Abstract
Background
Like many countries in Africa, Nigeria is improving the quality and coverage of its cancer surveillance. This work is essential to address this growing category of chronic diseases, but is made difficult by economic, geographic and other challenges.
Purpose
To evaluate the completeness, comparability and diagnostic validity of Nigeria’s cancer registries.
Methods
Completeness was measured using children’s age-specific incidence (ASI) and an established metric based on a modified Poisson distribution with regional comparisons. We used a registry questionnaire as well as percentages of death-certificate-only cases, morphologically verified cases, and case registration errors to examine comparability and diagnostic validity.
Results
Among the children’s results, we found that over half of all cancers were non-Hodgkin lymphoma. There was also evidence of incompleteness. Considering the regional completeness comparisons, we found potential evidence of cancer-specific general incompleteness as well as what appears to be incompleteness due to inability to diagnose specific cancers. We found that registration was generally comparable, with some exceptions. Since autopsies are not common across Nigeria, coding for both them and death-certificate-only cases was also rare. With one exception, registries in our study had high rates of morphological verification of female breast, cervical and prostate cancers.
Conclusions
Nigeria’s registration procedures were generally comparable to each other and to international standards, and we found high rates of morphological verification, suggesting high diagnostic validity. There was, however, evidence of incompleteness.
Keywords: Cancer, Surveillance, Cancer registration, Nigeria, Africa, validity, morphological verification, comparability
1. Introduction
Nigeria is the most populous country in Africa, and is also unique on the continent for having begun cancer registration in 1960, soon after independence [1, 2]. Today, there are at least 24 cancer registries in Nigeria. Of these, one (Ibadan) has had data published in the International Agency for Research on Cancer (IARC) publication Cancer on Five Continents [3, 4, 5, 6].
As in other countries, many Nigerian cancer registries are attempting to become population-based or, if they already consider themselves population-based, they are working on improving case ascertainment within catchment areas. This, however, is difficult because of weak health-sector infrastructure and access, challenging geographies, inadequate case ascertainment in older populations, insufficient diagnostic facilities, and poor collaboration among reporting sources.
In this paper we study comparability, diagnostic validity and completeness of participating Nigerian cancer registries [7, 8, 9, 10]. These qualities are essential for characterizing cancer epidemiology in a given population.
2. Materials and methods
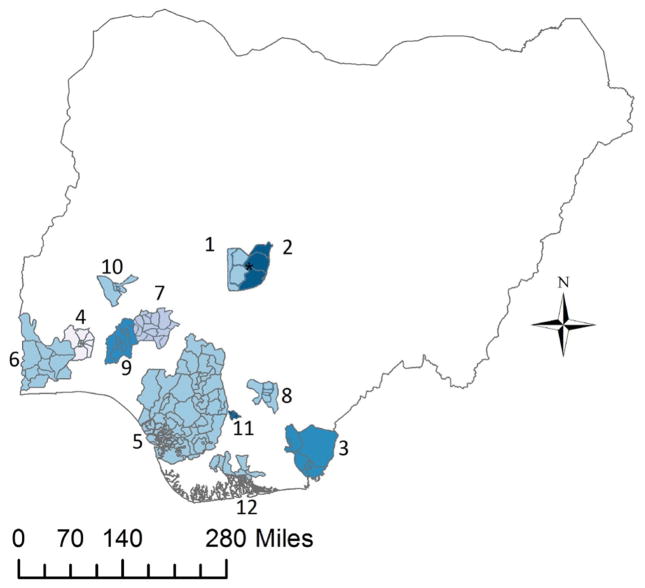
In collaboration with the Institute of Human Virology, Nigeria [11], and the Federal Ministry of Health [12], ethical approval was sought and granted from the University of Minnesota Institutional Review Board [13] and the National Health Research Ethics Committee of Nigeria [14]. Twenty-four Nigerian cancer registries were invited to participate regardless of whether they considered themselves population- or hospital-based. Fourteen of these received permission to participate from their local authorities. Two submitted data with 4.5 and 61.4 cases per year, with more than 88% of the cancer classification codes missing; these were excluded from the study. The remaining 12 registries were: (1) University of Abuja Teaching Hospital Cancer Registry; (2) The Abuja Cancer Registry, National Hospital, Abuja; (3) University of Calabar Teaching Hospital Cancer Registry; (4) The Ibadan Cancer Registry; (5) Professor Olikoye Ransome-Kuti (Midwestern Nigeria) Cancer Registry; (6) Abeokuta Cancer Registry; (7) Cancer Registry, Federal Medical Centre, Ido Ekiti; (8) University of Nigeria Teaching Hospital Cancer Registry; (9) Ife Ijesha Cancer Registry; (10) Ilorin Cancer Registry; (11) Nnewi Cancer Registry; and (12) University of Port Harcourt Teaching Hospital Cancer Registry (see Figure 1).
Figure 1.
Participating cancer registry coverage in Nigeria. (1) * University of Abuja Teaching Hospital Cancer Registry, (2) * The Abuja Cancer Registry, National Hospital, Abuja, (3) University of Calabar Teaching Hospital Cancer Registry, (4) The Ibadan Cancer Registry, (5) Professor Olikoye Ransome-Kuti (Midwestern Nigeria) Cancer Registry, (6) Abeokuta Cancer Registry, (7) Cancer Registry, Federal Medical Centre, Ido Ekiti, (8) University of Nigeria Teaching Hospital Cancer Registry, (9) Ife Ijesha Cancer Registry, (10) Ilorin Cancer Registry, (11) Nnewi Cancer Registry and (12) University of Port Harcourt Teaching Hospital Cancer Registry.
*For this work, these catchment areas were considered together as merged.
2.1. Data
Participating cancer registries submitted data from the years of their choice. Registries were also surveyed with a questionnaire to establish working catchment areas and to gather other pertinent metadata. Data were cleaned and standardized. Some registries included standardized case addresses, which were used for case inclusion in working catchment areas. If registries did not use CanReg4 (case registration software created and maintained by the International Agency for Research on Cancer, IARC) and did not code cases using any internationally recognized pathological classification system, a professional cancer registrar was hired to code these using the International Classification of Diseases for Oncology, third edition (ICD-O-3) [15]. Case data were then harmonized for subsequent calculations of age-standardized cancer rates (ASRs). The professional registrar also recoded a previously ICD-O-3-coded registry (Ibadan) that had been stripped of coding to examine fidelity.
Population data from the 1991 and 2006 censuses came from the National Bureau of Statistics [16] and the National Population Commission of Nigeria [17]. Geographic data came from the Global Administrative Areas project [18].
2.2. Population imputation, age-specific incidence and age-standardized rates
Using the population data at the state and local government area (LGA) level, a linear model was used to impute the population growth from 1989 to 2011 in 5-year age groups [19]. Cancer age-specific incidences (ASIs) by gender were calculated and then used for estimation of the ASRs using the World Standard Population [20].
2.3. Children’s ASIs
Completeness was first examined using childhood cancer rates. Although there are well-documented exceptions [21], these rates are relatively homogeneous across populations compared to adult rates [21, 7, 22]. As such, this measure estimates absolute completeness by examining individual registry rates for all cancers in boys and girls aged 0–4, 5–9 and 10–14 years. These were compared to the 10th and 90th percentiles of the global rates from Volume VIII of Cancer Incidence in Five Continents [7, 22].
2.4. Regional completeness
Registry completeness was examined by comparing registry ASRs for select cancers by measuring the individual registry rates’ distances from the mean rate of the GLOBOCAN 2012 estimated rates from The Gambia, Mali, Guinea Conakry and Niger in terms of a Z2 score distributed in a χ2 distribution using the method established by Bray and Parkin [23, 24, 25].
Registries with scores > 3.84 (corresponding to the probability distribution < 0.05) were considered as exhibiting evidence of incomplete recording [23, 24].
2.5. Comparability
Comparability of registry data was evaluated through: (1) the system used for classification and coding of neoplasms; (2) the definitions of incidence dates; (3) how primary cancers were differentiated from recurrent or metastatic cancer; and (4) the percentages of cancers detected through autopsy [15, 26, 27, 10].
2.6. Diagnostic validity
We used three previously published measures to assess cancer registry validity: (1) percentage of site-and sex-specific cases morphologically verified; (2) percentage of cases registered on the basis of death certificates alone; and (3) percentage of cases with IARC-CHECK errors [10].
2.6.1. Morphological verification
The percentages of morphologically verified lung, colorectum, liver and bile-duct cancers and non-Hodgkins lymphoma in both sexes, as well as female breast, cervical and ovarian cancers and prostate cancer were found by querying cancer registry databases [28]. To examine the percentages of morphologically verified cancers relative to other registries, we used a test statistic based on a binomial model which produces another Z2 score which is interpreted like those discussed above [24].
2.6.2. Death certificate only
Establishing the number of cases registered based on death certificate only (DCO) was done by querying registry CanReg4 databases for basis of diagnosis “death certificate”. The registry with the lowest percentage of DCO cases was chosen as the country reference. All registries were evaluated using ratios and associated 95% confidence intervals (95%CIs), with the registry with the lowest percentage in the denominator.
2.6.3. IARC-CHECK
IARC-CHECK is a software tool developed by the IARC that reviews registry data for internal consistency among variables. For example, it checks data to ensure that only females are recorded as having cervical cancer and that patient birth dates occur before date of incidence. Registry data were put into IARC-CHECK and errors were examined in two ways. First, all registries were checked for errors in all fields except grade (standard method). Second, registries were examined for errors only in fields that they recorded (particular method) in all years submitted. Using the registry with the lowest percentage of errors as the benchmark, other registries were evaluated using ratios and associated 95%CIs with the lowest registry percentage in the denominator [29].
2.7. Software
STATA release 12 was used for data management, rate calculations and statistics, population imputation and matrix and table management [30]. R and its associated lattice package were used to create graphics [31, 32]. ArcGIS was used to develop choropleth maps [33].
3. Results
We found that among the 12 registries in the study, eight considered themselves to be population-based (see Table 1). The University of Abuja Teaching Hospital and the Abuja Cancer Registry at the National Hospital Abuja split coverage of Local Government Areas of the Federal Capitol Territory (FCT) and are considered together as the FCT registries for the purposes of the completeness statistics as the imputed population denominator is necessarily at the state level.
Table 1.
Comparability Table I: Characteristics measured for registry comparability.
| Incidence Date Rule(s) | Years submitted | Population at risk † | Coding | Mult. Primary Rule | Autopsy Code | |
|---|---|---|---|---|---|---|
| Univeristy of Abuja | CC | 2007–2011 | 1,682,123* | ICD-O-3 | IARC | Yes |
| National Hospital Abuja | PD, CC | 2009–2011 | 1,682,123* | ICD-O-3 | IARC | No |
| Port Harcourt | PD, CC | 2007–2011 | 2,789,901 | ICD-O-3 | IARC | Yes |
| Nnewi | PD, CC | 2002–2011 | 568,272 | ICD-O-3 | IARC | No |
| Midwest | PD, CC | 2008–2009 | 8,034,695 | ICD-O-3 | IARC | No |
| Ido Ekiti | PD | 2000–2010 | 2,571,588 | ICD-O-3 | IARC | No |
| Ibadan | CC, PD, D | 2004–2010 | 2,753,067 | ICD-O-3 | IARC | Yes |
| Calabar | PD, CC | 2004–2011 | 1,258,604 | ICD9 | IARC | Yes |
|
| ||||||
| Ile Ife | PD | 1989–2010 | 1,485,519 | ICD-O-3 | IARC | No |
| Abeokuta | PD, CC | 2002–2010 | 2,699,593 | ICD9 | SEER | No |
| University of Nigeria | CC, PD | 2008–2011 | 1,369,136 | ICD-O-3 | IARC | No |
| Ilorin | CC | 2009–2011 | 973,655 | ICD-O-3 | IARC | Yes |
Notes: PD = Pathological Diagnosis, CC = Clinical Consultation, D = Death
The National Hospital Abuja and the University of Abuja jointly cover the Federal Capitol Territory, however for adminstrative reasons, the imputed population for 2010 cannot be separated.
Imputed populations at risk based on registry catchment or estimated catchment areas for 2010. Hospital-based registries below double horizontal lines.
With the exception of the FCT and Ibadan registries, it is not clear whether the registries which considered themselves population-based had superior case-finding abilities or whether their populations were more clearly defined than registries that considered themselves hospital-based. Accordingly, we used the same metrics for assessing completeness in all registries and we asked hospital-based (as well as population-based) registrars and registry directors to estimate their population catchment areas if they were not formally defined (as in the case of a population-based registry).
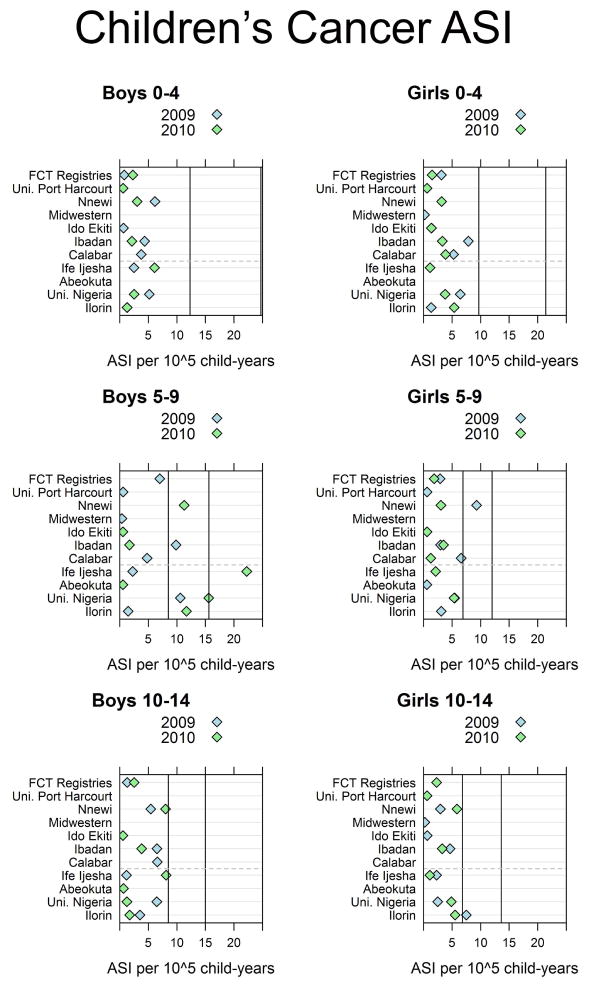
3.1. Children’s age-specific incidences
Results of the boys’ and girls’ ASI comparison studies are found in Figure 2. We found that over half of all children’s cancers were non-Hodgkin lymphoma. Considering the ASI of all cancers in boys 0–4 years old in Figure 2, it appears that in 2009 and 2010 all registries had ASIs well below the world 10th percentile at 12.3 per 105 child-years. The other panels can be appreciated in a similar manner.
Figure 2.
Age-specific incidence of cancer in boys and girls from Nigerian cancer registries compared to the world 10th and 90th percentiles (vertical lines). Registries which consider themselves population-based are above the gray dashed line and hospital-based registries are below it. Federal Capitol Territory (FCT) Registries combine both the University of Abuja and the National Hospital Abuja.
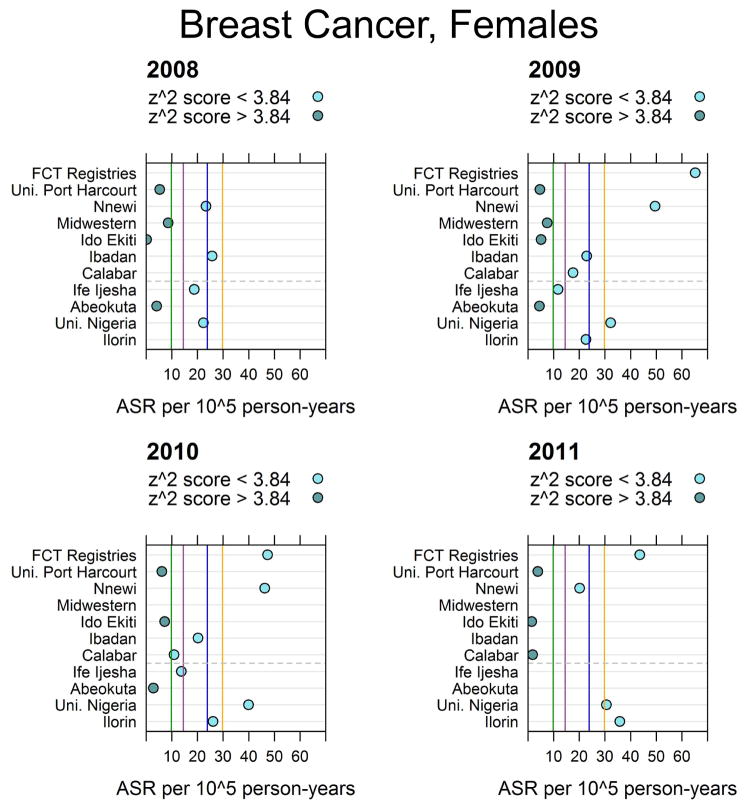
3.2. Regional completeness
The results for the rate completeness statistics for female breast cancer are given in Figure 3. The vertical lines in the figure allow comparison with regional registry rates. The corresponding figures for male and female lung, cervical, prostate, male esophagus, male and female liver, and male and female non-Hodgkin lymphoma are given in the appendix.
Figure 3.
Female breast age-standardized cancer rates (ASRs) in Nigeria. The vertical green, orange, violet and blue lines represent the estimated GLOBOCAN 2012 rates from The Gambia, Mali, Guinea Conakry and Niger respectively [23, 25]. Federal Capitol Territory (FCT) Registries combine both the University of Abuja and the National Hospital Abuja. Registries which consider themselves population-based are above the grey dashed line and hospital-based registries are below it.
3.3. Comparability
We found that of the 12 participating registries, eight had primary and secondary incidence date rules, and one of these also had a tertiary incidence date rule. Among all registries, eight used the date of pathological diagnosis as the incidence date of preference. The other four used the date of clinical consultation. Among the registries with secondary incidence date rules, if pathological diagnosis was used as the first rule, then clinical consultation was used secondarily, or vice versa.
All registries except for the Abeokuta Registry used the IARC rules to record multiple primary cancers; Abeokuta used SEER rules. Although five of the registries reported special coding for cases discovered during autopsy, no actual cases of autopsy-discovered cancer were found in any registry (see Table 1). Tables of select common routinely collected variables at each registry, as well as years of data submission, population at risk, and case sources and frequency of data collection can be found in Tables 2 and 3.
Table 2.
Select routinely collected variables in each registry 2009–2010.
| Sex | Birthdate | Age | Address | Incidence Date | Diagnosis Basis | Primary Site | Histology | Multiple Primary | |
|---|---|---|---|---|---|---|---|---|---|
| Univeristy of Abuja | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| National Hospital Abuja | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Port Harcourt | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Nnewi | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Midwest | ● | ● | ● | ● | ● | ● | |||
| Ido Ekiti | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Ibadan | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Calabar | ● | ● | ● | ● | ● | ● | ● | ● | ● |
|
| |||||||||
| Ile Ife | ● | ● | ● | ● | ●† | ||||
| Abeokuta | ● | ● | ● | ● | |||||
| University of Nigeria | ● | ● | ● | ● | ● | ● | ● | ● | |
| Ilorin | ● | ● | ● | ● | ● | ● | ● | ● | ● |
Although this registry recorded cancer histology, there was no variable for whether a cancer was morphologically verified.
Table 3.
Comparability Table II.
| Base | Case Finding | Data Sources | Frequency | |
|---|---|---|---|---|
| Univeristy of Abuja | P | Active | University, Hospitals of catchment area LGAs | 2X week |
| National Hospital Abuja | P | Active | National Hospital, Asokoro district hospital, Garki Hospital, State House Clinic, Primus Specialist Hospital | Weekly |
| Port Harcourt | P | Active | University of Port Harcourt Teaching Hospital, private hospitals within Port Harcourt and surrounding LGAs | Monthly |
| Nnewi | P | Active | Nnamdi Azikiwe University Teaching Hospital, all hospitals and pathology diagnostic centres in and around Nnewi | Daily |
| Midwest | P | Active | University of Benin Teaching Hospital, Central Hospital - Benin City, Stella Obasanjo Hospital, Ashama Foundation Pathology Laboratory, Central Hospital - Warri, FMC Asaba, Central Hospital - Agbor, Central Hospital - Sapele, Central Hospital - Ughell | Weekly |
| Ido Ekiti | P | Active | FMC Ido-Ekiti, Ekiti State University Teaching Hospital, almost all public and private hospitals in Ekiti state | Weekly |
| Ibadan | P | Active | 39 Government and Private Hospitals and Clinics in LGAs | Monthly |
| Calabar | P | Active | General Hospitals of LGAs, University of Calabar | Weekly |
| Ile Ife | H | Active | Obafemi Awolowo University Teaching Hospitals in Ife, Ilesha and Imesiile and the Adventist Hospital Ile-Ife, some cases from private institutions | Weekly |
| Abeokuta | H | Active | Pathology and Medical Records Departments of the FMC Abeokuta | Monthly |
| University of Nigeria | H | Active | Hospital pathology records, specialist clinics, wards and records department | 4X week |
| Ilorin | H | Active | pathology laboratories, hospital wards, outpatient clinics and mortuary register | Weekly |
Notes: A = Active Case Finding, H = Self Considered Hospital Based, P = Self Considered Population Based, FMC = Federal Medical Centre, LGA = Local Government Area
3.4. Diagnostic validity statistics
3.4.1. Morphological verification
Abeokuta and Ile Ife registries did not record whether cases were morphologically verified. Among the other ten registries, considering the cases of female breast cancer, percentages that were morphologically verified ranged from 40% to 100%. For prostate cancer, the percentages ranged from 37% to 100%. All of the registries with the exception of Nnewi had Z2 scores < 3.84 for all the cancers examined (see Table 4).
Table 4.
Percentage of Cancers Morphologically Verified in Participating Nigerian Cancer Registries.
| National % | UA %i | NHA %i | PH %i | Nn %i | MW %i | IE %i | Ib %i | Cal %i | UNTH %i† | Il %i† | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast | 78.9 | 100 | 93.3 | 100 | 40.0* | 81.1 | 100 | 71.7 | 99.1 | 87.9 | 97.9 |
| Cervix | 79.5 | 100 | 91.3 | 94.5 | 29.5* | 76.3 | 100 | 81.6 | 100 | 73.7 | 100 |
| Ovary | 74.8 | 100 | 87.5 | 100 | 26.5* | 53.4 | 100 | 85.2 | 100 | 79.4 | 100 |
| Prostate | 76.8 | 100 | 94.3 | 100 | 36.6* | 76.4 | 100 | 76.6 | 100 | 88.0 | 95.3 |
| Liver F | 55.6 | 83.3 | 100 | 100 | 40.0 | 26.1 | 100 | 25.3 | 72.7 | 90.9 | 88.9 |
| Liver M | 49.8 | 98.4 | 100 | 100 | 11.8 | 42.9 | 100 | 15.5 | 50.0 | 85.0 | 100 |
| Lung F | 73. | 1 | 71.4 | . | 0 | 50.0 | 100 | 62.5 | 100 | 100 | 100 |
| Lung M | 74.2 | . | 100 | 100 | 33.3 | 100 | 100 | 60.0 | 100 | 95.5 | 100 |
| NHL F | 82.3 | . | 100 | 100 | . | 78.3 | 100 | 73.8 | 75.0 | 90.9 | 100 |
| NHL M | 77.2 | 100 | 94.1 | 100 | . | 80.0 | 100 | 64.0 | 0 | 90.2 | 100 |
| Colorectum F | 79.7 | 100 | 100 | 100 | 33.3* | 95.8 | 100 | 77.5 | 100 | 84.2 | 100 |
| Colorectum M | 73.4 | 100 | 92.0 | 100 | 21.5* | 96.9 | 100 | 71.7 | 100 | 90.8 | 100 |
Cal = Calabar, Ib = Ibadan, IE = Ido Ekiti, Il = Ilorin, MW = Midwest, NHA = National Hospital Abuja, Nn = Nnewi PH = Port Harcourt, UA = University of Abuja, UNTH = University of Nigeria Teaching Hospital.
Astrix (*) indicates that Z2 scores >3.84.
indicates self-identified hospital-based registry
3.4.2. Death certificate only
Only University College Ibadan, the National Hospital Abuja and the Nnewi registries used specific coding for cases registered based exclusively on death certificates. The Ile-Ife registry has a policy of not allowing registration of cases on the exclusive basis of death certificates without a post-mortem examination. This registry did not have a variable for DCO cases. Ibadan had the lowest percentage of these to total cases, and the National Hospital Abuja and Nnewi cancer registries had 7.455 (95%CI 1.670, 33.28) and 38.92 (95%CI 20.69, 73.23) times higher DCO cases than Ibadan respectively (see Table 5).
Table 5.
Death Certificate Only Cases in Nigerian Cancer Registries
| % | Proportion Ratio | 95% | CI | |
|---|---|---|---|---|
| Ibadan | 0.0307 | 1 | . | . |
| National Hospital Abuja | 0.229 | 7.455 | 1.670 | 33.28 |
| Nnewi | 1.20 | 38.92 | 20.69 | 73.23 |
3.4.3. IARC-CHECK
The IARC-CHECK study was carried out in two ways. The first was standardized in that all registries were evaluated on the same criteria regardless of whether or not a given registry collected every item used for evaluation. As such, registries not recording incidence dates for any cases would have errors with every case in this method. The results of the standard method are available on request.
The second method evaluated registries only on the data that they collected. As such, the criteria used to measure the number of errors varies with each registry. These results are presented in Table 6.
Table 6.
Particular IARC Check Errors in Nigerian Cancer Registries
| % | Prop. Ratio | 95% | CI | |
|---|---|---|---|---|
| University of Abuja | 0.424 | 1.695 | 0.707 | 4.062 |
| National Hospital Abuja | 3.28 | 13.14 | 6.638 | 26.01 |
| Port Harcourt | 0.318 | 1.274 | 0.531 | 3.054 |
| Nnewi | 0.630 | 2.519 | 1.227 | 5.171 |
| Midwest | 0.685 | 2.738 | 1.352 | 5.548 |
| Ido Ekiti | 0.250 | 1 | . | . |
| Ibadan | 0.423 | 1.691 | 0.856 | 3.340 |
| Ibadan Recoded | 0.845 | 3.381 | 1.720 | 6.649 |
| Calabar | 75.2 | 300.8 | 153.6 | 589.0 |
|
| ||||
| Ile Ife | 5.78 | 23.13 | 11.80 | 45.32 |
| Abeokuta | 2.97 | 11.88 | 5.882 | 24.00 |
| University of Nigeria | 0.430 | 1.721 | 0.842 | 3.518 |
| Ilorin | 7.31 | 29.26 | 14.83 | 57.71 |
4. Discussion
4.1. Children’s age-specific incidences
The comparisons of registry children’s ASIs to the world 10th and 90th percentiles can be thought as an estimate of the absolute completeness of a registry [21, 7, 22]. Among the self-identified hospital-based registries, it is not surprising to see lower-than-expected children’s ASIs because these registries were not attempting to capture all cases of cancer in their estimated catchment areas. For the population-based registries, there are many potential reasons for the lower rates. It is possible, but unlikely, that rates in these registries were lower because there were fewer cases of childhood cancer in these populations. It is more likely that the lower rates were due to insufficient resources for case-seeking.
Many registries considered themselves population-based, but either covered too large an area to catch all cancers within their population (Midwestern Registry) or had modest-sized catchment areas but did not have the resources or infrastructure to seek out all cases within it (Calabar). These registries, with ASIs lower than the world 10th percentile, likely did not capture all of the cases that occurred within their populations.
Only for boys 5–9 years old were several registries (University of Nigeria, Nnewi, Ilorin and Ibadan) between the global 10th and 90th percentiles. It is possible that this is an effect of age-heaping where many parents of boys in this age range may state that their boy is 5 years old while in fact he is younger. This would increase the cases counted in the 5–9-year-old group. However, this is not seen among girls nor among boys 10–14 years old, making this seem unlikely.
It is not clear why the 2010 rate for Ife Ijesha is so much higher than the 90th global percentile.
We found that more than half of these children’s cancers were non-Hodgkin’s lymphoma which were likely Burkitt’s lymphoma. Data from the Ibadan registry in 1999 showed very high age-standardized rates of Burkitt’s and other non-Hodgkin lymphomas at that time as well [21].
4.2. Regional completeness
Similarly to the findings for children’s cancers, the regional comparison completeness statistics showed evidence of general incompleteness across multiple cancers (see supplementary figures in the appendix). Our average ASRs are, in general, lower than the estimated rates from GLOBOCAN as illustrated by the vertical lines in these figures and Z2 scores. The lower rates in our study in comparison may suggest incompleteness. However, it should be noted that some registries have rates that approach the GLOBOCAN rates and, more importantly, several registries have consistent rates over multiple years. In considering comparisons with registries in the region, it appears that rates are comparable for rarer cancers like female lung cancer and esophageal cancer in males. It appears that rates are notably lower for liver cancer in both males and females as well as for lung cancer in males. The lower rates of liver cancer may reflect difficulty in diagnosing these cancers, while the low rates of lung cancer could reflect genuinely lower rates due, in part, to the strong legislation in force prohibiting smoking in public places as well as the ban on direct and indirect tobacco marketing [34].
Registry completeness is a function of treatment-seeking on the part of the patient- and case-seeking by the cancer registry personnel. While there may be very little that registry personnel can do to increase treatment-seeking behavior and care access, completeness may be improved by changing how case addresses are recorded. In particular, we found that recording case addresses using local government areas (LGAs), Nigeria’s smallest census-based geographic unit, would facilitate more accurate rate calculations.
4.3. Comparability
The majority of Nigerian registries used the date of pathological diagnosis as the preferred incidence date. The Ibadan cancer registry employed a third incidence rule where if neither the date of clinical consultation nor the date of pathological diagnosis were available, they then used the date of death. However, despite the homogeneous training provided to almost all the registries, a third of registries preferred to use the date of clinical consultation. The implications of using one date over another are not clear; however, since for rate calculation purposes only the year is used, such differences, even occurring on annual margins, would not be expected to introduce an important magnitude of variation. The clinical consultations will almost always precede the pathological diagnosis except in cases where the cancer is discovered posthumously [35–38].
All registries, with the exception of Abeokuta, used IARC rules for multiple primary cancers. Abeokuta used the SEER rules. There is evidence that the use of the SEER multiple primary rules results in higher ASRs than those registered using the IARC/IACR multiple primary rule [39]. Our findings did not confirm this (results available on request).
Lastly, although some registries had specific coding for autopsy-discovered cancers, no actual cases were recorded. This may be due in large part to the relative rarity of autopsies in some parts of Nigeria because of taboo [40]. Despite these considerations, it appears that data across the country is relatively comparable for the majority of studied comparability characteristics.
4.4. Diagnostic validity
4.4.1. Morphologically verified
Among the ten registries, only the Nnewi registry showed evidence of low morphological verification for multiple cancers (see Table 4). Excluding this registry, these results lend confidence to an essential component of diagnostic validity in Nigerian cancer registries. While high levels of morphological verification are important from the perspective of validity, very high levels may indicate that a population-based registry is missing cases diagnosed clinically and therefore is incomplete [10].
In general, neither cancer-specific nor all-cancer measures of morphological verification are only infrequently reported in the literature. See references 41–44 for exceptions. This is because these measures require a regional or national context to be interpretable. In West Africa, the registry in Abidjan between 1995 and 1997 has been reported to have had an overall rate of morphological verification of around 80% for all sites combined in males and females [45]. At the lower end of the spectrum, the national registry in The Gambia between 1995 and 1999 had rates of around 18% and 30% for males and females respectively [45]. These numbers increased to around 39 % for each sex after the exclusion of liver cancers.
In Nigeria, in our sample of registries, 79% of breast cancers were morphologically verified, but only 50–56% of liver cancers were morphologically verified. These latter statistics may be due to late presentation of the malignancy resulting in poor surgical candidacy for biopsy and treatment.
4.4.2. Death certificate only
The DCO index has, in the past, been used as an indicator of completeness in population-based registries. In Nigeria, because of the heterogeneity of autopsy practice, many undiagnosed cancers leading to death are likely never discovered. It is therefore not surprising that only three registries had cases coded as registered from DCO. In all three, the percentage of DCO cases ranged from 0.031% to 1.2%. An unfavorable interpretation of this finding would be that registrars are missing many cancer mortalities and the opportunity to record these important mortality statistics. On the other hand, this could be interpreted favorably in the sense that it does not appear that registries are heavily relying on cause of death from death certificates to populate their registries. Evaluation of this particular method of validity is contentious because of wide variation in the use of death certificates, vital statistics, quality of cause of death statements, and other reasons [46]. The most favored approach seems to be comparison to other registries in the region [24,41]. Although the DCO percentage is not commonly reported in the literature, the national registry in The Gambia reports DCO percentages of 6.6% and 3.6% for males and females respectively [45].
4.4.3. IARC-CHECK
The registry with the lowest number of IARC-CHECK errors was Ido Ekiti with only 0.25% of cases with an error. Calabar’s data had the most cases with errors; however, this was likely due to having submitted multiple years before adoption of CanReg4 for case data management. Many of the errors would have presumably been detected and rectified if CanReg4 had been used rather than Microsoft Excel. However, there are other registries (Abeokuta and Midwest) who also did not use CanReg4 and still had low error percentages.
Importantly, case data from Abeokuta, Ile Ife and Midwest were recoded by a professional cancer registrar in the United States. The registrar also coded a version of the Ibadan data with the ICD-O-3 codes removed. Since the percentage of errors was higher in the recoded Ibadan version than the original version, it appears that some error was likely introduced into the coding of Abeokuta, Ile Ife and Midwest data.
4.5. Conclusions
We found consistently lower-than-expected children’s ASIs in almost all registries, which likely reflects inability to capture all the cases in catchment areas. We found that over half of these cases were non-Hodgkin lymphoma.
Considering the regional completeness comparisons, we found several estimations that were consistent across years, but also discovered evidence of cancer-specific general incompleteness as well as what may be incompleteness due to inability to diagnose specific cancers. Among the population-based registries with rigorous case-finding procedures and resources, consistent estimates across years for particular cancers suggested completeness for specific cancers. Rates for male esophageal cancer and female breast cancer were comparable with other registries in the region, while rates for liver cancer in men and women were lower than those of other registries in the region.
Standardizing patient addresses to only the smallest census-based geographic unit of residence in the year before seeking treatment is an essential next step towards improving cancer registry completeness in Nigeria. Similarly, regular reviews of registry case-finding procedures would likely help to improve completeness. The comparability metrics examined demonstrated that Nigerian registries are relatively comparable to each other and to international standards. Although one registry used SEER rules for multiple primaries, the majority used rules from the IARC. Differences in incidence date rules, while not desirable from a uniformity standpoint, may be unlikely to lead to severe estimation differences. Few registries had special coding for autopsy-detected cases or DCO cases.
With the exception of the cancer registry at Nnewi, all the cancer registries had high rates of morphologic verification for the most common cancers, including breast, cervix and prostate cancers. The percentage of morphological verification was lowest for male liver cancer. Although seven of the participating cancer registries considered themselves to be population-based, only four (Ibadan, National Hospital Abuja, University of Abuja and Calabar) are considered to have the experience, personnel and resources necessary to carry out case-finding with the rigor expected of a population-based registry.
4.6. Necessary assumptions & limitations
This work and these conclusions are based on several assumptions. First, there is the problem of defined population and catchment area. Second, while some registries had standardized methods for recording the address of a given case, most registries did not, leading to the assumption that all cases registered should fall into the associated catchment area. Even among Nigerian registries with defined populations, the regular and complete ascertainment of all cases is an ongoing challenge made more difficult by insufficient resources, personnel, geography and strife.
Supplementary Material
Acknowledgments
This work would not have been possible without the assistance of Dr Patience Osinubi at the Federal Ministry of Health in securing permissions. We would also like to thank Dr Titilola Akinremi, Professor Ima-Obong A. Ekanem, Dr Theresa Ize Otu, Dr Olagoke Erinomo, Professor Babatunde J Olasode, Dr E.A.O Afolayan, Mrs Agnes O. Fabowale, CCR, Mr B.B. Etim-Okon, Mr Henry Kumai, Miss Gloria Osubor, Ms Ovo Agofure, Adedeji Adeyanju, Mr Ojo Adebayo, Mrs Elizabeth Udeh, Mr O. Olajide, Dr R. Saidu, Miss Gift Oviemuno, Mr Akin Ladipo, Mr Joel Bassey, Mr Jerome Seyil, Mr Ubasi Agada Batholom, Mr Ilugbemi Segun, Mr Kehinde Oladele, Miss Ann Okorafor, Mr G.T. Ayilara, Ms G.C. Oyeka and Mr Dick Benson.
This work was supported by the University of Minnesota Medical Scientist Training Program (NIH MSTP grant T32 GM008244), an Interdisciplinary Doctoral Fellowship from the Minnesota Population Center and the Graduate School, the Hawley Award and the Wilfred Wetzel Fellowship. Drs Adebamowo, Jedy-Agba and Oga were supported by the IHV-UM Capacity Development for Research into AIDS Associated Malignancies (NIH/NCID43CA153792-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
B.J.S. al-Haddad, Medical Scientist Training Program, University of Minnesota, 420 Delaware St. SE, B681 Mayo Building, MMC 293, Minneapolis, MN, 55414 USA.
Elima Jedy-Agba, Institute of Human Virology, Nigeria, Pent House, Maina Court, Plot 252, Herbert Macaulay Way, Central Business District, P.O. Box 9396, Garki, Abuja, Nigeria.
Emmanuel Oga, Institute of Human Virology, Nigeria, Pent House, Maina Court, Plot 252, Herbert Macaulay Way, Central Business District, P.O. Box 9396, Garki, Abuja, Nigeria.
E.R. Ezeome, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu, Enugu State, Nigeria.
Christopher C. Obiorah, University of Port Harcourt Teaching Hospital, East-West Road, Port Harcourt, Rivers State, Nigeria.
Michael Okobia, Professor Olikoye Ransome-Kuti (Midwestern Nigeria) Cancer Registry, Department of Surgery, University of Benin Teaching Hospital, P.M.B. 1111, Benin City, Edo State, Nigeria.
J. Olufemi Ogunbiyi, Ibadan Cancer Registry, Department of Pathology, University College Hospital, P.M.B. 5116, Ibadan, Nigeria.
Cornelius Ozobia Ukah, Nnewi Cancer Registry, Nnamdi Azikiwe University Teaching Hospital, PMB 5025, Nnewi, Anambra State, Nigeria.
Abidemi Omonisi, Ife Ijesha Cancer Registry, Obafemi Awolowo University Teaching Hospitals. PO Box 1923, Ile Ife, Osun State, Nigeria.
A.M.E. Nwofor, Nnewi Cancer Registry, Nnamdi Azikiwe University Teaching Hospital, PMB 5025, Nnewi, Anambra State, Nigeria.
Festus Igbinoba, Abuja Cancer Registry, National Hospital, Abuja, Plot 132, Central District (Phase 11), P.M.B. 425, Garki-Abuja, Federal Capital Territory, Nigeria.
Clement Adebamowo, Institute of Human Virology, Nigeria, Pent House, Maina Court, Plot 252, Herbert Macaulay Way, Central Business District, P.O. Box 9396, Garki, Abuja, Nigeria.
References
- 1.Falola T, Heaton MM. A History of Nigeria. Cambridge University Press; New York: 2008. [Google Scholar]
- 2.Parkin DM, Ferlay J, Hamdi-Cherif M, Sitas F, Thomas JO, Wabinga H, Whelan SL. Cancer in Africa: Epidemiology and Prevention. 2003 [Google Scholar]
- 3.Doll R, Payne P, Waterhouse JAH. Cancer Incidence in Five Continents. 1966 http://ci5.iarc.fr/CI5i-ix/ci5i-ix.htm.
- 4.Doll R, Muir CS, Waterhouse JAH. Cancer Incidence in Five Continents. 1970 http://ci5.iarc.fr/CI5i-ix/ci5i-ix.htm.
- 5.Waterhouse J, Muir CS, Correa P, Powell J. Cancer Incidence in Five Continents. 1976 http://ci5.iarc.fr/CI5i-ix/ci5i-ix.htm.
- 6.IACR. IACR Members - Africa, Tech rep. 2011 http://www.iacr.com.fr/Africactoct09.pdf.
- 7.Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. European journal of cancer (Oxford, England: 1990) 2009;45(5):756–64. doi: 10.1016/j.ejca.2008.11.033. http://www.ncbi.nlm.nih.gov/pubmed/19128954. [DOI] [PubMed] [Google Scholar]
- 8.Curado MP, Voti L, Sortino-Rachou AM. Cancer registration data and quality indicators in low and middle income countries: their interpretation and potential use for the improvement of cancer care. Cancer Causes and Control. 2009;20:751–756. doi: 10.1007/s10552-008-9288-5. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM, Wabinga H, Nambooze S. Completeness in an African cancer registry. Cancer causes & control: CCC. 2001;12(2):147–52. doi: 10.1023/a:1008966225984. http://www.ncbi.nlm.nih.gov/pubmed/11246843. [DOI] [PubMed] [Google Scholar]
- 10.Bray F, Parkin DM. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. European journal of cancer (Oxford, England: 1990) 2009;45(5):747–55. doi: 10.1016/j.ejca.2008.11.032. http://www.ncbi.nlm.nih.gov/pubmed/19117750. [DOI] [PubMed] [Google Scholar]
- 11.N. Institute of Human Virology. About Institute of Human Virology. Nigeria: 2009. http://ihvnigeria.org/ihvnweb/webnew/index.php/about-ihvn/introduction.html. [Google Scholar]
- 12.Federal Ministry of Health. Federal Ministry of Health; 2012. http://www.fmh.gov.ng/ [Google Scholar]
- 13.Regents of the University of Minnesota. Institutional Review Board: HRPP: OVPR. University of Minnesota; 2012. http://www.irb.umn.edu/ [Google Scholar]
- 14.Federal Ministry of Health: Department of Health Planning and Research, National Code of Health Research Ethics Committee of Nigeria (NHREC) 2007 http://www.nhrec.net.
- 15.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. 3. International Agency for Research on Cancer; Lyon, France: 2000. [Google Scholar]
- 16.National Bureau of Statistics. National Bureau of Statistics; 2012. http://www.nigerianstat.gov.ng/ [Google Scholar]
- 17.National Population Commission of Nigeria. 2006 Census Priority Tables. 2011:1–15. http://www.population.gov.ng/
- 18.Global Administrative Areas. http://www.gadm.org/about.
- 19.Siegel JS, Swanson DA. The Methods and Materials of Demography. 2. Elsevier; San Diego: 2004. [Google Scholar]
- 20.Bray F. Age Standardization. In: Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer Incidence in Five Continents. VIII. IARC Scientific Publications; Lyon, France: 2002. pp. 87–89. [Google Scholar]
- 21.Stiller CA, Parkin DM. Geographic and ethnic variations in the incidence of childhood cancer. British Medical Bulletin. 1996;52(2):682–703. doi: 10.1093/oxfordjournals.bmb.a011577. [DOI] [PubMed] [Google Scholar]
- 22.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. 2002 [Google Scholar]
- 23.Parkin DM, Plummer M. Comparability and quality of data. In: Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer incidence in five continents. VIII. IARC; Lyon: 2002. pp. 57–73. [Google Scholar]
- 24.Bray F, Parkin DM. Evaluation of data quality in cancer registry: Principles and methods. Part I: Comparability, validity and timeliness. European Journal of Cancer. 2009;(45):747–755. doi: 10.1016/j.ejca.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] 2013 http://globocan.iarc.fr.
- 26.Schouten L, Jager J, van den Brandt P. Quality of cancer registry data: a comparison of data provided by clinicians with those of registration personnel. British Journal of Cancer. 1993;68(5):974–977. doi: 10.1038/bjc.1993.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson C, Adamo M, editors. SEER Program Coding and Staging Manual 2007. 07. National Institutes of Health; 2008. [Google Scholar]
- 28.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008: cancer incidence and mortality worldwide. 2010 doi: 10.1002/ijc.25516. http://globocan.iarc.fr. [DOI] [PubMed]
- 29.Ferlay J, Burkhard C, Whelan S, Parkin DM. Tech rep. Lyon, France: 2005. Check and Conversion Programs for Cancer Registries (IARC/IACR Tools for Cancer Registries) http://www.iacr.com.fr/TechRep42-MPrules.pdf. [Google Scholar]
- 30.StataCorp. Stata Statistical Software: Release. 2011;12 [Google Scholar]
- 31.R Core Team, R. A language and environment for statistical computing. 2012 http://www.r-project.org/
- 32.Sarkar D. Lattice: Multivariate Data Visualization with R. 2008. Springer; New York: http://lmdvr.r-forge.r-project.org. [Google Scholar]
- 33.ESRI. ArcGIS Desktop: Release. 2011;10 [Google Scholar]
- 34.Federal Government of Nigeria, National Tobacco Control Bill. 2009 [Google Scholar]
- 35.Adamo M, Johnson C, Ruhl J, Dickie L, editors. 2010 SEER Program Coding and Staging Manual. National Cancer Institute; Bethesda, Maryland: seer.cancer.gov/manuals/2010/SPCSM2010maindoc.pdf. [Google Scholar]
- 36.IACR. IARC, Cancer Incidence in Five Continents Volume X: Call for Data. 2011 www.iacr.com.fr/CI5/CI5X call for data-EN.pdf.
- 37.Jensen O, Parkin D, MacLennan R, Muir C, Skeet R, editors. Cancer Registration: Principles and Methods. IARC Scientific Publications; Lyon, France: 1991. www.iarc.fr/en/publications/pdfs-online/epi/sp95/SP95.pdf. [Google Scholar]
- 38.European Network of Cancer Registries. Recommendations for coding Incidence Date. 1999 www.encr.com.fr/incideng.pdf.
- 39.Hotes JL, Ellison LF, Howe HL, Friesen I, Kohler B. Variation in breast cancer counts using SEER and IARC multiple primary coding rules. Cancer Causes & Control. 2004;15(2):185–191. doi: 10.1023/B:CACO.0000019505.97836.7d. [DOI] [PubMed] [Google Scholar]
- 40.Oluwasola O, Fawole O, Otegbayo A, Ogun G, Adebamowo C, Bamigboye A. The autopsy: knowledge, attitude, and perceptions of doctors and relatives of the deceased. Archives of Pathology and Laboratory Medicine. 2009;133(1):78–82. doi: 10.5858/133.1.78. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Zheng R, Zhang S, Li N, Zhao P, Li G, Wu L, He J. Report of incidence and mortality in china (sic) cancer registries, 2008. Chinese Journal of Cancer Research. 2012;24(3):171–180. doi: 10.1007/s11670-012-0171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tumino R, Ferretti S. Quality and completeness indices. Epidemiologia e prevenzione. 2004;28(2 Supplement):17–21. [PubMed] [Google Scholar]
- 43.Stang A, Parkin D, Ferlay J, Jöckel K. International uveal melanoma incidence trends in view of a decreasing proportion of morphological verification. International Journal of Cancer. 2005;114(1):114–23. doi: 10.1002/ijc.20690. [DOI] [PubMed] [Google Scholar]
- 44.Enerly E, Bray F, Mellem C, Hansen BT, Kjølberg G, Dahl1 T, Johannesen TBr, Nygaard M. Quality assessment of the registration of vulvar and vaginal premalignant lesions at the Cancer Registry of Norway. Acta Oncologica. 2012;51(1):45–50. doi: 10.3109/0284186X.2011.624545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimakawa Y, Bah E, Wild CP, Hall AJ. Evaluation of data quality at the Gambia National Cancer Registry. International Journal of Cancer. 2012;132(3):658–665. doi: 10.1002/ijc.27646. [DOI] [PubMed] [Google Scholar]
- 46.Brenner H. Limitations of the death certificate only index as a measure of incompleteness of cancer registration. British Journal of Cancer. 1996;74(7):1155. doi: 10.1038/bjc.1995.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.