Abstract
Objective
Limited research has evaluated African American substance users’ response to evidence-based treatments. This study examined the efficacy of contingency management (CM) in African American and White cocaine users.
Method
A secondary analysis evaluated effects of race, treatment condition, and baseline cocaine urine sample results on treatment outcomes of African American (n = 444) and White (n = 403) cocaine abusers participating in one of six randomized clinical trials comparing CM to standard care.
Results
African American and White patients who initiated treatment with a cocaine-negative urine sample remained in treatment for similar durations and submitted a comparable proportion of negative samples during treatment regardless of treatment type; CM was efficacious in both races in terms of engendering longer durations of abstinence in patients who began treatment abstinent. Whites who began treatment with a cocaine positive sample remained in treatment longer and submitted a higher proportion of negative samples when assigned to CM than standard care. African Americans who initiated treatment with a cocaine positive sample, however, did not remain in treatment longer with CM compared with standard care, and gains in terms of drug use outcomes were muted in nature relative to Whites. This interaction effect persisted through the 9-month follow-up period.
Conclusions
CM is not equally effective in reducing drug use among all subgroups, specifically African American patients who are using cocaine upon treatment entry. Future research on improving treatment outcomes in this population is needed.
Keywords: contingency management, race, cocaine, outpatient substance abuse treatment
Cocaine use disorders are one of the most severe and destabilizing problems in segments of the African American community (Kramer, Bell-Tolliver, Tripathi, & Booth, 2011; Peters, Williams, Ross, Atkinson, & Yacoubain, 2007). Deleterious health effects of cocaine use, such as HIV (Tobin, German, Spikes, Patterson, & Latkin, 2011), coronary stenosis (Lai et al., 2012) and intracerebral hemorrhages (Martin-Schild et al. 2010; Qureshi et al., 2001), are particularly pronounced in African Americans, leading to high rates of morbidity and mortality (Bland et al., 2012; Kalokhe et al., 2012). Cocaine use is also strongly related to homicide (Chauhan et al., 2011) and incarceration (Hartley & Miller, 2010), sexual risk-taking behaviors (Gullette, Booth, Wright, Montgomery, & Stewart, 2013; Maranda, Han, & Rainone, 2004) and relationship conflicts (Golub, Dunlap, & Benoit, 2010) among African Americans. These negative cocaine-related consequences highlight the need for effective treatment for this population. However, a growing body of literature demonstrates that African Americans are more likely to drop out of substance abuse treatment (e.g., Campbell, Weisner, & Sterling, 2006; Davis & Ancis, 2012) and less likely to reduce drug use during treatment (Montgomery, Burlew, Kosinski, & Forcehimes, 2011; Montgomery, Petry, & Carroll, 2012) than their White counterparts.
Contingency management (CM) is a behavioral intervention that uses tangible reinforcers to promote abstinence from drugs. In exchange for negative urine samples, patients earn vouchers worth escalating monetary amounts (Higgins, Badger, & Budney, 2000; Higgins et al., 2007) or chances to win $1-$100 prizes (Petry, Alessi, Hanson, & Sierra, 2007; Petry, Alessi, & Ledgerwood, 2012; Petry, Alessi, Marx, Austin, & Tardif, 2005). In a meta-analysis of psychosocial treatments for substance use disorders, CM had the largest effect size in reducing drug use (Dutra et al., 2008), and it consistently reduces cocaine use (Farronato, Dursteler-Macfarland, Wiesbeck, & Petitjean, 2013; Lussier, Heil, Mongeon, Badger, & Higgins, 2006).
Several studies have examined effects of patient factors, such as age (Weiss & Petry, 2011; 2013), socioeconomic status (Secades-Villa et al., 2013), and income (Rash, Olmstead, & Petry, 2009; Rash, Andrade, & Petry, 2013), as influences on CM outcomes. These studies generally find few patient factors reliably impact response. However, one patient factor—baseline severity of drug use—has consistently been related to response to treatment in general (Alterman, McKay, Mulvaney, & McLellan, 1996) and CM (McLellan et al., 1994) in particular. Presence of a drug-negative urine sample at treatment initiation is associated with lower drug use severity and improved outcomes (Ahmadi, et al., 2009; Preston et al., 1998; Silverman et al., 1998). Further, CM is more efficacious in patients who begin outpatient treatment with a drug-negative sample than those who initiate treatment with a drug-positive sample (Stitzer et al., 2007).
Race is another patient factor that has been show to influence treatment outcomes generally (Guerrero et al., 2013; Hunter, Paddock, Zhou, Watkins, & Hepner, 2013). To our knowledge, only four studies have examined CM outcomes in African American patients in outpatient settings (Barry, Sullivan & Petry 2009; Bride & Humble, 2008; McKay et al., 2010; Montgomery et al., 2012). Bride and Humble (2008) found that CM was effective in increasing outpatient substance abuse treatment attendance and completion rates among African-American women on welfare. Barry et al. (2009) demonstrated comparable efficacy of CM in reducing cocaine use among African American, Hispanic and White methadone maintained clients. McKay et al. (2010) found that CM was more effective than cognitive-behavioral relapse prevention in producing lower rates of cocaine positive urine samples and self-reported cocaine use among a predominately African American cocaine-dependent sample who had achieved initial engagement in intensive outpatient (IOP) treatment. However, a recent study by Montgomery et al. (2012) revealed that African American marijuana-dependent young adults receiving outpatient treatment did not derive benefits from CM, while CM was effective in reducing the proportion of marijuana positive samples among their White counterparts.
Although these studies assessed outcomes among racially diverse populations, one was limited to women (Bride & Humble, 2008), one to court-referred young adults (Montgomery et al., 2012), another to adults who successfully completed IOP treatment (Mckay et al., 2010), and the fourth (Barry et al. 2009) to methadone maintenance patients, who have severe drug use problems and for whom treatment is a lifelong recommendation. Effects of race on response to CM have not been systematically evaluated in standard, outpatient psychosocial treatment facilities. Further, the small number of African Americans participating in some of the above studies limits the power to detect between group differences and generalization of the findings.
The present study was designed to address these gaps and determine if, in a larger sample, African American and White cocaine abusing patients responded similarly to CM, and if response to CM between racial groups is affected by initial abstinence status. Given past literature (Montgomery et al., 2011; Montgomery et al., 2012; Milligan et al., 2004; Stitzer et al., 2010), we hypothesized that African American patients may disengage from treatment more rapidly and have worse drug use outcomes, regardless of treatment condition and baseline drug use status. We also expected that baseline drug use status would impact treatment outcomes overall, with those with a positive sample at treatment initiation remaining in treatment for shorter periods and achieving less abstinence. CM was expected to positively impact outcomes, but whether effects varied by race and baseline drug use status was examined. Both short-term (during treatment) and long-term follow-up effects were evaluated.
Methods
Participants
Participants in these analyses were from one of six randomized studies (Petry, et al., 2004; Petry, Alessi, Tedford, Austin, & Tardif, 2005; Petry et al., 2006a; Petry, Weinstock, & Alessi, 2011; Petry, Barry, Alessi, Rounsaville, & Carroll; 2012a; Petry, Alessi, & Ledgerwood, 2012b). Inclusion criteria for all studies were 18 years or older, past year Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) criteria for cocaine dependence, and ability to understand study procedures. Exclusion criteria were uncontrolled psychopathology (e.g., active suicidal ideation, psychosis), or being in recovery for pathological gambling due to potential similarity with gambling and CM (but see Petry & Alessi, 2010; Petry et al., 2006b). All patients provided written informed consent, approved by the University and hospital (when applicable) Institutional Review Board.
Procedures
Participants completed a demographics questionnaire that included information about self-reported racial identity, checklists of drug use modules from the Structured Clinical Interview for the DSM-IV (First, Spitzer, Gibbon, & Williams, 1996), and the Addiction Severity Index (ASI; McLellan, Luborsky, Cacciola, & Griffin, 1985). The ASI assesses psychosocial functioning with respect to alcohol use, drug use, medical, employment, legal, family/social, and psychiatric domains. Composite scores range from 0.00-1.00, with higher scores in each domain indicating greater severity of symptoms.
Treatments
After completing the baseline evaluation, participants were randomly assigned to a treatment condition using a computerized urn randomization procedure (Stout, Wirtz, Carbonari, & Del Boca, 1994). Five of the six primary studies (Petry et al., 2004, 2005a, 2006a, and 2012ab) included three treatment conditions, one of which was Standard Care (SC), and the other two were CM interventions. The Petry et al. (2011) study had only one CM condition and a SC condition. All trials shared the primary goal of evaluating the efficacy of CM plus SC versus SC alone, and all found benefits of CM. A high level of consistency across studies (e.g., targeted population, community clinics, similar assessment measures and intervals) provided rationale for combining SC conditions and combining CM conditions for the analyses in the present report. As treatments are detailed in original reports, they are only briefly described below.
SC treatment
Participants assigned to SC condition in all studies received intensive outpatient substance abuse treatment services. Therapy consisted of group sessions covering coping and life skills, AIDS education, relapse prevention, and 12-step interventions. During the intensive phase of care (2-4 weeks), group sessions were held 3-5 days per week, followed by aftercare, consisting of one group session per week for up to 12 months. Patients also submitted up to 24 breath and urine samples over the 12-week treatment period. Breath samples were screened for alcohol using an Alcosensor IV Altometer (Intoximeters, St Louis, MO, USA), and urine specimens for opioids and cocaine using Ontrak TesTstiks (Roche, Somersville, NJ, USA).
CM treatment
Patients assigned to CM received SC as above, and they submitted breath and urine samples at the same schedule. They also earned reinforcers for substance negative urine and breath samples and/or for completing goal-related activities or attending treatment. Abstinence was reinforced for submission of samples testing negative for three substances concurrently: alcohol, cocaine, and opioids. Goal-related activities (e.g., attending a doctor’s appointment for a medical-related goal) were consistent with each patient’s treatment plan. Objective verification in the forms of receipts or brochures was needed for reinforcement (see Petry, Tedford, & Martin, 2001 for examples). In conditions in which both goal-related activities and abstinence were reinforced, the reinforcement schedules were independent (e.g., failure to complete scheduled activities did not affect reinforcement for abstinence). The Petry et al. (2011) trial reinforced group attendance along with abstinence, and one CM condition in the Petry et al. (2012a) study reinforced attendance alone (without reinforcing abstinence).
Data Analysis
Patients were classified based on self reports of race into African American (n = 444) or White (n = 403). Individuals who self-identified with other races (Native American, Asian, Pacific Islanders, etc.) were not included in these analyses. Demographics and baseline characteristics were compared across the two races using χ2 and independent t-tests.
Multivariate general linear models (GLM) evaluated the relationship between race, treatment condition, baseline cocaine urine toxicology result, and their interactions on primary study outcomes while controlling for baseline variables that differed between racial groups and were associated with outcomes (e.g., study, age, income, and ASI legal composite score). Other baseline variables that differed (or nearly differed) between the races (e.g., other ASI composite scores and gender) were not significantly associated with outcome measures, and their inclusion or exclusion from the model did not impact the overall pattern of results presented herein. Primary outcomes included weeks retained in treatment, longest duration of consecutive abstinence (LDA), and proportion of negative samples submitted. LDA was defined as the greatest number of consecutive weeks of objectively verified abstinence from alcohol, cocaine, and opioids during treatment (range 0 – 12 weeks). Positive samples for one or more drugs, missed samples, or unexcused absences on a testing day broke the string of abstinence. Proportions of samples negative for cocaine, alcohol, and opioids were calculated with the number of samples submitted in the denominator, so that missing samples (and treatment retention) did not impact this variable. Weeks retained in treatment varied from 0 to 12, and was based on clinic records. These measures were available from 100% of randomized patients.
Logistic regressions assessed long-term predictors of abstinence, as assessed by samples testing negative for alcohol, cocaine and opioids, at the Month 9 follow-up. Baseline variables that differed between the groups (race, baseline cocaine urine toxicology results, age, income, and ASI legal composite score) and study were included in step one, and in step two, treatment condition and the interactions between treatment condition, baseline urine toxicology result, and race were added in the model. These analyses were conducted twice—first only using patients who submitted a sample at the Month 9 follow-up (n = 622), and secondly using all randomized (n = 847) patients coding those without a sample at Month 9 as positive. Analyses were performed on SPSS for Windows (v 15). Two-tailed alphas < 0.05 were considered significant.
Results
Sample characteristics by race
As shown in Table 1, racial groups differed on several baseline characteristics. African Americans were more likely to submit a cocaine positive sample at treatment initiation than Whites (24% vs 18%). African Americans were also significantly older and reported more employment problems than Whites. Whites reported receiving higher incomes and having more legal and drug, family and psychological problems than their African American counterparts. The racial composition also differed across the six studies.
Table 1.
Baseline Characteristics by Race
| Variable | African America (n = 444) % |
White (n = 403) % |
χ 2 | Analysis df |
p | ||
|---|---|---|---|---|---|---|---|
| Women | 54.5 | 48.1 | 3.43 | 1 | 0.06 | ||
| Men | 45.5 | 51.9 | |||||
| Marital Status | |||||||
| Married | 10.1 | 9.7 | |||||
| Never married | 56.1 | 51.4 | 2.48 | 2 | 0.29 | ||
| Other | 33.8 | 39.0 | |||||
| Study | |||||||
| Petry et al. (2004) | 16.9 | 6.9 | |||||
| Petry et al. (2005) | 18.0 | 8.2 | |||||
| Petry et al. (2006) | 10.1 | 14.9 | 64.55 | 5 | <.01 | ||
| Petry et al. (2011) | 19.4 | 15.1 | |||||
| Petry et al. (2012a) | 11.5 | 10.4 | |||||
| Petty et al (2012b) | 24.1 | 44.4 | |||||
| Treatment Group | |||||||
| Contingency Management | 65.8 | 65.5 | 0.01 | 1 | 0.94 | ||
| Standard Care | 34.2 | 34.5 | |||||
| Alcohol Dependence Diagnosis | |||||||
| Yes | 52.0 | 54.8 | 0.67 | 1 | 0.45 | ||
| No | 48.0 | 45.2 | |||||
| Baseline Opiate Urine Screen | |||||||
| Negative | 96.4 | 95.5 | 1.30 | 1 | 0.52 | ||
| Positive | 3.6 | 4.2 | |||||
| Baseline Cocaine Urine Screen | |||||||
| Negative | 76.2 | 82.4 | 4.89 | 1 | <.05 | ||
| Positive | 23.8 | 17.6 | |||||
| M | SD | M | SD | t | df | p | |
|
|
|||||||
| Age (years) | 38.4 | 8.2 | 35.7 | 9.4 | 4.54 | 845 | <.01 |
| Education (years) | 11.9 | 1.8 | 12.0 | 1.9 | −0.86 | 845 | 0.39 |
| Earned income* | 7067.1 | 12620 | 10715.4 | 15634.3 | −3.75 | 843 | <.01 |
| Previous no. of drug treatments | 6.1 | 9.6 | 6.5 | 8.1 | −0.59 | 845 | 0.56 |
| Days of alcohol use in past 30 | 3.9 | 7.0 | 4.0 | 7.0 | −0.12 | 845 | 0.91 |
| Days of cocaine use in past 30 | 4.7 | 7.5 | 4.5 | 6.7 | 0.44 | 845 | 0.66 |
| Days of heroin use in past 30 | 0.6 | 3.2 | 0.9 | 3.7 | −1.08 | 845 | 0.28 |
| ASI employment composite | 0.78 | 0.26 | 0.69 | 0.28 | 5.05 | 845 | <.01 |
| ASI legal composite | 0.11 | 0.19 | 0.15 | 0.23 | −2.33 | 844 | <.05 |
| ASI drug use composite | 0.16 | 0.09 | 0.18 | 0.09 | −3.22 | 844 | <.01 |
| ASI medical composite | 0.23 | 0.35 | 0.27 | 0.36 | −1.51 | 845 | 0.13 |
| ASI family composite | 0.16 | 0.21 | 0.20 | 0.22 | −2.61 | 843 | <.01 |
| ASI psychological composite | 0.25 | 0.23 | 0.31 | 0.24 | −3.84 | 843 | <.01 |
Notes. ASI = Addiction Severity Index;
Income was log transformed prior to analyses.
Effects of race, baseline toxicology and treatment group on during treatment outcomes
Age, income and ASI-legal scores were associated with primary outcomes and included as covariates in analyses evaluating effects of race. Age was positively associated with LDA, F(1, 836) = 8.85, p < .02, and weeks in treatment, F(1, 836) = 9.36, p < .01. Income was positively related to proportion of negative samples submitted, F(1, 836) = 6.45 p < .01, and ASI-legal scores were negatively related to proportion of negative samples submitted, F(1, 836) = 7.31, p <.01.
Baseline cocaine urine toxicology results were related to percentage of negative samples submitted and the longest duration of abstinence from all substances, F(1, 836) = 244.78 and 39.36, respectively, ps < .01, but not time in treatment, F(1, 836) = 0.41, p = 0.52. Patients who started treatment with a cocaine negative sample submitted a higher percentage of negative samples during treatment (M = 90.9%, SE = 1.3) than patients who started treatment with a cocaine positive sample (M = 42.0%, SE = 2.4). Patients who started treatment with a cocaine negative sample had a longer duration of abstinence from all substances (M = 5.2 weeks, SE = 0.2) than their counterparts who tested cocaine positive at baseline (M = 2.0 weeks, SE = 0.4).
Treatment type was associated with all three primary outcomes: percentage of negative samples submitted during treatment, the longest duration of abstinence from all substances and weeks retained in treatment, F(1, 836) = 22.93, 26.60, and 4.17, respectively, ps < .05. Specifically, patients randomized to CM submitted a higher percentage of negative urine samples (M = 71.6%, SE = 1.4) than patients randomized to standard care (M = 61.3%, SE = 1.8). Patients in CM had a longer duration of abstinence (M = 4.5 weeks, SE = 0.2) than their SC counterparts (M = 2.7 weeks, SE = 0.3). Individuals assigned to CM also remained in treatment longer (M = 6.8 weeks, SE = 0.2) than SC patients (M = 6.0 weeks, SE = 0.3).
The GLM analyses also revealed a main effect of race with respect to the percentage of negative samples, F (1, 836) = 10.67, p <.01, with Whites (M = 70.0%, SE = 1.7) submitting a higher proportion of negative samples than African Americans (M = 62.9%, SE = 1.5). Additionally, two-way interaction effects were significant for race by treatment condition, race by baseline urine toxicology results, and treatment condition by baseline toxicology result for proportion negative samples, F (1, 836) = 4.24, 10.30, 33.55, respectively, ps < .05 (see Table 2).
Table 2.
Three-Way Interaction of Baseline Urine Toxicology Screens by Treatment Type by Race on Treatment Outcomes
| African American M(SE) | n |
White M
(SE) |
n | |
|---|---|---|---|---|
| Percentage of negative urine samples of total samples submitted | ||||
| Standard Care | ||||
| Cocaine Negative Screen | 91.6 (2.3) | 121 | 92.3 (2.5) | 110 |
| Cocaine Positive Screen | 28.3 (4.4) | 31 | 33.0 (4.6) | 29 |
| Contingency Management | ||||
| Cocaine Negative Screen | 90.1 (1.8) | 215 | 89.6 (1.9) | 222 |
| Cocaine Positive Screen | 41.7 (3.1) | 74 | 65.2 (4.1) | 42 |
| Longest duration of abstinence from all substance assessed (weeks) | ||||
| Standard Care | ||||
| Cocaine Negative Screen | 4.1 (0.4) | 121 | 4.0 (0.4) | 110 |
| Cocaine Positive Screen | 1.3 (0.7) | 31 | 1.1 (0.8) | 29 |
| Contingency Management | ||||
| Cocaine Negative Screen | 6.3 (0.3) | 215 | 6.2 (0.3) | 222 |
| Cocaine Positive Screen | 1.8 (0.5) | 74 | 3.6 (0.7) | 42 |
| Weeks retained in treatment | ||||
| Standard Care | ||||
| Cocaine Negative Screen | 5.9 (0.4) | 121 | 6.3 (0.4) | 110 |
| Cocaine Positive Screen | 6.2 (0.7) | 31 | 5.6 (0.8) | 29 |
| Contingency Management | ||||
| Cocaine Negative Screen | 7.3 (0.3) | 215 | 6.6 (0.3) | 222 |
| Cocaine Positive Screen | 5.7 (0.5) | 74 | 7.3 (0.7) | 42 |
After controlling for these main and interaction effects, analyses revealed a 3-way interaction between race, baseline toxicology results and treatment group on percentage of negative samples (F [1, 835] = 5.46, p < .05) and retention (F [1, 835] = 5.32, p < .05), but not longest duration of abstinence (F (1, 835) = 2.04, p = .15). As shown in Table 2, African Americans submitted as high a proportion of negative samples and remained in treatment for similar durations as Whites if they started treatment with a cocaine negative sample. Whites who began treatment with a cocaine positive sample and were assigned to CM submitted a higher proportion of negative samples and remained in treatment longer than their White counterparts who were assigned to SC. However, African Americans who started out with a cocaine positive sample also achieved some improvements in drug use outcomes with CM compared with SC, but the gains were relatively muted in nature, and in the case of retention non-existent.
Follow-up analyses
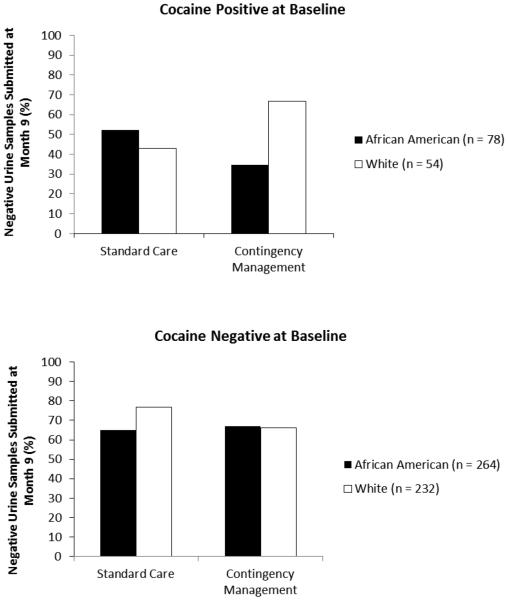
Logistic regressions evaluated whether this interaction effect persisted after treatment. Table 3 displays results from the final models. The analysis involving only follow-up completers (n = 622) was significant χ2 (14) = 53.02, p < .001. Older age was inversely related to submission of a negative sample at follow-up, and submission of a cocaine negative sample at baseline increased the probability of submitting a negative sample at follow-up by nearly 4-fold. Although study was a significant overall predictor, no study differed significantly from the Petry et al. (2004) study in terms of percentages of participants submitting negative samples. The 3-way interaction between race, baseline cocaine toxicology result, and treatment group significantly predicted submission of a negative sample at Month 9. As illustrated in Figure 1 (top panel), over 60% of patients who initiated treatment with a cocaine negative sample submitted a negative sample at Month 9, rates that did not vary by race or treatment assignment. Of those with cocaine positive samples at baseline, only about 45% submitted a negative sample at Month 9 if they were assigned to SC, regardless of race. However, Whites who began treatment cocaine positive were more likely than their African American counterparts to submit a negative sample at follow-up if they were assigned to CM; over 65% of Whites who received CM tested negative at Month 9 versus less than 35% of African Americans.
Table 3.
Predictors of a Negative Urine Toxicology Result at 9-month Follow-up Evaluation
| Variable | Wald | p | β (Standard error) | Odds ratio (95% Confidence interval) |
|---|---|---|---|---|
| Follow-up completers only (n = 622) | ||||
| Age | 5.97 | <.02 | −0.03 (0.01) | 0.98 (0.96-1.00) |
| Income | 1.24 | 0.27 | 0.00 (0.00) | |
| Baseline ASI legal composite score | 0.23 | 0.63 | −0.21 (0.44) | |
| Studya | 13.48 | <.02 | ||
| Petry et al. (2005) | 0.00 | 0.96 | 0.02 (0.44) | |
| Petry et al. (2006) | 0.14 | 0.70 | 0.16 (0.44) | |
| Petry et al. (2011) | 0.58 | 0.45 | 0.32 (0.42) | |
| Petry et al. (2012a) | 2.67 | 0.10 | −0.62 (0.38) | |
| Petry et al. (2012b) | 3.23 | 0.07 | −0.67 (0.37) | |
| Baseline cocaine negative sampleb | 9.62 | <.01 | 1.35 (0.44) | 3.86 (1.64-9.05) |
| Treatment groupc | 0.99 | 0.32 | −0.45 (0.45) | |
| Raced | 2.47 | 0.12 | 0.48 (0.31) | |
| Baseline cocaine negative sample x Treatment group | 0.72 | 0.40 | 0.42 (0.49) | |
| Treatment group x Race | 2.00 | 0.16 | 0.81 (0.57) | |
| Baseline cocaine negative sample x Treatment group X Race | 5.68 | <.02 | −1.28 (0.54) | 0.28 (0.10-0.80) |
| Missing data considered positive (n = 847) | ||||
| Age | 1.79 | 0.18 | −0.01 (0.01) | |
| Income | 0.00 | 0.99 | 0.00 (0.00) | |
| Baseline ASI legal composite score | 3.54 | 0.06 | −0.65 (0.35) | |
| Studya | 4.64 | 0.46 | ||
| Petry et al. (2005) | 0.84 | 0.36 | −0.28 (0.31) | |
| Petry et al. (2006) | 0.50 | 0.48 | 0.21 (0.30) | |
| Petry et al. (2011) | 4.17 | <.05 | 0.74 (0.36) | 2.10 (1.03-4.27) |
| Petry et al. (2012a) | 0.82 | 0.37 | 0.25 (0.27) | |
| Petry et al. (2012b) | 1.75 | 0.19 | 0.37 (0.28) | |
| Baseline cocaine negative sampleb | 6.64 | <.02 | 0.94 (0.37) | 2.56 (1.25-5.25) |
| Treatment groupc | 1.11 | 0.29 | −0.43 (0.41) | |
| Raced | 0.00 | 0.99 | −0.00 (0.24) | |
| Baseline cocaine negative sample x Treatment group | 0.88 | 0.35 | 0.40 (0.43) | |
| Treatment group x Race | 4.62 | <.05 | 1.04 (0.48) | 2.82 (1.10-7.24) |
| Baseline cocaine negative sample x Treatment | 8.20 | <.01 | −1.32 (0.46) | 0.27 (0.11-0.66) |
Notes.
No β can be calculated when three groups are present; Petry et al. 2004 is the reference study.
Cocaine positive sample at baseline is the reference group.
Standard treatment is the reference group.
African American is the reference group.
ASI = Addiction Severity Index
Figure 1.
Three way-interaction between race, treatment type and baseline cocaine urine sample result on percentage of negative urine samples submitted at month 9 (completers only).
In the second analysis, patients who failed to provide a urine toxicology screen at the follow-up were coded as positive. The overall model was again significant, χ2 (14) = 33.72, p < .001, and the 3-way interaction between race, baseline cocaine toxicology result, and treatment group significantly predicted whether or not patients submitted a positive or negative sample at the 9 month follow-up (Table 3). The proportions of negative samples were lower overall when patients who failed to provide a urine sample at Month 9 were coded as positive, but the general pattern of results was similar to that shown in Figure 1 (data not shown; available upon request).
Discussion
Results from this secondary analysis suggest that initial abstinence status differentially influences outcomes in African American and White patients receiving CM. Patients who begin treatment with a cocaine-negative sample respond equally well to CM, regardless of race. However, of those who initiate treatment cocaine-positive, Whites assigned to CM remain in treatment longer and submit a higher proportion of negative samples than those assigned to SC. African Americans who begin treatment cocaine positive do not remain in treatment longer with CM relative to SC, and gains in terms of drug use outcomes are minimized compared to Whites.
Consistent with existing literature (Ahmadi et al., 2009), individuals with a negative urine sample at treatment initiation remain in treatment longer and achieve more abstinence than those who enter treatment with a positive sample. Negative urine screens at baseline are indicative of a good prognosis in treatment overall (Shah et al., 2013; Sofuoglu, Gonzalez, Poling, & Kosten, 2003) and in predominately African American samples as well (Petry, Rash, & Easton, 2011; Tzilos, Rhodes, Ledgerwood, & Greenwald, 2009). However, the current study found differential outcomes in baseline urine results and treatment outcomes by treatment type and race.
A possible explanation for the three-way interaction between baseline sample results, race and treatment type may relate to the severity of drug use. More African Americans began treatment with a positive sample, suggesting more severe cocaine use compared to Whites. Although the racial groups overall did not differ in frequencies of cocaine use at baseline (Table 1), African Americans who tested positive for cocaine and reported recent use at baseline had trends toward higher frequencies of cocaine use in the past month than their White counterparts, 13.2 ± 9.1 days versus 10.6 ± 8.3 days, p = .08. CM may be less effective in patients with more severe drug use (Stitzer, Petry, Peirce et al., 2007; Preston, Silverman, Higgins et al., 1998), and higher magnitude reinforcement may be needed to impact drug use in patients with more severe substance use (Petry et al., 2004, 2012). This and other theories for this interaction effect require further study to determine how best to apply CM, or other evidence-based practices, to African Americans initiating treatment with cocaine positive samples. For example, CM may be effective if combined with other intensive and practical skills-based interventions (Schumacher, 2003) in the subgroup of African Americans who are actively using when they start treatment.
This study has several strengths. It is the first to examine systematically the influence of race on response to CM. The large number of African American participants is especially noteworthy, given that most studies (Bride & Humble, 2008; Burlew et al., 2011; Burlew, Kosinski, & Forcehimes, 2011) have relatively few African Americans. Further, we used objective indicators of treatment outcomes (Higgins, Badger, & Budney, 2000; Rousanville, Petry & Carroll, 2003) and included long-term post-treatment indices of relapse.
However, some limitations to this study are also noteworthy, including those outlined in the original reports (Petry et al., 2004, 2005a, 2006a, 2011, & 2012ab). Additionally, the present report represents a retrospective analysis that was not designed or powered to detect interaction effects. A smaller percentage of participants initiated treatment with a cocaine positive urine sample (21%) than with a cocaine negative sample (79%), resulting in a relatively small sample size in some of the groups when divided by baseline sample result, race, and treatment condition.
Despite limitations, this study extends the literature in examining effects of race on treatment outcomes. Similarly to other studies (e.g., Acevedo et al., 2012; Montgomery, Burlew, Kosinski, & Forcehimes, 2011), the current study suggests that African American and White substance users may have different treatment needs that may call for culturally-tailored interventions (Burlew, Copeland, Ahuama-Jonas, & Calsyn, 2013). For example, Brown, Hill and Giroux (2004) conducted a focus group with rural African American cocaine users who suggested that interventions should involve friends and family and provide direct resources (job, car, housing) or assistance in receiving these resources in treatment. Future studies could evaluate whether the inclusion of these elements in treatment, with or without CM, improves outcomes especially in this subset of African Americans with a poor overall prognosis.
In summary, CM is effective for both African Americans and Whites who enter treatment with a cocaine negative sample. However, African Americans who begin treatment with a cocaine positive sample do not benefit from CM as much as their White counterparts, and this subgroup in particular is in great need of effective treatment that will both initiate abstinence and prevent relapse once abstinence is achieved. Future research directed at improving outcomes in this population is criticalfor reducing the personal and societal consequences of cocaine use in this highly disadvantaged and disenfranchised segment of the African American community.
PUBLIC HEALTH SIGNIFICANCE: Contingency management, an empirically-based treatment that rewards clinically appropriate behaviors (e.g., submission of drug-negative urine samples), does not appear equally effective in reducing drug use among all subgroups, specifically African Americans who are using cocaine upon treatment entry. This study highlights the need for additional research on effective treatments for this population
ACKNOWLEDGEMENTS
The authors thank the research team for assisting with the successful planning and execution of the clinical trials.
FUNDING: Funding for this study was provided by National Institute on Drug Abuse (P30-DA023918, REWARD Center). NIDA had no further role in study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Contributor Information
LaTrice Montgomery, University of Cincinnati, School of Human Services, Mental Health and Substance Abuse Counseling, 2160 McMicken Circle, P.O. Box 210068, Cincinnati, Ohio 45221, Phone: 513-556-3344.
Kathleen M. Carroll, Yale University School of Medicine, VA Connecticut Healthcare System, 950 Campbell Avenue (151D), West Haven, CT 06516
Nancy M. Petry, University of Connecticut Health Center, Department of Medicine, 263 Farmington Avenue, Farmington, CT 06030
References
- Ahmadi J, Kampman KM, Oslin DM, Pettinati HM, Dackis C, Sparkman T. Predictors of treatment outcome in outpatient cocaine and alcohol dependence treatment. American Journal on Addictions. 2009;18:81–86. doi: 10.1080/10550490802545174. doi: 10.1080/10550490802545174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman AI, McKay JR, Mulvaney FD, McLellan AT. Prediction of attrition from day hospital treatment in lower socioeconomic cocaine-dependent men. Drug and Alcohol Dependence. 1996;40:227–233. doi: 10.1016/0376-8716(95)01212-5. [DOI] [PubMed] [Google Scholar]
- Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic and White methadone maintenance clients. Psychology of Addictive Behaviors. 2009;23:168–174. doi: 10.1037/a0014575. doi: 10.1037/a0014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland SE, Mimiaga MJ, Reisner SL, White JM, Driscoll MA, Isenberg D, Mayer KH. Sentencing risk: History of incarceration and HIV/STD transmission risk behaviors among Black men who have sex with men in Massachusetts. Culture, Health and Sexuality. 2012;14:329–345. doi: 10.1080/13691058.2011.639902. doi: 10.1080/13691058.2011.639902. [DOI] [PubMed] [Google Scholar]
- Bride BE, Humble MN. Increasing retention of African-American women on welfare in outpatient substance user treatment using low-magnitude incentives. Substance Use and Misuse. 2008;43:1016–1026. doi: 10.1080/10826080801914154. doi: 10.1080/10826080801914154. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Hill MA, Giroux SA. “A 28-day program ain’t helping the crack smoker”—perceptions of effective drug abuse prevention interventions by north central Florida African Americans who use cocaine. Journal of Rural Health. 2004;20:286–295. doi: 10.1111/j.1748-0361.2004.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Burlew AK, Copeland VC, Ahuama-Jonas C, Calsyn DA. Does cultural adaptation have a role in substance abuse treatment? Social Work in Public Health. 2013;28:440–460. doi: 10.1080/19371918.2013.774811. doi: 10.1080/19371918.2013.774811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlew AK, Feaster D, Brecht ML, Hubbard R. Measurement and data analysis in research addressing health disparities in substance abuse. Journal of Substance Abuse Treatment. 2009;36:25–43. doi: 10.1016/j.jsat.2008.04.003. doi: 10.1016/j.jsat.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlew AK, Montgomery L, Kosinski AS, Forcehimes A. Does treatment readiness enhance the response of African American substance users to Motivational Enhancement Therapy? Psychology of Addictive Behaviors. 2013;27:744–753. doi: 10.1037/a0031274. doi: 10.1037/a0031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlew AK, Weekes JC, Montgomery L, Feaster DJ, Robbins MS, Rosa C, Wu LT. Conducting research with racial/ethnic minorities: Methodological lessons from the NIDA Clinical Trials Network. American Journal of Drug and Alcohol Abuse. 2011;37:324–332. doi: 10.3109/00952990.2011.596973. L. doi: 10.3109/00952990.2011.596973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CI, Weisner C, Steling S. Adolescents entering chemical dependency treatment in private managed care: Ethnic differences in treatment initiation and retention. Journal of Adolescent Health. 2006;38:343–350. doi: 10.1016/j.jadohealth.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Chauhan P, Cerda M, Messner SF, Tracy M, Tardiff K, Galea S. Race/ethnic specific homicide rates in New York City: Evaluating the impact of broken windowns policing and crack cocaine markets. Homicide Studies. 2011;15:268–290. doi: 10.1177/1088767911416917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Ancis J. Look to the relationship: A review of African American women substance users’ poor treatment retention and working alliance development. Substance Use and Misuse. 2012;47:662–672. doi: 10.3109/10826084.2012.654882. doi: 10.3109/10826084.2012.654882. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Farronato NS, Dursteler-Macfarland KM, Wiesbeck GA, Petitjean SA. A systematic review comparing cognitive behavioral therapy and contingency management for cocaine dependence. Journal of Addictive Diseases. 2013;32:274–287. doi: 10.1080/10550887.2013.824328. doi: 10.1080/10550887.2013.824328. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV –TR Axis I Disorders, Clinician Version (SCID-CV) American Psychiatric Press, Inc.; Washington, D.C.: 1996. [Google Scholar]
- Golub A, Dunlap E, Benoit E. Drug use and conflict in inner-city African-American relationships in the 2000s. Journal of Psychoactive Drugs. 2010;42:327–337. doi: 10.1080/02791072.2010.10400695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero EG, Marsh JC, Duan L, Oh C, Perron B, Lee B. Disparities in completion of substance abuse treatment between and within racial and ethnic groups. Health Services Research. 2013;48:1450–1467. doi: 10.1111/1475-6773.12031. doi: 10.1111/1475-6773.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullette D, Booth BM, Wright PB, Montgomery BE, Stewart KE. Sexual sensation seeking, transactional sex, and rural African American cocaine users. Journal of the Association of Nurses in AIDS Care, S1055-3290. 2013:00146–5. doi: 10.1016/j.jana.2013.07.008. doi: 10.1016/j.jana.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Experimental and Clinical Psychopharmacology. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Hunter SB, Paddock SM, Zhou A, Watkins KE, Hepner KA. Do client attributes moderate the effectiveness of a group cognitive behavioral therapy for depression in addiction treatment? Journal of Behavioral Health Services and Research. 2013;40:57–70. doi: 10.1007/s11414-012-9289-8. doi: 10.1007/s11414-012-9289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalokhe AS, Paranjape A, Bell CE, Cardenas GA, Kuper T, Metsch LR, del Rio C. Intimate partner violence among HIV-infected crack cocaine users. AIDS Patient Care and STDS. 2012;26:234–240. doi: 10.1089/apc.2011.0275. doi: 10.1089/apc.2011.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer TL, Bell-Toliver L, Tripathi SP, Booth BM. Stimulant use by young adult African Americans in a rural community: A pipeline to prison? Substance Use and Misuse. 2011;46:716–727. doi: 10.3109/10826084.2010.526981. doi: 10.3109/10826084.2010.526981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Gerstenblith G, Fishman EK, Brinker J, Kickler T, Tong W, Lai S. Vitamin D deficiency is associated with silent coronary artery disease in cardiovascularly asymptomatic African Americans with HIV infection. Clinical Infectious Diseases. 2012;54:1747–1755. doi: 10.1093/cid/cis306. doi: 10.1093/cid/cis306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance abusers. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Maranda MJ, Han C, Rainone GA. Crack cocaine and sex. Journal of Psychoactive Drugs. 2004;36:315–322. doi: 10.1080/02791072.2004.10400032. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Albright KC, Hallevi H, Barreto AD, Phillip M, Mistra V, Savitz SI. Intracerebral hemorrhage in cocaine users. Stroke. 2010;41:680–684. doi: 10.1161/STROKEAHA.109.573147. doi: 10.1161/STROKEAHA.109.573147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Lynch KG, Coviello D, Morrison R, Cary MS, Skalina L, Plebani J. Randomized trial of continuing care enhancements for cocaine-dependent patients following initial engagement. Journal of Consulting and Clinical Psychology. 2010;78:111–120. doi: 10.1037/a0018139. doi: 10.1037/a0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Metzger DS, Grissom GR, Woody GE, Luborsky L, O’Brien CP. Similarity of outcome predictors across opiate, cocaine, and alcohol treatments: Role of treatment services. Journal of Consulting and Clinical Psychology. 1994;62:1141–1158. doi: 10.1037//0022-006x.62.6.1141. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborksy L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Milligan CO, Nich C, Carroll KM. Ethnic differences in substance abuse treatment retention, compliance and outcome from two clinical trials. Psychiatric Services. 2004;55:167–173. doi: 10.1176/appi.ps.55.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery L, Burlew AK, Kosinski AS, Forcehimes A. Motivational Enhancement Therapy for African American substance users: A randomized clinical trial. Cultural Diversity and Ethnic Minority Psychology. 2011;17:357–365. doi: 10.1037/a0025437. doi: 10.1037/a0025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery L, Petry N, Carroll KM. Moderating effects of race in clinical trial participation and outcomes among marijuana-dependent adults. Drug and Alcohol Dependence. 2012;126:333–339. doi: 10.1016/j.drugalcdep.2012.05.033. doi: 10.1016/j.drugalcdep.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stizer ML, Blaine J, Kellogg S, Satterfield F, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Peters RJ, Williams M, Ross MW, Atkinson J, Yacobian GS. Codeine cough syrup use among African-American crack cocaine users. Journal of Psychoactive Drugs. 2007;39:97–102. doi: 10.1080/02791072.2007.10399868. [DOI] [PubMed] [Google Scholar]
- Petry NP. A comparison of African American and non-Hispanic Caucasian cocaine-abusing outpatients. Drug and Alcohol Dependence. 2003;69:43–49. doi: 10.1016/s0376-8716(02)00255-7. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. Journal of Substance Abuse Treatment. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, MacKinnon S, Rounsaville B, Sierra S. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology. 2006;74:592–601. doi: 10.1037/0022-006X.74.3.592. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. Journal of Consulting and Clinical Psychology. 2007;75:983–991. doi: 10.1037/0022-006X.75.6.983. M. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. Journal of Consulting and Clinical Psychology. 2012b;80:286–298. doi: 10.1037/a0026826. doi: 10.1037/a0026826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: Contingency management treatment of substance abusers in community treatment settings. Journal of Consulting and Clinical Psychology. 2005;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology. 2012a;80:276–285. doi: 10.1037/a0026883. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Pierce JM, Roll JM, Stitzer ML, Hamilton JA. Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence. 2006;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry N, Rash CJ, Easton CJ. Contingency management treatment in substance abusers with and without legal problems. Journal of the American Academy of Psychiatry and the Law. 2011;39:37–378. [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Martin B. Reinforcing compliance with non-drug-related activities. Journal of Substance Abuse Treatment. 2001;20:33–44. doi: 10.1016/s0740-5472(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. Journal of Consulting and Clinical Psychology. 2011;79:686–696. doi: 10.1037/a0024813. doi: 10.1037/a0024813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, Cone EJ. Cocaine use early in treatment predicts outcome in a behavioral treatment program. Journal of Consulting and Clinical Psychology. 1998;66:691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Qureshi A, Mohammad Y, Suri MF, Braimah J, Janardhan V, Guterman LR, Frankel MR. Cocaine use and hypertension are major risk factors for intracerebral hemorrhage in young African Americans. Ethnicity and Disease. 2001;11:311–319. [PubMed] [Google Scholar]
- Rash CJ, Andrade LF, Petry NM. Income received during treatment does not affect response to contingency management treatments in cocaine-dependent outpatients. Drug and Alcohol Dependence. 2013;3:528–534. doi: 10.1016/j.drugalcdep.2013.03.020. doi: 10.1016/j.drugalcdep.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash CJ, Olmstead TA, Petry NM. Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug and Alcohol Dependence. 2009;104:249–253. doi: 10.1016/j.drugalcdep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher JE, Milby JB, Wallace D, Simpson C, Frison S, McNamara C, Usdan S. Diagnostic compared with abstinence outcomes of day treatment and contingency management among cocaine dependent homeless persons. Experimental and Clinical Psychopharmacology. 2003;11:146–157. doi: 10.1037/1064-1297.11.2.146. [DOI] [PubMed] [Google Scholar]
- Secades-Villa R, Garcia-Fernandez G, Pena-Suarez E, Garcia-Rodriguez O, Sanchez-Hervas E, Fernandez-Hermida JR. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. Journal of Substance Abuse Treatment. 2013;44:349–354. doi: 10.1016/j.jsat.2012.08.018. doi: 10.1016/j.jsat.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Shah S, DeMatteo D, Keesler M, Davis J, Heilbrun K, Festinger DS. Addiction Severity Index scores and urine drug screens at baseline as predictors of graduation from drug court. Crime and Delinquency. 2013:59. [Google Scholar]
- Sofuoglu M, Gonzalez G, Polling J, Kosten TR. Prediction of treatment outcome by baseline urine cocaine results and self-reported cocaine use for cocaine and opioid dependence. American Journal of Drug and Alcohol Abuse. 2003;29:713–727. doi: 10.1081/ada-120026256. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Petry N, Peirce J, Kirby K, Killeen T, Roll J, Li R. Effectiveness of abstinence-based incentives: Interaction with intake stimulant test results. Journal of Consulting and Clinical Psychology. 2007;75:805–811. doi: 10.1037/0022-006X.75.5.805. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Polk T, Bowles S, Kosten T. Drug users’ adherence to a 6-month vaccination protocol: Effects of motivational incentives. Drug and Alcohol Dependence. 2010;107:76–79. doi: 10.1016/j.drugalcdep.2009.09.006. doi: 10.1016/j.drugalcdep.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Tobin KE, German D, Spikes P, Patterson J, Latkin C. A comparison of the social and sexual network of crack-using and non-crack using African American men who have sex with men. Journal of Urban Health. 2011;88:1052–1062. doi: 10.1007/s11524-011-9611-4. doi: 10.1007/s11524-011-9611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzilos G, Rhodes GL, Ledgerwood DM, Greenwald MK. Predicting cocaine group treatment outcome in cocaine-abusing methadone patients. Experimental and Clinical Psychopharmacology. 2009;17:320–325. doi: 10.1037/a0016835. [DOI] [PubMed] [Google Scholar]
- Walton MA, Blow FC, Booth BM. Diversity in relapse prevention needs: Gender and race comparisons among substance abuse treatment patients. American Journal of Drug and Alcohol Abuse. 2001;27:225–240. doi: 10.1081/ada-100103707. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Petry NM. Interaction effects of age and contingency management treatments in cocaine-dependent outpatients. Experimental and Clinical Psychopharmacology. 2011;19:173–181. doi: 10.1037/a0023031. doi: 10.1037/a0023031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LM, Petry NM. Older methadone patients achieve greater durations of cocaine abstinence with contingency management than younger patients. American Journal of Addictions. 2013;22:119–126. doi: 10.1111/j.1521-0391.2013.00306.x. doi: 10.1111/j.1521-0391.2013.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]