Abstract
Evolutionary-minded developmentalists studying predictive-adaptive-response processes linking childhood adversity with accelerated female reproductive development and health scientists investigating the developmental origins of health and disease (DOoHaD) may be tapping the same process, whereby longer-term health costs are traded off for increased probability of reproducing before dying via a process of accelerated reproductive maturation. Using data from 73 females, we test the following propositions using path analysis: (a) greater exposure to prenatal stress predicts greater maternal depression and negative parenting in infancy, (b) which predicts elevated basal cortisol at 4.5 years, (c) which predicts accelerated adrenarcheal development, (d) which predicts more physical and mental health problems at age 18. Results prove generally consistent with these propositions, including a direct link from cortisol to mental health problems. DOoHaD investigators should consider including early sexual maturation as a core component linking early adversity and stress physiology with poor health later in life in females.
Keywords: predictive adaptive response, developmental origins of health and disease, adrenarche, prenatal stress, stress physiology, health
Research on the developmental origins of health and disease indicates that adverse developmental experiences and environmental exposures early in life undermine later physical health and well-being (Evans, 2003; Hertzman & Power, 2004) and mental health (e.g., Anda et al., 2006; Poulton et al., 2002; Schilling, Aseltine, & Gore, 2007). Thus, investigators have chronicled links between low childhood socioeconomic status and increased morbidity in mid-life (Melchior, Moffitt, Milne, Poulton, & Caspi, 2007; Poulton et al., 2002), between childhood maltreatment and compromised adolescent health (Flaherty et al., 2013), and between cumulative contextual risk and allostatic load in adulthood (Brody et al., 2013). Health-minded scholars have even begun to illuminate plausible mediating biological mechanisms—or at least biomarkers of the presumed health-deterioration process (Carroll et al., 2013)--including inflammation (Chen, Miller, Kobor, & Cole, 2011; Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Miller, Chen, & Parker, 2011), immune competence (e.g., O'Connor et al., 2013; Shirtcliff, Coe, & Pollak, 2009), metabolic functioning (Lehman, Taylor, Kiefe, & Seeman, 2005) and chromosomal integrity as indexed by telomere length (e.g., Entringer, Buss, & Wadhwa, 2012; Epel et al., 2004; Kiecolt-Glaser, Jaremka, Derry, & Glaser, 2013; Shalev, 2012). Here we seek to extend such work when linking prenatal stress with physical and mental health at age 18—by considering the role of stress physiology and sexual maturation.
Given our view that two different models of accelerated aging may reflect a single evolved process of accelerated development (Belsky & Shalev, in press)--the developmental-origins-of-health-and-disease framework linking early adversity with increased morbidity and early mortality later in life (Epel, Blackburn, Lin, Dhabhar, Adler, Morrow & Cawthon, 2004; Shalev, 2012; Shalev, Caspi, Ambler, D.Belsky, D. W., Chapple, S., Cohen et al., 2014) and a reproductive-strategy one linking similar early experiences with earlier sexual maturation in females (Belsky, 2012; Belsky, Steinberg & Draper, 1991)--we seek to test the following Propositions (a) that greater prenatal stress exposure will predict greater maternal depression and negative parenting in infancy—both known to forecast more problematic child functioning (NICHD Early Child Care Research Network, 1999) and to be interrelated (Lovejoy, Graczyk, O'Hare, & Neuman, 2000); and (b) that such early experiences will themselves predict elevated basal cortisol at age 4.5 years, (c) which itself will predict accelerated adrenarcheal development in first grade, (d) which itself will predict poorer physical and mental health at age 18. We also evaluate whether pubertal status at age 11 mediates the association between adrenarche and later health.
The Role of Stress Physiology
Stress physiology is presumed to play an important role in the development process just outlined. Indeed, there is extensive evidence that early exposures to environmental stressors, both within the family and larger social and physical contexts, predict systematic differences in stress physiology later in childhood, most notably, the hypothalamic-pituitary-adrenal (HPA) axis (Ellis, Essex, & Boyce, 2005; Essex, Klein, Cho, & Kalin, 2002; Mackrell et al., 2014). Just as important is evidence showing that elevated levels of basal cortisol—the stress-physiology index used in this inquiry--are associated with increased risk for maladaptive development and perturbations of both mental and physical health (e.g., Doom & Gunnar, 2013; Essex, Klein, et al., 2002). And this is true even though some theory and empirical findings suggest that stress-physiology linkages may be non-linear and/or bidirectional in character (Boyce & Ellis, 2005; Ellis et al., 2005; Parker, Buckmaster, Sundlass, Schatzberg, & Lyons, 2006) research.
The Role of Sexual Maturation
Where we break new ground in investigating the developmental origins of health and disease is by considering the role of sexual maturation, a process regulated in part by the HPA axis (Ellis, 2004) and thus linked to cortisol-related indices of stress physiology (Saxbe, Negriff, Susman, & Trickett, in press). In fact, we focus on adrenarche, considered the first stage of pubertal development occurring around 6-8 years of age (Campbell, 2006; McClintock & Herdt, 1996), for two reasons. The first is that cortisol and dehydroepiandrosterone (DHEA), a hormonal index of adrenarcheal development, share the same hormone that triggers their synthesis and release, ACTH. The second is that DHEA plays a role in the stress system (Saczawa, Graber, Brooks-Gunn & Warren, 2013).
To our knowledge, no prior work, including that of the larger project from which the data for this report derive, has sought to link, in a single inquiry, exposure to adversity, stress physiology, sexual maturation and physical and mental health. What has been examined in prior reports using the current sample are (a) links between early family stress exposure and later mental—but not physical--health problems, with childhood cortisol sometimes treated as a mediating mechanism (e.g., Essex et al, 2002), and, separately, (b) those between early adversity and sexual maturation, including adrenarche (Ellis et al., 2007, 2012). Thus, what we endeavor to do herein is novel: Evaluate, using data from an 18-year longitudinal study, whether there is a shared pathway whereby early family stress, measured for the first time during pregnancy, forecasts mental and physical health problems at age 18 via childhood cortisol and adrenarche.
It is noteworthy, given this developmental model, that higher levels of DHEA are positively related to mental health problems (Dmitrieva, Oades, Hauffa & Eggers, 2001; van Goozen et al., 2000), with Dorn and colleagues (1999, 2008) reporting that children with premature adrenarche manifest increased levels of depression, anxiety and behavioral problems relative to their normatively developing peers. And this may be because higher levels of DHEA are associated with neurological processes reflective of emotion dysregulation; in fact, fMRI measurements to this effect mediated the link between higher DHEA levels and externalizing problems in Whittle and associates (in press) work with nine-year olds, a result which remained even with Tanner Stage controlled. Seemingly in line with such results are Klauser and colleagues' (2015) data indicating that higher levels of DHEA in nine-year olds are associated with delayed development of frontal white matter in the left corona radiate, as anomalies in this brain region have been linked to irritability symptoms in adolescent depression (Henderson et al., 2013).
An Evolutionary Trade-Off Model
Whereas most theory and research on the developmental origins of health and disease is based on the view that exposure to adversity early in life generates “wear and tear” on developing systems, thereby undermining health in the long-term (Evans, 2003; Hertzman & Power, 2004), evolutionary theorizing calls attention to a process of “predictive adaptive response” which serves to “program” the developing organism to fit the context that is likely to be encountered later in life (Bateson et al., 2004; Belsky et al., 1991; Ellis & Del Giudice, 2014; Gluckman, Hanson, & Spencer, 2005). This framework stipulates that natural selection shaped child development to be sensitive to environmental cues pertaining to risk and opportunity, treating these as a “weather forecast” (Bateson, 2008) to regulate development. And because the central goal of all living things is to pass genes on to future generations, developmental experiences that reflect increased risk of dying before reproducing—as poverty, maltreatment, neglect, and harsh parenting are presumed to do—should accelerate reproductive maturation (Belsky et al., 1991; Chisholm, 1993). Extensive evidence proves consistent with this view—in the case of females—as they evince earlier sexual maturation than age-mates when exposed to sexual abuse, harsh parenting, father absence, conflicted marriages and related adverse family experiences (Belsky, 2012; Ellis, 2004). Indeed, prior research on the children studied for this report links unsupportive family relationships with earlier ardrenarche (Ellis & Essex, 2007).
To summarize, two separate lines of research independently link early adversity with stress physiology, accelerated reproductive development (in females) and/or poor health later in life, considered to reflect accelerated aging. This raises the prospect that evolutionary-minded scholars who study the predictive-adaptive-response process and focus on reproductive maturity and health scientists who investigate the developmental origins of health and disease and focus on poor health in midlife may well be tapping into the same evolutionary developmental process, one in which longer-term health costs are traded off for increased probability of reproducing before dying via a process of accelerated reproductive maturation (Belsky, 2014; Belsky & Shalev, in press; Ellis, Del Giudice, & Shirtcliff, 2013; Rickard, Frankenhuis, & Nettle, 2014). Not inconsistent with such a view is extensive evidence that early sexual maturation in females is associated with increased morbidity in later life (Ellis, 2004).
Method
Participants
When children originally recruited at birth to participate in the Wisconsin Study of Families and Work were in grade 1, a subsample of 120 participants (73 female) was re-recruited for additional study, consisting of individuals scoring either high or low on mental health problems. For details on the overall sample, recruitment procedures, and eligibility criteria, see Hyde, Klein, Essex, and Clark (1995); for subsample details, see Ellis and Essex, (2007). None (0%) of the female participants were missing prenatal maternal stress, infancy maternal depression, negative parenting, or child adrenarche data; 13 (17.8%) were missing childhood cortisol data; and 7 (9.6%) were missing mental and physical health data.
At recruitment, the 73 mothers ranged in age from 22-41 years (Mdn = 30) and the majority were Caucasian (86%), well-educated (e.g., 80% had some level of post-secondary education) and married (94%). Family incomes ranged from less than $10,000 to $120,000 (Mdn = $50,000). Importantly, there were no significant differences on these demographic characteristics or the specific variables of interest in the present study between the 73 participating families and the remainder of the 570 original families.
Measures
Prenatal Maternal Stress
Prenatal maternal stress scores were composites of maternal reports of 1) maternal depression symptoms, 2) marital conflict, and 3) financial stress, created on the basis of Principal Components Analysis. Maternal depression was assessed by the 20-item Center for Epidemiologic Studies—Depression scale (CES-D, Radloff, 1977). Marital conflict was assessed with the average of three items from the Partner Role Quality scale (Barnett & Marshall, 1989) tapping overt marital conflict (e.g., concerned about “arguing or fighting”). Financial stress was the average of four items (e.g., “how much difficulty making monthly payments”).
Infancy Maternal Depression
Maternal report of depression symptoms was assessed repeatedly in infancy with the CES-D in infancy. Scores obtained at 1-, 4- and 12-month measurement occasions were averaged to create a composite measure.
Infancy Negative Parenting
Maternal report of negative parenting was assessed with the Sense of Competence and Child Reinforces Parent scales of the Parental Stress Index (PSI) (Abidin, 1986) and a Negative Affect scale from the Block Childrearing Practices Report (CRPR) (Block, 1965); see Ellis and Essex (2007) for details. Measures were obtained in infancy (child ages 1, 4, and 12 months for the PSI, 12 months for the CRPR). PSI scores were averaged over time and then combined with CRPR Negative Affect based on Principal Components Analysis.
Basal Cortisol
Child salivary cortisol (mean age = 4.64 years, SD = 0.05 years) was sampled at home over 3 days, at a target time between 3:00 p.m. and7:00 p.m., prior to dinner, to capture cortisol levels reflecting more environmental than genetic influences (Schreiber et al., 2006). Additional details have beenreported previously (Essex, Klein, et al., 2002; Smider et al., 2002).
Adrenarche
Saliva was sampled four times over a home visit following grade 1, and dehydroepiandrosterone (DHEA) was assayed in duplicate. Participants were coded as preadrenarcheal (41%) if six or more of their eight DHEA assays were below the detection threshold (10.0 pg/ml) and all DHEA scores were <16 pg/ml. All other participants were coded as adrenarcheal (59%). Assay (Salimetrics, State College, PA) specifications and additional details are reported elsewhere (Ellis & Essex, 2007).
Pubertal Status
In the summer following 5th grade (mean age = 11.25 years, SD = 0.30 years) pubertal status was assessed via child report of Tanner stages using standard line drawings (Morris & Udry, 1980) and mother report of both Tanner stages and a questionnaire version (Carskadon & Acebo, 1993) of the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988). A single score combined ratings across measures and respondents as detailed elsewhere (Ellis & Essex, 2007; Ellis, Shirtcliff, Boyce, Deardorff, & Essex, 2011).
Adolescent Mental and Physical Health Problems
Adolescents (mean age = 17.84 years, SD = 0.30 years) completed an age-appropriate version (Burk et al., 2011) of the MacArthur Health and Behavior Questionnaire (Boyce et al., 2002; Essex, Boyce, et al., 2002) to assess 1) overall mental health symptoms (i.e., the average of scores for internalizing (depression, anxiety) and externalizing (conduct problems, oppositional-defiance, aggression)), and 2) global physical health problems (e.g., “I am/am not healthy enough to do the things I want to do”). For both variables, higher scores reflect more health problems.
Results
Descriptive statistics are presented in Table 1. Bivariate Pearson-r (two-tailed) correlations indicate that all anticipated associations were significant (ps ≤ .05) and in the expected direction, except for those involving negative parenting in infancy. A path analysis was constructed using Mplus version 5.2 (Muthén & Muthén, 1998-2012) to examine the hypothesized temporal ordering of the variables via an indirect pathway from prenatal stress to the health outcomes via maternal depression and negative parenting in infancy, childhood cortisol and adrenarche. In addition to modeling temporal associations, Mplus affords the opportunity to impute missing data using full information maximum likelihood estimation. Little's Missing Completely At Random test revealed that the data were missing at random; thus we imputed at the data analysis stage, permitting the inclusion of all 73 female participants in the path analyses. The resulting model demonstrated acceptable fit: χ2 (11) = 12.63, p = .32; root-mean-square error of approximation (RMSEA) = .04 (90% CI = [.00, .14]); standardized root mean residual (SRMR) = .09; comparative fit index (CFI) = 0.98).
Table 1. Descriptive Statistics and Correlations among Predictor and Outcome Variables in Girls.
| Descriptive Statistics | Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean | SD | Prenatal stress | Infancy maternal depression | Infancy negative parenting | Cortisol | Adrenarche | Mental health symptoms | Physical health problems |
| 1. Prenatal stress | 0.09 | 1.10 | -- | .64*** | .12 | .22t | .06 | .23t | .22t |
| 2. Infancy maternal depression | 6.76 | 5.36 | -- | .42*** | .35** | .20t | .38** | .33** | |
| 3. Infancy negative parenting | -0.18 | 0.92 | -- | .21 | .26* | .17 | .14 | ||
| 4. Cortisol | 0.04 | 0.31 | -- | .25* | .29* | .02 | |||
| 5. Adrenarche | 0.59 | 0.50 | -- | .32** | .25* | ||||
| 6. Mental health symptoms | 1.86 | 0.47 | -- | .60*** | |||||
| 7. Physical health problems | 4.87 | 0.95 | -- | ||||||
p ≤ .10;
p ≤ .05;
p ≤ .01;
p ≤ .001
Note: Adrenarche was coded as 0 for preadrenarcheal and +1 for adrenarcheal.
Inspection of Figure 1 reveals that results proved generally consistent with our hypothesized multi-stage developmental model after adding a direct path linking basal cortisol with mental-health problems, with the exception of the role of negative parenting in infancy, and accounted for 15.9% of the variance in mental health symptoms and 6.4% of the variance in physical health problems. In a secondary analysis, the addition of pubertal status at age 11 as a mediator between adrenarche and age-18 health outcomes failed to improve model fit (χ2 (4) = 1.77, p = 0.78). Moreover, age 11 pubertal status did not significantly mediate the association between adrenarche and either age-18 mental health (Est. = .05, p = .18) or physical health problems (Est. = .04, p = .45); therefore, only the original, more parsimonious model (excluding puberty) is displayed.
Fig. 1.
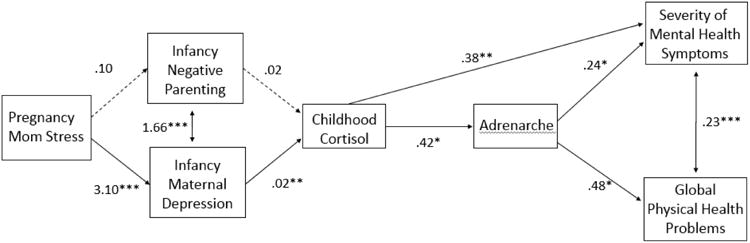
Path analysis demonstrating longitudinal associations between negative early environments, altered cortisol, early adrenarche, and health outcomes in girls. Asterisks indicate statistically significant paths (* p ≤ .05, ** p ≤ .01, *** p ≤ .001).
Discussion
Many scholars have long regarded experiences in the family early in life as formative in shaping psychological and behavioral development. More recently, health scientists have documented the influence of such developmental experiences on adult morbidity and mortality. The fact that many of the same environmental exposures found to link early-life adversity with compromised mental and physical health in adulthood also predict accelerated sexual maturation in females, as theorized some time ago (Belsky et al., 1991), raised the prospect that that those studying the developmental origins of health and disease and those investigating contextually regulated reproductive maturation were tapping into the very same evolutionary-developmental process—whereby compromised health in later life is traded off against increased probability of reproducing before dying, which is instantiated via accelerated reproductive maturation (Belsky & Shalev, in press).
Results presented in this longitudinal and observational study proved tolerably consistent with this trade-off view while highlighting a plausible mediating stress-physiology process involving elevated basal cortisol in early childhood—and thus one involving the HPA axis which has previously been implicated in linking early adversity with both accelerated reproductive development and compromised health (Ellis & Del Giudice, 2014). Thus, the longitudinal modeling revealed that greater prenatal stress exposure predicted greater maternal depression—but not negative parenting—in infancy, which itself predicted elevated basal cortisol levels at age 4.5 years, which itself predicted earlier age of adrenarche, which itself predicted poorer physical and mental health at age 18. As Figure 1 indicates, it was also the case that greater basal cortisol itself directly predicted poorer mental health. This likely reflects the fact that additional mechanisms not included in this inquiry merit future study.
The fact that the effect of adrenarche on mental and physical health was not mediated by pubertal development may appear surprising, but the fact remains that the relation between these two phases of pubertal development “has been debated controversially for decades” (Remer, Shi, Buyken, Maser-Gluth, Hartmann & Wudy, 2010, p. 3002). Conceivably, the reason we did not detect any association between the two phases of pubertal development was because of the differential precision and parameterization of the two measures used in this inquiry. Recall that adrenarche was assessed by means of a laboratory assay of salivary DHEA, whereas Tanner Stage was based on consensus estimates of secondary sex characteristics by children and their mothers. In any event, one should not lose sight of the fact that pubertal development is characterized by a series of hormonal events leading to the attainment of adult reproductive capacity. What this means, then, is that it should not be presumed that one index of this complex process is a better reflection of it than another, especially with regard to the issues addressed herein.
Due to the observational and correlational nature of these longitudinal results, as well as the modest sample size, the findings do not necessarily document causal effects. Moreover, it would be a mistake to infer that either exposure to prenatal stress or maternal depression in infancy are the only, or even most important, adverse early experiences regulating reproductive development and, thereby, physical and mental health. And the same goes for stress physiology as operationalized by means of basal cortisol when considering mediating biological mechanisms. It would also be misguided to conclude that parenting plays no role whatsoever in the developmental process under consideration. The null findings involving negative parenting in infancy could be a result of the particular measures of parenting included in our model and/or their timing of assessment. Investigators should thus expand targets of inquiry when testing the hypothesized evolutionary trade off central to this study. Despite these limits, the reported findings document, at the very least, proof of principle—namely, that the acceleration of reproductive development may be an important, but as yet, unaddressed component in the developmental origins of health and disease among females. Additionally, to our knowledge this is the first study to chronicle links between cortisol and adrenarche.
Whether or not one embraces the trade-off view that gave birth to the research reported herein, implying that scholars studying the developmental origins of health and disease would benefit from viewing the subject from an evolutionary and not just medical, wear-and-tear perspective, the implications of the results are in line with those which typically derive from the traditional view. That is, later-life health is, at least in part, forged much earlier in life than once thought. In consequence, mid-life health costs would likely be reduced by intervening early in life, long before health problems emerge. But to the extent that the process linking early adversity with poor health in adulthood is part and parcel of an evolutionary trade-off process crafted by natural selection, then it may be the case that health-related interventions implemented after the onset of reproductive maturation could be especially unlikely to succeed.
Acknowledgments
This research was supported by National Institutes of Health grants R01-MH044340, P50-MH052354, P50-MH069315, P50-MH084051, and R21-MH082705; the HealthEmotions Research Institute at the University of Wisconsin–Madison; and the John D.and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development.
Footnotes
Author Contributions: J. Belsky first proposed the study concept. All authors contributed to the study design. Data collection was planned by M.J. Essex, J.M. Armstrong, and W.T. Boyce and directed by M.J. Essex. P.L. Ruttle performed the data analyses under the direction of M.J. Essex, and with feedback from J. Belsky and W.T. Boyce. J. Belsky drafted the introduction and discussion, J.M. Armstrong the methods, and P.L. Ruttle the results, with all authors providing feedback and editing in the creation of the final version. All authors approved the final version of the manuscript for submission.
References
- Abidin RR. Parenting Stress Index. 2nd. Charlottesville, VA: Pediatric Psychology Press; 1986. [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:174–86. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett RC, Marshall NL. Preliminary manual for the Role-Quality Scales. Wellesley College Center for Research on Women; Wellesley, MA: 1989. [Google Scholar]
- Bateson P. Preparing offspring for future conditions is adaptive. Trends in Endocrinology and Metabolism. 2008;19:111. doi: 10.1016/j.tem.2008.02.001. author reply 112. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, et al. Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–21. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Belsky J. The development of human reproductive strategies: Progress and prospects. Current Directions in Psychological Science. 2012;21:310–316. [Google Scholar]
- Belsky J. Toward an evo-devo theory of reproductive strategy, health, and longevity: Commentary on Rickard et al. (2014) Perspectives on Psychological Science. 2014;9:16–18. doi: 10.1177/1745691613513471. [DOI] [PubMed] [Google Scholar]
- Belsky J, Shalev I. Contextual adversity, telomere erosion, pubertal development and health: Two models of accelerated aging--or one? Development and Psychopathology. doi: 10.1017/S0954579416000900. in press. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy and evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Block JH. The Child-Rearing Practices Report (CRPR): A set of Q items for the description of parental socialization attitudes and values. Berkeley, CA: Institute of Human Development; 1965. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. I: Exploring the “headwaters” of early life morbidities. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:580–587. doi: 10.1097/00004583-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen YF, Kogan SM, Evans GW, Windle M, et al. Philibert RA. Supportive family environments, genes that confer sensitivity, and allostatic load among rural African American emerging adults: a prospective analysis. Journal of Family Psychology. 2013;27:22–9. doi: 10.1037/a0027829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk LR, Armstrong JM, Park JH, Zahn-Waxler C, Klein MH, Essex MJ. Stability of early identified aggressive victim status in elementary school and associations with later mental health problems and functional impairments. Journal of Abnormal Child Psychology. 2011;39:225–238. doi: 10.1007/s10802-010-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. Adrenarche and the evolution of human life history. Am J Hum Biol. 2006;18:569–89. doi: 10.1002/ajhb.20528. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17149–53. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. Journal of Adolescent Health. 1993;14:190–5. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16:729–37. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm JS. Death, hope, and sex: Life-history theory and the development of reproductive strategies. Current Anthropology. 1993;34:1–24. [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Hitt SF, Rotenstein D. Biopsychological and cognitive differences In children with premature vs on-time adrenarche. Archives of pediatrics & adolescent medicine. 1999b;153(2):137–46. doi: 10.1001/archpedi.153.2.137. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Rose SR, Rotenstein D, Susman EJ, Huang B, Loucks TL, et al. Differences in endocrine parameters and psychopathology in girls with premature adrenarche versus on-time adrenarche. Journal of Pediatric Endocrinology and Metabolism. 2008;21(5):439–48. doi: 10.1515/jpem.2008.21.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva T, Oades RD, Hauffa B, Eggers C. Dehydroepiandrosterone sulphate and corticotropin levels are high in young male patients with condulct disorder. Neuropsychobiology. 2001;43:134–140. doi: 10.1159/000054881. [DOI] [PubMed] [Google Scholar]
- Doom JR, Gunnar MR. Stress physiology and developmental psychopathology: past, present, and future. Development and Psychopathology. 2013;25:1359–73. doi: 10.1017/S0954579413000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M. Beyond allostatic load: rethinking the role of stress in regulating human development. Development and Psychopathology. 2014;26:1–20. doi: 10.1017/S0954579413000849. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Shirtcliff EA. Beyond allostatic load: The stress response system as a mechanism of conditional adaptation. In: Beauchaine TP, Hinshaw SP, editors. Child and Adolescent Psychopathology. 2nd. New York: Wiley; 2013. pp. 251–284. [Google Scholar]
- Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: A longitudinal test of a life history model. Child Development. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary–developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, Essex MJ. Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology. 2011;23:85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Science Signaling. 2012;5:pt12. doi: 10.1126/scisignal.2003580. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Flaherty EG, Thompson R, Dubowitz H, Harvey EM, English DJ, Proctor LJ, Runyan DK. Adverse childhood experiences and child health in early adolescence. JAMA Pediatr. 2013;167:622–9. doi: 10.1001/jamapediatrics.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–33. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Frontiers of Psychiatry. 2013;4:152. doi: 10.3389/fpsyt.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman C, Power C. Child development as a determinant of health across the life course. Current Paediatrics. 2004;14:438–443. [Google Scholar]
- Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women's mental health. Psychology of Women Quarterly. 1995;19:257–285. [Google Scholar]
- Kiecolt-Glaser JK, Jaremka LM, Derry HM, Glaser R. Telomere length: a marker of disease susceptibility? Brain, Behavior and Immunity. 2013;34:29–30. doi: 10.1016/j.bbi.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauser P, Whittle S, Simmons JG, Byrne ML, MUndy LK, Patton GC, et al. Reduced frontal white matter volume in children with early onset ofadrenarche. Psychoneuroendocrinology. 2015;52:111–118. doi: 10.1016/j.psyneuen.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine. 2005;67:846–54. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Mackrell SV, Sheikh HI, Kotelnikova Y, Kryski KR, Jordan PL, Singh SM, Hayden EP. Child temperament and parental depression predict cortisol reactivity to stress in middle childhood. Journal of Abnormal Psychology. 2014;123:106–16. doi: 10.1037/a0035612. [DOI] [PubMed] [Google Scholar]
- McClintock MK, Herdt G. Rethinking puberty: The development of sexual attraction. Current Directions in Psychological Science. 1996;5:178–183. [Google Scholar]
- Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. American Journal of Epidemiology. 2007;166:966–974. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Muthén L, Muthén B. Mplus User's Guide. 7th. Los Angeles: Muthén & Muthén; 1998-2012. [Google Scholar]
- NICHD Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Developmental Psychology. 1999;35:1297–1310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Winter MA, Hunn J, Carnahan J, Pressman EK, Glover V, et al. Caserta MT. Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain, Behavior and Immunity. 2013;32:21–8. doi: 10.1016/j.bbi.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3000–5. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, Moffitt TE. Association between children's experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–5. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Remer T, Shi L, Buyken AE, Maser-Gluth C, Hartmann Mf, Wundy SA. Prepubertal adrenarchal androgens and animal protein intake independently and differentially influence pubertal timing. Journal of Clinical Endocrinology and Metabolism. 2010;95:3002–3009. doi: 10.1210/jc.2009-2583. [DOI] [PubMed] [Google Scholar]
- Rickard IJ, Frankenhuis WE, Nettle D. Why are childhood family factors associated with timing of maturation? A role for internal prediction. Perspectives on Psychological Science. 2014;9:3–15. doi: 10.1177/1745691613513467. [DOI] [PubMed] [Google Scholar]
- Saczawa ME, Graber JA, Brooks-Gunn J, Warren MP. Methodlogical considerations in use of the cortisol/DHEA(S) ratio in adolescent populations. Psychoneuroendorcinology. 2013;38:2815–2819. doi: 10.1016/j.psyneuen.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Negriff S, Susman EJ, Trickett PK. Attenuated hypothalamic-pituitary-adrenal axis functioning predicts accelerated pubertal development in girls 1 year later. Development and Psychopathology. doi: 10.1017/S0954579414000790. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, Jr, Gore S. Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health. 2007;7:30. doi: 10.1186/1471-2458-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I. Early life stress and telomere length: investigating the connection and possible mechanisms: a critical survey of the evidence base, research methodology and basic biology. Bioessays. 2012;34:943–52. doi: 10.1002/bies.201200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Caspi A, Ambler A, Belsky DW, Chapple S, Cohen HJ, et al. Moffitt TE. Perinatal complications and aging indicators by midlife. Pediatrics. 2014;134(5):e1315–e1323. doi: 10.1542/peds.2014-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: a comparison with psychiatric and normal controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1446–51. doi: 10.1097/00004583-200011000-00020. [DOI] [PubMed] [Google Scholar]
- Whittle S, Simmons JG, Byrne ML, Strikwerda-Brown R, Kerestes R, Seal ML, et al. Associations between early adrenarche, affective brain function and mental health in children. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsv014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


