Abstract
Transgenic expression of neurotrophic factors in skeletal muscle has been found to protect mice from neuromuscular disease, including spinal bulbar muscular atrophy (SBMA), triggering renewed interest in neurotrophic factors as therapeutic agents for treating neuromuscular disease. Because SBMA is an androgen-dependent disease, and brain-derived neurotrophic factor (BDNF) mediates effects of androgens on neuromuscular systems, we asked whether BDNF expression is impaired in two different transgenic (Tg) mouse models of SBMA, the so called “97Q” and “myogenic” SBMA models. The 97Q model globally overexpresses a full length human AR with 97 glutamine repeats whereas the myogenic model of SBMA overexpresses a wild-type rat androgen receptor (AR) only in skeletal muscle fibers. Using quantitative PCR, we find that muscle BDNF mRNA declines in an androgen-dependent manner in both models, paralleling changes in motor function, with robust deficits (6-8 fold) in both fast and slow twitch muscle of impaired Tg males. Castration rescues or reverses disease-related deficits in muscle BDNF mRNA in both models, paralleling its effect on motor function. Moreover, when disease is acutely induced in Tg females, both motor function and muscle BDNF mRNA expression plummet, with the deficit in muscle BDNF emerging before overt motor dysfunction. That androgen-dependent motor dysfunction is tightly associated with a robust and early down-regulation of muscle BDNF mRNA suggests that BDNF delivered to muscle may have therapeutic value for SBMA.
Keywords: motoneuron disease, neuromuscular disease, neurodegenerative disease, neurotrophic factor, neurotrophin
1. Introduction
Spinal bulbar muscular atrophy (SBMA) is an androgen-dependent neuromuscular disorder linked to a polyglutamine expansion mutation in the androgen receptor (AR) gene (La Spada et al., 1991). The disease affects middle-aged men and is characterized by progressive proximal muscle weakness and reduced androgen sensitivity. Muscle-derived neurotrophic factors (e.g., vascular endothelial growth factor (VEGF), insulin-like growth factor-1, glial cell line-derived neurotrophic factor, neurotrophin-4) have been implicated in SBMA (Mo et al., 2010; Monks et al., 2007; Sopher et al., 2004; Yamamoto et al., 1999; Yu et al., 2006); their expression is often reduced by disease and experimentally bolstering their expression in muscle can rescue SBMA mice from disease (Palazzolo et al., 2009). One possible mechanism by which muscle-derived neurotrophic factors combat disease is by correcting disease-related impairments in retrograde axonal transport (Kemp et al., 2011).
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, is also produced by muscle and implicated in neuromuscular disease (Korkmaz et al., 2014; Kust et al., 2002; Mitsumoto et al., 1994; Sagot et al., 1998) but has not been investigated as a possible player in SBMA. Because BDNF mediates effects of androgens on neuromuscular systems (Ottem et al., 2013; Verhovshek et al., 2013), and SBMA is an androgen-dependent neuromuscular disease, we examined BDNF expression and its androgen-dependence using quantitative polymerase chain reaction (qPCR) in two transgenic (Tg) mouse models of SBMA, the “97Q” and “myogenic” models.
While both models exhibit marked androgen-dependent motor dysfunction that affects only males, recapitulating core features of SBMA (Kennedy et al., 1968; Kinirons & Rouleau, 2008), the genetics behind each model are distinct. Disease in the 97Q model is triggered by global overexpression of a full length human AR containing a 97 polyglutamine tract (Katsuno et al., 2002) whereas disease in the myogenic model is triggered by overexpression of a wild-type (Wt) rat AR exclusively in muscle cells (Monks et al., 2007). While not fully understood, Wt and polyglutamine expanded AR appear to trigger disease via the same toxic mechanisms (Nedelsky et al., 2010). Thus, the myogenic model offers an opportunity for identifying muscle-specific components which critically drive SBMA (Cortes et al., 2014; Lieberman et al., 2014). Studying this model may also shed light on why some men with SBMA symptoms (muscle weakness, elevated creatine kinase levels, and gynecomastia) lack the disease-associated CAG expansion in their AR gene (Ferlini et al., 1995; Jobsis et al., 1995; Mariotti et al., 2000). Our goal is to identify common pathogenic processes across diverse models of SBMA, reasoning that such processes are also likely to critically mediate SBMA in humans.
The BDNF gene undergoes alternative splicing to produce multiple transcript variants. Each variant contains a non-coding exon (I – XIII) and a common coding exon (IX), with non-coding exons differentially expressed across tissue types (Aid et al., 2007). We chose to examine the historically better characterized exons: noncoding exons II, IV, and VI and the coding exon IX. We also examined expression of BDNF receptors TrkB (full length and truncated) and pan-neurotrophin p75, since complement changes in the level of full length TrkB, for example, could effectively maintain BDNF signaling at a normal level in the face of changes in BDNF expression itself. Both the full length TrkB and p75 receptors signal upon ligand binding (Reichardt, 2006), but the truncated TrkB isoform does not and may serve to concentrate BDNF at critical sites (Huang & Reichardt, 2003).
We find that BDNF mRNA expression in skeletal muscle tightly correlates with motor dysfunction in two SBMA mouse models; levels of muscle BDNF mRNA wax and wane in an androgen-dependent manner, paralleling the effects of androgen on motor function. Furthermore, we find that the deficit in muscle BDNF precedes overt expression of motor symptoms. We do not find that disease affects the expression of TrkB and p75 mRNA in either muscle or lumbar spinal cord nor does it affect BDNF mRNA expression in the lumbar spinal cord. These data suggest that deficits in muscle BDNF may critically underlie the loss of motor function in SBMA.
2. Materials and Methods
2.1 Animals
Animal colonies were held on a 12h:12h light:dark cycle, group housed, and provided food and water ad libitum. All animal procedures were approved and performed in compliance with the Michigan State University Institutional Animal Care and Use Committee in accordance with the standards in the NIH Guide for the Care and Use of Laboratory Animals.
97Q model
Tg animals ubiquitously overexpressing a full length human AR with a 97 glutamine repeat and Wt age-matched controls were maintained on a C57Bl/6J genetic background. Mice were genotyped using PCR at weaning as previously described (Katsuno et al., 2002).
Myogenic model
Tg animals overexpressing the rat Wt AR exclusively in skeletal muscle fibers and Wt age-matched controls were maintained on a C57Bl/6J genetic background. Mice were genotyped using PCR at weaning as previously described (Monks et al., 2007). Tg and Wt males were exposed prenatally to flutamide, an antiandrogen, to block the effects of prenatal endogenous androgens to enhance survival rate of Tg males neonatally (Johansen et al., 2011). Because survival of Tg females is not affected, females used in our studies were from litters not exposed to flutamide prenatally. In each case, Wt controls used in a given study were treated in the same way as the Tg mice.
2.2 Gonadally intact males
We examined BDNF mRNA expression in the fast twitch extensor digitorum longus (EDL) and slow twitch soleus muscles of adult, gonadally intact age-matched Wt and Tg males (mean age and range in postnatal days: 97Q: 69.1, 59-76; myogenic: 180.4, 171-194). We also assessed BDNF mRNA in lumbar spinal cords of the same Tg and Wt males of the two models. Tissue was harvested from chronically impaired myogenic males with severe motor dysfunction, comparable to what has previously been reported for this Tg model (Johansen et al., 2011; Monks et al., 2007). Tissue was harvested from 97Q males once they reached end-stage, defined as hang time < 30 sec (see description of test below).
2.3 Castrated males
97Q model
Tg and Wt males were castrated (Tg+Castrate, Wt+Castrate) or sham castrated (Tg+Sham, Wt+Sham) on postnatal day 28-32, before symptoms emerged as previously described (Renier et al., 2014). We monitored motor function twice weekly starting on the day of but prior to surgery, until gonadally intact (Tg+Sham) males reached end-stage (hang time <30 sec), at which point it and its age-matched controls (Tg+Castrate, Wt+Castrate and Wt+Sham) were sacrificed and muscles harvested (mean age and range in postnatal days: 77.8, 55-104).
Myogenic model
Since chronically affected adult myogenic males recover motor function after castration (Kemp et al., 2011; Monks et al., 2007), we castrated a cohort of age-matched adult Tg and Wt brothers to assess whether expression of BDNF mRNA in skeletal muscle of diseased males also recovers. Tg and Wt males were either castrated (Tg+Castrate, Wt+Castrate) or sham castrated (Tg+Sham, Wt+Sham)(mean age and range of postnatal days at castration: 106.3, 87-133). Motor function (described below) was monitored starting on the day of but just before surgery and on days 2, 4, 6, 8, 14, and 21 after surgery. Animals were sacrificed after the last motor test.
2.4 Androgen treated females
Tg females offer the experimental advantage in that disease is triggered in this model by exposure to male levels of testosterone (T). Thus, the time of disease onset is known and readily controlled, making this model ideal for examining the time course of change in muscle BDNF mRNA as disease develops. We first examined whether acutely diseased females (age matched to Wt females) exposed to five days of T show the same deficit in muscle BDNF as chronically diseased myogenic males (mean age and range in postnatal days: 117.7, 101-178). Treatment was delivered via Silastic capsules containing either crystalline T (effective release length: 6mm, inner diameter: 1mm, outer diameter: 3mm; soaked in sterile phosphate-buffered saline overnight) or nothing (“Blank”) implanted subcutaneously just caudal to the scapula under deep isoflurane anesthesia. Such T capsules produce T levels slightly below normal circulating levels for adult male mice (Johansen et al., 2009). Motor function of female mice was assessed immediately prior to surgery and daily during the five day treatment period, with tissue harvested on the final day of motor testing.
Because acutely disease myogenic (Tg) females also show a deficit in muscle BDNF mRNA after five days of T exposure, we next asked how soon the deficit emerges. We used a separate cohort of age-matched Tg and Wt females to assess BDNF mRNA levels in muscle after 1, 2, 3, 4, or 5 days of T exposure, monitoring motor function daily based on the hang test (mean age and range in postnatal days: 97.5, 81-130).
2.5 Motor function tests
Motor function was assessed using three different measures: number of rears in an open field, grip strength, and hang time as previously described (Johansen et al., 2011). The hang test provides a measure of overall limb strength. If the hang time on the first try was less than the maximum time possible, 120 sec, then mice were given two more tries with the highest score recorded for that session on that day.
2.6 Tissue collection
The EDL and/or soleus were collected on both sides from deeply anesthetized mice. Lumbar spinal cords were quickly extracted with pressurized air. All tissue was weighed fresh and immediately frozen in RNase-free tubes on dry ice, and held at −80°C until processed. All instruments were sprayed with RNaseZap (Sigma-Aldrich) between animal harvests. Additionally, blood was collected intracardially and plasma isolated to measure circulating plasma T levels as previously described (Johansen et al., 2009).
2.7 RNA extraction and quantitative real-time PCR
RNA extraction was performed for muscles with RNeasy Fibrous Tissue Mini Kit (Qiagen). To improve RNA yield for spinal cords, we used TRIzol reagent (Ambion). Tissue was mechanically homogenized with a PRO200 homogenizer (Pro Scientific). RNA was purified for each type of tissue according to the respective manufacturer directions. Spinal cord RNA samples were treated with DNase I (Invitrogen). A DNase treatment step is included as part of the Qiagen protocol for muscles. Following extraction, RNA was quantified on a spectrophotometer (Beckman DU 530) by measuring 260 nm absorbance values. RNA for both muscles and lumbar spinal cord was then reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) with the following thermocycle: 25°C for 10 min, 37°C for 2 h, 85°C for 5 min. Each sample for quantitative real-time PCR assay included 2.5 ng of cDNA, primers, and Power SYBR Green PCR Master Mix (Applied Biosystems). Thermocycle for the quantitative step on the ABI PRISM 7000 Sequence Detection System was as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 sec and 60°C for 1 min. A dissociation curve was determined for each well to confirm that the correct gene was being amplified. Each sample was run in triplicate. Samples without reverse transcriptase during the cDNA conversion were also assessed to ensure that there was no DNA contamination. The reference genes were Cyclophilin A (200nM primers: ccactgtcgcttttcgccgc and cccaagggctcgtcatcggc), GAPDH (200nM primers: ccccagcaaggacactgagcaag and tctgggatggaaattgtgagggaga), β-actin (200nM primers: ttcgttgccggtccacaccc and tttgcacatgccggagccgt), and 18s (100nM primers: either ggaccagagcgaaagcatttg and gccagtcggcatcgtttatg or ttgacggaagggcaccaccag and gcaccaccacccacggaatcg). The reference gene was chosen for each experiment by confirming that levels were equivalent between treatment groups. Transcripts of two non-coding and one coding (i.e., total) BDNF exons were quantified: BDNF IV (200nM primers: ctccgccatgcaatttccac and cgagtctttggtggccgata), BDNF VI (200nM primers: gtgacaacaatgtgactccac and gccttcatgcaaccgaagta), and BDNF IX (200nM primers: gcggcagataaaaagactgc and tcagttggcctttggatacc). We measured an additional non-coding transcript in spinal cord, BDNF II (100nM primers: aaccttttcctcctcctgcg and gagccgaacctcggaaaaga), as this transcript is highly expressed in neural tissue but not in muscle (Aid et al., 2007). We also measured levels of BDNF’s receptors in muscle and spinal cord: full length TrkB (400nM primers: ggcaacttcgggaaaggaga and gtaaacccctcaccgcctac), truncated TrkB (600nM primers: ccattgccctctgctaacca and gagatctgaggtgctctcgc), and p75 (200 nM primers: cgtgaccatctcaggccttt and ggtgcccctgttaccttctc). Optimal concentrations and amplification efficiencies were calculated for each primer set.
2.8 Statistical analysis
Relative Expression Software Tool (REST) was used to assess statistical significance and fold change of genes (Pfaffl et al., 2002). Specifically, this software uses the non-parametric Pair-Wise Fixed Reallocation Randomisation Test to account for amplification efficiencies when determining fold change. It measures relative expression of a target gene (BDNF, TrkB, p75) between two experimental groups following the normalization of the target gene to a reference gene (Cyclophilin A, GAPDH, β-actin, or 18s).
We performed a two-factor between subjects analysis of variance to test whether genotype (Tg or Wt) or treatment (sham versus castrate (in males); T versus blank (in females)) influenced motor ability on the day of tissue harvest. Post-hoc pairwise comparisons with a Bonferroni correction were used to determine significant differences between planned comparisons.
3. Results
3.1 Diseased 97Q and myogenic male mice show comparable deficits in muscle BDNF mRNA
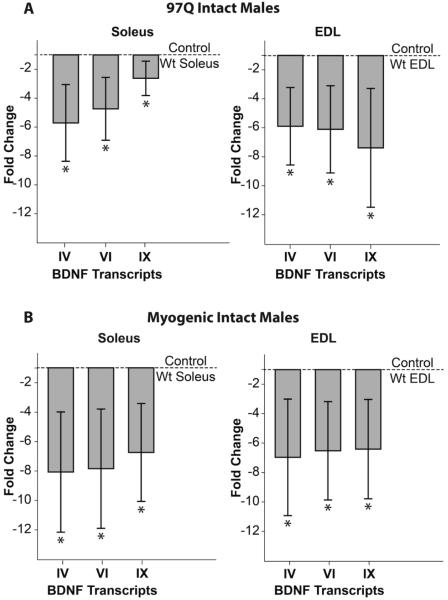
We find significant deficits in both the coding transcript IX and the noncoding transcripts IV and VI in both the EDL and soleus muscles of symptomatic 97Q and myogenic males (Fig. 1). On average, we observe a ~6-7 fold deficit in BDNF transcripts in both the EDL and soleus of both models compared to muscles from their respective Wt controls. However, we find no significant changes in BDNF mRNA levels in the lumbar spinal cord (all p>.05 for transcripts II, IV, VI, and IX; 97Q: n=6/group and myogenic: n=5/group) where EDL and soleus motoneurons are located, nor did we detect changes in BDNF receptors TrkB (truncated or full-length) or p75 in muscles or the lumbar spinal cord (p>0.05) of either model except for a slight increase (1.8 fold) in truncated TrkB in the EDL of 97Q males (p=0.026). We found no differences in circulating T levels between gonadally intact Tg and Wt males for either the 97Q or myogenic models (data not shown), indicating that differences in BDNF expression between Tg and Wt males are not due to differences in circulating T levels.
Figure 1. 97Q and myogenic transgenic (Tg) males show comparable disease-related deficits in BDNF transcripts in skeletal muscle.
Both slow twitch soleus and fast twitch extensor digitorum longus (EDL) muscles of motor-impaired mice show significant deficits in all three BDNF transcripts (noncoding IV and VI and the coding IX) based on qPCR (A, B). Since the disease-causing AR is expressed only in muscle fibers of myogenic mice, these data indicate that AR can act directly in muscle fibers to down-regulate BDNF mRNA (A), possibly triggering dysfunction not only in skeletal muscles, but in the motoneurons too. All plotted values are relative to Wt controls (dashed line). Error bars represent standard error of the mean. n=6-7/group. * p<0.01 from Wt control
3.2 Castration rescues BDNF mRNA expression in muscles of Tg 97Q and myogenic males as well as motor function
To examine whether the disease-related loss in muscle BDNF transcripts respects the same rules as motor function itself, we asked whether castration of Tg male mice improves BDNF expression in muscle, comparable to its effect on motor function. Because motor dysfunction emerges after the onset of puberty in 97Q males, we took advantage of this model to ask whether castration before symptom onset can protect BDNF expression from decline, like its protective effect on motor function. In contrast, because myogenic males are chronically impaired from birth, we took advantage of this model to ask whether castration of impaired adult males can reverse a disease-related deficit in muscle BDNF mRNA. Given that the effect of disease on BDNF expression did not depend on fiber type composition of the muscle, we examined this question only in the soleus.
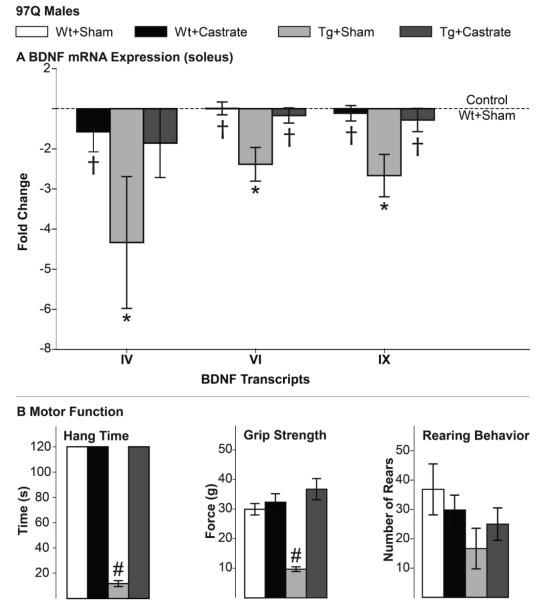
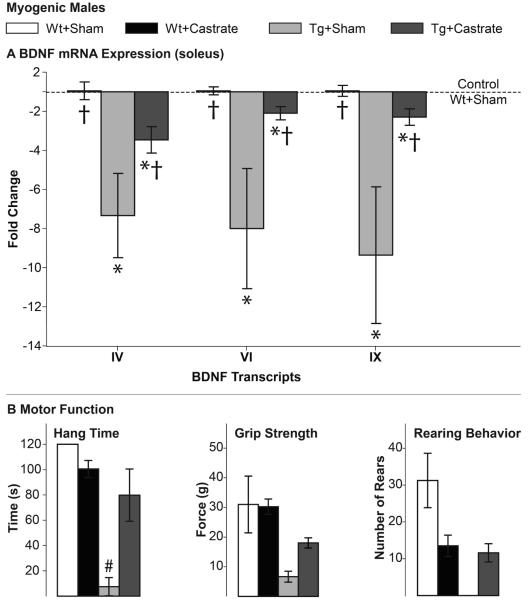
We find that castration of asymptomatic 97Q males at the start of puberty protects animals from impairments in BDNF mRNA expression in muscle, paralleling its effect on motor function, maintaining both at Wt levels (Fig. 2). These data show that the transgene alone does not cause the deficit in muscle BDNF mRNA. We note that castration at puberty had no effect on either muscle BDNF expression or motor function in Wt males. We also find that after three weeks, castration of chronically diseased adult myogenic males largely reverses both the deficit in motor function and BDNF mRNA (Fig. 3), while having no effect on levels of BDNF mRNA in muscle of Wt males. Although neither motor function nor expression of BDNF mRNA fully recovered in castrated myogenic Tg males, both measures approached Wt levels. These data also make it clear that the AR transgene alone does not cause the BDNF deficit. In sum, BDNF mRNA expression in muscles of 97Q and myogenic SBMA males depends on androgens and is tightly correlated with disease symptoms, suggesting that the loss of muscle-derived BDNF may trigger a loss in function. To further explore this possibility, we next asked whether the loss in muscle BDNF mRNA precedes the loss of motor function using a model in which disease is acutely induced in adulthood.
Figure 2. Presymptomatic castration rescues 97Q males from deficits in both muscle BDNF mRNA and motor function.
Deficits in muscle BDNF mRNA and motor function of gonadally-intact, end-stage 97Q Tg males are not evident in 97Q males castrated at puberty. Both BDNF expression and motor function of castrated Tg males is comparable to Wt males. Note that we find the same deficits in BDNF mRNA in gonadally-intact 97Q males as in the first experiment (Fig. 1A) and that castration of Wt males has no effect on BDNF mRNA (A), nor motor function. Error bars represent standard error of the mean. n=6/group. A) * p<0.01 from Wt+Sham, † p<0.05 from Tg+Sham. B) # p<0.05 Tg+Castrate versus Tg+Sham.
Figure 3. Castration reverses deficits in both muscle BDNF mRNA expression and motor function of chronically diseased myogenic males.
Deficits in muscle BDNF (A) and motor function (B) found in gonadally intact Tg males are largely reversed by castration, indicating that both defects are androgen-dependent. Note that we find comparable BDNF deficits in gonadally-intact Tg males as seen in first experiment (Fig 1B) and that castration of Wt males affects neither BDNF expression nor motor function based on hang times and grip strength but does lower rearing behavior, an androgen-dependent measure of anxiety. That castration did not reverse the rearing deficit in Tg males may reflect increased anxiety-like behavior and not an effect on motor function per se. Error bars represent standard error of the mean. n=4-5/group. A) * p<0.01 from Wt+Sham, † p<0.05 from Tg+Sham. B) # p<0.05 Tg+Castrate versus Tg+Sham.
3.3 Deficit in muscle BDNF mRNA levels in acutely diseased myogenic females
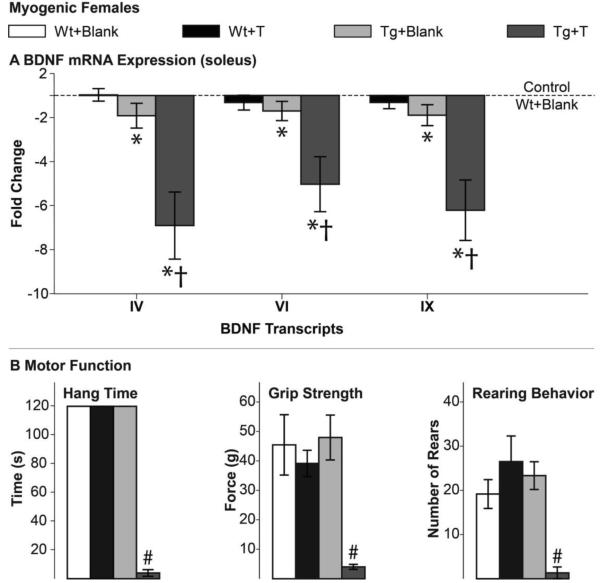
Five days of T exposure induced a > 6 fold drop in BDNF mRNA in the soleus of acutely diseased Tg females (Fig. 4A), comparable to that seen in chronically diseased myogenic males (Fig. 1B). The effect of androgens on expression of BDNF mRNA in muscle again shows a striking correlation with motor function; each are severely compromised after five days of T treatment (Fig. 4B). We also found that the level of BDNF mRNA level in the soleus of control Tg females is reduced (<2 fold, Fig. 4A) relative to Wt controls, albeit much less than in diseased muscle. While such females exhibit normal motor function (Fig. 4B), this somewhat compromised expression of BDNF may account for why motor dysfunction develops so rapidly in Tg females exposed to androgens. These data indicate that the system can tolerate some loss of BDNF expression without deleterious effects on motor function. Treatment with androgens had no influence on BDNF mRNA levels in Wt muscle (p>0.05 for all transcripts), as seen previously in Wt males (Figs. 2 and 3).
Figure 4. Androgen treatment of myogenic females induces significant losses in both muscle BDNF mRNA and motor function.
Exposing asymptomatic myogenic Tg females to testosterone (T) for only 5 days induces a significant, more than 6-fold, decrease in muscle BDNF transcript levels (A) compared to either T-treated Wt controls or asymptomatic control-treated Tg females (Tg+Blank). Androgen’s effect on BDNF expression in muscle of Tg females correlates with a robust induction of motor dysfunction (B). Control treated Tg females show a small but significant deficit (< 2 fold) in muscle BDNF mRNA compared to Wt controls (Wt+Blank, dashed line, A) despite having normal motor function, suggesting that a small deficit in BDNF mRNA in asymptomatic Tg females may prime the system for rapid collapse once exposed to male levels of androgens. Error bars represent standard error of the mean. A) * indicates p<0.05 from Wt+Blank, † indicates p<0.05 from Tg+Blank. B) # p<0.05 Tg+Blank versus Tg+T.
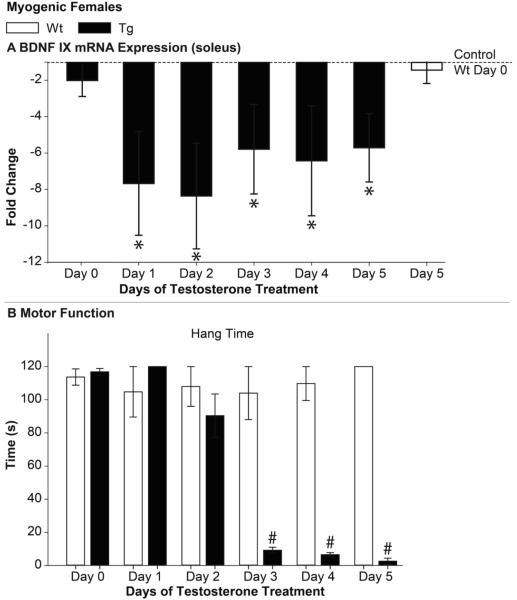
Given that motor function declines in a highly predictable manner in this acute model, we next determined how soon the >6 fold deficit in muscle BDNF would emerge by examining BDNF mRNA expression in the soleus of Tg females after 1 to 5 days of T treatment. Of note, we included a 5-day treated group in this experiment to confirm whether we could replicate the finding of reduced BDNF at this time point from the previous experiment. Because disease had comparable effects on all three transcripts in the first experiment, we measured only the levels of the coding transcript IX in this experiment. Not only did we replicate the same deficit after 5 days of androgen treatment, but we find the same > 6 fold deficit after only 24 hours of T exposure (Fig. 5A), a full 48 hours prior to any overt motor dysfunction. A deficit in hang time became apparent only after 3 days of T treatment (Fig. 5B). The decline in BDNF expression also preceded the decline in open field performance and grip strength (data not shown). Interestingly, we did not find a significant deficit in muscle BDNF mRNA in control treated myogenic females in this cohort of females (p=0.179). This may be because this cohort was slightly younger than in the previous experiment (97.5±1.7 days vs. 117.7 ± 4.2 days).
Figure 5. Androgen induces the deficit in muscle BDNF mRNA before detectable motor dysfunction in acutely diseased myogenic females.
Muscle BDNF mRNA was significantly reduced after only one day of testosterone (T) treatment (A). Importantly, this deficit preceded the loss in overt motor function; deficits in hang time were evident starting at three days of T treatment but not before. The magnitude of the deficit at 24 hours was not only comparable to that at 5 days of T treatment in Tg females but also comparable to that of chronically affected myogenic and 97Q Tg males (Fig. 1), suggesting that a defect in BDNF mRNA expression in muscle is an early event in disease progression and might trigger the loss in overt motor function. Error bars represent standard error of the mean. n=4-6/group. A) * indicates p<0.05 from Wt Day 0. B) # p<0.05 Wt versus Tg.
4. Discussion
We find that BDNF mRNA levels are robustly down-regulated in muscles of motor-impaired male mice in both the 97Q and myogenic models of SBMA. Because motor symptoms in these models are androgen-dependent, we asked whether castration of Tg males could also rescue BDNF mRNA expression, comparable to its beneficial effect on motor function (Katsuno et al., 2002; Kemp et al., 2011; Monks et al., 2007). We also asked the complement question of whether the deficit in BDNF expression in muscle could be acutely induced by androgens in myogenic females, like motor dysfunction in this model, and if so, whether the deficit emerged before overt motor function. We find that changes in muscle BDNF mRNA parallel changes in motor function in both male Tg models. In the acute female model, we find that the deficit in muscle BDNF mRNA develops within 24 hours of androgen exposure, emerging before overt motor dysfunction, supporting the idea that BDNF may critically underlie the loss in motor function.
BDNF expression in skeletal muscle has been shown to be controlled by factors other than disease, including circulating T levels and muscle activity levels (i.e., exercise). Unexpectedly, we find no effect of androgens on the expression of BDNF mRNA in muscles of either male or female Wt mice, contrary to previously published results in rats (Osborne et al., 2007; Ottem et al., 2007; Verhovshek et al., 2010). Nonetheless, the pattern of results in myogenic and 97Q Tg mice underscores the fact that only the combination of T and the disease allele leads to impairments in both BDNF mRNA expression and motor function in these SBMA models. Because diseased mice are less active, inactivity may play some role in the loss of BDNF in motor-impaired mice. However, the effect of being less active is likely minor given that complete inactivity of the soleus for one week results in only a 30% decline in the amount of muscle BDNF mRNA (Gomez-Pinilla et al., 2002) whereas disease leads to a deficit in muscle BDNF mRNA that is far greater, a 6-8 fold deficit. Moreover, we find that the deficit in muscle BDNF mRNA is independent of activity since T-treated myogenic females show the deficit well before any loss in motor function. While the BDNF gene contains an estrogen response element (Sohrabji et al., 1995), there is no known androgen response element. One possible mechanism by which BDNF expression may be dysregulated in SBMA is through disruption of an AR regulated cyclic AMP-mediated pathway (reviewed in Verhovshek et al., 2013).
There is some dispute over whether the myogenic model is a legitimate mouse model for SBMA since motor dysfunction in this model is triggered by Wt AR rather than polyglutamine-expanded AR (La Spada et al., 1991). There are several compelling reasons to think that the myogenic model has clinical relevance to SBMA. First and foremost, the disease phenotype is expressed only in Tg males and not Tg females, showing the same male-bias as for SBMA in humans and as seen in other mouse models recapitulating this disease (Chevalier-Larsen et al., 2004; Katsuno et al., 2002; Monks et al., 2007; Yu et al., 2006). Disease in the myogenic mice is also androgen-dependent (Johansen et al., 2009; Kemp et al., 2011; Monks et al., 2007; Renier et al., 2014), exhibiting another core trait of SBMA. Moreover, results of two recent studies fully support conclusions based on the myogenic model that muscle AR critically underlies SBMA pathology in both muscles and the motoneurons (Cortes et al., 2014; Lieberman et al., 2014). There is also growing precedence linking both mutant and Wt alleles of the same gene to the same neurodegenerative disease. Notable examples in the human population include superoxide dismutase 1 linked to amyotrophic lateral sclerosis (Bosco et al., 2010) and α-synuclein linked to Parkinson’s disease (Singleton et al., 2003). Wt AR has also been shown to exert comparable androgen-dependent toxicity as the polyglutamine-expanded AR in both mouse and fly models of SBMA, and seems to engage common toxic pathways (Mo et al., 2010; Nedelsky et al., 2010). Results of the myogenic model also help to explain the >17% of affected men diagnosed with SBMA that show the expected cluster of symptoms (e.g., slowly progressing motor dysfunction coupled with signs of partial androgen insensitivity (e.g., gynecomastia) and elevated serum creatine kinase levels) but lack the polyglutamine expansion in their AR gene (Ferlini et al., 1995; Mariotti et al., 2000). Finally, because we find comparable defects in BDNF expression in a mouse model that expresses a polyglutamine-expanded AR, it is clear that the deficit in muscle BDNF mRNA is not unique to the myogenic model, and thus likely a more general pathogenic mechanism underlying the loss of motor function in SBMA.
As SBMA is an androgen-dependent disease, we asked whether castration of Tg male mice could rescue BDNF expression, akin to its effects on motor function. Castration of presymptomatic 97Q males fully protects the expression of muscle BDNF mRNA, , paralleling the protective role of castration on motor function (Fig. 2). These data make it clear that nonspecific effects of the transgene are minimal if nonexistent. Note, however, that it is not clear whether castration prevents or reverses the decline, as prepubertal levels of BDNF are unknown in these animals. Moreover, after three weeks of castration, chronically impaired myogenic males show noticeably improved motor function and BDNF mRNA expression, each nearly at Wt levels (Fig. 3). While it is possible that the transgene partially impairs BDNF expression independent of androgens in this model, the fact that neither BDNF expression nor motor function fully recover argues against this possibility. Finding that androgen-dependent loss of muscle BDNF mRNA strongly correlates with motor dysfunction in two SBMA models suggests that defects in the expression of muscle BDNF mRNA may trigger neuromuscular dysfunction in SBMA.
To further probe the androgen dependence of the BDNF deficit, we treated myogenic females with T. When adult asymptomatic myogenic females are exposed to male levels of androgens for five days, both motor function and BDNF expression rapidly decline. Androgen-treated Tg females show severe deficits in grip strength, hang time, and rearing ability (Fig. 4B), comparable to the severity of deficits seen in chronically impaired (gonadally intact) myogenic males (Fig. 3B). Notably, we also saw comparable deficits in BDNF mRNA. Muscles from acutely impaired myogenic females showed ~6 fold deficit in BDNF mRNA after only five days of T exposure, comparable to that in chronically impaired Tg males, indicating that disease-related deficits in BDNF can be rapidly induced. While control-treated myogenic females (Tg+Blank) had a small, but significant decrease (<2 fold) in BDNF mRNA, despite normal motor function, we did not replicate this deficit in the second experiment, questioning its significance. We also found that this same deficit in muscle BDNF mRNA emerges within 24 hours of T exposure, a full two days before motor dysfunction emerged. We were surprised to see the full magnitude of the deficit, about an ~8 fold deficit, in muscle BDNF mRNA at the one day mark. These data indicate that significant dysregulation in BDNF expression can occur early in the disease process and may in fact be a proximate event in disease, critically mediating the progressive loss of motor function.
To understand whether BDNF signaling per se might be altered, we also examined the expression of BDNF receptors, the high-affinity TrkB receptor (full length and truncated forms) and the pan-neurotrophin p75 receptor. We find no evidence of compensatory expression for these receptors at the mRNA level in either muscle or lumbar spinal cord, apart from a small increase in the truncated form of TrkB in one muscle of one model. While it is possible that other parts of the signaling pathway have compensated for the likely loss of BDNF protein, these results are consistent with the idea that both diseased muscles and motoneurons are deprived of the beneficial effects of BDNF.
The literature provides ample examples of how muscle-derived BDNF maintains key cellular functions including synaptic strength and axonal transport (Koliatsos et al., 1993; Lohof et al., 1993; Sagot et al., 1998; Stoop & Poo, 1996). We previously showed that axonal transport is perturbed in this myogenic SBMA model and can be rescued by exogenous VEGF locally applied to muscle (Kemp et al., 2011). The results of the current study point to BDNF as another potential neurotrophic factor that may underlie deficits in axonal transport. BDNF has also been shown to overcome retrograde transport dysfunction caused by motoneuron disease (Sagot et al., 1998). Because factors other than BDNF are also perturbed in SBMA, it is always possible that deficits in some combination of neurotrophic factors underlie the overt loss in motor function. Nonetheless, it important to determine what part BDNF might play in the demise of neuromuscular function in SBMA. For example, because neuromuscular junctions are much weaker in diseased Tg males of both models (Jordan, unpublished data), the loss of muscle-supplied BDNF may be behind this deficit. Since TrkB receptors are present in all three cell types (motoneuron, Schwann cell, and muscle fiber) of the neuromuscular junction (Garcia et al., 2010; reviewed in Pitts et al., 2006), BDNF may act at any of these locations to enhance synaptic communication. Presynaptically, BDNF might accomplish this through local protein translation, promoting actin polymerization and/or enhancing calcium influx, all known to enhance neurotransmitter release (reviewed in Park & Poo, 2013). Additionally, BDNF may promote TrkB signaling to maintain acetylcholine receptor clustering at the neuromuscular junction (Gonzalez et al., 1999; Kulakowski et al., 2011). Interestingly, our models show the same fragmentation of the junction (Jordan, unpublished data) as when TrkB signaling is blocked (Gonzalez et al., 1999; Kulakowski et al., 2011). Moreover, TrkB signaling also appears to be involved in maintaining the expression of the adult isoform of the voltage-gated sodium channel. When TrkB signaling is perturbed, muscles express the neonatal isoform of the sodium channel (Kulakowski et al., 2011), which leads to slower and less reliable conduction of the action potential along muscle fibers. In sum, muscle-derived BDNF may act presynaptically on motor nerve terminals to mobilize neurotransmitter stores as well as postsynaptically to ensure high fidelity synaptic transmission and efficient excitation of muscle fibers, all of which contribute to high quality motor function. Moreover, because muscle-derived BDNF also promotes myofiber regeneration via the satellite cell population (Clow & Jasmin, 2010), the loss of muscle BDNF in SBMA may also cause fibers to be particularly prone to damage, consistent with the elevated levels of creatine kinase in SBMA patients (Chahin & Sorenson, 2009).
5. Conclusions
Motor dysfunction in SBMA may critically depend on reduced levels of muscle-derived BDNF, suggesting that neuromuscular function can be rescued in disease simply by replenishing the supply of muscle BDNF. Potential sites of BDNF action for promoting neuromuscular function include motoneurons, muscles, and Schwann cells. Despite a loss of muscle fibers and/or motoneurons, BDNF has the potential to not only promote recovery of function of remaining motoneurons and muscle fibers, but also to promote the rebuilding of diseased muscle fibers by mobilizing the satellite cell population in muscle.
Highlights.
Level of muscle BDNF mRNA correlates with disease symptoms in two SBMA mouse models
Expression of muscle BDNF mRNA is androgen-dependent only in transgenic SBMA mice
Castration rescues both muscle BDNF expression and motor function in affected males
Loss of muscle BDNF mRNA precedes expression of motor symptoms
Impaired muscle BDNF expression may underlie motor dysfunction in SBMA
Acknowledgements
We wish to thank Diane Redenius, Kate Mills, Qi Ding, and Donald Zeolla for their technical expertise. We also thank the Wade lab for use of their Sequence Detection System.
This work was supported by NIH NS045195 (CLJ) and NSERC PGS-M (KH).
Abbreviations
- AR
androgen receptor
- BDNF
brain-derived neurotrophic factor
- BC/LA
bulbocavernosus/levator ani
- EDL
extensor digitorum longus
- SBMA
spinal bulbar muscular atrophy
- T
testosterone
- Tg
transgenic
- VEGF
vascular endothelial growth factor
- Wt
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007;85(3):525–535. doi: 10.1002/jnr.21139. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Brown RH., Jr. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 2010;13(11):1396–1403. doi: 10.1038/nn.2660. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahin N, Sorenson EJ. Serum creatine kinase levels in spinobulbar muscular atrophy and amyotrophic lateral sclerosis. Muscle Nerve. 2009;40(1):126–129. doi: 10.1002/mus.21310. doi: 10.1002/mus.21310. [DOI] [PubMed] [Google Scholar]
- Chevalier-Larsen ES, O'Brien CJ, Wang H, Jenkins SC, Holder L, Lieberman AP, Merry DE. Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J. Neurosci. 2004;24(20):4778–4786. doi: 10.1523/JNEUROSCI.0808-04.2004. doi: 10.1523/JNEUROSCI.0808-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow C, Jasmin BJ. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Molecular biology of the cell. 2010;21(13):2182–2190. doi: 10.1091/mbc.E10-02-0154. doi: 10.1091/mbc.E10-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes CJ, Ling SC, Guo LT, Hung G, Tsunemi T, Ly L, La Spada AR. Muscle expression of mutant androgen receptor accounts for systemic and motor neuron disease phenotypes in spinal and bulbar muscular atrophy. Neuron. 2014;82(2):295–307. doi: 10.1016/j.neuron.2014.03.001. doi: 10.1016/j.neuron.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlini A, Patrosso MC, Guidetti D, Merlini L, Uncini A, Ragno M, et al. Androgen receptor gene (CAG)n repeat analysis in the differential diagnosis between Kennedy disease and other motoneuron disorders. Am J Med Genet. 1995;55(1):105–111. doi: 10.1002/ajmg.1320550125. [DOI] [PubMed] [Google Scholar]
- Garcia N, Tomas M, Santafe MM, Lanuza MA, Besalduch N, Tomas J. Localization of brain-derived neurotrophic factor, neurotrophin-4, tropomyosin-related kinase b receptor, and p75 NTR receptor by high-resolution immunohistochemistry on the adult mouse neuromuscular junction. Journal of the peripheral nervous system : JPNS. 2010;15(1):40–49. doi: 10.1111/j.1529-8027.2010.00250.x. doi: 10.1111/j.1529-8027.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton R. Voluntary Exercise Induces a BDNF-Mediated Mechanism That Promotes Neuroplasticity. J. Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, Kraner S, Balice-Gordon RJ. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron. 1999;24(3):567–583. doi: 10.1016/s0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Jobsis GJ, Louwerse ES, de Visser M, Wolterman RA, Bolhuis PA, Busch HF, et al. Differential diagnosis in spinal and bulbar muscular atrophy clinical and molecular aspects. J. Neurol. Sci. 1995;129(Suppl):56–57. doi: 10.1016/0022-510x(95)00064-9. [DOI] [PubMed] [Google Scholar]
- Johansen JA, Troxell-Smith SM, Yu Z, Mo K, Monks DA, Lieberman AP, Jordan CL. Prenatal flutamide enhances survival in a myogenic mouse model of spinal bulbar muscular atrophy. Neurodegener Dis. 2011;8(1-2):25–34. doi: 10.1159/000313682. doi: 10.1159/000313682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JA, Yu Z, Mo K, Monks DA, Lieberman AP, Breedlove SM, Jordan CL. Recovery of function in a myogenic mouse model of spinal bulbar muscular atrophy. Neurobiol Dis. 2009;34(1):113–120. doi: 10.1016/j.nbd.2008.12.009. doi: 10.1016/j.nbd.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, Sobue G. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35(5):843–854. doi: 10.1016/s0896-6273(02)00834-6. [DOI] [PubMed] [Google Scholar]
- Kemp MQ, Poort JL, Baqri RM, Lieberman AP, Breedlove SM, Miller KE, Jordan CL. Impaired motoneuronal retrograde transport in two models of SBMA implicates two sites of androgen action. Hum. Mol. Genet. 2011;20(22):4475–4490. doi: 10.1093/hmg/ddr380. doi: 10.1093/hmg/ddr380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18(7):671–680. doi: 10.1212/wnl.18.7.671. [DOI] [PubMed] [Google Scholar]
- Kinirons P, Rouleau GA. Administration of testosterone results in reversible deterioration in Kennedy's disease. J. Neurol. Neurosurg. Psychiatry. 2008;79(1):106–107. doi: 10.1136/jnnp.2006.101899. doi: 10.1136/jnnp.2006.101899. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- Korkmaz OT, Aytan N, Carreras I, Choi JK, Kowall NW, Jenkins BG, Dedeoglu A. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci. Lett. 2014;566:286–291. doi: 10.1016/j.neulet.2014.02.058. doi: 10.1016/j.neulet.2014.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulakowski SA, Parker SD, Personius KE. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. J Appl Physiol (1985) 2011;111(3):844–852. doi: 10.1152/japplphysiol.00070.2011. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- Kust BM, Copray JC, Brouwer N, Troost D, Boddeke HW. Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp. Neurol. 2002;177(2):419–427. doi: 10.1006/exnr.2002.8011. [DOI] [PubMed] [Google Scholar]
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352(6330):77–79. doi: 10.1038/352077a0. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Yu Z, Murray S, Peralta R, Low A, Guo S, Hung G. Peripheral androgen receptor gene suppression rescues disease in mouse models of spinal and bulbar muscular atrophy. Cell reports. 2014;7(3):774–784. doi: 10.1016/j.celrep.2014.02.008. doi: 10.1016/j.celrep.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363(6427):350–353. doi: 10.1038/363350a0. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Mariotti C, Castellotti B, Pareyson D, Testa D, Eoli M, Antozzi C, Donato SD. Phenotypic manifestations associated with CAG-repeat expansion in the androgen receptor gene in male patients and heterozygous females: a clinical and molecular study of 30 families. Neuromuscul Disord. 2000;10(6):391–397. doi: 10.1016/s0960-8966(99)00132-7. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Ikeda K, Klinkosz B, Cedarbaum JM, Wong V, Lindsay RM. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. 1994;265(5175):1107–1110. doi: 10.1126/science.8066451. [DOI] [PubMed] [Google Scholar]
- Mo K, Razak Z, Rao P, Yu Z, Adachi H, Katsuno M, Monks DA. Microarray analysis of gene expression by skeletal muscle of three mouse models of Kennedy disease/spinal bulbar muscular atrophy. PLoS One. 2010;5(9):e12922. doi: 10.1371/journal.pone.0012922. doi: 10.1371/journal.pone.0012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks DA, Johansen JA, Mo K, Rao P, Eagleson B, Yu Z, Jordan CL. Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(46):18259–18264. doi: 10.1073/pnas.0705501104. doi: 10.1073/pnas.0705501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelsky NB, Pennuto M, Smith RB, Palazzolo I, Moore J, Nie Z, Taylor JP. Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron. 2010;67(6):936–952. doi: 10.1016/j.neuron.2010.08.034. doi: 10.1016/j.neuron.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB immunolabeling in spinal motoneurons. J. Neurosci. Res. 2007;85(2):303–309. doi: 10.1002/jnr.21122. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Bailey DJ, Jordan CL, Breedlove SM. With a little help from my friends: androgens tap BDNF signaling pathways to alter neural circuits. Neuroscience. 2013;239:124–138. doi: 10.1016/j.neuroscience.2012.12.019. doi: 10.1016/j.neuroscience.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148(8):3655–3665. doi: 10.1210/en.2007-0308. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- Palazzolo I, Stack C, Kong L, Musaro A, Adachi H, Katsuno M, Pennuto M. Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron. 2009;63(3):316–328. doi: 10.1016/j.neuron.2009.07.019. doi: 10.1016/j.neuron.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts EV, Potluri S, Hess DM, Balice-Gordon RJ. Neurotrophin and Trk-mediated signaling in the neuromuscular system. International anesthesiology clinics. 2006;44(2):21–76. doi: 10.1097/00004311-200604420-00004. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier KJ, Troxell-Smith SM, Johansen JA, Katsuno M, Adachi H, Sobue G, Jordan CL. Antiandrogen flutamide protects male mice from androgen-dependent toxicity in three models of spinal bulbar muscular atrophy. Endocrinology. 2014;155(7):2624–2634. doi: 10.1210/en.2013-1756. doi: 10.1210/en.2013-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot Y, Rosse T, Vejsada R, Perrelet D, Kato AC. Differential effects of neurotrophic factors on motoneuron retrograde labeling in a murine model of motoneuron disease. J. Neurosci. 1998;18(3):1132–1141. doi: 10.1523/JNEUROSCI.18-03-01132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(24):11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopher BL, Thomas PS, Jr., LaFevre-Bernt MA, Holm IE, Wilke SA, Ware CB, La Spada AR. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 2004;41(5):687–699. doi: 10.1016/s0896-6273(04)00082-0. [DOI] [PubMed] [Google Scholar]
- Stoop R, Poo MM. Synaptic modulation by neurotrophic factors: differential and synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor. J. Neurosci. 1996;16(10):3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, Osborne MC, Sengelaub DR. Androgen Regulates Brain-Derived Neurotrophic Factor in Spinal Motoneurons and Their Target Musculature. Endocrinology. 2010;151(1):253–261. doi: 10.1210/en.2009-1036. doi: Doi 10.1210/En.2009-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Rudolph LM, Sengelaub DR. Brain-derived neurotrophic factor and androgen interactions in spinal neuromuscular systems. Neuroscience. 2013;239:103–114. doi: 10.1016/j.neuroscience.2012.10.028. doi: 10.1016/j.neuroscience.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Mitsuma N, Inukai A, Ito Y, Li M, Mitsuma T, Sobue G. Expression of GDNF and GDNFR-alpha mRNAs in muscles of patients with motor neuron diseases. Neurochem. Res. 1999;24(6):785–790. doi: 10.1023/a:1020739831778. [DOI] [PubMed] [Google Scholar]
- Yu Z, Dadgar N, Albertelli M, Gruis K, Jordan C, Robins DM, Lieberman AP. Androgen-dependent pathology demonstrates myopathic contribution to the Kennedy disease phenotype in a mouse knock-in model. J. Clin. Invest. 2006;116(10):2663–2672. doi: 10.1172/JCI28773. doi: 10.1172/JCI28773. [DOI] [PMC free article] [PubMed] [Google Scholar]