Abstract
The skeleton, populated by large numbers of osteoblasts and long-lived osteocytes, requires a constant supply of energy-rich molecules to fuel the synthesis, deposition, and mineralization of bone matrix during bone modeling and remodeling. When these energetic demands are not met, bone acquisition is suppressed. Recent findings suggest that key developmental signals emanating from WNT- low-density lipoprotein-related receptor 5 and Hypoxia-inducible factor pathways impact osteoblast bioenergetics to accommodate the energy requirements for bone cells to fulfill their function. In vivo studies in several mutant mouse strains have confirmed a link between bone cells and global metabolism, ultimately leading to the identification of hormonal interactions between the skeleton and other tissues. The hormones insulin and leptin affect postnatal bone acquisition, while osteocalcin produced by the osteoblast in turn stimulates insulin secretion by the pancreas. These observations have prompted additional questions regarding the nature of the mechanisms of fuel sensing and processing in the osteoblast and their contribution to overall energy utilization and homeostasis. Answers to such questions should advance our understanding of metabolic diseases and may ultimately improve management of affected patients. In this review we highlight recent studies in this field and offer a perspective on the evolutionary implications of bone as a metabolic endocrine organ.
Keywords: genetic mouse models, insulin, leptin, osteoblasts
Introduction
The endochondral skeleton evolved over 300 million years ago during the late Devonian period when the first animals colonized land. Survival of terrestrial vertebrates in this new environment required wholesale adaptations in their anatomy and physiology for respiration, osmoregulation, and reproduction. In particular, a much larger and energy-expensive musculoskeletal system was needed for ambulation against increased gravitational forces on land. In addition, terrestrial animals needed new strategies to regulate extracellular mineral ion levels and energy homeostasis in an environment with limited minerals and nutrients. The parathyroid gland emerged at this evolutionary stage [1]; this enabled homeostatic control of extracellular calcium and phosphate concentrations, a feature that also required the evolution of more specialized bone cells capable of resorbing bone minerals. To more efficiently manage fuel homeostasis, skeletal muscle and adipose tissue evolved into robust ‘factories’ for acquiring and storing fuel, which in turn required new endocrine networks that could accurately inventory and report fuel status among these tissues. It is clear that bone is also exquisitely sensitive to changes in nutrient status as longitudinal bone growth ceases and osteopenia develops in cases of extreme energy deprivation such as anorexia nervosa [2, 3]. Recent findings have begun to identify new pathways that link bone cells to energy metabolism and reproduction. These physiological processes are controlled in a classical endocrine fashion through bone-derived hormones that act on distal target organs and are subject to feed-forward and negative-feedback control. From this perspective, we discuss historical and current studies of bioenergetics and fuel metabolism in bone (with a focus on osteoblasts and osteocytes) and attempt to fit the results into a working model that rationalizes bone as a metabolic organ.
Overview of bone development and remodeling
During endochondral bone development, osteoblasts differentiate from mesenchymal precursors at the peripheral edge of the cartilage anlagen (perichondrium). Osteoblast differentiation and lineage allocation is tightly coordinated by the sequential activities of transcriptional regulators (e.g. Runx2 and osterix) and morphogens (e.g. sonic hedgehog and Wnts) (for details, see [4, 5]). Some periosteal osteoblasts [6, 7] migrate into the hypertrophic region of the growth plate under the influence of vascular endothelial growth factor, which is upregulated due to hypoxia-driven Hypoxia-inducible fator -1 (HIF-1) signaling. The osteoblasts remaining at the periosteum form the template for cortical bone. At the distal ends of long bones and the metaphysis, hypertrophic chondrocytes are resorbed by osteochondroclasts and new bone is formed giving rise to trabecular (cancellous) bone inside the cortical bone shell.
Throughout postnatal life, bone is remodeled via the replacement of old bone by new tissue such that a complete adult skeleton is regenerated every 10 years [8]. This process is achieved by the coordinated activities of three bone cell types: osteoclasts, osteoblasts, and osteocytes. Osteoclasts, which are derived from circulating hematopoietic monocyte precursors, resorb bone. The resorption process in turn generates signals, which recruit stromal-derived osteoblasts to the freshly excavated site where they secrete and mineralize the bone matrix [9]. A portion of mature osteoblasts differentiate into osteocytes and become entrapped in lacunae within the bone matrix. These osteocytes are connected to other osteocytes, osteoblasts, and osteoclasts by an extensive network of neuron-like cell projections that form the canalicular network [10]. The precise function of osteocytes is still unclear, but they are widely assumed to have a role in mineralization and transduction of mechanical signals into anabolic events. Osteocytes are indeed the most abundant type of bone cell, with approximately 10,000 cells per mm3 in humans and an estimated lifespan of 10–20 years. In addition to their homotypic interactions through the canalicular network, osteocytes also contact blood vessels, nerves, and bone-lining cells on trabecular and cortical bone [10]. Considering their long lifespan and interconnectivity, osteocytes are particularly well suited to sense changes in circulating mineral levels, energy status and the general ‘health’ of the skeleton. In accordance with this notion, the osteocyte is the primary source of the phosphate-regulating hormone fibroblast growth factor (FGF)23 and osteocalcin; the latter has been shown to regulate insulin production by the pancreas (see below).
Energy substrate utilization by bone cells
Terrestrial animals regulate the flux of energy sources in accordance with the changing metabolic rates that result from variation in physiological conditions (for review, see [11]). For instance, exercising mammals are able to maintain a balanced ratio of lipids to carbohydrates at a given maximal oxygen consumption. During highly aerobic activity, skeletal muscle uses stored intramuscular fuels because energy supply from the circulation is constrained by trans-sarcolemmal transfer. Conversely, energy-dense lipids can be more effectively compartmentalized for storage and are ideal fuel substrates for prolonged moderate-intensity work. The cellular and molecular mechanisms that function to balance global fuel selection and utilization have been extensively characterized in skeletal muscle and adipose and other tissues, but analogous data are very limited for bone (Fig. 1).
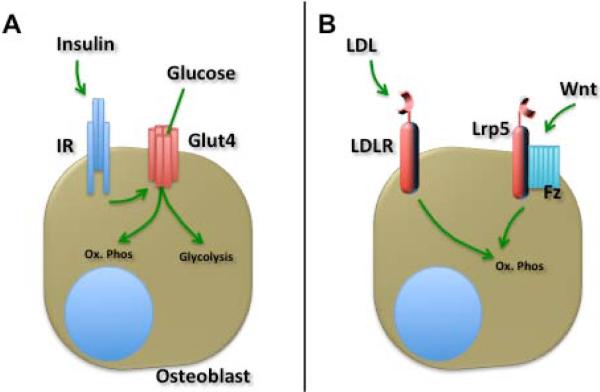
Fig. 1.
Metabolic flexibility of osteoblasts. Much remains to be learned about the metabolic requirements of bone-forming osteoblast. Firstly (A), insulin and insulin receptor (IR) signaling stimulates the glucose transporter (Glut)4-mediated uptake of glucose, which can be utilized via oxidative phosphorylation (Ox.Phos) or aerobic glycolysis. Secondly (B), the Low-density lipoprotein receptor (LDLR) facilitates the uptake of a significant fraction of postprandial lipoproteins (LDL) by bone. Free fatty acids and triglycerides that can then be oxidized to generate ATP. Our recent findings suggest that activation of the Frizzled (Fz)-Lrp5 signaling complex by Wnt-ligands favors lipid oxidation in the osteoblast.
Glucose
Glucose is the primary energy substrate for most cells. Oxidation of glucose via oxidative phosphorylation or glycolysis provides the ATP needed to maintain cell homeostasis, cell growth, and differentiation. Glucose uptake and transportation are mediated by a family of high-affinity glucose transporter (GLUT) proteins. Three GLUTs (GLUT1, GLUT2, and GLUT4) are expressed by immature osteoblasts, but GLUT4 expression uniquely increases as osteoblasts mature [12, 13]. Parathyroid hormone, triiodothyronine, insulin-like growth factor-1, and insulin stimulate glucose transport in bone cells [14-16]; we have recently shown that insulin-dependent glucose uptake in primary mouse osteoblasts requires GLUT4 [13].
The findings from early studies of fuel metabolism using ill-defined cell populations can be summarized as follows: (i) osteoblasts express the enzymatic requirements for both aerobic and anaerobic glycolysis; (ii) glucose metabolism by osteoblasts is primarily glycolytic [17, 18] (i.e. glucose is converted to lactate); and (iii) both insulin and parathyroid hormone affect glucose oxidation [19-21]. Two more recent studies [22, 23] using more modern methods to measure osteoblast bioenergetics over the course of differentiation in vitro have yielded similar results. At early time points when cells are actively proliferating, cellular respiration increases and oxidative phosphorylation predominates. As osteoblasts begin to mineralize, ATP levels peak in association with the accumulation of abundant mitochondria with high-transmembrane-potentials. At later stages when mineralization is complete, osteoblasts convert to glycolytic pathways to maintain ATP production. These findings indicate that osteoblasts adapt their bioenergetic machinery in order to adapt to transient challenges such as changes in either oxygen supply to bone or increases in the acute and transient demands for energy. The results of other studies suggest the intriguing possibility that this glycolytic switch is coupled to key osteoblast developmental signaling pathways. Thus, it has recently been reported that signals downstream of WNT-LRP5 converge on mammalian target of rapamycin (mTOR) complex 2 and AKT pathways to facilitate aerobic glycolysis during WNT-induced osteoblast differentiation [24]. In addition, hypoxia and HIF-1 generated signals induce aerobic glycolysis during osteoblast differentiation by activating glycolytic enzymes, including pyruvate dehydrogenase kinase 1, lactate dehydrogenase A, and hexokinase II [25]. If indeed there is a functional linkage between osteoblast developmental signals and bioenergetics, this might contribute to the metabolic disturbances observed in mice lacking genes involved in osteoblast development. Conversely, defects in bone development might be expected in mice lacking key metabolic proteins in osteoblasts.
Lipids
Lipid molecules represent another important fuel for metabolically active tissues. Lipid molecules are delivered to cells in the form of either free fatty acids that are taken up by cell surface transporters [26] or lipoprotein particles that are bound by LDL receptor family members to facilitate endocytosis [27]. Once inside the cell, the initial and rate setting enzyme, CPT1, generates acyl-carnitines that can traverse the mitochondrial membranes via specific transporters. Once inside the mitochondrial matrix, CPT2 generates acyl-CoAs from acyl-carnitines to initiate the beta-oxidation of long chain fatty acids to acetyl-CoA which enters the citric acid cycle and is used to produce NADH and FADH2 for oxidative phosphorylation.
The factors and metabolic pathways that mediate lipid uptake and regulate its oxidation in osteoblasts are still poorly understood. Osteoblasts can oxidize fatty acids, which potentially account for 40–80% of the energy needs of these cells [28]. In support of this, a significant fraction of postprandial lipoproteins is taken up by bone (approximately one-fifth as much as by liver, but more than heart and muscle) [29], and osteoblasts produce apolipoprotein E [30, 31]. Likewise, osteoblasts grown in the absence of lipoproteins exhibit severe proliferation defects that are not rescued by growth factor stimulation [32], and enhancing lipid oxidation capacity is associated with an increase in collagen synthesis [33]. Such observations are consistent with the perceived need for the osteoblast to maintain a high level of ATP to support matrix production [34] as lipid oxidation yields twice as much energy as oxidation of glucose.
Based on very recent work [RCR, 2014 (unpublished data)], we have implicated Wnt-LRP5 signaling in lipid oxidation by osteoblasts. We observed that mice lacking the LRP5 specifically in osteoblasts exhibited the anticipated deficits in bone mass, but also developed increased peripheral fat with a corresponding reduction in whole-body energy expenditure. Surprisingly, mice lacking the closely related LRP6 co-receptor exhibited reduced bone volume but did not become fat. Subsequent studies revealed that WNT signaling through LRP5 (but not LRP6) was required for normal oxidation of fatty acids. This finding appears to represent another example in which key osteoblast developmental signals simultaneously alter metabolic machinery to fulfill the bioenergetic needs of differentiated osteoblast functions. The concept that LRP5 might have a role in fuel metabolism is further supported by evidence linking polymorphisms in human LRP5 gene (A1330V, Q89R) with increased total and LDL cholesterol levels, hypertension, increased body mass index, and obesity [35-39]. Similarly, mice globally deficient in Lrp5 are glucose intolerant and exhibit increased plasma cholesterol levels when fed a high-fat diet; this phenotype results from reduced clearance of chylomicron remnants from the circulation [40, 41].
Amino acids
Given the critical role of protein as a structural component of bone, it is surprising that most studies have focused on the detrimental effects of high-protein diets on bone. Ingestion of large amounts of sulfur-rich amino acids disturbs acid–base balance [42]. However, recent studies have highlighted the importance of essential amino acids as signals that cause changes in the levels of hormones that modulate digestion, absorption, satiety and appetite, nutrient disposal, metabolic rate, and fuel selection. Identifying amino acids as signals in this way is analogous to the role of glucose in signaling the state of whole-body carbohydrate stores. Certain amino acids are now known to play important nutrient-sensing roles involving the mTOR-mediated signaling pathway [43]. A possible association between amino acid transport and osteoblast-dependent collagen synthesis was identified in mice homozygous for ablation of the transcription factor ATF4; amino acid transporter expression is defective in these mice, which exhibit delayed skeletal development and high levels of fetal wastage. It is interesting that high-protein diets normalized the phenotype and promoted survival [44, 45]. The amino acid L-type transport system, responsible for sodium-independent transport of neutral amino acids, is expressed in human osteoblasts [46].
Endocrine-integration of bone and global metabolism
Until recently, studies of bone as an endocrine organ have focused exclusively on its role in mineral ion homeostasis. Evidence that bone might contribute to global energy homeostasis was first reported by Ducy and colleagues [47], who demonstrated that the adipokine leptin controlled bone mass by acting in the brain (see below). At the time, bone scientists were puzzled by this finding , but in subsequent studies additional factors produced by adipocytes and osteoblasts have been identified that appear to function interactively to coordinate global energy balance.
Leptin
Leptin is produced by adipocytes and regulates food intake by stimulating its receptor in the hypothalamus to suppress satiety signaling. Genetic studies in mice lacking either leptin (ob/ob) or its receptor (db/db) have shown that these animals develop high-bone mass due to a massive increase in bone formation [47]. This phenotype is evident despite the fact that these mice are hypogonadic, a condition known to increase bone resorption and decrease bone mass. Mice deficient in leptin exhibit a metabolic phenotype characterized by increased appetite, obesity, and increased bone formation [47]. Leptin exerts these effects indirectly by activating sympathetic nerves whose efferent outputs target β2-adrenergic receptors on osteoblasts [48] to regulate the proliferation and differentiation of these cells. Upregulation of sympathetic tone by leptin has also been shown to indirectly inhibit insulin secretion by inhibiting the production of osteocalcin, a newly recognized hormone that controls insulin secretion [49]. Thus, leptin signaling in the brain negatively impacts bone through separate pathways that both inhibit bone formation and increase bone resorption. These studies have firmly established bone as part of an endocrine loop linking adipocytes, neurons, and osteoblasts to energy metabolism.
Osteocalcin
Another factor implicated in endocrine control of metabolism by the skeleton is osteocalcin (Fig. 2). Osteocalcin is synthesized and secreted by mature, mineralizing osteoblasts and osteocytes and is widely used as a marker of osteoblast activity. The protein undergoes post-translational γ-carboxylation, which enhances its affinity for hydroxyapatite [50], but its function in bone is not fully understood. To study the function of osteocalcin, Ducy and co-workers characterized a mouse lacking osteocalcin that was described as having over-mineralized bone [51]. This mouse also exhibited other characteristics that were not initially described by these authors, including peripheral adiposity, which was unexpected as osteocalcin was thought to function exclusively in bone [52]. However, in light of new information about the skeletal actions of leptin, these investigators re-examined the osteocalcin-deficient mouse to search for other osteoblast-derived proteins that might explain dysfunctional glucose homeostasis/regulation [52]. In a series of elegant experiments, they identified a new osteoblast-specific gene Esp. This gene encoded a tyrosine phosphatase termed OST-PTP, which altered osteocalcin production and post-translational γ-carboxylation. Deletion of Esp (Esp−/−) produced a metabolic phenotype exactly opposite to that of the osteocalcin knockout mice. Additional studies by this group established that OST-PTP dephosphorylates the insulin receptor, and its genetic removal leads to increased insulin signaling and osteocalcin production by osteoblasts as well as enhanced liberation of undercarboxylated osteocalcin from the bone matrix by osteoclasts [53]. This undercarboxylated form of osteocalcin promotes pancreatic β-cell proliferation and insulin production, and stimulates production of the insulin-sensitizing adipokine adiponectin [54-56].
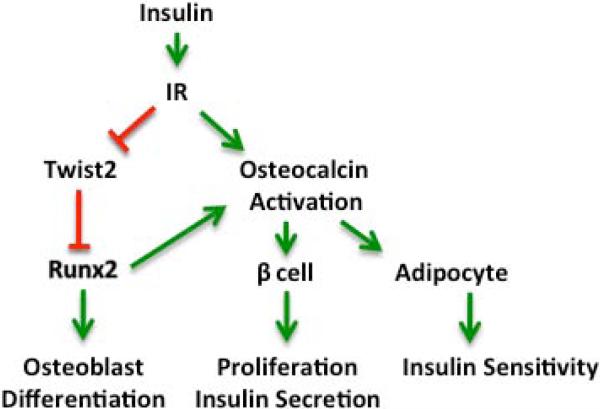
Fig. 2.
Osteoblast differentiation and osteocalcin activation in response to insulin. The activation of the insulin receptor (IR) expressed by osteoblasts stimulates both osteoblast differentiation and the activation of the hormone osteocalcin. By triggering the downregulation of Twist2, insulin signaling relieves suppression on the transcription factor Runx2, required for osteoblast differentiation. Once activated, undercarboxylated osteocalcin stimulates proliferation and insulin secretion by the pancreatic β-cell and increases insulin sensitivity in adipocytes and other target cells for insulin. Red lines indicate inhibitory interactions. Green arrows indicate activating interactions.
In independent studies, we serendipitously came across this same insulin–osteocalcin pathway as we analyzed mice lacking the insulin receptor in osteoblasts [57]. We noticed that these mice had reduced bone formation due to impairments in osteoblast differentiation, but also displayed a metabolic profile similar to that of the osteocalcin-deficient mice of Karsenty's group; these animals developed progressive peripheral adiposity, with insulin resistance and glucose intolerance, and low circulating levels of undercarboxylated osteocalcin [57]. It eventually became apparent that osteocalcin might represent the common factor linking insulin signaling in the osteoblast to whole-body metabolic abnormalities. This idea was tested directly by infusing osteocalcin into insulin receptor-deficient mutant mice, which improved insulin sensitivity. The results from these two independent but highly complementary studies [53, 57], provide strong evidence for a novel endocrine loop in which insulin signaling in osteoblasts regulates the production and bioavailability of osteocalcin, which in turn acts in an endocrine manner to regulate pancreatic insulin secretion and peripheral insulin responsiveness.
It is well established that patients who receive high doses of glucocorticoids have reduced bone formation and frequently develop glucose intolerance, insulin resistance, diabetes, and dyslipidemia. A recent study by Brennan-Speranza et al. suggest that the osteoblast and osteocalcin are involved in the development to these metabolic disturbances [58]. These authors demonstrated that mice overexpressing the glucocorticoid-inactivating enzyme 11β-hydroxysteroid dehydrogenase type 2 in osteoblasts were resistant to glucocorticoid-induced bone loss and do not develop the metabolic abnormalities seen in similarly treated wild-type mice [58]. Moreover, the profound reductions in undercarboxylated osteocalcin following corticosteroid treatment in the wild-type mice did not occur in transgenic mice with disrupted glucocorticoid signaling in osteoblasts. Further, ectopic re-expression of osteocalcin from a liver transgene greatly improved the metabolic abnormalities induced by glucocorticoids in wild-type mice. These observations strongly suggest that suppression of osteocalcin production is a major mediator of the adverse effect of glucocorticoids on energy metabolism [59].
Direct evidence for the role of osteocalcin in glucose metabolism in humans is still lacking, but the results of a number of cross-sectional studies show that total and/or undercarboxylated osteocalcin levels are negatively associated with body mass index, fat mass, insulin secretion, and insulin resistance [60-62]. In the most direct attempt to examine the effects of insulin on osteocalcin and bone turnover in humans, Basu and colleagues performed an insulinemic–euglycemic clamp in healthy subjects [63]. The authors showed that serum osteocalcin levels were not significantly affected by increasing insulin concentrations. However, the level of the C-terminal telopeptide of type 1 collagen, a bone resorption marker, was correlated with measures of insulin sensitivity, including glucose disposal rates. These data may indicate that insulin stimulates the release of other hormones from the skeleton. Furthermore, pregnant women with gestational diabetes mellitus had much higher osteocalcin levels that were correlated with increased insulin secretion compared with women with normal glucose tolerance, though the importance of osteocalcin in this condition could not be ascertained [64]. Thus, further studies are required to firmly establish a role for osteocalcin in glucose homeostasis in humans.
Adiponectin
Involvement of adiponectin in the reciprocal regulation of bone and fuel metabolism seemed plausible given its established role as an insulin-sensitizing factor [65, 66] and its regulation by osteocalcin in adipocytes [55, 56]. However, initial studies in mice deficient in or overexpressing adiponectin indicated no major abnormalities in bone mass or turnover [67]. In general, elucidation of the mechanisms and mode of adiponectin action has been complicated by the existence of different circulating forms of the protein and the presence of multiple receptors [65]. However, more recent studies using genetic and biochemical approaches have begun to elucidate the complex nature of the effects of adiponectin and suggest a mechanism whereby it attenuates the inhibitory actions of leptin in bone [68]. Like leptin, adiponectin affects bone by acting both locally and in the brain. But unlike leptin, the local and central actions of adiponectin produce opposite effects on bone mass in an age-dependent manner. In young mice, adiponectin inhibits osteoblast proliferation and increases apoptosis to reduce bone mass whereas in older animals, adiponectin, acting on the sympathetic neurons in the locus coeruleus, opposes leptin activity and decreases sympathetic output to peripheral osteoblasts.
Whether and to what extent adiponectin regulates bone in humans remains unclear at present. The results of clinical studies suggest that circulating adiponectin levels are inversely correlated with bone mineral density [69-71]. These findings may represent a direct link between adipose tissue and bone as a positive correlation between adiponectin levels and bone turnover markers has been reported; however others studies have identified only a modest correlation [72, 73]. Interpretation of changes in circulating adiponectin is complicated by many confounding variables including age, gender, race, smoking, diabetes status, and hormone levels. The only indisputable fact from these studies is that adiponectin levels rise with age while bone mass decreases.
Summary and perspectives
In this brief overview, we have highlighted selected examples of contemporary work, in the emerging field of bone science, exploring the factors and mechanisms that enable bone cells to participate in the regulation of global energy metabolism. Like other metabolically active tissues, the cells of bone require substantial amounts of energy to perform their different functions particularly during periods of active bone formation and remodeling. Osteoblasts appear to have evolved mechanisms to assess fuel status and communicate metabolic demands to other metabolically active tissues via circulating factors. It is likely that these skeletal, energy-managing pathways emerged in early terrestrial species when muscle and fat were evolving analogous pathways for fuel production, storage, and expenditure.
The findings discussed herein raise many additional questions for future studies. In particular, the metabolic demands and fuel-utilizing machinery of the osteoblast lineage will need to be characterized. Do osteoblasts simply burn glucose or do bone cells also utilize lipids and amino acids as fuel? Can bone store fuel in the same way as muscle and fat, and what role might marrow adipocytes play in this process? In addition, it will be important to determine whether fuel preferences vary according to different functional demands of osteoblasts at different stages of their life cycle or in a pathophysiological setting, such as during fracture repair. Furthermore, it can be expected that many other factors produced by fat and muscle (soluble FGF, resistin, adipsin, irisin, etc.) will also interact with bone cells to affect metabolism. From a clinical perspective, studies investigating the possibility that metabolic disturbances underlying the pathogenesis of diabetes and obesity might also affect the skeleton and vice versa are already underway. Answers to such questions will certainly expand our understanding of the biology of the skeleton and might ultimately aid in the diagnosis and management of patients with a broad range of metabolic diseases.
Acknowledgments
Funding sources
RCR is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK099134) and a Career Development Award (BX001284) from the Biomedical Laboratory Research and Development Service of the Veterans Administration Office of Research & Development. TLC is supported by a Merit Review grant (BX001234) and is the recipient of a Research Career Scientist Award from the Veterans Administration.
Footnotes
Conflict of interest statements
No conflicts of interest were declared.
References
- 1.Das KC, White CW. Redox systems of the cell: possible links and implications. Proc Natl Acad Sci U S A. 2002;99:9617–8. doi: 10.1073/pnas.162369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolton JG, Patel S, Lacey JH, White S. A prospective study of changes in bone turnover and bone density associated with regaining weight in women with anorexia nervosa. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16:1955–62. doi: 10.1007/s00198-005-1972-7. [DOI] [PubMed] [Google Scholar]
- 3.Nussbaum M, Baird D, Sonnenblick M, Cowan K, Shenker IR. Short stature in anorexia nervosa patients. Journal of adolescent health care : official publication of the Society for Adolescent Medicine. 1985;6:453–5. doi: 10.1016/s0197-0070(85)80052-8. [DOI] [PubMed] [Google Scholar]
- 4.Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328:658–65. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 5.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 6.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–9. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono N, Ono W, Mizoguchi T, Nagasawa T, Frenette PS, Kronenberg HM. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell. 2014;29:330–9. doi: 10.1016/j.devcel.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolagas SC, Weinstein RS. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res. 1999;14:1061–6. doi: 10.1359/jbmr.1999.14.7.1061. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–65. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JM. Metabolic fuels: regulating fluxes to select mix. J Exp Biol. 2011;214:286–94. doi: 10.1242/jeb.047050. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DM, Rogers SD, Sleeman MW, et al. Modulation of glucose transport by parathyroid hormone and insulin in UMR 106-01, a clonal rat osteogenic sarcoma cell line. Journal of molecular endocrinology. 1995;14:263–75. doi: 10.1677/jme.0.0140263. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Leslie JM, Wong GW, Kahn BB, Riddle RC, Clemens TL. Expression of Glucose Transporter-4 by the osteoblast is required for global glucose metabolism. J Bone Miner Res. 2013;28:1044. [Google Scholar]
- 14.Fulzele K, DiGirolamo DJ, Liu Z, Xu J, Messina JL, Clemens TL. Disruption of the insulin-like growth factor type 1 receptor in osteoblasts enhances insulin signaling and action. J Biol Chem. 2007;282:25649–58. doi: 10.1074/jbc.M700651200. [DOI] [PubMed] [Google Scholar]
- 15.Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Molecular and cellular biochemistry. 2011;348:33–42. doi: 10.1007/s11010-010-0634-z. [DOI] [PubMed] [Google Scholar]
- 16.Zoidis E, Ghirlanda-Keller C, Schmid C. Triiodothyronine stimulates glucose transport in bone cells. Endocrine. 2012;41:501–11. doi: 10.1007/s12020-012-9594-2. [DOI] [PubMed] [Google Scholar]
- 17.Borle AB, Nichols N, Nichols G., Jr. Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. J Biol Chem. 1960;235:1211–4. [PubMed] [Google Scholar]
- 18.Borle AB, Nichols N, Nichols G., Jr Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960;235:1206–10. [PubMed] [Google Scholar]
- 19.Schmid C, Steiner T, Froesch ER. Parathormone promotes glycogen formation from [14C]glucose in cultured osteoblast-like cells. FEBS Lett. 1982;148:31–4. doi: 10.1016/0014-5793(82)81236-2. [DOI] [PubMed] [Google Scholar]
- 20.Schmid C, Steiner T, Froesch ER. Insulin-like growth factors stimulate synthesis of nucleic acids and glycogen in cultured calvaria cells. Calcif Tissue Int. 1983;35:578–85. doi: 10.1007/BF02405097. [DOI] [PubMed] [Google Scholar]
- 21.Felix R, Neuman WF, Fleisch H. Aerobic glycolysis in bone: lactic acid production by rat calvaria cells in culture. Am J Physiol. 1978;234:C51–5. doi: 10.1152/ajpcell.1978.234.1.C51. [DOI] [PubMed] [Google Scholar]
- 22.Guntur AR, Le PT, Farber CR, Rosen CJ. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology. 2014;155:1589–95. doi: 10.1210/en.2013-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000;279:C1220–9. doi: 10.1152/ajpcell.2000.279.4.C1220. [DOI] [PubMed] [Google Scholar]
- 24.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17:745–55. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, Long F. Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proc Natl Acad Sci U S A. 2014;111:8673–8. doi: 10.1073/pnas.1324290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimeno RE. Fatty acid transport proteins. Current opinion in lipidology. 2007;18:271–6. doi: 10.1097/MOL.0b013e3281338558. [DOI] [PubMed] [Google Scholar]
- 27.Herz J, Chen Y, Masiulis I, Zhou L. Expanding functions of lipoprotein receptors. Journal of lipid research. 2009;50(Suppl):S287–92. doi: 10.1194/jlr.R800077-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamek G, Felix R, Guenther HL, Fleisch H. Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. The Biochemical journal. 1987;248:129–37. doi: 10.1042/bj2480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niemeier A, Niedzielska D, Secer R, et al. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43:230–7. doi: 10.1016/j.bone.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Bachner D, Schroder D, Betat N, Ahrens M, Gross G. Apolipoprotein E (ApoE), a Bmp-2 (bone morphogenetic protein) upregulated gene in mesenchymal progenitors (C3H10T1/2), is highly expressed in murine embryonic development. Biofactors. 1999;9:11–7. doi: 10.1002/biof.5520090103. [DOI] [PubMed] [Google Scholar]
- 31.Schilling AF, Schinke T, Munch C, et al. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res. 2005;20:274–82. doi: 10.1359/JBMR.041101. [DOI] [PubMed] [Google Scholar]
- 32.Catherwood BD, Addison J, Chapman G, Contreras S, Lorang M. Growth of rat osteoblast-like cells in a lipid-enriched culture medium and regulation of function by parathyroid hormone and 1,25-dihydroxyvitamin D. J Bone Miner Res. 1988;3:431–8. doi: 10.1002/jbmr.5650030410. [DOI] [PubMed] [Google Scholar]
- 33.Chiu KM, Keller ET, Crenshaw TD, Gravenstein S. Carnitine and dehydroepiandrosterone sulfate induce protein synthesis in porcine primary osteoblast-like cells. Calcif Tissue Int. 1999;64:527–33. doi: 10.1007/s002239900644. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro I, Haselgrove J. Energy Metabolism in Bone. In: Hall B, editor. Bone. CRC Press; 1991. pp. 99–140. [Google Scholar]
- 35.Suwazono Y, Kobayashi E, Uetani M, et al. G-protein beta 3 subunit polymorphism C1429T and low-density lipoprotein receptor-related protein 5 polymorphism A1330V are risk factors for hypercholesterolemia in Japanese males--a prospective study over 5 years. Metabolism: clinical and experimental. 2006;55:751–7. doi: 10.1016/j.metabol.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Suwazono Y, Kobayashi E, Uetani M, et al. Low-density lipoprotein receptor-related protein 5 variant Q89R is associated with hypertension in Japanese females. Blood pressure. 2006;15:80–7. doi: 10.1080/08037050600650191. [DOI] [PubMed] [Google Scholar]
- 37.Suwazono Y, Kobayashi E, Dochi M, et al. Combination of the C1429T polymorphism in the G-protein beta-3 subunit gene and the A1330V polymorphism in the low-density lipoprotein receptor-related protein 5 gene is a risk factor for hypercholesterolaemia. Clinical and experimental medicine. 2007;7:108–14. doi: 10.1007/s10238-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 38.Guo YF, Xiong DH, Shen H, et al. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. Journal of medical genetics. 2006;43:798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lappalainen S, Saarinen A, Utriainen P, Voutilainen R, Jaaskelainen J, Makitie O. LRP5 in premature adrenarche and in metabolic characteristics of prepubertal children. Clin Endocrinol. 2009;70:725–31. doi: 10.1111/j.1365-2265.2008.03388.x. [DOI] [PubMed] [Google Scholar]
- 40.Fujino T, Asaba H, Kang MJ, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 2003;100:229–34. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magoori K, Kang MJ, Ito MR, et al. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein E. J Biol Chem. 2003;278:11331–6. doi: 10.1074/jbc.M211987200. [DOI] [PubMed] [Google Scholar]
- 42.Patience JF. A review of the role of acid-base balance in amino acid nutrition. Journal of animal science. 1990;68:398–408. doi: 10.2527/1990.682398x. [DOI] [PubMed] [Google Scholar]
- 43.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–9. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Karsenty G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J Biol Chem. 2004;279:47109–14. doi: 10.1074/jbc.M410010200. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Matsuda K, Bialek P, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117:387–98. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 46.Kim SG, Kim HH, Kim HK, et al. Differential expression and functional characterization of system L amino acid transporters in human normal osteoblast cells and osteogenic sarcoma cells. Anticancer research. 2006;26:1989–96. [PubMed] [Google Scholar]
- 47.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 48.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 49.Hinoi E, Gao N, Jung DY, et al. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–42. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 51.Ducy P, Desbois C, Boyce B, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 52.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vestri HS, Lara-Castro C, Moellering DR, Gundberg CM, Garvey WT. Osteocalcin is not just for bones: effects on adipocytes and role in human metabolism. Diabetes. 2008;57:A29. [Google Scholar]
- 55.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2011;50:568–75. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–19. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brennan-Speranza TC, Henneicke H, Gasparini SJ, et al. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Invest. 2012;122:4172–89. doi: 10.1172/JCI63377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henneicke H, Gasparini SJ, Brennan-Speranza TC, Zhou H, Seibel MJ. Glucocorticoids and bone: local effects and systemic implications. Trends in endocrinology and metabolism: TEM. 2014;25:197–211. doi: 10.1016/j.tem.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellstrom D. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24:785–91. doi: 10.1359/jbmr.081234. [DOI] [PubMed] [Google Scholar]
- 61.Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP, Norman PE. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol. 2010;163:265–72. doi: 10.1530/EJE-10-0414. [DOI] [PubMed] [Google Scholar]
- 62.Gower BA, Pollock NK, Casazza K, Clemens TL, Goree LL, Granger WM. Associations of Total and Undercarboxylated Osteocalcin With Peripheral and Hepatic Insulin Sensitivity and beta-Cell Function in Overweight Adults. The Journal of clinical endocrinology and metabolism. 2013;98:E1173–80. doi: 10.1210/jc.2013-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basu R, Peterson J, Rizza R, Khosla S. Effects of physiological variations in circulating insulin levels on bone turnover in humans. The Journal of clinical endocrinology and metabolism. 2011;96:1450–5. doi: 10.1210/jc.2010-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winhofer Y, Handisurya A, Tura A, et al. Osteocalcin is related to enhanced insulin secretion in gestational diabetes mellitus. Diabetes Care. 2010;33:139–43. doi: 10.2337/dc09-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 67.Shinoda Y, Yamaguchi M, Ogata N, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- 68.Kajimura D, Lee HW, Riley KJ, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 2013;17:901–15. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okuno S, Ishimura E, Norimine K, et al. Serum adiponectin and bone mineral density in male hemodialysis patients. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23:2027–35. doi: 10.1007/s00198-011-1789-5. [DOI] [PubMed] [Google Scholar]
- 70.Zillikens MC, Uitterlinden AG, van Leeuwen JP, et al. The role of body mass index, insulin, and adiponectin in the relation between fat distribution and bone mineral density. Calcif Tissue Int. 2010;86:116–25. doi: 10.1007/s00223-009-9319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. The Journal of clinical endocrinology and metabolism. 2007;92:1517–23. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- 72.Jurimae J, Jurimae T. Adiponectin is a predictor of bone mineral density in middle-aged premenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18:1253–9. doi: 10.1007/s00198-007-0365-5. [DOI] [PubMed] [Google Scholar]
- 73.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. Relationships between serum adiponectin levels versus bone mineral density, bone metabolic markers, and vertebral fractures in type 2 diabetes mellitus. Eur J Endocrinol. 2009;160:265–73. doi: 10.1530/EJE-08-0642. [DOI] [PubMed] [Google Scholar]