Abstract
Objective
Our aim was to determine the relationship of various thoracic fat depots to the presence and extent of coronary artery plaque and circulating biomarkers.
Methods
In 342 patients (52±11 years, 61% male, BMI 29.1±5.9 kg/m2) with coronary CT angiography, we measured the fat volume in four thoracic depots (pericoronary, epicardial, periaortic, extracardiac), assessed coronary plaque and determined the circulating level of C-reactive protein, tumor necrosis factor alpha, plasminogen activator inhibitor-1, monocyte chemoattractant-1, and adiponectin. Extent of coronary plaque was classified into 3 groups: 0, 1–3 and >3 segments.
Results
Patients with plaque (n=169, 49%) had higher volumes of all 4 fat depots as compared to patients without plaque (all p<0.01), despite similar BMI (p=0.18). Extracardiac fat was most strongly correlated with BMI (r=0.45, p<0.001), while pericoronary fat was least (r=0.21, p<0.001). Only pericoronary fat remained associated with coronary plaque in adjusted analyses. Inflammatory biomarkers showed a positive correlation with pericoronary fat (all p<0.0001), whereas adiponectin was not associated to this fat compartment (p=0.60) and showed a negative correlation with all other fat depots (all p<0.001).
Conclusion
Pericoronary fat is independently associated with CAD. Its correlation with inflammatory biomarkers suggests that while systemic inflammation plays a role in the pathogenesis of CAD, there are additional local effects that may exist.
Keywords: pericoronary fat, coronary atherosclerosis, cardiac computed tomography
Introduction
An influence of various thoracic fat depots on development of coronary artery disease (CAD) has been suggested, as elevated visceral fat volume is closely associated to cardiovascular risk factors1 and the presence of cardiovascular disease2. A variety of fat depots have been found to be associated with coronary atherosclerotic disease burden including epicardial, periaortic, intrathoracic fat, and visceral abdominal fat3–10. It has been suggested that regional fat depots may have a greater influence on the development of CAD rather than overall measures of adiposity2, 10–12. Although perivascular fat depots may be smaller in volume compared to overall subcutaneous fat tissue, their close proximity to the vessel intima may lead to increased risk of atherogenesis through paracrine inflammatory effects6, 13, 14.
Pericoronary fat is part of the epicardial adipose tissue that directly surrounds the coronary arteries. It has been suggested that pro-inflammatory cytokines produced by pericoronary fat might amplify vascular inflammation in the local environment leading to atherogenesis, plaque instability, and neovascularization13, 15, 16. We recently described a new volumetric method of measuring pericoronary fat quantity and in a pilot study we found that pericoronary fat volume is increased in patients and surrounding vessels with coronary plaque17.
Coronary computed tomography angiography (CT) allows for simultaneous assessment of coronary atherosclerosis (non-calcified and calcified plaques) and thoracic fat volumes12. It is not yet known which thoracic fat depot is most strongly associated with the presence of CAD. To better understand the relationship of fat and CAD, we aimed to determine the association of four different thoracic fat depots including pericoronary, epicardial, periaortic, and extracardiac fat to the presence and extent of CAD as measured by contrast-enhanced CT.
Inflammatory processes have evolved as important mediators of all stages of atherosclerosis18. To assess systemic inflammation we determined the circulating levels of C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), plasminogen activator inhibitor-1 (PAI-1), and monocyte chemoattractant protein-1 (MCP-1). In addition we measured adiponectin, which plays a role in the development of insulin resistance and atherosclerosis through its potent anti-inflammatory and anti-atherogenic effects19.
Methods
Study population
From May 2005 to May 2007 consecutive subjects were prospectively enrolled as part of the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial (NCT00990262). Details of the study have been previously reported20. Briefly, the main inclusion criteria were: patients with age >18 years and admitted to rule out myocardial infarction through standard care protocols. The main exclusion criteria were: Elevated troponin I or CK-MB levels in the initial blood sample obtained in the emergency department; new diagnostic ECG changes for myocardial infarction; hemodynamic or clinical instability; history of established CAD, defined as stent implantation or coronary artery bypass grafting. From the 368 ROMICAT patients who underwent 64-slice multi-detector CT, only patients where pericoronary, epicardial, periaortic, and intrathoracic fat (Figure 1) were available for measurements were included in this analysis. We excluded a total of 26 patients who did not have axial images extending caudally to allow for measurement of periaortic fat and thus included a total of 342 patients.
Figure 1.
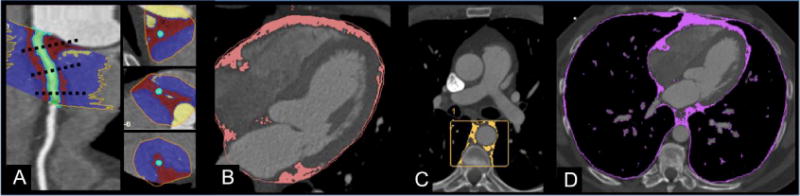
Depiction of thoracic adipose tissue depots on contrast enhanced cardiac computed tomography: A) Pericoronary, B) Epicardial, C) Periaortic, D) Intrathoracic fat depots. A. Pericoronary fat is indicated by red voxels. Green voxels represent the coronary vessel lumen. B. Epicardial fat (pink) includes all fat contained within the visceral pericardium. Epicardial fat includes all pericoronary fat. C. Periaortic fat (yellow) includes fat surrounding the descending thoracic aorta. D. Intrathoracic fat (purple) is entirety of fat within the thorax including the areas of fat within the pericardium and external to the pericardium is intrathoracic fat.
Computed Tomography Imaging Protocol
CT imaging was performed using a standard coronary artery 64-slice multidetector CT (Sensation 64, Siemens Medical Solutions, Forchheim, Germany) imaging protocol using a 330 ms rotation time, 32 × 0.6 mm collimation, tube voltage of 120 kVp, and maximum effective tube current-time product of 850 mAs, which has been published previously20.
Adipose Tissue Assessment
Pericoronary Adipose Tissue
Pericoronary fat volume (Figure 1A) was measured using a method of threshold-based volumetric pericoronary fat volume (cm3) assessment based on a modified application of software for coronary plaque quantification17. Briefly, pericoronary fat measurements started at the ostium of the left main (LM)/left anterior descending coronary artery (LAD), left circumflex artery (LCx), and right coronary artery (RCA) and continued to a distance of 40 mm. Manual tracing was used to circle the region containing pericoronary fat in cross-sectional images perpendicular to the vessel centerline in every 5 mm. The exact pericoronary fat volume within the manually traced region was calculated by the software using Hounsfield unit (HU) based thresholds. Voxels with values between the minimum setting of the SUREPlaque (Vitrea 2, Version 3.9.0.1, Vital Images Inc, Plymouth, MN) tool (−149HU) and an upper threshold of −30HU were used to represent adipose tissue, and the total pericoronary volume was calculated by summing these voxels along the course of each coronary artery.
Epicardial Adipose Tissue (Excluding Pericoronary)
Epicardial fat volume (Figure 1B), defined as adipose tissue contained within the visceral pericardium, in cm3 was measured according to the method previously described3. Briefly, measurements were made on axial CT images using a semiautomatic software program (Volume Viewer, Siemens Medical Solutions, Forchheim, Germany) at 10 mm intervals with interpolation of fat volume between the planar regions of interest. Manual adjustment was made when necessary to correct for interpolation errors and tracings were confirmed through use of sagittal and coronal planes. Pixels with HU values of −190 to −30 within the selected region were defined as adipose tissue. Epicardial fat volume used for analysis was calculated as the absolute difference between the measured epicardial fat and pericoronary fat volumes.
Periaortic Adipose Tissue
Periaortic fat volume (Figure 1C) was measured in accordance with the previously published methods using a semiautomated method on a dedicated offline workstation (Volume Viewer, Siemens Medical Solutions, Forchheim, Germany)8, 21. Briefly, the volume of interest was defined by an approximately 7.0 cm vertical column of fat surrounding the thoracic aorta between the pulmonary artery bifurcation and the diaphragm21. Periaortic fat was defined by voxels between −190 and −30 HU within this columnar region of interest, and total periaortic fat volume was determined.
Extracardiac Adipose Tissue
For the calculation of the extracardiac fat volume in cm3 we have subtracted epicardial fat volume from the sum of intrathoracic fat and periaortic fat volumes. Intrathoracic fat volume (Figure 1D) defined as all fat contained within the mediastinum3. For intrathoracic fat measurements, the mediastinum was defined as the area bordered by the sternum anteriorly, anterior wall of the descending aorta posteriorly, the center of the right pulmonary artery superiorly, and the diaphragm inferiorly. Pixels from −190 to −30 HU within the mediastinal boundaries were defined as intrathoracic fat.
Coronary Artery Plaque Assessment
Presence of coronary artery plaque by CT was assessed based on a 17-segment model20, 22. Extent of coronary artery plaque burden was examined by stratifying patients into 3 groups, those with 0 segments containing plaque, 1–3 segments containing plaque, or >3 segments containing plaque.
Biomarker Testing
Peripheral venous samples for biomarker testing were collected at the time of the CT scan. Samples were collected into ethylenediaminetetraacetic acid (EDTA) coated tubes and non-coated tubes, and immediately centrifuged. The aliquoted plasma and serum were stored in microcentrifuge tubes at −80°C until assayed. Specimens were tested on the first freeze thaw cycle. All analyses were performed in an independent laboratory (Biomarker Laboratory at the Department of Cardiology, University of Ulm, Germany) in a blinded fashion, irrespective of the clinical and CT findings. Concentration of hs-CRP was measured nephelometrically on a BN II analyzer (Dade-Behring, Marburg, Germany). Enzyme-linked immunosorbent assays (ELISA) from R&D Systems (Wiesbaden, Germany) were used to measure TNF-α, PAI-1, MCP-1, and adiponectin. The intra-assay coefficient of variation (CV) and inter-run CV were ≤10% for all markers.
Statistical Analysis
Continuous variables are reported as mean ± standard deviation (SD) or median and interquartile range (IQR), as appropriate. Discrete variables are given in frequency and percentiles. To compare the differences in characteristics between patients with and without CAD, we used t-test or Wilcoxon rank sum test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables as appropriate. We used Pearson’s correlation to compare the normally distributed fat depots with each other and to BMI. We used the partial Spearman’s correlation to assess the strength of association between non-normally distributed biomarker levels and fat compartments, adjusting for presence of coronary plaque. For the association of each of the fat depots to the presence of coronary artery plaque as well as the extent of plaque, we used logistic regression based on a per 10 cm3 increase. Ordinal logistic regression analysis was adjusted for age, gender, diabetes, hypertension, dyslipidemia, smoking, BMI, aspirin use, and statin use. A two-tailed p-value of <0.05 was considered significant. All analyses were performed using the SAS software (Version 9.2, SAS Institute Inc, Cary, North Carolina).
Results
The demographic characteristics of the 342 patients are described in Table 1. Patients with coronary plaque were predominately male and had an increased rate of cardiovascular risk factors such as hypertension, dyslipidemia, and smoking compared to those without CAD, all p values p≤0.01. There was no significant difference in race, BMI, prevalence of diabetes, or family history of premature CAD between patients with and without CAD.
Table 1.
Patient Characteristics of Cohort and of Subjects With Versus Without CAD.
| Overall Cohort n=342 |
No Plaque n=173 |
Plaque n=169 |
P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years (±SD) | 52.5±11.5 | 47.6±9.5 | 57.6±10.4 | < 0.0001 |
| Male (%) | 210 (61%) | 94 (54%) | 116 (69%) | 0.008 |
| White Race (%) | 294 (86%) | 143 (83%) | 151 (89%) | 0.09 |
| BMI, kg/m2 (±SD) | 29.1±5.9 | 28.7±6.0 | 29.5±5.7 | 0.18 |
|
| ||||
| Risk factors | ||||
| Diabetes (%) | 35 (10%) | 12 (7%) | 23 (14%) | 0.05 |
| Hypertension (%) | 133 (39%) | 44 (25%) | 89 (53%) | < 0.0001 |
| Dyslipidemia (%) | 128 (37%) | 40 (23%) | 88 (52%) | < 0.0001 |
| Smoking (%) | 171 (50%) | 72 (42%) | 99 (59%) | 0.002 |
| FH of CAD (%) | 85 (25%) | 37 (21%) | 48 (28%) | 0.17 |
|
| ||||
| Medication | ||||
| Aspirin (%) | 108 (32%) | 46 (27%) | 62 (37%) | 0.05 |
| Statins (%) | 99 (29%) | 31 (18%) | 68 (40%) | < 0.01 |
|
| ||||
| Biomarkers | ||||
| hsCRP (IQR) | 1.4 (0.6–2.9) | 1.1 (0.5–2.4) | 1.7 (0.9–3.4) | 0.0004 |
| TNFα (IQR) | 1.1 (0.7–1.9) | 1.0 (0.6–1.8) | 1.1 (0.8–2.1) | 0.24 |
| PAI-1 (IQR) | 12.3 (6.1–24.0) | 11.8 (5.6–24.1) | 13.1 (7.1–23.7) | 0.22 |
| Adiponectin (IQR) | 4.9 (2.9–7.7) | 4.9 (3.1–7.9) | 4.8 (2.6–7.4) | 0.33 |
| MCP-1 (IQR) | 248.5 (181.0–348.0) | 248.0 (178.0–316.0) | 252.0 (183.0–367.0) | 0.34 |
| Fat Measures in cm3 | ||||
| Pericoronary (±SD) | 29.9±17.1 | 24.0±12.9 | 35.7±18.8 | < 0.0001 |
| Epicardial* (±SD) | 74.4±37.3 | 63.5±31.4 | 85.5 ±39.6 | < 0.0001 |
| Periaortic (±SD) | 15.5±9.0 | 12.3±6.7 | 18.7±10.0 | < 0.0001 |
| Extracardiac*(±SD) | 99.9±63.2 | 83.3±59.2 | 117.0±62.7 | < 0.0001 |
CAD denotes coronary artery disease; SD, standard deviation; BMI, body mass index; FH, family history; IQR, interquartile range
Epicardial fat compartment excluded pericoronary fat. Extracardiac fat compartment is defined as the thoracic fat volume without epicardial or periaortic fat.
Correlation of fat depots to BMI
Table 2 demonstrates that all four fat depots were highly correlated with each other and showed a modest positive correlation with BMI. The largest adipose tissue depot, extracardiac fat (volume 99.9±63.2 cm3), was most strongly correlated with BMI, (r=0.45, p<0.001). The pericoronary fat depot (volume 29.9±17.1 cm3) was least correlated to BMI (r=0.21, p<0.001).
Table 2.
Correlation Among Fat Measures and Body Mass Index (BMI)
| BMI | Pericoronary | Epicardial | Periaortic | Extracardiac | |
|---|---|---|---|---|---|
| BMI | – | 0.21 | 0.44 | 0.44 | 0.45 |
| Pericoronary | 0.21 | – | 0.70 | 0.54 | 0.49 |
| Epicardial | 0.44 | 0.67 | – | 0.69 | 0.70 |
| Periaortic | 0.44 | 0.54 | 0.69 | – | 0.75 |
| Extracardiac | 0.45 | 0.49 | 0.70 | 0.75 | – |
All p<0.0001
Association of fat depots to presence of coronary plaque
Despite no difference in BMI (p=0.18), patients with coronary plaque had higher volumes of all fat depots as compared to patients without plaque (all p<0.01). We used logistic regression to determine the association between fat depots and the presence of plaque on a per patient basis. All four fat depots were associated with the presence of any coronary artery plaque in unadjusted analysis, all p <0.001 (Table 3). In adjusted analyses only pericoronary fat were found to be independently associated to the presence of coronary artery plaque (p=0.006), while epicardial, periaortic and extracardiac fat depots were not (all p≥0.08), Figure 2A.
Table 3.
Unadjusted and Adjusted Analysis of Pericoronary Fat Volume to Presence of any Plaque on a per Patient Basis per 10cm3 Increase in Fat Volume
| Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) 1 | p-value | |
|---|---|---|---|---|
| Pericoronary | 1.66 (1.41–1.97) | < 0.0001 | 1.31 (1.08–1.59) | 0.006 |
| Epicardial | 1.21 (1.12–1.29) | < 0.0001 | 1.09 (0.99–1.19) | 0.08 |
| Periaortic | 2.74 (1.98–3.78) | < 0.0001 | 1.40 (0.87–2.23) | 0.16 |
| Extracardiac | 1.10 (1.06–1.15) | < 0.0001 | 1.04 (0.99–1.10) | 0.13 |
OR denotes odds ratio; CI, confidence interval
Adjusted for age, gender, diabetes, hypertension, dyslipidemia, smoking, BMI, aspirin use, statin use
Figure 2.
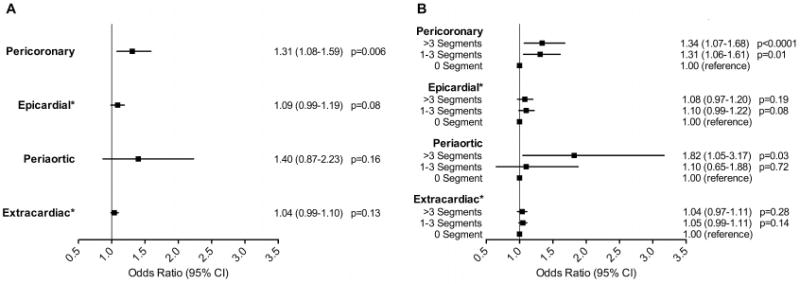
The relationship of thoracic adipose tissue volumes (per 10 cm3 increase) to the presence of coronary atherosclerotic plaque (A) and the extent of atherosclerotic plaque by number of coronary segments (B). Adjusted for age, gender, diabetes, hypertension, dyslipidemia, smoking, body mass index, aspirin use and statin use. *Epicardial fat compartment excluded pericoronary fat. Extracardiac fat compartment is defined as the thoracic fat volume without epicardial or periaortic fat. OR denotes odds ratio; CI, confidence interval.
Association of fat depots to extent of coronary plaque
We examined the association between the four fat depots to the extent of plaque and found that pericoronary fat remained associated in adjusted analysis in patients with at least one segment of plaque as compared to those without plaque, irrespective of amount of plaque burden (Figure 2B and Table 4). In addition, periaortic fat showed an association with CAD that affects more than 3 segments of the coronaries (p=0.03).
Table 4.
Relation of Adipose Tissue Volume (per 10cm3 Increase) to Extent of Plaque by Number of Segments.
| Unadjusted | Adjusted1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 1–3 Segments vs. 0 Segment | >3 Segments vs. 0 Segment | 1–3 Segments vs. 0 Segment | >3 Segments vs. 0 Segment | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Pericoronary | 1.57 (1.31–1.89) | <0.0001 | 1.76 (1.46–2.12) | <0.0001 | 1.31 (1.06–1.61) | 0.01 | 1.34 (1.07–1.68) | <0.0001 |
| Epicardial | 1.18 (1.09–1.28) | <0.0001 | 1.23 (1.14–1.34) | <0.0001 | 1.10 (0.99–1.22) | 0.08 | 1.08 (0.97–1.20) | 0.19 |
| Periaortic | 2.04 (1.41–2.96) | 0.0002 | 3.69 (2.53–5.38) | <0.0001 | 1.10 (0.65–1.88) | 0.72 | 1.82 (1.05–3.17) | 0.03 |
| Extracardiac | 1.09 (1.04–1.14) | 0.0003 | 1.11 (1.06–1.17) | <0.0001 | 1.05 (0.99–1.11) | 0.14 | 1.04 (0.97–1.11) | 0.28 |
OR, odds ratio; CI, confidence interval.
Adjustment for age, gender, diabetes, hypertension, dyslipidemia, smoking, BMI, aspirin use, statin use
Association of fat depots to circulating levels of biomarkers
We also examined the correlation between the various fat depots and markers of inflammation independent of CAD. Table 5 demonstrates that the circulating hsCRP and PAI-1 levels showed a modest positive correlation with all fat depots (all p≤0.003). Whereas, TNFα level showed a modest positive correlation only with the perivascular fat depots, such as the pericoronary and periaortic fat compartments (p<0.0001 and p=0.02, respectively). MCP-1 correlated with the fat compartments closest to the heart, pericoronary and epicardial fat compartments (p<0.0001 and p=0.006, respectively). On the other hand, adiponectin was not associated with the pericoronary fat depot. However, it showed a modest negative correlation with epicardial (p=0.001), periaortic (p<0.0001) and extracardiac (p<0.000) fat compartments.
Table 5.
Partial Correlation Among Biomarkers and Various Adiopose Tissue Depots Adjusted for the Presence of Coronary Artery Plaque.
| Pericoronary | Epicardial | Periaortic | Extracardiac | |
|---|---|---|---|---|
| hsCRP | 0.21 0.0002 |
0.22 <0.0001 |
0.29 <0.0001 |
0.21 0.0003 |
| TNFα | 0.25 <0.0001 |
0.10 0.07 |
0.132 0.02 |
0.07 0.21 |
| PAI-1 | 0.22 <0.0001 |
0.27 <0.0001 |
0.20 0.0003 |
0.24 <0.0001 |
| Adiponectin | −0.03 0.60 |
−0.15 0.001 |
−0.28 <0.0001 |
−0.27 <0.0001 |
| MCP-1 | 0.30 <0.0001 |
0.16 0.006 |
0.08 0.14 |
0.09 0.14 |
Discussion
The study provides a mechanistic view on various fat compartments located in the thorax and their relationship to coronary artery plaque and systemic markers of inflammation. We found that all four thoracic fat depots were higher in patients with coronary plaque compared to those without despite no difference in BMI. Correlation of the fat depots to BMI was moderate for epicardial, periaortic, and extracardiac fat depots and it was modest for the pericoronary fat compartment. The strength of association to coronary plaque was dependent on the proximity of the fat depot to the coronary arteries. Furthermore, there was an association between higher volumes of perivascular fat depots to the presence of plaque, and more specifically pericoronary fat to presence of CAD irrespective of the extent of CAD. Despite being the least correlated to BMI, pericoronary fat, which is one of the smallest fat depots yet closest in proximity to the coronary vasculature, was most consistently associated with CAD. Interestingly, the fat depots farther from the coronary vasculature (epicardial, periaortic and extracardiac) attenuated in their association to CAD after adjustment for cardiovascular risk factors. Furthermore, circulatory biomarkers of inflammation showed the strongest positive correlation with fat compartments closest to the coronary arteries. Interestingly, adiponectin was not associated with pericoronary adipose tissue, and it showed a negative correlation with the other intrathoracic fat depots.
It has long been understood that increased adipose tissue volume and elevated BMI is associated with increase in cardiovascular disease risk23. Our study further extends to the relationship of the local fat volumes closest to the heart and their relationship to CAD. Our findings that increased volume of thoracic fat depots closest to the coronary vessels are associated to presence of coronary plaque are consistent with previous studies that showed that pericoronary fat is associated with coronary atherosclerosis in the local underlying coronary segment in patients with known or suspected CAD9.
We found that the adipose tissue depot in closest proximity to the coronary artery vessels (pericoronary fat compartment) remained independently associated to the presence of coronary plaque even following adjustment for BMI and other CAD risk factors. Notably, pericoronary adipose tissue was found to be the least correlated to BMI in our analysis. This further suggests the presence of a local atherogenic effect of adipose tissue. These results suggest that coronary atherosclerosis might be influenced by the fat depot in closest proximity to the coronary vasculature. Furthermore, to account for systemic inflammation, which is a well known risk factor of CAD, we have assessed the levels of several inflammatory biomarkers. The intrathoracic fat depots showed an association with circulating inflammatory biomarker levels irrespective of CAD. The strongest correlations were present between hsCRP and PAI-1 and the fat depots. These findings are consistent with previous studies describing increased inflammatory status and the predisposition of thrombosis in patients with increased adipose tissue volumes24. Adiponectin was not associated to the pericoronary fat tissue and it showed an inverse relationship with all other intrathoracic fat compartments. Mechanistically, this finding is consistent with the results of a recently published meta-analysis, which showed no association between adiponectin and CAD25.
It has been suggested that locally acting perivascular fat depots such as pericoronary fat may contribute to the development of cardiovascular disease through the modulation of vascular tone, oxidative stress, and inflammation26, 27. Thoracic fat located close to the coronary arteries has also been shown to be associated to the presence of calcified plaque in large population based studies such as the Multi-Ethnic Study of Atherosclerosis4. Importantly, EAT and PCAT are most probably consisting of the same type of metabolically active adipose tissue, however their difference in proximity from the coronary wall what renders potentially different pathophysiologic roles in the process of atherogenesis. The mechanism of action of local perivascular fat depots on the development of CAD is currently under investigation, and multiple studies have shown that visceral fat secretes a variety of inflammatory cytokines including interleukin-6, adiponectin, and TNFα13, 28–31. Adipocytokines secreted by local fat tissue may diffuse into the vessel wall promoting the development of atherosclerosis independent of the effects of total body fat stores or systemic levels of inflammation. In addition, a genome wide association study has recently shown a specific genetic locus to be associated with the ectopic deposition of fat, further emphasizing the unique role of the adipose tissue located within the pericardium32.
Taken together, our results support the current literature suggesting that there is a local effect of pericoronary adipose tissue on the development of CAD. Our results also suggest that that there is a gradient in terms of CAD risk from extracardiac fat compartment towards the pericoronary adipose tissue depot. Furthermore, the circulatory markers of inflammation were correlated to the intrathoracic fat compartments irrespective of CAD, which underscores the endocrine organ-like functions of adipose tissue.
Limitations
There are several limitations to the current study. The ROMICAT cohort included patients with no prior history of CAD who presented to the emergency department with acute chest pain, thus the results of this study may not be applicable to patients with pre-existing CAD. Differences in Hounsfield unit cut off values for measuring pericoronary and the other adipose tissue depots due to software limitations needs to be addressed in future studies where all four perivascular fat depots are measured with the same software program and HU thresholds. In the future, measurement of visceral fat depots would be of interest in addition to thoracic fat depots.
Conclusions
Of the thoracic fat depots, pericoronary fat is associated with coronary atherosclerosis independently of the standard measures of obesity such as BMI. Furthermore, its correlation with inflammatory biomarkers but not adiponectin, a marker of visceral fat, suggests that while systemic inflammation plays a role in the pathogenesis of coronary atherosclerosis, there are additional local effects that exist.
What is already known about this subject
Increased adipose tissue volume and elevated BMI is associated with increase in cardiovascular disease risk.
A variety of fat depots have been found to be associated with coronary atherosclerotic disease burden including epicardial, periaortic, intrathoracic fat, and visceral abdominal fat.
It has been suggested that pro-inflammatory cytokines produced by pericoronary fat might amplify vascular inflammation in the local environment leading to atherogenesis, plaque instability, and neovascularization.
What this study adds
Of the thoracic fat depots, pericoronary fat shows the strongest association with coronary atherosclerosis.
The adipose tissue depot in closest proximity to the coronary artery vessels (pericoronary fat compartment) is independently associated to the presence of coronary plaque even following adjustment for BMI and other cardiovascular risk factors.
The correlation of pericoronary fat with inflammatory biomarkers suggests that while systemic inflammation plays a role in the pathogenesis of coronary atherosclerosis, there are additional local effects that may exist.
Acknowledgments
The author would like to thank Mrs Gerlinde Trischler (University of Ulm) for excellent technical assistance.
Sources of Funding: This work was supported by the NIH R01 HL080053, and in part supported by Siemens Medical Solutions and General Electrics Healthcare. Dr. Truong received support from NIH grant K23HL098370 and L30HL093896. Additional funds for biomarker determination came from the University of Ulm Medical Centre.
Abbreviations and Acronyms
- BMI
body mass index
- CAD
Coronary artery disease
- CRP
C-reactive protein
- CT
Computed tomography
- HU
Hounsfield unit
- LAD
left anterior descending coronary artery
- LCX
left circumflex coronary artery
- LM
left main coronary artery
- MCP-1
monocyte chemoattractant-1
- PAI-1
plasminogen activator inhibitor-1
- RCA
right coronary artery
- ROMICAT
Rule Out Myocardial Infarction using Computer Assisted Tomography
- TNFα
tumor necrosis factor alpha
Footnotes
Competing interests: the authors have no competing interests.
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
References
- 1.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–13. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 2.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. European heart journal. 2009;30:850–6. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols JH, Samy B, Nasir K, Fox CS, Schulze PC, Bamberg F, et al. Volumetric measurement of pericardial adipose tissue from contrast-enhanced coronary computed tomography angiography: a reproducibility study. Journal of cardiovascular computed tomography. 2008;2:288–95. doi: 10.1016/j.jcct.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) The American journal of clinical nutrition. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MF, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. The American journal of cardiology. 2008;102:380–5. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American College of Cardiology. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. The American journal of cardiology. 2008;102:1602–7. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Massaro JM, Schlett CL, Lehman SJ, Meigs JB, O’Donnell CJ, et al. Periaortic fat deposition is associated with peripheral arterial disease: the Framingham heart study. Circulation Cardiovascular imaging. 2010;3:515–9. doi: 10.1161/CIRCIMAGING.110.958884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–9. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis G, Lonn E, Lamy A, Singh N, Sharma AM. Epicardial fat thickness and coronary artery disease correlate independently of obesity. Int J Cardiol. 2011;146:452–4. doi: 10.1016/j.ijcard.2010.10.117. [DOI] [PubMed] [Google Scholar]
- 11.Poirier P, Despres JP. Waist circumference, visceral obesity, and cardiovascular risk. Journal of cardiopulmonary rehabilitation. 2003;23:161–9. doi: 10.1097/00008483-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H, et al. Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol. 2012;161:45–9. doi: 10.1016/j.ijcard.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–5. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, et al. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. The Journal of clinical investigation. 1996;97:769–76. doi: 10.1172/JCI118476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorter PM, van Lindert AS, de Vos AM, Meijs MF, van der Graaf Y, Doevendans PA, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Maurovich-Horvat P, Kallianos K, Engel LC, Szymonifka J, Fox CS, Hoffmann U, et al. Influence of pericoronary adipose tissue on local coronary atherosclerosis as assessed by a novel MDCT volumetric method. Atherosclerosis. 2011;219:151–7. doi: 10.1016/j.atherosclerosis.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 19.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–50. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlett CL, Massaro JM, Lehman SJ, Bamberg F, O’Donnell CJ, Fox CS, et al. Novel measurements of periaortic adipose tissue in comparison to anthropometric measures of obesity, and abdominal adipose tissue. Int J Obes (Lond) 2009;33:226–32. doi: 10.1038/ijo.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bamberg F, Dannemann N, Shapiro MD, Seneviratne SK, Ferencik M, Butler J, et al. Association between cardiovascular risk profiles and the presence and extent of different types of coronary atherosclerotic plaque as detected by multidetector computed tomography. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:568–74. doi: 10.1161/ATVBAHA.107.155010. [DOI] [PubMed] [Google Scholar]
- 23.Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arteriosclerosis, thrombosis, and vascular biology. 1996;16:1509–15. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 24.Darvall KA, Sam RC, Silverman SH, Bradbury AW, Adam DJ. Obesity and thrombosis. Eur J Vasc Endovasc Surg. 2007;33:223–33. doi: 10.1016/j.ejvs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–9. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 26.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28(Suppl 4):S58–65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- 27.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–70. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 28.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–4. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 29.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovascular diabetology. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. The Journal of clinical endocrinology and metabolism. 2006;91:4620–7. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 31.Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovascular research. 2007;75:690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Fox CS, White CC, Lohman K, Heard-Costa N, Cohen P, Zhang Y, et al. Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat. PLoS genetics. 2012;8:e1002705. doi: 10.1371/journal.pgen.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]


