Abstract
Fibulin-1 is a member of a growing family of proteins that includes eight members and is involved in cellular functions such as adhesion, migration and differentiation. Fibulin-1 has also been implicated in embryonic development of the heart and neural crest-derived structures. It is an integral part of the extracellular matrix (ECM) and has been shown to bind to a multitude of ECM proteins. However, fibulin-1 was first identified as a protein purified from placental extracts that binds to the cytoplasmic domain of integrin β1. Human fibulin-1 is alternatively spliced into four different isoforms namely A-D. These isoforms share a common N-terminus sequence that contains a secretion sequence but differ in their carboxy-terminal fibulin-1 module. In this report we identify a new splice variant of fibulin-1 that differs from all other fibulin-1 variants in the N-terminus sequence and has a similar carboxy-terminus sequence as fibulin-1D. This variant that we named fibulin-1D prime (fibulin-1D’) lacks a secretion sequence and the anaphlatoxin region of fibulin-1 variants. The protein has an apparent molecular weight of 70.5 kDa. Herein we show that fibulin-1D’ binds to the intracellular domain of integrin β1 as well as to integrin α5β1. The protein was localized intracellularly in CHO cells transfected with a pEF4 plasmid containing full-length coding sequence of fibulin-1D’. We also localized the protein in human placenta. We propose that the fibulin-1D’ variant might play a role in early embryo development as well as in modulating integrin β1 functions including adhesion and motility.
Keywords: Integrin β1, Integrin alpha 5 beta 1, Intracellular localization, Fibulin-1D isoform, Placenta
INTRODUCTION
Fibulin-1 is a member of a growing family of proteins that includes eight members 1, 2. Most fibulins have functions that relate to the assembly of tropoelastin into elastic fibers and lamellae, since defective elastic fibers are observed in mice deficient in fibulin-2, 3, 4 or 5 3–7. Fibulin-1, the prototypic member of the fibulin family, is characterized by its rod-like shape, presence of multiple calcium-binding epidermal growth factor (EGF)-like repeats and a unique carboxy domain 8, 9. Fibulin-1 is also associated with elastin fibers, but its role in elastin assembly has not been established 10. Fibulin-1 binds to a multitude of molecules including itself and many extracellular matrix (ECM) molecules in tissues such as fibronectin (FN), nidogen, laminin and versican, which led to the hypothesis that fibulin-1 acts as an ECM structure stabilizer 1. Fibulin-1 was specifically found to be a FN-binding protein and a component of ECM of blood vessels 11. Fibulin-1 has also been reported to regulate adhesion and migration of cells 12 as well as hemostasis and thrombosis 13, 14.
The human fibulin-1 mRNA transcript is comprised of 20 exons 15. The transcript is spliced into 4 different variants that share the first 14 exons and differ only in the carboxy terminus fibulin-1 module 15. The fibulin-1A transcript is comprised of exons 1–15. Fibulin-1B RNA splicing occurs from exon 14 to exon 17, whereas fibulin-1C RNA splicing occurs from exon 14 to exon 16. Fibulin-1D, the longest fibulin-1 variant comprised of 703 amino acids, is translated from exons 1–14 and 18–20 15. The mouse fibulin-1 transcript is comprised of 18 exons that have similar exon-intron organization as human fibulin-1 transcript and is spliced into fibulin-1C and -1D variants 15.
Despite the large amount of evidence indicating extracellular functions for fibulin-1, it was first identified as a protein that binds to a synthetic peptide representing the cytoplasmic domain of the FN receptor integrin β1 subunit 16. Also, the immunohistochemical localization of fibulin-1 was found to overlap with that of the FN receptor β1 subunit in gingival fibroblasts 16. This discrepancy between the ability of fibulin-1 to bind to the intracellular domain of integrin β1 and its extracellular localization has not been resolved. Herein, we report the finding of a novel fibulin-1D variant, which we name fibulin-1D prime (fibulin-1D’) comprised of 638 amino acids and apparent molecular weight of 70.5 kDa. Fibulin-1D’ lacks a secretion sequence, has a truncated N-terminus domain and binds to the intracellular domain of integrin β1. This discovery could explain and resolve the discrepancy of fibulin-1 intracellular localization and association with integrin β1 cytoplasmic domain as well as playing a possible role in integrin-mediated cell adhesion and motility.
RESULTS
Identification of the new fibulin-1D’ protein
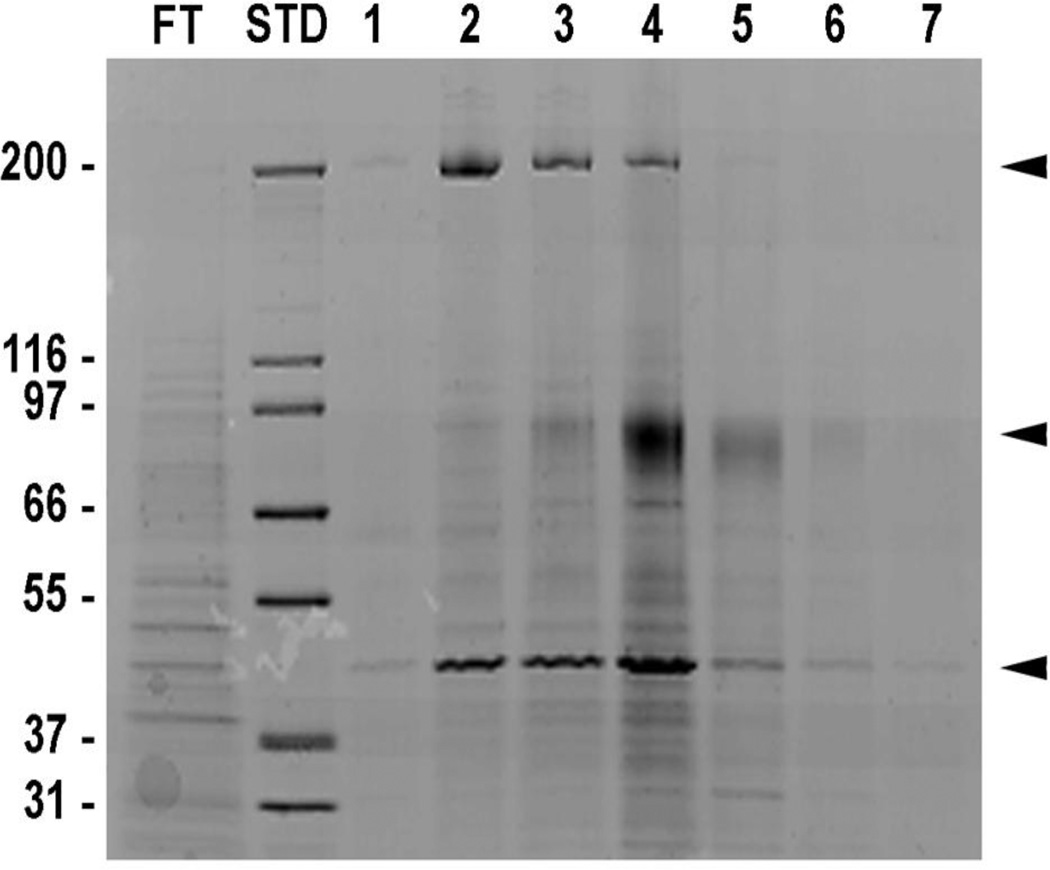
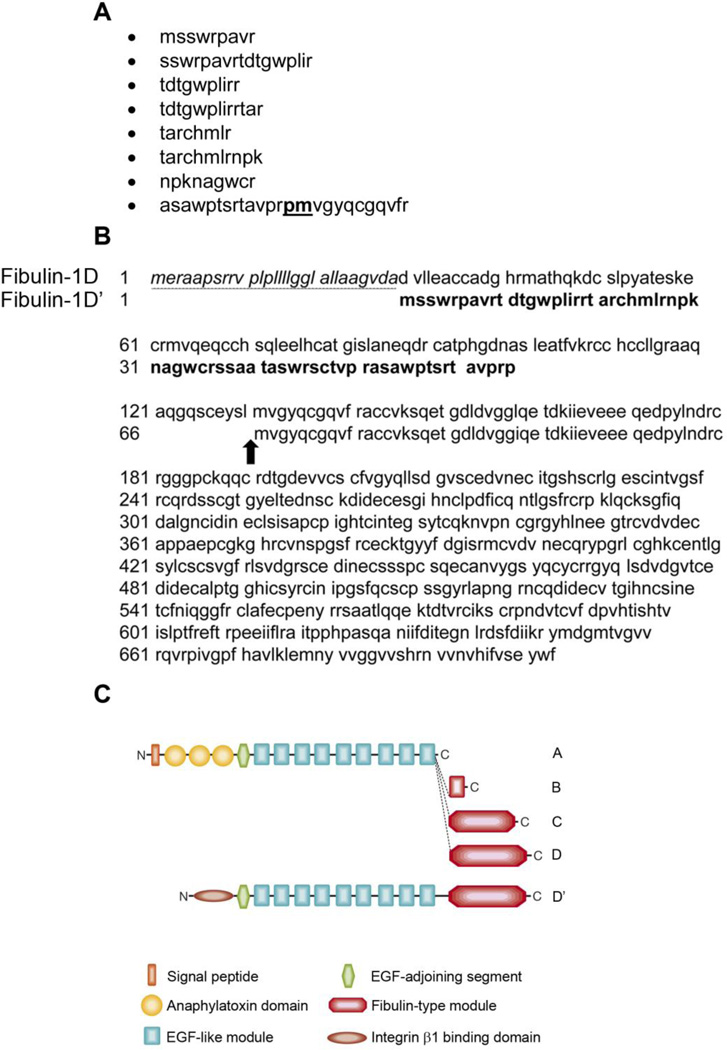
In an effort to identify fibulin-1 binding partners on the surface of the cell, A431 cells were extracted and the extract was passed over a human placental fibulin-1-conjugated sepharose column. After elution, the fractions were electrophoresed on 4–12% Tris Glycine SDS-PAGE gel; then stained with Coomassie blue (Figure 1). Major gel bands identified in the Coomassie blue stained gel were approximately 200, 75 and 42 kDa molecular weight (Figure 1, arrow heads). The three Coomassie blue-stained bands were sequenced at MUSC Mass Spectroscopy Facility. The 200-kDa band was identified as myosin heavy chain 9 (MYH9) and the 42 kDa band was identified as actin. The most abundant band at about 75 kDa was identified as fibulin-1 variants A, B, C, D and an unknown protein that had similar sequence to fibulin-1D. The sequences of the polypeptides reported from the Mass Spectroscopy Facility, which did not match any of the fibulin-1 variant’s A-D sequences, are listed in Figure 2A. Search of the protein database identified a protein that contains these sequences as a fibulin-1D-like protein but with different amino acid sequence at the N-terminus 17. This variant, which we name fibulin-1D prime (fibulin-1D’), was identified from in silico translation of the sequenced genome of human teratocarcinoma cell line NT2 treated with retinoic acid 17 (accession number AK075566.1). In addition, purified human placental fibulin-1 (pFBLN1) sequenced at the MUSC Mass Spectroscopy Facility contained all 4 isoforms of fibulin-1 (A-D) as well as fibulin-1D’. Alignment of the N-terminus amino acid sequence of fibulin-1D’ and fibulin-1D revealed the absence of the secretion sequence (underlined, italics) and a deletion of 65 amino acids (Figure 2B). However, amino acid sequences starting at methionine 66 in fibulin-1D’ (methionine 131 in fibulin-1D) to the end of the transcript were identical in both variants (Figure 2B, arrow). Schematic representation of all the fibulin-1 variants depicted in Figure 2C shows that the signal sequence and anaphlatoxin regions (round shaped) are missing in fibulin-1D’ and replaced by an integrin β1 binding domain (elliptical shaped).
Figure 1.
Proteins from A431 cell extracts bind to fibulin-1. A431 cell extract was passed over fibulin-1 sepharose column. Eight-molar urea was used to elute the fractions. Fractions were run on 4–12% Tris Glycine SDS-PAGE gel then stained with Coomassie blue. The three major bands were sent for sequencing at the MUSC Mass Spectroscopy Facility and identified as MYH9 (200 Kda), fibulin-1 (75 Kda) and actin (42 Kda). FT, flow-through; STD, molecular weight standards; arrowheads depict major eluted proteins.
Figure 2.
Identification of fibulin-1D’ protein. The 75 kDa band from the Coomassie stained gel was excised and sequenced by the MUSC Mass Spectroscopy Facility. A) Peptides identified from the band that are not found in fibulin-1 variants A-D. The sequences were queried in the protein database and were identified as part of an unknown protein that is similar to fibulin-1D. B) Amino acid alignment of the N-terminus sequence of fibulin-1D splice variant and the new fibulin-1D’. The amino acids sequence of fibulin-1D’ variant N-terminus region (shown in block letters), lacked the secretion sequence in fibulin-1D (amino acids 1–29, underlined italics) and the anaphlatoxin sequence (amino acids 30–152 in fibulin-1D). The sequence of fibulin-1D’ matches that of fibulin-1D starting at amino acid 66 in fibulin-1D’ (amino acid 131 in fibulin-1D, arrow). Note that the sequence of the last peptide reported from the sequencing facility contains the proline and methionine amino acids resulting from the splice junctions in exons 3 and 4 of fibulin-1D’ (proline 65 and methionine 66, bold underlined in A. C) The schematic representation of all members of the fibulin-1 family. The anaphlatoxin region of fibulin-1D (round shapes) was replaced with a 65 amino acid segment that binds to integrin β1 cytoplasmic domain (elliptical shape). The EGF-like and fibulin-1D modules, which comprise the remainder of both fibulin-1D and -1D’ proteins, are identical.
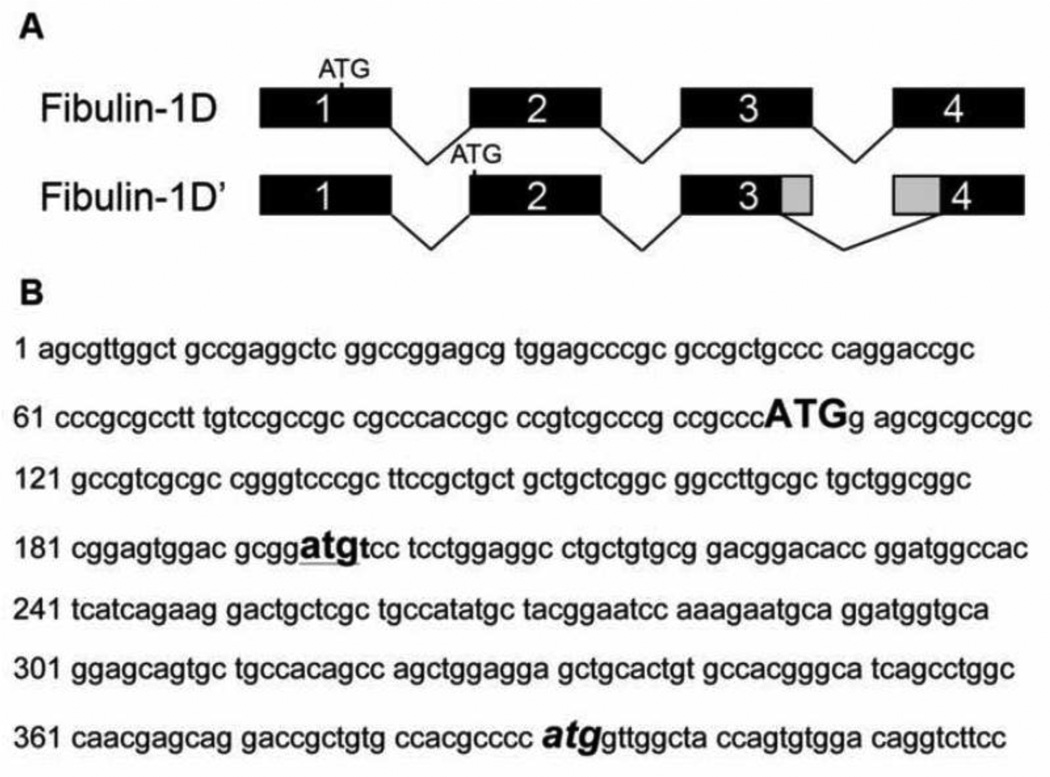
Analysis of the N-terminus sequence of fibulin-1D revealed that, in order to obtain this transcript two events must occur. The first is an alternative start site in exon 2 and the second is alternative splice junctions in exons 3 and 4. Translation of nucleotides at the N-terminus of fibulin-1D identified the alternative start site in the second exon and new splice sites in exons 3 and 4 (Figure 3A). These two events resulted in deletion of the 65 amino acids from the N-terminus of fibulin-1D’ compared to fibulin-1D. The splice site in exon 4 returns the translation back into frame for the remaining 13 exons. In Figure 3B the N-terminus nucleotide sequence of fibulin-1 is presented showing the start sites for all fibulin-1 variants (A-D) (upper case bold letters) and that of fibulin-1D’ (lower case bold letters, underlined). The atg splice site in exon 4 where fibulin-1D’ and -1D amino acid sequence starts to be identical is depicted in Figure 3B (bold italic letters).
Figure 3.
The alternative start site and exon splicing of fibulin-1D’ variant mRNA transcript at the N-terminus. Fibulin-1D transcript is comprised of 17 exons (1–14 and 18–20). A) Fibulin-1D’ alternative start site is located in exon 2 instead of the start site in fibulin-1D located in exon 1. This alternative start site leads to an mRNA with no signal sequence and out of frame translation of the N-terminus. Deletion of part of exon 3 and exon 4 results in the return of translation back into frame as in fibulin-1D. B) The N-terminus nucleotide sequence of fibulin-1 transcript showing the different ATG start sites of fibulin-1D (bold upper case) and fibulin-1D’ (bold underlined lower case). The third atg (bold italics) delineates the start of the amino acid sequence in exon 4 that is identical in both fibulin-1D and -1D’ for the remaining 13 exons (5–14 and 18–20).
A search in the protein database for similar proteins identified two primate species that have similar protein sequence to fibulin-1D’ (Figure 4). Figure 4A compares the amino acid sequence alignment of the N-terminus of human fibulin-1D’ (middle row, underlined) with Macaca mulatta (Rhesus monkey, upper row, accession number XP_001109928.1) and Pongo abelii (Sumatran orangutan, accession number XP_002831310.1, lower row). Arrow in Figure 4A indicates the ATG site in exon 4 after which the sequence of the fibulin-1D’ is almost identical in all three species. Figure 4B depicts the phylogenic tree of humans and the closely related primates that are reported to express fibuin-1D’ (boxed). No other species were reported to express this protein in the NCBI database. Attempts to translate fibulin-1D message in other species such as the mouse resulted in either premature stop codons or a protein sequence that does not match the fibulin-1D’ N-terminus sequence.
Figure 4.
Amino acid N-terminus sequence alignment of human fibulin-1D’ with two primate species. A) Literature search in protein database for similar proteins to human fibulin-1D’ N-terminus domain (middle row underlined) identified two primates, the Macac monkey (upper row) and Sumatran orangutan (lower row). Sequence alignment of both primates fibulin-1D’ with the human variant is shown and the arrow delineates the end of the fibulin-1D’ segment after which the sequence of fibulin-1D’ matches that of fibulin-1D. No other organism was identified as having the fibulin-1D’ variant. B) The Phylogenic tree of the human closely related primates reported to express fibulin-1D’-like protein are depicted in the dash-line rectangle.
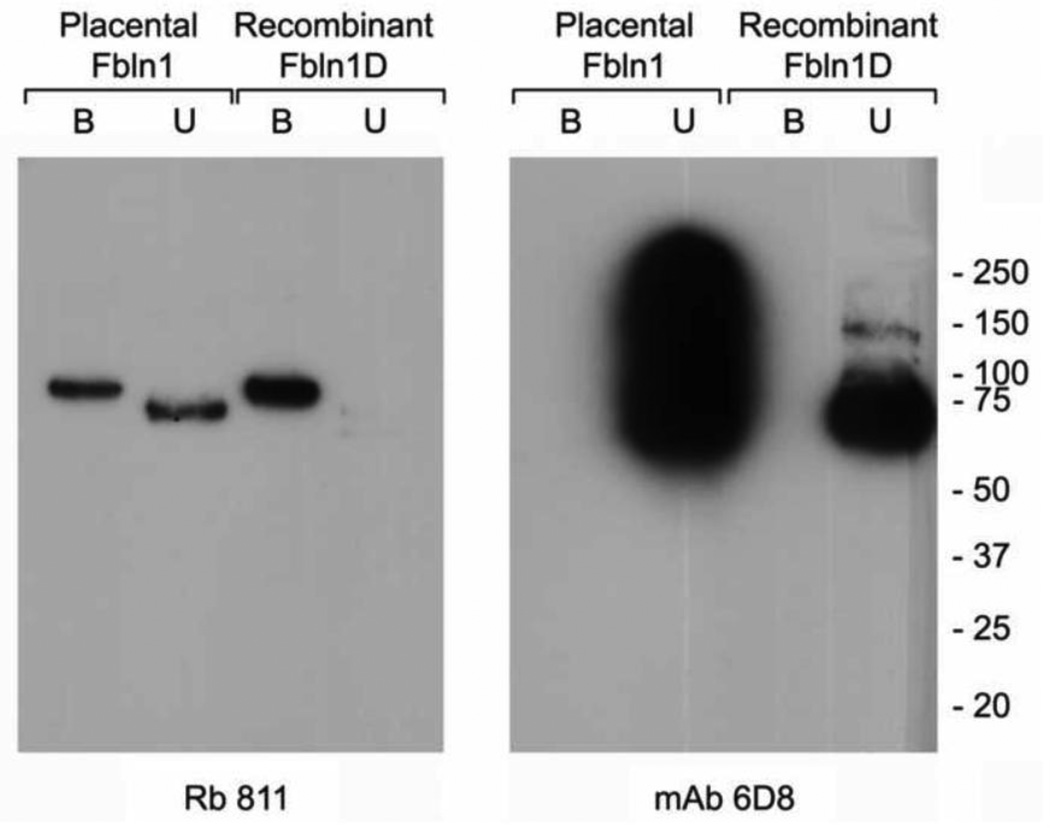
Fibulin-1D’ in human placental fibulin-1 binds to integrin α5β1-conjugated sepharose beads
Placental fibulin-1 is comprised of a mixture of fibulin-1 variants that form homodimers or heterodimers in solution 18. We incubated pFBLN1 and rFBLN1D with integrin α5β1-conjugated sepharose beads to investigate if the fibulin-1D’ variant is present in both preparations and binds to integrin α5β1. As shown in Figure 5A, a polypeptide binding to integrin α5β1-coated beads was identified by immunoblotting using a polyclonal antibody that recognizes fibulin-1D and -1D’ variants (Rb 811). However, the monoclonal antibody 6D8 that recognizes an epitope in the N-terminus anaphlatoxin region of fibulin-1 variants A-D, did not recognize fibulin-1D’-bound to integrin α5β1 since it lacks this sequence (Figure 5B). The apparent differences in molecular weight between fibulin-1D’ (Rb 811 bound fraction) versus fibulin-D (Rb 811 unbound fraction), is probably due to the change in the conformation of the fibulin-1D’. Fibulin-1 is a dumbbell shaped protein that runs at about 75–77 kDa under non-reduced conditions. Under reduced conditions the protein runs at a slower rate with apparent molecular weight of 100 kDa. Changes in the N-terminus sequence probably eliminated the globular region at one end of fibulin-1D’ variant, which could have resulted in an aberrant mobility.
Figure 5.
Placental fibulin-1 and recombinant fibulin-1 D bind to integrin α5β1. Purified placental fibulin-1 or recombinantly expressed fibulin-1D was allowed to bind to magnetic beads covalently bound to integrin α5β1. Both bound and unbound fractions were resolved on a 4–12% SDS-PAGE gel, and separated proteins were transferred to a PVDF membrane and immunoblotted with anti fibulin-1D antibody (Rb 811, left panel) or a pan fibulin-1 monoclonal antibody (mAb 6D8, right panel) that have an epitope mapping to the anaphlatoxin region. Note that the mAb 6D8 does not recognize the integrin α5β1-bound fibulin-1D’ due to lack of the anaphlatoxin region. Fbln, fibulin; B, bound fraction; U, unbound fraction
Fibulin-1D’ is localized to the inside of the cell
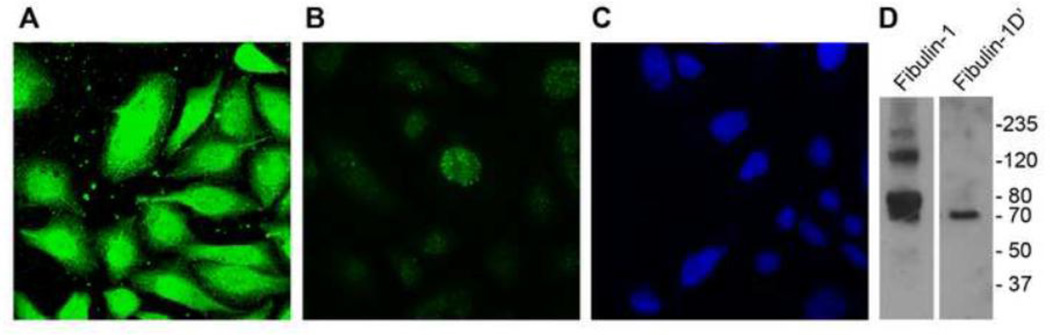
We then sought to characterize the localization of fibulin-1D’. As shown in Figure 6A, overexpression of fibulin-1D’ in CHO cells followed by immuno-staining with fibulin-1D’ specific antibody, revealed that the protein is localized inside the cell since permeabilization with Triton-X100 during fixation was needed to detect the protein (Figure 6A). Little or no protein was detected when cells were not permeabilized (Figure 6B). Immunoblot analysis of cell extracts of the transfected CHO cells using Rb 1323 anti fibulin-1 antibody detected multiple bands including a band at 70 kDa molecular weight (Figure 6D, left lane). When fibulin-1D’ antibody (Rb1603) was used to detect fibulin-1D’ in pFBLN1 (positive control), a single immunoreactive band at 70 kDa molecular weight was detected (Figure 6D, right lane).
Figure 6.
Localization of fibulin-1D’ in transfected CHO cells. A) Fibulin-1D’ protein from CHO cells transfected with pEF4 plasmid containing the full length fibulin-1D’ coding sequence is localized inside the cell using fibulin-1D’ specific antibody (Rb1603, green) after permeabilization of the cells. B) Little or no detection was observed in cells that were not permeabilized. C) Nuclei were counterstained with TOTO 3 (blue). D) Left lane, immunodetection of fibulin-1 variants in transfected CHO cells using Rb1323 antibody showing multiple immune-reactive bands including the 70-kDa fibulin-1D’ band. Right lane, immuno-detection of fibulin-1D’ variant in placental fibulin-1 using the specific antibody Rb1603 showing a single 70- kDa fibulin-1D’ band (positive control)
We also performed an immunoblot on the conditioned culture medium (CCM) of fibulin-1D’-transfected CHO cells. The immunoblot was probed with an anti-HIS antibody, anti fibulin-1 antibody (3A11), or anti fibulin-1D’ antibody (Rb1603). We did not detect fibulin-1D’ protein using the anti HIS antibody (the fibulin 1D’ in the pEF4 plasmid has a 6X HIS tag, positope reagent (Life Technologies) was used as control). The anti fibulin-1 monoclonal antibody 3A11 did not detect any fibulin-1 protein in the CCM indicating that CHO cells do not secrete any of the fibulin-1 variants in the medium. The fibulin-1D’ Rb1603 antibody detected two non-specific bands that were present in all lanes including the empty vector-transfected cells (data not shown).
Fibulin-1D’ in pFBLN1 binds to the 37 amino acid cytoplasmic domain of integrin β1
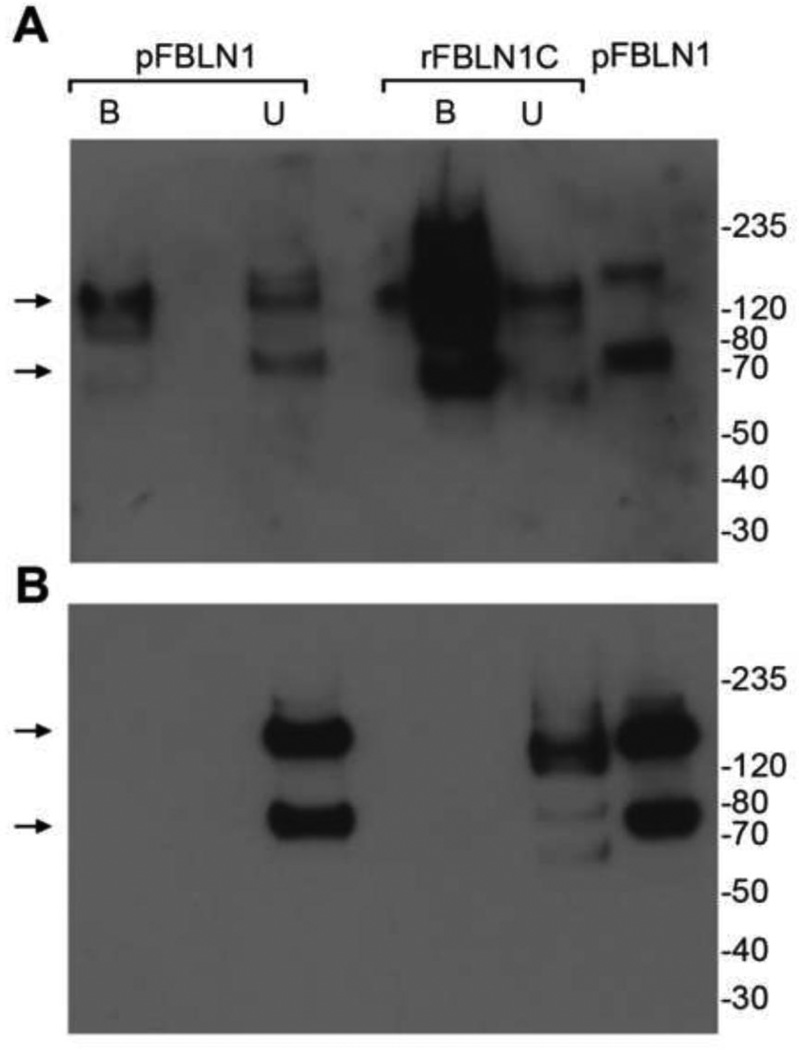
To verify whether the intracellularly localized fibulin-1D’ binds to the cytoplasmic domain of integrin β1, human pFBLN1 was incubated with the cytoplasmic domain of integrin β1-conjugated sepharose beads. Immunoblotting of the bound proteins identified two bands at a size of about 70 and 140 kDa using the fibulin-1D’-specific antibody (Rb1603) (Figure 7A). When recombinant fibulin-1C was used as a control, a significant reactivity with the fibulin-1D’ antibody was apparent in the integrin-bound fraction (Figure 7A). Re-probing the blot with anti fibulin-1C-specific antibody 5D12, revealed that fibulin-1C is detected only in the unbound fractions of both pFBLN1 and rFBLN1C (Figure 7B), indicating that the intense reactivity seen in the bound fraction of rFBLN1C was fibulin-1D’ immune-reactive polypeptide. It is possible that transfection of HT 1080 cells with fibulin-1C plasmid induced the expression of fibulin-1D transcript and favored the splicing of fibuln-1D’ in these cells.
Figure 7.
Fibulin-1D’ binds to the cytoplasmic domain peptide of integrin β1. The 37 mer cytoplasmic domain peptide was covalently linked to sepharose beads and incubated with placental fibulin-1 (pFBLN1) or recombinant fibulin-1C (rFBLN1C) overnight at 4°C. A) Immunoblot analysis of the bound and unbound fractions of pFBLN1 shows that fibulin-1D’ binds to the integrin β1 cytoplasmic domain as detected by the fibulin-1D’ specific polyclonal antibody Rb1603. Note the 70-kDa monomer and 140-kDa dimer of fibulin1-D’ in fractionated and unfractionated pFBLN1 (arrows). rFBLN1C (negative control) contained an appreciable amount of reactivity to fibulin-1D’ antibody in the bound fraction. B) Re-probing with the fibulin-1C-specific monoclonal antibody 5D12 identified the monomer and/or dimer fibulin-1C in unbound fractions of pFBLN1 and rFBLN1C as well as unfractionated pFBLN1 (arrows). No detection of fibulin-1C was evident in integrin β1 cytoplasmic domain-bound fractions.
Fibulin-1D’ is localized in the human placental tissue
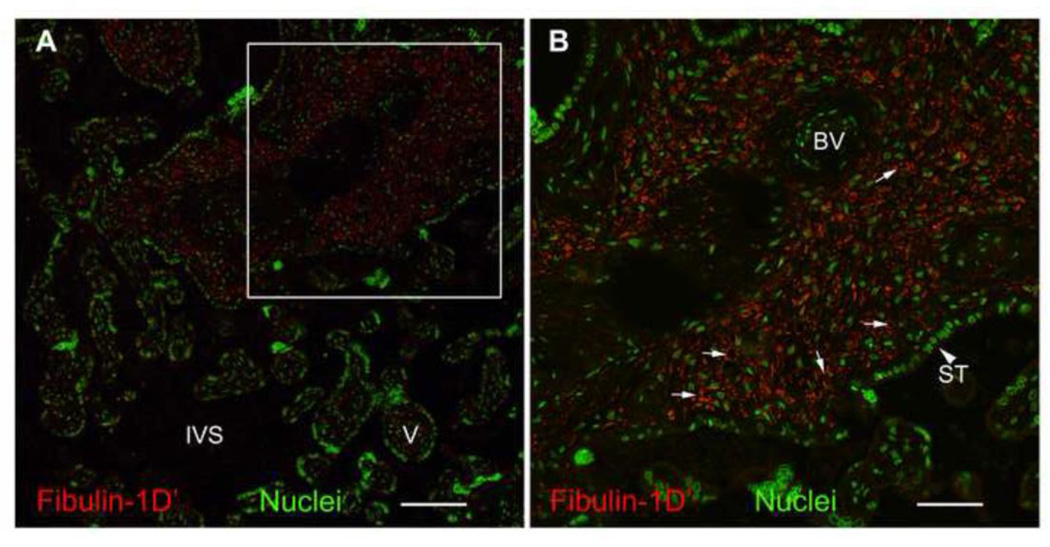
To localize fibulin-1D’ in the human placenta, we performed immunohistochemistry of fibulin-1D’ protein in paraffin embedded placental tissue (Figure 8). Fibulin-1D’ appeared to be localized in the villi, punctate and intracellular (small arrows). No fibulin-1D’ was detected in the matrix.
Figure 8.
Immunohistochemical detection of fibulin-1D’ protein in human term placenta. Paraffin-embedded sections from human term placenta were probed with Rb1603 antibody (fibulin-1D’, 50 µg/ml, 18 hrs) (red). Fluorescent secondary antibodies used were Alexa 568-conjugated anti-rabbit IgG at 10 µg/ml. Nuclei were counterstained with Draq 5 at 5 µM (green). Fibulin-1 staining appears confined to villi of the placenta (A) and exhibits a punctate appearance predominantly inside the cells (B, small arrows). No matrix-associated fibulin-1D’ staining was observed. Inset in A is magnified in B. BV, blood vessel; ST, syncytiotrophoblast (large arrowhead); IVS, intra-villous space; V, Villous. Bar in A represents 100 µm and in B represents 200 µm.
Fibulin-1D’ mRNA is expressed at different stages of placental development
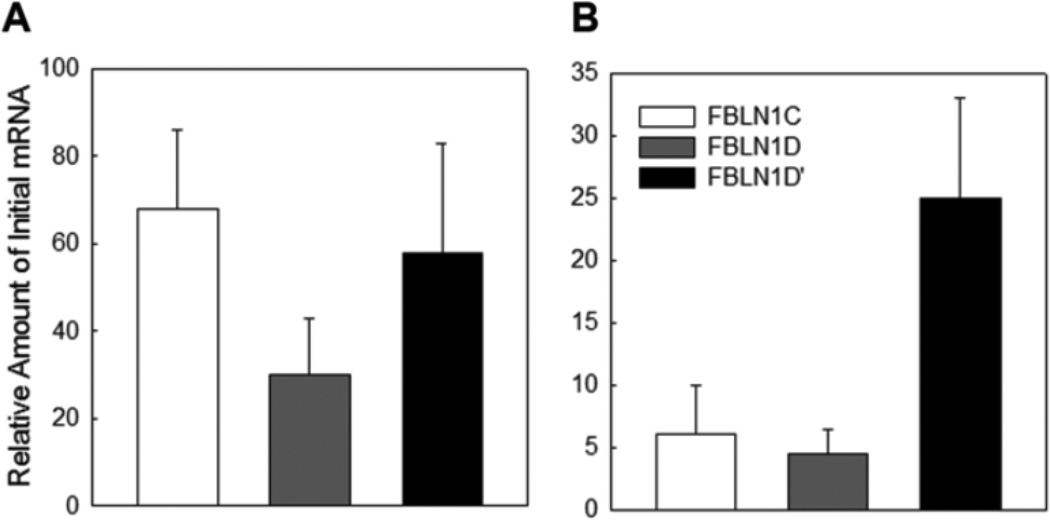
To detect fibulin-1D’ transcript in human placenta, we used real-time PCR with specifically designed primers for fibulin-1D’. The reverse primer was designed to bind to the alternative splice region of fibulin-1D’ in exons 3 and 4, and cannot bind to any of the other fibulin-1 variants. The sequences of exon 3 and 4 splice regions in all other fibulin-1 variants (A-D) are separated by 107 bp. We detected fibulin-1D’ in both first trimester and full-term placental tissues (Figure 9); however, the first trimester levels showed two fold higher expression of fibulin-1D’ than full term placenta (Figure 9A and B). Fibulin-1D mRNA expression was about 50% less than fibulin-1C and -1D’ in the first trimester placental tissue (Figure 9A). In full term placenta, fibulin-1D’ mRNA levels were about five fold higher than both fibulin-1C and -1D (Figure 9B). Interestingly, full term placenta showed decreased levels (about 10%) of fibulin-1C and -1D compared to first trimester levels (Figure 9A, B).
Figure 9.
Fibulin-1D’ mRNA is detected in human placental tissue. Panel A, real time PCR analysis of mRNA expression of fibulin-1D’ (FBLN1D’), fibulin-1C (FBLN1C) and -1D (FBLN1D) transcripts in the first trimester placenta. Panel B, Full term placental mRNA levels of fibulin-1 transcripts C, D and D’. Data presented as mean ± range of duplicate samples, and a representative of two independent experiments.
DISCUSSION
In this report we present the characterization of a novel fibulin-1D variant that binds to the intracellular domain amino acid sequence of integrin β1. It is possible that fibulin-1D’ protein characterized in this manuscript is the same variant isolated and localized intracellularly in the original study by Argraves et al., 1989 16. The protein sequencing data supports the presence of an alternative start site in exon 2 and alternative splicing sites in exons 3 and 4. Fibulin-1D’ was expressed in the human placenta as well as in the human epidermoid carcinoma cell line A431 and in fibulin-1C and -1D transfected HT 1080 cells. Ota et al. reported an unknown protein that is similar to fibulin-1D in the human teratocarcinoma cell line NT2 that were treated for two weeks with retinoic acid 17. The sequence of the unknown protein (termed here fibulin-1D’) was deduced from the whole genome sequencing project of the NT2 human cell line and the in silico translation of putative genes. The sequence of the reported unknown protein matched the fibulin-1D’ protein reported in this manuscript which we discovered using a proteomics approach. Since fibulin-1 was first identified as a protein binding to the cytoplasmic domain peptide of integrin β1 16, all subsequent reports have shown that fibulin-1 is an ECM glycoprotein. We report here that fibulin-1D’ protein is an intracellular variant of fibulin-1 and is not as abundant as other fibulin-1 variants as evident by immunoblot analysis. Although message levels of fibulin-1D’ in placental tissue are higher than the levels of both fibulin-1C and -1D, it is possible that the translation of the fibulin-1D’ mRNA is not efficient, or the mRNA message of fibulin-1D’ is not stable, favoring the more stable transcript of fibulin-1D.
We have provided evidence that the fibulin-1D’ protein expression can be induced by transfection of HT1080 cells with either fibulin-1D or fibulin-1C full coding sequences, as shown by experiments outlined in Figures 5 and 7. It is possible that fibulin-1C transfection induces the expression of fibulin-1D transcript, which can then be spliced into either fibulin-1D or -1D’. Likewise, the presence of high levels of fibulin-1D transcript after transfection with plasmid containing fibulin-1D coding sequence might induce splicing into the fibulin-1D’ as well as fibulin-1D transcripts. It is not known why or when the cell decides to make fibulin-1D or 1-D’ are since they are made from the same transcript. The phenomena of one transcript effect on the induction of another transcript has been previously reported for transient receptor potential cation channel, subfamily A, member 1 protein (TRPA1) in dorsal root ganglion neurons 19 and FERM domain-containing protein 7 (FRMD7) in human developing brain tissue 20.
Actin was co-eluted from the fibulin-1-conjugated sepharose column along with fibulin-1D’. Actin is a protein that is known to be associated with focal adhesions and involved in force transduction of migrating cells 21. Argraves et al. (1989) previously found that the integrin β1-bound fibulin-1 co-localized with focal adhesions of gingival fibroblasts 16. We speculate that fibulin-1D’ could be part of a complex of integrin β1, actin, and myosin that interact intracellularly to modulate spreading and cell motility.
Another protein that co-purified from the fibulin-1-bound sepharose column was MYH9 (Figure 1), a non-muscle myosin involved in cell motility, maintenance of cell shape, and cytokinesis. Toren et al. reported that people affected with autosomal-dominant giant platelet syndromes, a multitude of diseases affecting humans, were related to several mutations in the MYH9 gene 22. When several individuals from eight different families afflicted with this syndrome were examined for MYH9 mutations, seven out of eight families had MYH9 mutations, while one family did not have any MYH9 mutation identified. Instead of an MYH9 gene mutation on chromosome 22, a different mutation in chromosome 22 was identified in the Israeli Fechtner family, specifically in exon 19 of fibulin-1, which affects the expression of fibulin-1D and -1D’ variants. This mutation caused these individuals to lack the expression of fibulin-1D protein and surprisingly, the authors found an expression of anti-sense fibulin-1D transcript that abolished the expression of fibulin-1D variant. The authors concluded that fibulin-1D might be acting as a modifier in this group of syndromes. It is possible that some of the phenotypes seen in the Fechtner family autosomal-dominant giant platelet syndrome might be related to lack of both fibulin-1D and -1D’. Moreover, mutations in the MYH9 gene affecting other families with this syndrome, might be interfering with MYH9/fibulin-1D’or MYH9/fibulin-1D interaction and manifesting some aspects of the phenotype.
We have previously shown that ECM fibulin-1/FN complexes had no effect on FN binding to integrin α5β1 12. We have shown however that extracellular fibulin-1 bound to FN reduces the phosphorylation of intracellular myosin-II heavy chain (MHC) and ERK phosphorylation 12, which are involved in cell spreading 23 and integrin-mediated cell survival 24, respectively. It has been postulated that MHC phosphorylation initiates cell spreading by releasing F-actin from actinomyosin complexes, allowing it to reassemble within lamellipodial protrusions 23. We have previously shown that cells attached to matrices of fibulin-1 bound to FN have a rounded morphology, lack lamellipodia, and display a lower rate of spreading than cells grown on FN substrata 12. We also reported that fibulin-1 inhibits FN-mediated activation of ERK 12. It is not known however whether there is a relationship between the phosphorylation of ERK and MHC. MHC phosphorylation has been shown to involve an influx of extracellular calcium and activation of a calmodulin-dependent kinase (CaM kinase) 23. Franklin et al. have shown that increases in intracellular calcium induce activation of ERK1/2 via activation of CaM kinase 25, and inhibition of CaM kinase has been shown to prevent ERK1/2 activation 26. Therefore, we postulate that the intracellular fibulin-1D’ might reduce intracellular calcium levels, thus reducing the activation of both ERK and MHC. Fibulin-1D’ might accomplish this by modulating integrin α5β1-FN signaling, which has been shown to regulate a tyrosine phosphorylation cascade regulating the function of the L-type calcium channel 27. Further investigations will be needed to elucidate the effects of fibulin-1D’ on cell signalling of integrin α5β1 bound to FN.
In summary, the finding of the novel fibulin-1D variant, which we termed fibulin-1D’, could explain and resolve the discrepancy of fibulin-1 intracellular localization and association with integrin β1 cytoplasmic domain as well as playing a possible role in integrin mediated cell adhesion and motility. Further studies are needed to elucidate the effect of fibulin-1D’ on α5β1 functions in the cell.
MATERIALS AND METHODS
Antibodies
Polyclonal anti-fibulin-1 Rb811 recognizes fibulin-1D/D’ variants, Rb1323 recognizes all fibulin-1 variants, and Rb2914 recognizes FN 8, 16, 18. Monoclonal antibodies 3A11 and 6D8, which recognize fibulin-1 A-D variants, with epitope mapping to the anaphlatoxin region; and 5D12, which recognizes fibulin-1C variant, were described earlier 8, 28. The polyclonal anti-fibulin-1D’ antibody (Rb1603) was commercially generated against the unique N-terminus protein sequence TGWPLIRRTARCHML (amino acid residues 12–26) by QED Bioscience (San Diego, CA) and recognizes the un-reduced form of fibulin-1D’ only.
Cell culture and affinity chromatography of A431 cell extract
A431 cells (Human epidermoid carcinoma cell line) were cultured in 150-mm culture dishes and grown to 80% confluence in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were dissociated non-enzymetically with 2 mM EDTA, rinsed twice with non-serum-supplemented DMEM, and re-suspended in extraction buffer (50 mM HEPES, pH 7.4 containing 0.5 M NaCl, 1% Triton X-100, 0.5% Tween-20 and 2 mM phenylmethyl sulfonyl floride (PMSF)). The extract was centrifuged at 16,000 × g at 4°C and the supernatant was passed over a CL4B sepharose column (bed volume 5 ml) then over a placental fibulin-1-coupled sepharose column (bed volume 2.6 ml). Columns were rinsed with 50 ml of extraction buffer and eluted with 8 M urea. Eight 0.65 ml fractions were collected. A fixed volume (300 µl) of each fraction was mixed with 500 µl of Tris buffered saline (TBS, pH 7.4) then a 10-µl aliquot of Strataclean resin (Strategene, Santa Clara, CA) was added to bind proteins in each eluted fraction. After centrifugation, supernatant was removed and resin was washed twice with TBS then 10 µl of 2X reducing (β mercaptoethanol) sample buffer was added. Fractions were then boiled and resolved on 4–12% Tris glycine SDS-PAGE gel (Novex, Life Technologies, Carlsbad, CA). Gels were stained with Coomassie blue dye.
Sequencing and identification of fibulin-1D’ protein
Coomassie blue-stained bands at approximately 200 kDa, 75 kDa, and 40 kDa molecular weights were excised and analyzed at MUSC Mass Spectrometry Facility for protein identification. In addition, a purified sample of human placental fibulin-1 (pFBLN1) was sequenced.
Purification of integrin α5β1, coupling to sepharose and fibulin-1 binding
Integrin α5β1 was purified as described previously 29 and coupled to Dynabeads (Life Technologies, ≈ 2.0 µg/107 beads) according to the manufacturer protocol. pFBLN1 (2.7 µg) or equivalent amount of recombinant fibulin-1D (rFBLN1D), purified from transfected HT1080 cells CCM 28, was incubated with integrin α5β1-coupled beads and allowed to bind for 18 hrs at 4°C.
Immunodetection of integrin α5β1-bound fibulin-1D’
At the end of the incubation period (in section 4.4), beads were collected by centrifugation and rinsed four times with TBS containing 25mM n-octyl-B-D-glucopyranoside (OG) and 1 mM MgCl2. Bound proteins were released from the beads with 2X SDS reducing sample buffer. Unbound protein fractions (15 µl) were mixed with 4X reducing sample buffer and both fractions (bound and unbound) were resolved on 4–12 % SDS-PAGE NUPAGE gel (Novex, Life Technologies). Proteins were transferred to polyvinylidene fluoride (PVDF) membrane and blocked with 5% non-fat milk. Bound and unbound proteins were visualized using antibodies against fibulin-1D/D’ and then re-probed with anti-fibulin-1 antibody and chemiluminescent reagent (ECL or ECL+, GE Healthcare Life Sciences, Pittsburgh, PA). Ovalbumin-coupled Dynabeads were used as negative control. Detection of integrin α5β1-bound fibulin-1D’ was performed using polyclonal antibody against fibulin-1 D/D’ (Rb811) at 2 µg/ml and monoclonal anti fibulin-1 antibody (6D8) at 0.2 µg/ml. Secondary antibodies (anti rabbit IgG or anti mouse IgG HRP-conjugated) were used at 0.2 µg/ml. Visualization was performed using ECL+ chemiluminescent reagent for Rb811 antibody and ECL chemiluminescent reagent for 6D8 antibody.
Binding of fibulin-1D’ to the cytoplasmic domain of Integrin β1 peptide
The 37 amino acid cytoplasmic domain peptide of integrin β1 was coupled to CNBR-activated sepharose beads (GE Biosciences, Pharmacia). Beads were incubated overnight at 4 °C with 5 µg of pFBLN1 or recombinant fibulin-1C (rFBLN1C), purified from conditioned media of transfected HT1080 cells 28, in TBS binding buffer containing 25 mM OG and 1mM of CaCl2 and MgCl2. The supernatant (unbound fraction) was removed and beads were washed 4 times with binding buffer and 2X non-reducing sample buffer was added. Bound and unbound fractions were resolved on 4–12% NUPAGE gel, transferred to PVDF membrane and probed with anti fibulin-1D’ antibody (Rb1603). Visualization was performed using ECL prime chemiluminescent reagent (GE Healthcare Life Sciences). Re-probing with the 5D12 antibody was done after washing the membrane with 50 mM glycine pH 2.3 for 10 minutes to remove bound antibodies then rinsing with copious amounts of TBS buffer containing 0.1% Tween 20 (TBST).
Cloning of fibulin-1D’ transcript into pEF4 plasmid
The fibulin-1D’ whole coding sequence was cloned into pEF4/V5-His version A (Invitrogen, Carlsbad Ca) plasmid using EcoR1 and Xba1 enzyme restriction sites. The N-terminus 195 bp coding fragment was amplified using primers containing EcoR1 and Nco1 enzyme restriction sites. The kozak sequence before the ATG start site was also added. The fibulin-1D’ carboxy-terminus 1443 bp fragment (bp 196-1637, starting at the splice junction in exon 4) was cloned with Nco1and Xba1 enzyme restriction sites. The plasmid was cut with EcoR1 and Xba1 restriction enzymes. The fibulin-1D’ 195 and 1443 fragments were cut with restriction enzymes EcoR1/Nco1 and Nco1/Xba1, respectively. Ligation of inserts and plasmid was performed and the ligated plasmid was transformed into JM109 bacterial cells. Transformed bacterial cultures were grown on agar containing 100 µg/ml of ampicillin. Positive clones were picked and grown in 4 ml of LB broth. The plasmid was purified from bacterial cultures using NucleoSpin Plasmid purification Kit (Macherey-Nagel, Germany) according to manufacture instructions. Purified plasmids were sequenced and their sequence verified. Expressed fibulin-1D’ protein has a 6X His tag at the carboxy terminus.
Transfection of fibulin-1D’ plasmid and detection of fibulin-1D’ protein in CHO cells
CHO-K1 cells were cultured in HAM’S/F12 medium supplemented with 10% FBS. Cells were dissociated and 9.0×105 cells per treatment were transfected with 3 µg of fibulin-1D’ plasmid using Amaxa T kit (Lonza, Switzerland) and program U-023. GFPmax plasmid was used as the positive control and no added plasmid as the negative control treatment. Cells (9.0 ×104) were cultured on 8-cell chambered slide (Nunc, Denmark) for immunohistochemistry. Cells were fixed with 3% paraformaldehyde for 30 minutes and blocked with 5% goat serum for 1 hr at room temperature with or without added Triton-X100, then incubated with anti fibulin-1D’ antibody (Rb1603) at 1:25 dilution of serum for 18 hours at 4°C. After rinsing three times with TBST, the cells were incubated with Alexa 488-conjugated anti rabbit secondary antibody (Life Technologies) at 50 µg/ml for 18 hrs at 4°C, then rinsed three times with TBST and mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Nuclei were counter stained with TOTO3 for 5 minutes during the last wash.
The remainder of the transfected cells was cultured in a 6-well plate for western blotting. Cells were extracted with Radioimmunoprecipitation Assay (RIPA) buffer containing enzyme inhibitors (Roche), and protein concentration was determined using BCA assay (Pierce, Rockford, IL). Ten micrograms of the extract were loaded in each lane; pFBLN1 was used as a positive control (0.9 µg/well). After transfer of proteins to PVDF, membrane were blocked with 5 % non-fat milk and then incubated with anti-fibulin-1D’ antibody (Rb1603) at 1 µg/ml for 18 hours at 4°C. The membrane was then washed three times with TBST and incubated with horseradish peroxidase-conjugated secondary antibody at a 1:5000 dilution. ECL+ chemiluminescent reagent was used for protein detection. Subsequently the membrane was re-probed as mentioned above with Rb1323 antibody at 0.2 µg/ml to detect all variants of fibulin-1.
Immunohistochemical detection of fibulin-1D’ in human placental tissue
Paraffin embedded sections of human term placenta were rehydrated using xylene and ethanol series of treatments. Sections were treated with unmasking solution (Vector Laboratories) under pressure for 5 minutes to unmask the fibulin-1 binding sites. Sections were then blocked with 1% bovine serum albumin in phosphate buffered saline (PBS) for 3 hours at room temperature. Sections were probed with Rb1603 polyclonal antibody against fibulin-1D’ (50 µg/ml, 18 hrs at 4°C) then incubated with the fluorescent secondary antibodies Alexa 568-conjugated anti-rabbit IgG at 20 µg/ml for 90 minutes at room temperature. Nuclei were counterstained with Draq5 at 5 µM for 5 minutes and then sections were rinsed twice with PBS and mounted in Vectashield mounting media.
Detection of fibulin-1D’ transcripts in human placenta using real-time PCR
To detect the message of fibulin-1D’ variant in human placenta, we designed a set of primers that specifically identifies this mRNA transcript. The reverse primer sequence that spans the splice site between human FBLN1 exon 3 and exon 4, is unique for this variant and amplifies a 164 bp amplicon in fibulin-1D’ (see Table 1 for sequences). The reverse primer cannot bind to any of the other fibulin-1 variants because the sequences of exon 3 and 4 splice regions in all other fibulin-1 variants are separated by 107 bp. Total RNA was purified from first trimester placenta villi and decidua tissues using RNAeasy plus mini kit (Qiagen, Germany) according to manufacturer protocol. Quality of RNA was verified using an Agilent 2100 system. Full term placental total RNA was purchased from Biochain (San Francisco, CA) and 1 µg of each total RNA was used to synthesize cDNA using iScript cDNA synthesis kit (BioRad, Hercules, CA). For detection of fibulin-1 C, D and D’ variants, the cDNA was diluted 1:1 in water. Housekeeping genes TATA Box binding protein and GAPDH cDNA were diluted at 1:1 and 1:100, respectively. The real-time polymerase chain reaction (qPCR) reactions were carried out using SsoAdvanced SyberGreen qPCR reagent (BioRad) in a total volume of 10 µl. The cycling parameters were 95°C for 2 minutes, 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds. Verification of the specificity of the primers was performed using a melt curve from 65°C to 95°C in 0.5°C increments and the size of the amplicon in the fibulin-1D’ reactions was verified using the Agilent 2100 system and a DNA 1000 chip. Primers specific for fibulin-1 variants C and D were also used to detect fibulin-1C and -1D transcripts (Table 1). Expression data was analyzed using the PCR miner method 30.
Table 1.
Human primer sequences used for real-time PCR
| Gene | Type | Sequence | Reference Sequence Number |
|---|---|---|---|
| Fibulin-1C | Forward | caactgctccatcaacgaga | NM_001996.3 |
| Reverse | attctcagaggcagcttgga | ||
| Fibulin-1D | Forward | cgagtgccctgagaactacc | NM_006486.2 |
| Reverse | gagatgacggtgtgggagat | ||
| Fibulin-1D’ | Forward | atggccactcatcagaagga | AK075566.1 |
| Reverse | caaccatggggcgtgg | ||
| GAPDH | Forward | atgttcgtcatgggtgtgaa | NM_001256799.1 |
| Reverse | ggtgctaagcagttggtggt | ||
| TATA Box BP | Forward | cggctgtttaacttcgcttc | BT0 19657.1 |
| Reverse | ctgggtcactgcaaagatca |
Highlights.
We identify a new splice variant of fibulin-1 named fibulin-1D prime (fibulin-1D’)
Fibulin-1D’ differs from fibulin-1 variants in the N-terminus amino acid sequence
Fibulin-1D’ binds to the intracellular domain of integrin β1
Fibulin-1D’ lacks a secretion sequence and the anaphlatoxin region of fibulin-1
We localized the intracellular fibulin-1D’ protein in human placenta
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants NIH/NHLBI 5R01 HL09506 and NIH/NHLBI P01 HL52813 to WSA, and South Carolina Space Grant Consortium (Palmetto Academy) NNG05GI68G to SG. This study used the services of the Morphology, Imaging and Instrumentation Core, which is supported by NIH-NIGMS P30 GM103342 to the South Carolina COBRE for Developmentally Based Cardiovascular Diseases. The authors would like to thank Dr. Bryan Toole (MUSC) for helpful discussions.
Abbreviations
- qPCR
Quantitative real-time polymerase chain reaction
- pFBLN1
placental fibulin-1
- rFBLN1D
recombinant fibulin-1D
- rFBLN1C
recombinant fibulin-1C
- MYH9
myosin heavy chain 9
- MHC
myosin-II heavy chain
- EGF
epidermal growth factor
- FN
fibronectin
- ECM
extracellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Waleed O. Twal, Email: twalwo@musc.edu.
Samar M. Hammad, Email: hammadsm@musc.edu.
REFERENCES
- 1.Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4:1127–1131. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooley MA, Argraves WS. In: The fibulins. In Biology of Extracellular Matrix: The Extracellular Matrix: an Overview. Mecham RP, editor. Berlin, Heidelberg: Springer; 2011. pp. 337–367. [Google Scholar]
- 3.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted Disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol. Cell. Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin PJ, Bakall B, Choi J, Liu Z, Sasaki T, Davis EC, Marmorstein AD, Marmorstein LY. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum. Mol. Genet. 2007;16:3059–3070. doi: 10.1093/hmg/ddm264. [DOI] [PubMed] [Google Scholar]
- 6.Sicot FX, Tsuda T, Markova D, Klement JF, Arita M, Zhang RZ, Pan TC, Mecham RP, Birk DE, Chu ML. Fibulin-2 is dispensable for mouse development and elastic fiber formation. Mol. Cell Biol. 2008;28:1061–1067. doi: 10.1128/MCB.01876-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papke CL, Yanagisawa H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: Insights from mouse and human studies. Matrix Biol. 2014;37:142–149. doi: 10.1016/j.matbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argraves WS, Tran H, Burgess WH, Dickerson K. Fibulin is an extracellular matrix and plasma glycoprotein with repeated domain structure. J. Cell Biol. 1990;111:3155–3164. doi: 10.1083/jcb.111.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balbona K, Tran H, Godyna S, Ingham KC, Strickland DK, Argraves WS. Fibulin binds to itself and to the carboxyl-terminal heparin-binding region of fibronectin. J. Biol. Chem. 1992;267:20120–20125. [PubMed] [Google Scholar]
- 10.Roark EF, Keene DR, Haudenschild CC, Godyna S, Little CD, Argraves WS. The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J. Histochem. Cytochem. 1995;43:401–411. doi: 10.1177/43.4.7534784. [DOI] [PubMed] [Google Scholar]
- 11.Argraves WS, Tanaka A, Smith EP, Twal WO, Argraves KM, Fan D, Haudenschild CC. Fibulin-1 and fibrinogen in human atherosclerotic lesions. Histochem. Cell Biol. 2009;132:559–565. doi: 10.1007/s00418-009-0628-7. [DOI] [PubMed] [Google Scholar]
- 12.Twal WO, Czirok A, Hegedus B, Knaak C, Chintalapudi MR, Okagawa H, Sugi Y, Argraves WS. Fibulin-1 suppression of fibronectin-regulated cell adhesion and motility. J. Cell Sci. 2001;114:4587–4598. doi: 10.1242/jcs.114.24.4587. [DOI] [PubMed] [Google Scholar]
- 13.Tran H, Tanaka A, Litvinovich SV, Medved LV, Haudenschild CC, Argraves WS. The interaction of fibulin-1 with fibrinogen. A potential role in hemostasis and thrombosis. J. Biol. Chem. 1995;270:19458–19464. doi: 10.1074/jbc.270.33.19458. [DOI] [PubMed] [Google Scholar]
- 14.Godyna S, Diaz-Ricart M, Argraves WS. Fibulin-1 mediates platelet adhesion via a bridge of fibrinogen. Blood. 1996;88:2569–2577. [PubMed] [Google Scholar]
- 15.Pan TC, Kostka G, Zhang RZ, Timpl R, Chu ML. Complete exon-intron organization of the mouse fibulin-1 gene and its comparison with the human fibulin-1 gene. FEBS Lett. 1999;444:38–42. doi: 10.1016/s0014-5793(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 16.Argraves WS, Dickerson K, Burgess WH, Ruoslahti E. Fibulin, a novel protein that interacts with the fibronectin receptor beta subunit cytoplasmic domain. Cell. 1989;58:623–629. doi: 10.1016/0092-8674(89)90097-4. [DOI] [PubMed] [Google Scholar]
- 17.Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, Kimura K, Makita H, Sekine M, Obayashi M, Nishi T, Shibahara T, Tanaka T, Ishii S, Yamamoto J, Saito K, Kawai Y, Isono Y, Nakamura Y, Nagahari K, Murakami K, Yasuda T, Iwayanagi T, Wagatsuma M, Shiratori A, Sudo H, Hosoiri T, Kaku Y, Kodaira H, Kondo H, Sugawara M, Takahashi M, Kanda K, Yokoi T, Furuya T, Kikkawa E, Omura Y, Abe K, Kamihara K, Katsuta N, Sato K, Tanikawa M, Yamazaki M, Ninomiya K, Ishibashi T, Yamashita H, Murakawa K, Fujimori K, Tanai H, Kimata M, Watanabe M, Hiraoka S, Chiba Y, Ishida S, Ono Y, Takiguchi S, Watanabe S, Yosida M, Hotuta T, Kusano J, Kanehori K, Takahashi-Fujii A, Hara H, Tanase TO, Nomura Y, Togiya S, Komai F, Hara R, Takeuchi K, Arita M, Imose N, Musashino K, Yuuki H, Oshima A, Sasaki N, Aotsuka S, Yoshikawa Y, Matsunawa H, Ichihara T, Shiohata N, Sano S, Moriya S, Momiyama H, Satoh N, Takami S, Terashima Y, Suzuki O, Nakagawa S, Senoh A, Mizoguchi H, Goto Y, Shimizu F, Wakebe H, Hishigaki H, Watanabe T, Sugiyama A, Takemoto M, Kawakami B, Yamazaki M, Watanabe K, Kumagai A, Itakura S, Fukuzumi Y, Fujimori Y, Komiyama M, Tashiro H, Tanigami A, Fujiwara T, Ono T, Yamada K, Fujii Y, Ozaki K, Hirao M, Ohmori Y, Kawabata A, Hikiji T, Kobatake N, Inagaki H, Ikema Y, Okamoto S, Okitani R, Kawakami T, Noguchi S, Itoh T, Shigeta K, Senba T, Matsumura K, Nakajima Y, Mizuno T, Morinaga M, Sasaki M, Togashi T, Oyama M, Hata H, Watanabe M, Komatsu T, Mizushima-Sugano J, Satoh T, Shirai Y, Takahashi Y, Nakagawa K, Okumura K, Nagase T, Nomura N, Kikuchi H, Masuho Y, Yamashita R, Nakai K, Yada T, Nakamura Y, Ohara O, Isogai T, Sugano S. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat. Genet. 2004;36:40–45. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- 18.Tran H, VanDusen WJ, Argraves WS. The self-association and fibronectinbinding sites of fibulin-1 map to calcium-binding epidermal growth factor-like domains. J. Biol. Chem. 1997;272:22600–22606. doi: 10.1074/jbc.272.36.22600. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Suzuki Y, Uchida K, Tominaga M. Identification of a splice variant of mouse TRPA1 that regulates TRPA1 activity. Nat. Commun. 2013;4:2399. doi: 10.1038/ncomms3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Pu J, Liu Z, Xu S, Jin F, Zhu L, Tian J, Luo J, Zhang B. Identification of a novel FRMD7 splice variant and functional analysis of two FRMD7 transcripts during human NT2 cell differentiation. Mol. Vis. 2011;17:2986–2996. [PMC free article] [PubMed] [Google Scholar]
- 21.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 22.Toren A, Rozenfeld-Granot G, Heath KE, Amariglio N, Rocca B, Crosson J, Epstein CJ, Laghi F, Landolfi R, Carlsson LE, Argraves S, Bizzaro N, Moxey-Mims M, Brok-Simoni F, Martignetti JA, Greinacher A, Rechavi G. MYH9 spectrum of autosomal-dominant giant platelet syndromes: unexpected association with fibulin-1 variant-D inactivation. Am. J. Hematol. 2003;74:254–262. doi: 10.1002/ajh.10425. [DOI] [PubMed] [Google Scholar]
- 23.van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- 24.Fornaro M, Steger CA, Bennett AM, Wu JJ, Languino LR. Differential role of beta(1C) and beta(1A) integrin cytoplasmic variants in modulating focal adhesion kinase, protein kinase B/AKT, and Ras/Mitogen-activated protein kinase pathways. Mo. Biol. Cell. 2000;11:2235–2249. doi: 10.1091/mbc.11.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin RA, Atherfold PA, McCubrey JA. Calcium-induced ERK activation in human T lymphocytes occurs via p56(Lck) and CaM-kinase. Mol. Immunol. 2000;37:675–683. doi: 10.1016/s0161-5890(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 26.Rosengart MR, Arbabi S, Garcia I, Maier RV. Interactions of calcium/calmodulin-dependent protein kinases (CaMK) and extracellular-regulated kinase (ERK) in monocyte adherence and TNFalpha production. Shock. 2000;13:183–189. doi: 10.1097/00024382-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by alpha 5beta 1 integrin requires signaling between focal adhesion proteins. J. Biol. Chem. 2001;276:30285–30292. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- 28.Tran H, Mattei M, Godyna S, Argraves WS. Human fibulin-1D: molecular cloning, expression and similarity with S1-5 protein, a new member of the fibulin gene family. Matrix Biol. 1997;15:479–493. doi: 10.1016/s0945-053x(97)90021-4. [DOI] [PubMed] [Google Scholar]
- 29.Argraves WS, Tran H. Purification of a5b1 integrin by ligand affinity chromatography. J. Tissue Cult. Methods. 1994;16:243–247. [Google Scholar]
- 30.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005;12:1045–1062. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]