. 3.42 ± 1.71 Nm, respectively; P = 0.008). For the dynamic stimuli, glaucoma patients also had larger overall STD compared with control group for both translational (5.12 ± 2.39 Nm vs. 3.85 ± 1.82 Nm, respectively; P = 0.005) as well as rotational (5.60 ± 3.82 Nm vs. 3.93 ± 2.07 Nm, respectively; P = 0.022) stimuli. As shown in Table 2, differences between glaucomatous and controls subjects were seen in postural stability for both the anteroposterior as well as the mediolateral axes.
Table 2.
Results of postural assessment metrics (standard deviation of torque moments) in glaucoma patients and control subjects.
| STD | Glaucoma (n = 42) | Control (n = 38) | P-value* |
|---|---|---|---|
| Static stimulus | |||
| Overall, Nm | 4.69 ± 2.26 | 3.42 ± 1.71 | 0.008 |
| Anteroposterior, Nm | 2.71 ± 1.22 | 1.96 ± 1.00 | 0.001 |
| Mediolateral, Nm | 1.98 ± 1.14 | 1.46 ± 0.86 | 0.025 |
| Dark field | |||
| Overall, Nm | 4.84 ± 2.83 | 3.94 ± 1.96 | 0.123 |
| Anteroposterior, Nm | 2.82 ± 1.39 | 2.28 ± 1.10 | 0.064 |
| Mediolateral, Nm | 2.02 ± 1.57 | 1.66 ± 1.16 | 0.177 |
| Rotational stimulus | |||
| Overall, Nm | 5.60 ± 3.82 | 3.93 ± 2.07 | 0.022 |
| Anteroposterior, Nm | 3.05 ± 1.68 | 2.24 ± 1.05 | 0.012 |
| Mediolateral, Nm | 2.55 ± 2.30 | 1.69 ± 1.23 | 0.043 |
| Translational stimulus | |||
| Overall, Nm | 5.12 ± 2.39 | 3.85 ± 1.82 | 0.005 |
| Anteroposterior, Nm | 3.10 ± 1.41 | 2.36 ± 1.04 | 0.011 |
| Mediolateral, Nm | a2.02 ± 1.22 | 1.49 ± 1.06 | 0.017 |
STD = standard deviations of the torque moments; Nm = Newton meters.
Values are presented as mean ± standard deviation.
Wilcoxon rank-sum test.
From the 80 subjects in the study, 32 (40%) had history of falling in the previous year. Sixteen subjects had 1 fall, 10 had 2 falls, 1 had 3 falls, 4 had 4 falls and 1 had 6 falls. A diagnosis of glaucoma was significantly associated with falling (IRR = 2.54; 95% CI: 1.44 – 4.50; P = 0.001). Table 3 shows results of univariable and multivariable models evaluating the association between postural metrics and falls in glaucoma patients. Although all postural metrics were associated with falls in univariable models, only the metrics during dynamic translational stimulus were associated with falls in the multivariable models accounting for the potential confounding factors of age, gender, BMI, disease severity, visual acuity and STD during dark field condition. Table 4 shows results of the best multivariable model for association with falls, which included STD values in the mediolateral direction during dynamic translational stimulus. In this model, each 1 Nm larger STD was associated with an IRR of 1.85 (95% CI: 1.30 – 2.63; P = 0.001). Figure 4 illustrates the relationship between values of the STD parameter and falls in glaucoma patients. For healthy subjects, none of the postural metrics obtained during dynamic or static visual stimuli presentation were significantly associated with falls.
Table 3.
Results of the univariable and multivariable* Poisson regression models for explaining history of falls in glaucoma patients
| Characteristic | Univariable Model | Multivariable Model | |||
|---|---|---|---|---|---|
| IRR (95% CI) | P-value | IRR (95% CI) | P-value | BIC | |
| Anteroposterior STD during static stimulus, per 1 Nm increase | 1.33 (1.07 – 1.65) | 0.009 | 0.94 (0.59 – 1.49) | 0.787 | 137.7 |
| Mediolateral STD during static stimulus, per 1 Nm increase | 1.44 (1.18 – 1.76) | <0.001 | 1.26 (0.74 – 2.14) | 0.394 | 137.1 |
| Anteroposterior STD during rotational stimulus, per 1 Nm increase | 1.19 (1.04 – 1.37) | 0.012 | 1.20 (0.98 – 1.46) | 0.070 | 134.9 |
| Mediolateral STD during rotational stimulus, per 1 Nm increase | 1.17 (1.07 – 1.29) | 0.001 | 1.15 (1.00 – 1.33) | 0.054 | 134.3 |
| Anteroposterior STD during translational stimulus, per 1 Nm increase | 1.42 (1.20 – 1.68) | <0.001 | 1.43 (1.01 – 2.04) | 0.046 | 133.8 |
| Mediolateral STD during translational stimulus, per 1 Nm increase | 1.57 (1.30 – 1.88) | <0.001 | 1.85 (1.30 – 2.63) | 0.001 | 126.5 |
| Age, per 1 year older | 0.99 (0.97 – 1.02) | 0.619 | 1.02 (0.98 – 1.06) | 0.306 | NA |
| Gender, female | 1.84 (1.02 – 3.31) | 0.042 | 2.04 (1.07 – 3.86) | 0.030 | NA |
| BMI, per 1 kg/m2 higher | 1.08 (1.02 – 1.14) | 0.013 | 0.98 (0.90 – 1.07) | 0.730 | NA |
| Binocular MS, per 1 dB lower | 1.08 (0.96 – 1.21) | 0.437 | 1.06 (0.92 – 1.23) | 0.394 | NA |
| Binocular visual acuity, per 0.1 logMAR higher | 0.57 (0.04 – 7.51) | 0.673 | 0.61 (0.03 – 13.88) | 0.756 | NA |
| Overall STD in dark field condition, per 1 Nm increase | 1.16 (1.09 – 1.25) | <0.001 | 1.21 (1.07 – 1.36) | 0.002 | 134.0 |
IRR = incidence-rate ratio; CI = confidence interval; BIC = Bayesian information criterion; STD = standard deviations of the torque moments; Nm = Newton meter; NA = not apply; BMI = body mass index; kg/m2 = kilogram per square meter; MS = mean sensitivity; dB = decibel; logMAR = logarithm of the minimum angle of resolution.
Multivariable models were adjusted for age, gender, BMI, severity of visual field defect, best-corrected binocular visual acuity, and STD on dark field.
Table 4.
Results of the best multivariable* Poisson regression model for explaining history of falls in glaucoma patients
| Characteristic | IRR | 95% CI | P-value |
|---|---|---|---|
| Mediolateral STD during translational stimulus, per 1 Nm increase | 1.85 | 1.30 – 2.63 | 0.001 |
| Age, per 1 year older | 1.03 | 0.99 – 1.07 | 0.162 |
| Gender, female | 3.29 | 1.53 – 7.07 | 0.002 |
| BMI, per 1 kg/m2 higher | 1.00 | 0.91 – 1.09 | 0.953 |
| Binocular MS, per 1 dB lower | 1.04 | 0.90 – 1.20 | 0.575 |
| Binocular visual acuity, per 0.1 logMAR higher | 0.25 | 0.01 – 5.79 | 0.389 |
| Overall STD in dark field condition, per 1 Nm increase | 1.02 | 0.86 – 1.20 | 0.849 |
IRR = incidence-rate ratio; CI = confidence interval; STD = standard deviations of the torque moments; Nm = Newton meter; BMI = body mass index; kg/m2 = kilogram per square meter; MS = mean sensitivity; dB = decibel; logMAR = logarithm of the minimum angle of resolution.
Multivariable model was adjusted for age, gender, BMI, severity of visual field defect, best-corrected binocular visual acuity, and STD on dark field.
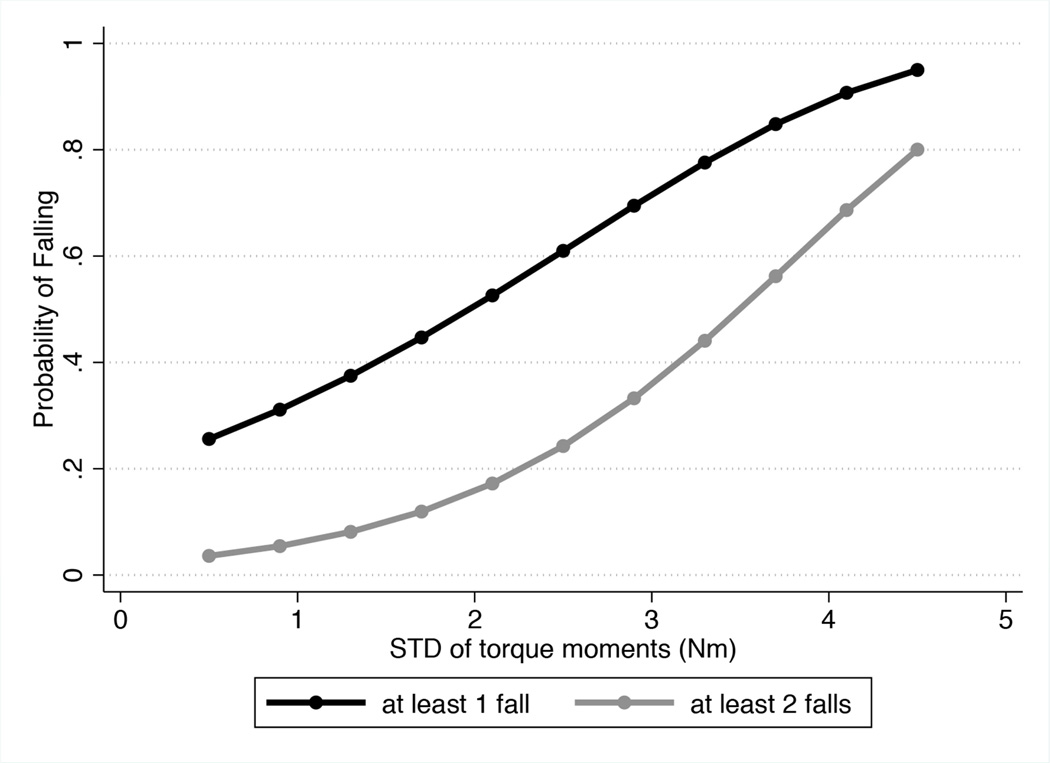
Figure 4.
Relationship between standard deviations of the torque moments in the mediolateral direction during translational stimulus (i.e. orthogonal to stimulus direction) and history of falls.
DISCUSSION
In the present study, we proposed a novel paradigm for evaluation of postural control in glaucoma patients. The testing paradigm was based on the evaluation of postural reactivity induced by presenting peripheral dynamic visual stimuli in an immersive virtual environment using stereoscopic goggles. The proposed metrics were significantly associated with history of falls and may help to better understand balance control and risk of falls in glaucoma patients.
Both the translational, as well as the rotational peripheral stimuli, w ere effective in producing postural perturbations, as shown by the significantly larger average STD values compared to the static condition. This is an expected result. The motion of the environment creates an illusion of self-motion (vection), which, in turn, induces compensatory postural responses, an effect well demonstrated in the literature.22 However, we hypothesize that if visual information processing is slow, these compensatory responses may be deficient or inappropriate, leading to postural instability. When the subject perceives a displacement of the background relative to the displacement of the body, he or she uses the discrepant information (error) to make adjustments. However, depending on the visual processing delay, it may take the subject longer or shorter time to register the error and make the correction. If the processing delay is longer, then the body will have been perturbed more and the background will have moved further. The correction that is applied is not enough and the next perception will register a larger error. This results in greater postural instability, as measured by the force platform. Due to loss of retinal ganglion cells, it is possible that visual processing speed may be slower and motion detection may be deficient in glaucoma patients, leading to ineffective compensatory responses to the peripheral perturbations.32, 33 In fact, we found that glaucoma patients had significantly larger STD in the presence of optic flow stimuli compared to healthy subjects. On average, STDs were approximately 30% and 40% greater for the translational and rotational stimuli, respectively, as compared to healthy subjects. To our knowledge, this is the first study in the literature to evaluate postural responses to optic flow in glaucoma patients. It is important to emphasize, however, that the actual mechanisms explaining these findings remain to be demonstrated.
A few previous studies have evaluated balance control in glaucoma patients. Kotecha et al21 evaluated balance control using a force platform in a group of glaucomatous subjects and healthy controls. Subjects were tested with eyes closed or open and fixating on a static stimulus. The authors found a reduced visual contribution to balance in glaucoma subjects, a s indicated by a greater relative sway with eyes open versus closed, as compared to healthy subjects, a finding similar to that of Shabana et al.19 However, none of the previous investigations evaluated the relationship between balance metrics and risk of falls in glaucomatous patients. Previous studies have suggested that differences in postural control may only be detectable when the inducing environment is dynamic, rather than static.34–36 In our study, even though force platform assessments in both static and dynamic visual stimuli conditions were associated with falls in univariable models, only the metrics obtained during dynamic visual stimuli retained the significance in multivariable models adjusting for confounding factors. Although glaucoma patients in our study had a broad range of disease severities, they all had remaining useful peripheral vision (binocular MS ranged from 18.7 to 31.8 dB), which allowed them to be perturbed by the peripheral optic flow. It would be expected that as the disease reaches its final stages, the response to the peripheral perturbations would likely decrease and the postural stability metrics would then become similar to those obtained in the dark field condition.
The best model in our study included the postural perturbations in the mediolateral direction during translational stimulus. In this model, each 1 Nm larger STD was associated with an 85% increase in the rate of falls 1.85 (95% CI: 1.30 – 2.63; P = 0.001). This model had a Nagelkerke R2 of 58.2% and performed significantly better than any other model, as assessed by BIC measures. For comparison, a model including visual field severity as well as age, gender, BMI and visual acuity had R2 of only 26.9%, indicating that the severity of visual field loss by SAP seems to be a weak indicator of the probability of falling. As shown on Figure 4, the probability of reporting a fall increased dramatically with an increase in STD in response to dynamic translational stimuli. For subjects with STD of 4 Nm, the probability of reporting at least 1 fall in the previous year was close to 90% compared to only 20% for a subject of similar age, gender, BMI and disease severity but who had STD of 0.5 Nm, for example. It is important to note that the multivariable model also adjusted for the postural stability measured in the dark field condition, which would represent the somatosensory and vestibular contributions to balance.
An interesting finding was that postural perturbations in the mediolateral direction during the translational stimulus were more strongly associated with history of falls than those measured in the anteroposterior direction. This may seem paradoxical, as the tunnel characteristic of the translational stimulus induced significantly larger perturbations in the anteroposterior direction. However, increased postural perturbations in the direction orthogonal to the visual stimuli may actually be a more important indicator of overall destabilization of the subject. That is, instead of swaying mostly along the direction of the stimuli to reduce vection, unstable individuals also show large perturbations in the orthogonal direction as a result of inappropriate compensatory responses. In contrast to the translational stimulus, during rotational stimulus the values of STD parameter in the orthogonal (i.e., anteroposterior) direction did not show improved ability to explain history of falls compared to those in the mediolateral direction. This is probably explained by the fact that the control of lateral stability is more important in explaining differences between fallers and nonfallers.5,37 In upright stance, an individual can more easily control posture in the anteroposterior direction than in the mediolateral one, by using simple movements such as extension-flexion in the knees and ankles.
Previous studies have also used virtual reality settings to evaluate postural reactivity to induced optic flow, although none in glaucoma patients.38, 39 Piponnier et al23 used a tunnel paradigm consisting of a CAVE (cave automatic virtual environment) room where the subject was immersed in a 3D displayed virtual environment to study the roles of central and peripheral vision in the control of posture in healthy individuals. The authors showed that, under static conditions, central and peripheral visual fields appear to have equal importance in the control of stance. However, in the presence of optic flow, peripheral vision plays a crucial role in the control of stance by processing visual information on location and velocity and allowing an adapted postural response to the perceived perturbation.25, 40 As glaucoma has a relatively larger effect on peripheral compared to central vision, this may help explain the better performance of postural metrics obtained in dynamic versus static visual stimuli conditions in our study. Our methodology for assessment of postural reactivity to optic flow has major differences compared to that employed by Piponnier et al.23 We used an Oculus Rift stereoscopic head-mounted display for stimuli presentation. This allows for a much simpler and less costly setup, albeit preserving the ability to present ecologically valid perturbations. Use of a head-mounted display is also likely to provide a more immersive visual experience without false visual anchors such as screen edges. In addition, we employed a force platform to evaluate postural control, which provides much more accurate and precise assessment than a motion-tracking device placed on glasses, which would be susceptible to confounding effects of head movements.
Our study has limitations. The history of falls was collected for the year before postural assessments were obtained, making it difficult to establish a causal relationship. Changes in postural control may have occurred after the fall episodes without necessarily being a risk factor for future falls. Prospective studies should be performed to clarify this issue. As another limitation, the study had a relatively small sample size and we did not perform specific musculoskeletal or vestibular tests to assess the influence of these systems in the risk of falls in our population. However, models adjusted for force platform measurements obtained in a dark field condition, which should reflect the somatosensory and vestibular contributions to balance. We also did not assess balance control during gait, which could be relevant for explaining risk of falls. However, despite these limitations, we demonstrated a significant and clinical relevant ability to explain history of falls by the metrics introduced in our study.
In conclusion, a novel paradigm for evaluation of balance control in glaucoma patients was proposed. The method involves the assessment of postural perturbations to peripherally presented dynamic visual stimuli in a virtual reality environment. The newly developed metrics were able to successfully explain history of falls and may help to provide a better understanding of postural control in this population.
Acknowledgments
Financial Disclosure(s):
The author(s) have made the following disclosure(s): Felipe A. Medeiros: Financial support – Alcon Laboratories (Fort Worth, TX), Bausch & Lomb (Garden City, NY), Carl Zeiss Meditec (Jena, Germany), Heidelberg Engineering (Heidelberg, Germany), Merck (White House Station, NJ), Allergan (Irvine, CA), Sensimed (Lausanne, Switzerland), Topcon (Livermore, CA), Reichert (Dewey, NY), National Eye Institute (Bethesda, MD); Research support – Alcon Laboratories (Fort Worth, TX), Allergan (Irvine, CA), Carl Zeiss Meditec (Jena, Germany), Reichert (Dewey, NY); Consultant – Allergan (Irvine, CA), Carl-Zeiss Meditec, (Jena, Germany), Novartis (Basel, Switzerland).
Supported in part by National Institutes of Health/National Eye Institute grant EY021818 (F.A.M), core grant P30EY022589; an unrestricted grant from Research to Prevent Blindness (New York, NY); grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck and Santen; fellowships from Brazilian National Research Council-CAPES 12309-13-3 (C.P.B.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fatalities and injuries from falls among older adults--United States, 1993–2003 and 2001–2005. MMWR Morb Mortal Wkly Rep. 2006;55:1221–1224. [PubMed] [Google Scholar]
- 2.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261:2663–2668. [PubMed] [Google Scholar]
- 3.O'Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–354. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- 4.Maki BE, McIlroy WE. Postural control in the older adult. Clin Geriatr Med. 1996;12:635–658. [PubMed] [Google Scholar]
- 5.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–M84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 6.Stel VS, Smit JH, Pluijm SM, Lips P. Balance and mobility performance as treatable risk factors for recurrent falling in older persons. J Clin Epidemiol. 2003;56:659–668. doi: 10.1016/s0895-4356(03)00082-9. [DOI] [PubMed] [Google Scholar]
- 7.Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44:M118–M127. doi: 10.1093/geronj/44.4.m118. [DOI] [PubMed] [Google Scholar]
- 8.Paulus WM, Straube A, Brandt T. Visual stabilization of posture. Physiological stimulus characteristics and clinical aspects. Brain. 1984;107:1143–1163. doi: 10.1093/brain/107.4.1143. [DOI] [PubMed] [Google Scholar]
- 9.Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev. 1998;22:465–472. doi: 10.1016/s0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 10.Wade MG, Jones G. The role of vision and spatial orientation in the maintenance of posture. Phys Ther. 1997;77:619–628. doi: 10.1093/ptj/77.6.619. [DOI] [PubMed] [Google Scholar]
- 11.Sloane PD, Baloh RW, Honrubia V. The vestibular system in the elderly: clinical implications. Am J Otolaryngol. 1989;10:422–429. doi: 10.1016/0196-0709(89)90038-0. [DOI] [PubMed] [Google Scholar]
- 12.Haymes SA, Leblanc RP, Nicolela MT, et al. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1149–1155. doi: 10.1167/iovs.06-0886. [DOI] [PubMed] [Google Scholar]
- 13.Lamoureux EL, Chong E, Wang JJ, et al. Visual impairment, causes of vision loss, and falls: the singapore malay eye study. Invest Ophthalmol Vis Sci. 2008;49:528–533. doi: 10.1167/iovs.07-1036. [DOI] [PubMed] [Google Scholar]
- 14.Tanabe S, Yuki K, Ozeki N, et al. The association between primary open-angle glaucoma and fall: an observational study. Clin Ophthalmol. 2012;6:327–331. doi: 10.2147/OPTH.S28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramulu PY, van Landingham SW, Massof RW, et al. Fear of falling and visual field loss from glaucoma. Ophthalmology. 2012;119:1352–1358. doi: 10.1016/j.ophtha.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glynn RJ, Seddon JM, Krug JH, Jr, et al. Falls in elderly patients with glaucoma. Arch Ophthalmol. 1991;109:205–210. doi: 10.1001/archopht.1991.01080020051041. [DOI] [PubMed] [Google Scholar]
- 17.Friedman SM, Munoz B, West SK, et al. Falls and fear of falling: which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. J Am Geriatr Soc. 2002;50:1329–1335. doi: 10.1046/j.1532-5415.2002.50352.x. [DOI] [PubMed] [Google Scholar]
- 18.Lamoureux E, Gadgil S, Pesudovs K, et al. The relationship between visual function, duration and main causes of vision loss and falls in older people with low vision. Graefes Arch Clin Exp Ophthalmol. 2010;248:527–533. doi: 10.1007/s00417-009-1260-x. [DOI] [PubMed] [Google Scholar]
- 19.Shabana N, Cornilleau-Peres V, Droulez J, et al. Postural stability in primary open angle glaucoma. Clin Experiment Ophthalmol. 2005;33:264–273. doi: 10.1111/j.1442-9071.2005.01003.x. [DOI] [PubMed] [Google Scholar]
- 20.Black AA, Wood JM, Lovie-Kitchin JE, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008;85:489–497. doi: 10.1097/OPX.0b013e31817882db. [DOI] [PubMed] [Google Scholar]
- 21.Kotecha A, Richardson G, Chopra R, et al. Balance control in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:7795–7801. doi: 10.1167/iovs.12-10866. [DOI] [PubMed] [Google Scholar]
- 22.Previc FH, Kenyon RV, Boer ER, Johnson BH. The effects of background visual roll stimulation on postural and manual control and self-motion perception. Percept Psychophys. 1993;54:93–107. doi: 10.3758/bf03206941. [DOI] [PubMed] [Google Scholar]
- 23.Piponnier JC, Hanssens JM, Faubert J. Effect of visual field locus and oscillation frequencies on posture control in an ecological environment. J Vis. 2009;9:13.1–13.10. doi: 10.1167/9.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Hanssens JM, Allard R, Giraudet G, Faubert J. Visually induced postural reactivity is velocity-dependent at low temporal frequencies and frequency-dependent at high temporal frequencies. Exp Brain Res. 2013;229:75–84. doi: 10.1007/s00221-013-3592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habak C, Casanova C, Faubert J. Central and peripheral interactions in the perception of optic flow. Vision Res. 2002;42:2843–2852. doi: 10.1016/s0042-6989(02)00355-3. [DOI] [PubMed] [Google Scholar]
- 26.Cwikel JG, Fried AV, Biderman A, Galinsky D. Validation of a fall-risk screening test, the Elderly Fall Screening Test (EFST), for community-dwelling elderly. Disabil Rehabil. 1998;20:161–167. doi: 10.3109/09638289809166077. [DOI] [PubMed] [Google Scholar]
- 27.Lawson SN, Zaluski N, Petrie A, et al. Validation of the Saskatoon Falls Prevention Consortium's Falls Screening and Referral Algorithm. Physiother Can. 2013;65:31–39. doi: 10.3138/ptc.2011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caldwell GE, Robertson DGE, Whittlesey SN. Forces and their measurement. In: Robertson DGE, Caldwell GE, Hamill J, Kamen G, Whittlesey SN, editors. Research methods in biomechanics. Champaign, IL: Human Kinetics; 2004. pp. 73–102. [Google Scholar]
- 29.Nelson-Quigg JM, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000;41:2212–2221. [PubMed] [Google Scholar]
- 30.Hilbe JM. Modeling count data. New York, NY: Cambridge University Press; 2014. pp. 59–70. [Google Scholar]
- 31.Raftery AE. Bayesian model selection in social research. In: Marsden PV, editor. Sociological Methodology. Cambridge, MA: Blackwell Publishers; 1995. pp. 111–163. [Google Scholar]
- 32.Shabana N, Cornilleau Peres V, Carkeet A, Chew PT. Motion perception in glaucoma patients: a review. Surv Ophthalmol. 2003;48:92–106. doi: 10.1016/s0039-6257(02)00401-0. [DOI] [PubMed] [Google Scholar]
- 33.Karwatsky P, Bertone A, Overbury O, Faubert J. Defining the nature of motion perception deficits in glaucoma using simple and complex motion stimuli. Optom Vis Sci. 2006;83:466–472. doi: 10.1097/01.opx.0000225107.38719.0d. [DOI] [PubMed] [Google Scholar]
- 34.Peterka RJ, Black FO. Age-related changes in human posture control: sensory organization tests. J Vestib Res. 1990;1:73–85. [PubMed] [Google Scholar]
- 35.Peterka RJ, Black FO. Age-related changes in human posture control: motor coordination tests. J Vestib Res. 1990;1:87–96. [PubMed] [Google Scholar]
- 36.Ring C, Nayak US, Isaacs B. Balance function in elderly people who have and who have not fallen. Arch Phys Med Rehabil. 1988;69:261–264. [PubMed] [Google Scholar]
- 37.Tanaka H, Uetake T. Characteristics of postural sway in older adults standing on a soft surface. J Hum Ergol (Tokyo) 2005;34:35–40. [PubMed] [Google Scholar]
- 38.Tossavainen T, Juhola M, Pyykko I, et al. Development of virtual reality stimuli for force platform posturography. Int J Med Inform. 2003;70:277–283. doi: 10.1016/s1386-5056(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 39.Sparto PJ, Redfern MS, Jasko JG, et al. The influence of dynamic visual cues for postural control in children aged 7–12 years. Exp Brain Res. 2006;168:505–516. doi: 10.1007/s00221-005-0109-8. [DOI] [PubMed] [Google Scholar]
- 40.Berencsi A, Ishihara M, Imanaka K. The functional role of central and peripheral vision in the control of posture. Hum Mov Sci. 2005;24:689–709. doi: 10.1016/j.humov.2005.10.014. [DOI] [PubMed] [Google Scholar]