Abstract
Pioneers in congenital heart surgery observed that exercise capacity did not return to normal levels despite successful surgical repair, leading some to cite a “myocardial factor” playing a role. They conjectured that residual alterations in myocardial function would be significant for patients’ long-term outlook. In fulfillment of their early observations, today’s adult congenital heart disease (ACHD) population shows well-recognized features of heart failure, even among patients without clear residual anatomic or hemodynamic abnormalities, demonstrating the vital role of the myocardium in their morbidity and mortality. Whereas the ‘myocardial factor’ was an elusive concept in the early history of congenital heart care, we now have imaging techniques to detect and quantify one such factor – myocardial fibrosis. Understanding the importance of myocardial fibrosis as a final common pathway in a variety of congenital lesions provides a framework for both the study and treatment of clinical heart failure in this context. While typical heart failure pharmacology should reduce or attenuate fibrogenesis, efforts to show meaningful improvements with standard pharmacotherapy in ACHD repeatedly fall short. This paper considers the importance of myocardial fibrosis and function, the current body of evidence for myocardial fibrosis in ACHD, and its implications for research and treatment.
Keywords: Heart defects, Congenital, Heart failure, Myocardial fibrosis, Cardiac magnetic resonance
Introduction
Heart failure is an increasingly common cause of late morbidity and mortality among the rising number of adults with congenital heart disease (ACHD), which affects over 1,000,000 adults in the United States,1 with similarly large populations throughout the world.2, Patients with cyanotic heart disease, tetralogy of Fallot, systemic right ventricle (RV) related to transposition of the great arteries and single ventricle patients after Fontan palliation are at particularly high risk for heart failure and its complications. Physiologic definitions of heart failure focus on the heart’s inability to meet the requirements of the metabolizing tissues. How to define heart failure in ACHD is a challenge, particularly since many younger patients do not exhibit decompensated HF despite significant alterations in myocardial structure and function. (Burchill, in press) This discussion focuses specifically on myocardial dysfunction in ACHD and what is now known about its causes and treatments.
The “Myocardial Factor”
In the 1960s, successful application of cardiopulmonary bypass paved the way for surgical repair of congenital heart defects.3 Success was indisputable, giving rise to a new generation of congenital heart survivors. Yet as more patients underwent repair, several groups made the early observation that cardiovascular performance in operative survivors was still below expectations for normal individuals. The first case report of this nature involved a man with pulmonary stenosis who presented six years after surgical repair with atrial flutter and “grossly impaired cardiac function, despite satisfactory relief of pulmonary valvular obstruction.”4 This publication, entitled, “The importance of the myocardial factor in determining the clinical course and surgical results” (italics added), was perhaps the first report to highlight the discrepancy between anatomic repair and cure, including a discussion on the vulnerability of the myocardium despite successful relief of obstruction. The authors postulated that “the status of the myocardium is the final determinant of the clinical course and prognosis.” Although acknowledging their patient was older at the time of repair, they wisely surmised, “it is unjust to conclude that youth protects the myocardium.”
Further reports of reduced exercise capacity in young congenital patients emerged in the years that followed.5 Focused studies in patients with atrial septal defects and tetralogy of Fallot in particular demonstrated poor exercise capacity and/or exercise hemodynamics after corrective surgery, despite excellent function at rest.6 Others demonstrated lower left ventricular ejection fraction in corrected tetralogy of Fallot,7 even though the left ventricle was not as directly affected by the initial anatomic defect. Borrowing the term that had often been used in conjunction with mitral valve disease, there was growing recognition of the importance of the “myocardial factor” as a determinant of postoperative functional capacity.8 They emphasized that repair of the anatomic defect could not be equated with a normally functioning heart and that correction of the hemodynamics was not in itself sufficient to restore full cardiovascular function.
In pursuit of the ‘myocardial factor,’ researchers employed available techniques such as electron microscopy to study pathologic changes occurring in the myocardium of these CHD survivors.9 Comparing histology sections from young and old patients, a 1977 study found interstitial fibrosis and myofibrillar changes in older patients after repair.8 Like those before them, they concluded that these surgeries, despite successful repair, “fail to obtain functionally satisfactory results because of poor myocardial function, arrhythmias or inadequately explained sudden death.”8
While these observations made at the dawn of congenital heart intervention may seem self-evident to providers today, they were astute, valuable, and even prophetic of future insight into myocardial performance and its central importance in protecting against arrhythmia and sudden death. One statement seems particularly relevant: “Myocardial function is becoming a most important factor in determining the long-term prognosis of patients with such lesions.”8
The heart failure problem in today’s ACHD population
In the contemporary era we now recognize the myocardium as a major determinant of late morbidity and mortality in congenital heart survivors, whose prognosis is tied to the myocardial function as foretold. ACHD patients demonstrate many of the defining features of heart failure, including reduced NYHA functional class, ventricular dysfunction, reduced peak oxygen uptake, and neurohormonal activation.10, 11 Among the 137,000 ACHD patients hospitalized in the United States between 1998 and 2005, 28% carried a diagnosis of heart failure. Dubbed “the original heart failure syndrome,”12 congenital heart disease continues to be increasingly recognized as a potential cause of myocardial dysfunction, clinical heart failure, and early death.
A congenital heart failure phenotype was brought into focus over a decade ago with the published report of neurohormonal activation in ACHD, namely elevated levels of renin, aldosterone, norepinephrine, and endothelin.10 Contemporary research continues to shed new light on heart failure and how it manifests in ACHD patients, including changes in exercise capacity, ventricular function, neurohormone release, and the propensity for arrhythmias.[Stout et al, in press] The interplay between these changes is complex and emphasizes the importance of recognizing heart failure as a manifestation of a series of maladaptive processes, all of which need to be addressed to improve myocardial performance, functional capacity, and health outcomes.
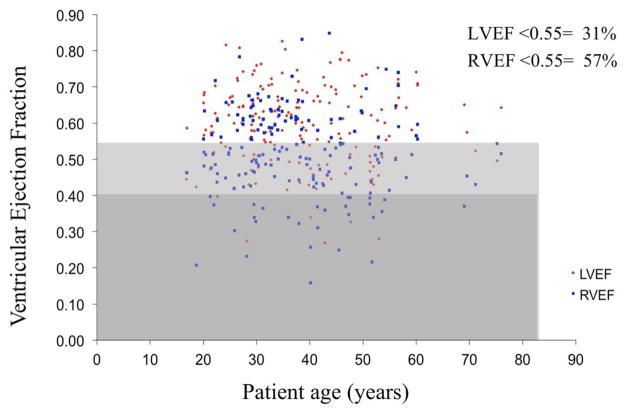
Several clinical features of the ACHD population that mirror other heart failure cohorts are worth highlighting. First, exercise capacity is reduced. Measured peak oxygen consumption in ACHD patients are similar to CHF patients, despite the fact that the ACHD patients are 25 years younger on the whole.13 Second, systolic dysfunction is reduced, especially in certain populations.14 At our institution, RV and LV ejection fraction measured by MRI in 188 consecutive unselected ACHD patients was were below normal in 57% and 31% of patients respectively (Figure 1). Third, biomarker elevations are present, most notably related to brain natriuretic peptide elevations across the spectrum of congenital defects.10, 15–18 Fourth, both supraventricular and ventricular tachycardia are common19, 20 and likely relate in part to ventricular dysfunction.21, 22 In particular, a relationship between ventricular function and arrhythmia has been shown in the atrial switch population23 and in tetralogy of Fallot. In fact, ventricular arrhythmias in tetralogy have a stronger relationship to left ventricular dysfunction than to a prior right ventriculotomy incision or even prior syncope. 21, 22 Hence, in addition to scar-induced arrhythmia, there is an ever-increasing arrhythmic burden related directly to ventricular dysfunction. Fifth, the majority of cardiovascular deaths in ACHD have been due to heart failure.24, 25 At a single referral center, 21% of ACHD deaths were secondary to heart failure, defined as “progressive myocardial failure of either systemic or venous ventricle.”25 Yet additional deaths were also likely heart failure-related. Three of four aortic coarctation patients who experienced sudden cardiac death had a LVEF < 40%, and all six patients with congenitally-corrected transposition who died suddenly had a RVEF < 45%. Along the same lines, 42% (15/36) of perioperative deaths involved cardiogenic shock. Combining deaths from heart failure as well as the sudden cardiac and perioperative deaths with significant ventricular systolic dysfunction, the proportion of death attributable to heart failure could be as high as 35%.
Figure 1.
Scatterplot of ejection fraction for the left (LVEF) and right (RVEF) ventricles in consecutive patients with congenital heart disease referred for cardiac magnetic resonance. The light gray bar represents those with mild systolic dysfunction, and the dark gray those with moderate/severe systolic dysfunction. Percentages of patients have reduced ejection fraction are given. Systolic dysfunction spans the spectrum of age.
The importance of myocardial fibrosis in the heart failure context
Normal myocardial structure and function relies heavily upon a collagen network which inhabits the extracellular space and serves to maintain tissue architecture and chamber geometry. In the healthy heart, collagen provides both tensile strength and elasticity, both of which are vital for systolic and diastolic ventricular function.26 Far from being static, myocardial collagen turnover is a dynamic process governed by local tissue and circulatory hormonal systems, including the renin-angiotensin-aldosterone system (RAAS). When dysregulated, as occurs in RAAS activation, collagen synthesis may outpace degradation leading to collagen accumulation and distortion of myocardial architecture.27 Distinct from myocyte hypertrophy, fibrosis is defined by this increase in myocardial collagen concentration. The functional consequence is an increase in myocardial stiffness and loss of normal architecture leading to adverse ventricular remodeling, including wall thinning and chamber dilation. Pharmacologic agents targeting the RAAS can halt and even reverse this process.28, 29
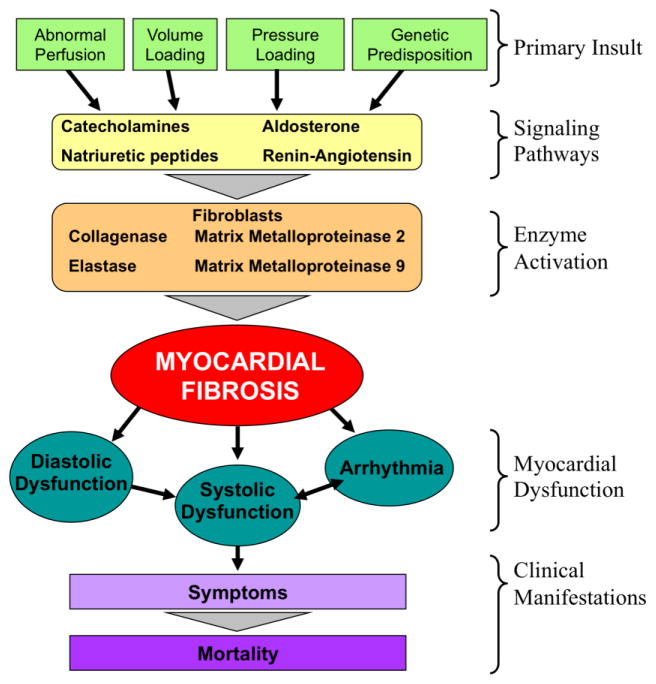
In a schematic of the heart failure process (Figure 2), one can conceptualize that the process begins with some precipitating insult such as ischemia, pressure or volume loading, cyanosis, inflammation, or perhaps a genetic predisposition. These stimuli activate a myriad of cell signaling pathways such as the RAAS which, in addition to their other effects, activate myocardial fibroblast activity. This activation begins to alter the intermyofibrillar space with a turnover of collagen, the net result of which is myocardial fibrosis, specifically diffuse microscopic fibrosis as opposed to replacement fibrosis and discrete scarring that follows myocardial infarction. Fibrosis, in turn, is associated with clinical manifestations such as diastolic dysfunction, systolic dysfunction, and arrhythmia, all of which may contribute to exercise dysfunction, venous congestion, and eventually death.30, 31
Figure 2.
Simplified schema of heart failure. Primary insults lead to various signaling pathways involving common cytokines and neurohormonal activators that in term signal enzymatic reactions. These enzymatic reactions, namely performed by myofibroblasts, lead eventually to myocardial fibrosis. The presence of fibrosis causes myocardial diastolic and systolic function, as well as arrhythmia, all of which are clinical features of heart failure. While there are multiple additional activities involved in the process of heart failure, this demonstrates the central role of myocardial fibrosis between preclinical events and clinical manifestations.
Treatments that target some of the proposed cellular signaling pathways lead to improvement in all downstream processes. As an example, aldosterone antagonism reverses fibrosis and leads to structural, functional, and clinical improvement. Spironolactone has specifically been shown to reduce collagen volume fraction in ex-vivo hearts32 and endomyocardial biopsies;28 reduce levels of end-products from collagen synthesis;33 improve cyclic variation of integrated backscatter by echocardiography (an indirect gage of fibrosis); 34, 35 improve diastolic function;28 reduce left ventricular size;35, 36 raise ejection fraction;36 lower natriuretic peptides; and improve exercise capacity.36 Many of these studies involved fewer than 20 subjects,28, 32, 34 an important consideration for a patient population that is relatively small, such as ACHD. In addition aldosterone inhibition improves mortality37 and lowers the incidence of sudden arrhythmogenic death.38 Given that many studies now demonstrate geometric and histologic improvement in the absence of significant hemodynamic change,34, 35 it is likely that the beneficial effects of aldosterone antagonism are mediated by a reduction of myocardial fibrosis, rather than solely through alternative actions such as afterload reduction, diuresis, or prevention of arrhythmia.29
The schema as shown intentionally demonstrates myocardial fibrosis at the center point between the inciting etiologies and the clinical manifestations of heart failure. Thanks in part to imaging techniques that allow for fibrosis detection and quantification, and to pharmacotherapy that prevents or reduces fibrosis, fibrosis has become a focus for studying and treating the problem specifically in ACHD patients. No longer an elusive “factor”, myocardial fibrosis is now seen to be a marker of advanced myocardial injury and remodeling.39 An accumulation of extracellular material composed largely of collagen, myocardial fibrosis is an early process incited by a series of events prevalent in ACHD.40
Histopathology descriptions from congenital patients decades ago demonstrated both intracellular and extracellular changes such as, “Marked interstitial fibrosis, cellular atrophy, myofibrillar disorganization, myelin figures, myofibrillar lysis, proliferation of smooth endoplasmic reticulum, lipid deposition, spherical microparticles associated with the plasma membranes of the muscle cells, intracytoplasmic junctions and thickening of the basal laminae of the muscle cells.”41 Similarly, in tetralogy of Fallot patients undergoing surgery (mean age only 9 years), biopsies from the RVOT demonstrated myocardial fibrosis in 65% and myocytolysis in 68%.42 Most research has focused on extracellular changes, specifically myocardial fibrosis, since these are arguably more detectable and quantifiable, as will be shown, although intracellular changes are just as relevant.
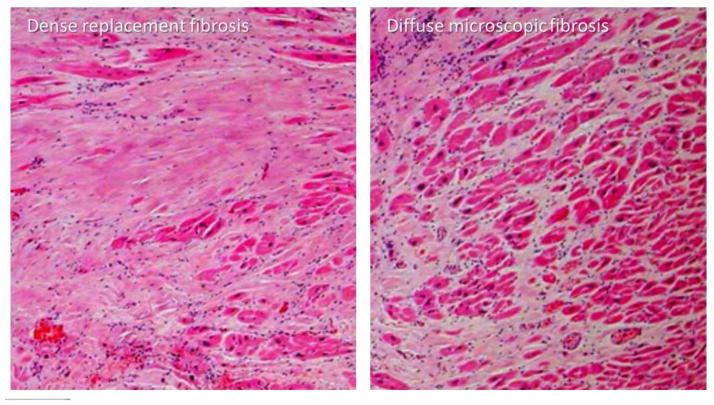
An important characteristic of fibrosis in CHD specifically is that it is often diffuse in nature. Patterns of fibrosis differ. Larger areas of patchy fibrosis, or scar, are typically seen following myocardial infarction where there is near total disruption of myofibrillar function and cellular integrity, a process sometimes referred to as “replacement fibrosis.” While such areas can be encountered in ACHD (Figure 3), a separate pattern of more diffuse fibrosis can be seen where the extracellular matrix is more subtly affected.
Figure 3.
Hematoxylin and eosin stain (400× magnification) of a right ventricular myocardial sample from a single patient with tetralogy of Fallot. Myocardial fibrosis in some areas is patchy “replacement” fibrosis with dense extracelullar material (left) as may be viewed by late gadolinium enhancement. Other areas show much more microscopic, diffuse fibrosis (right), more common in congenital heart disease, which is detectable by methods that measure the extraceullular volume fraction. Both types of fibrosis are present in the same patient (Courtesy of Dr. Henryk Kafka and Mary Shepperd, Royal Brompton Hospital).
Current methods to detect and quantify myocardial fibrosis
Interest in fibrosis has been spurned by imaging techniques allowing for its detection. Using delayed or “late” enhancement after gadolinium administration, several groups have demonstrated the presence of myocardial fibrosis in tetralogy of Fallot,43 systemic right ventricle,23 single ventricle patients after a Fontan palliation,44 and Eisenmenger syndrome.45 While generally in small quantities, ACHD-associated myocardial fibrosis is found within both the right and left ventricles and is not limited to areas of surgical scar or within a coronary distribution. Collectively these studies show convincing associations between fibrosis and other clinical evidence of heart failure, including reduced systolic function, poorer functional class, increased BNP and arrhythmia, and lower submaximal or maximal exercise capacity and ventricular size and function.23, 44–46
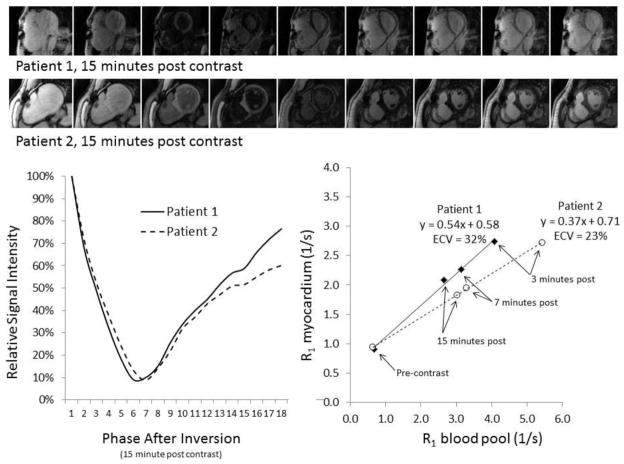
Late gadolinium enhancement is largely binary in that it identifies discrete areas of dense macroscopic “replacement” fibrosis (i.e. scar) on a per-pixel basis, as opposed to fibrosis that may be much more diffuse and microscopic in CHD (Figure 3). Both types of fibrosis may coexist in the same patient. Newer cardiovascular magnetic resonance techniques use quantification of T1. T1 is a measure of the time required for proton spins to return to their original magnetization after a radiofrequency pulse which is altered in states with myocardial pathology. This time is shortened in tissue that is more densely extracellular and is shortened by gadolinium that largely affects extracellular areas. By comparing T1 times before and after administration of gadolinium-based contrast (Figure 4), or during a steady-state infusion of gadolinium,47 and plotting the relationship of this response against that of the blood pool, one can mathematically calculate the partition coefficient of gadolinium. Then, by accounting for the plasma concentration of gadolinium in plasma, one can calculate the extracellular volume fraction (ECV). 48 In longitudinal studies using dog models, ECV more closely reflects changes in collagen volume fraction than T1 measures alone.49
Figure 4.
Examples of extracellular volume (ECV) assessment in two patients with tetralogy of Fallot. A T1 inversion recovery sequence through the mid myocardium is obtained before and after administration of gadolinium contrast. Phases 3–10 of images taken 15 minutes after gadolinium are shown (top). These images are used to plot a time signal intensity curve from which exponential fitting is used to define T1 times (left graph). The reciprocal of each T1 time (R1) before and after gadolinium (three time points after injection are shown here) is plotted against corresponding times for the blood pool in the left ventricle (right graph). The linear slope of this relationship is used to quantify ECV. Compared to patient 2, patient 1 reaches a low signal (dark myocardium) sooner (top bar), consistent with a shorter T1 time (left graph), and a steeper slope (right graph), coinciding with a higher ECV.
Significant momentum for ECV quantification is growing across cardiology. Emerging studies show consistently that ECV demonstrates fibrosis in many disease states including dilated cardiomyopathy,50 atrial fibrillation,51 aortic stenosis,47 hypertrophic cardiomyopathy,47, 52 and muscular dystrophy. 53 ECV correlates with other markers of fibrosis including collagen volume fraction by endomyocardial biopsy54 as well as with clinical events.53, 55 Importantly, ECV has been shown as a predictor of mortality.39 In many respects it is the imaging equivalent of a troponin assay, though its sensitivity to change has not been studied. While there are some differences amongst published methods, the ECV allows for measurement that is independent of heart rate, cardiac output, or Tesla strength.
These methods have been utilized to demonstrate diffuse fibrosis in ACHD patients of various types, showing that ECV correlates with ventricular size and function.48 As expected, the amount of fibrosis detected by ECV is more abundant than the quantity appreciable by late gadolinium enhancement alone. Applications of these techniques in pediatric and congenital heart disease have been recently summarized, including some of the caveats of their application specifically in these patients.40 Diffuse fibrosis has been recently demonstrated in focused studies in congenital aortic stenosis56 and in the systemic right ventricle57 through these methods.
While the mounting enthusiasm for T1 mapping generates a focus on fibrosis specifically, and adds support to the focus on fibrosis and its prominent role as at least one “myocardial factor, generally there are limitations to applying these techniques to CHD specifically.40 For example, most studies focus on a single mid-ventricular plane which may or may not be representative of the entire heart. Also, spatial resolution is limited, which compromises assessment of thinner walls such as the right ventricle or of trabeculations where blood pooling may compromise the measurement. T1 mapping cannot be universally applied to the congenital population since many patients have contraindications to CMR scanning. T1 mapping methods may serve as an important surrogate endpoint for future pharmacologic studies,40 though the variance in T1 quantification has not been formally evaluated and may be insufficient to detect changes in an individual over time. Finally, this fibrosis-centric model of heart failure pathogenesis ignores metabolics/energetics of the myocyte itself, which are more difficult to study with today’s tools. Still, the established reliability of these methods overall now provides an additional tool for studying the heart failure process.58
Myocardial fibrosis as a potential therapeutic target
In many forms of heart disease our understanding and treatment of myocardial dysfunction has advanced considerably. One notes, for example, the focus on intervention for valve disease before adverse myocardial remodeling occurs, more intense cardio-protection during surgery, and avoidance of surgery in situations where advanced dysfunction limits the likelihood of ventricular recovery. But so much more is needed in order to see improvements in ACHD outcomes secondary to adequate myocardial preservation.
A critical implication of the role of myocardial fibrosis in ACHD is as a target for attenuating it via pharmacotherapy. In ACHD, current strategies for treatment are empirically borrowed from established therapies in idiopathic and ischemic cardiomyopathy. As such, there is an obligation to understand if ACHD heart failure mechanisms are similar, to lend credibility to the use of effective pharmacotherapy. For other acquired models of heart failure, there is clear evidence that drugs that target the RAAS and neurohormonal activation reduce fibrosis and improve mortality.28, 32, 33 Given the evidence for neurohormonal up-regulation and myocardial fibrosis in ACHD-related heart failure, such drugs offer significant therapeutic potential in ACHD, particularly if they are applied early in the process.
Studies to date have unfortunately failed to show persistent and convincing effects of standard heart failure therapy in CHD. The central importance of the RAAS as a mediator of myocardial fibrosis and ventricular dysfunction has led a number of researchers to investigate blockade of the angiotensin pathway in CHD populations. Small retrospective studies of lisinopril in transposition did not show improvement in systolic function, peak VO2, or cardiac index.59,60,61 A small, prospective cross-over study of losartan in transposition showed improved ejection fraction and exercise duration in an adolescent and young adult population.62 However, in a larger randomized study found exercise duration, VO2 max, or NT-pro BNP levels were not improved with angiotensin receptor blockade.63 Another larger and longer randomized trial of angiotensin blockade in transposition similarly showed no benefit.64 A randomized controlled trial of ramipril in tetralogy of Fallot patients with pulmonary regurgitation demonstrated no improvement in right or left ventricular function or exercise capacity.65 Enalapril therapy in pediatric patients with single-ventricle physiology provided no benefit in HF symptoms, growth, ventricular systolic function or death.66 In a smaller randomized trial, enalapril did not alter systemic vascular resistance, resting cardiac index, diastolic function, or exercise capacity.67
By counteracting the pathogenic effects of sympathetic activation, beta-blockers may also be helpful for reducing myocardial fibrosis. Studies for beta-blockade in congenital heart disease are intriguing but limited due to small patient numbers and observational design. In a retrospective study among a heterogeneous group of children, adolescents and young adults with a single ventricle, carvedilol improved NYHA functional class, diuretic requirements, and ejection fraction in the Fontan patients,68 and several small single center reports show a potential use of beta-blockers in those with systemic right ventricles.69–71,72 But these studies are largely underpowered, biased, and observational. An important role for beta-blockers in CHD may be a reduction in arrhythmic events, such as in transposition patients with pacemakers/defibrillators.73 Yet bisoprolol in repaired tetralogy of Fallot patients with reduced RV function, elevated BNP and impaired VO2 showed no improvement in NYHA class, exercise capacity or systolic function of either ventricle. In fact, the patients randomized to receive bisoprolol actually demonstrated a statistically significant increase in BNP compared to the placebo group.74
In summary, despite plausible data demonstrating similar patterns of ventricular dysfunction as per non-congenital heart failure, pharmacotherapy is still largely unproven. However, these small studies chose clinical endpoints, for which they may have been greatly underpowered. It may be that a surrogate such as myocardial fibrosis quantification may provide a vital intermediate step toward demonstrating efficacy, though the therapy would need to target individuals early, even before fibrotic changes are detectable since late fibrosis may not be reversible.
A new paradigm
It is becoming increasingly clear that for patients with congenital heart disease, protection of the myocardium should receive as much attention as the hemodynamics early in the course of a patient’s life for preservation of both ventricular function and the patient. Granted, this attention has been ongoing; already earlier surgery and better cardio-protection have provided substantial improvements in patients’ functional capacity after repair. Focusing further on myocardial preservation will require continued efforts from the congenital heart community at large to consider, study, and treat myocardial dysfunction early on. Further improvements in timing of surgery, better anatomic repairs, and cardio-protection methods will slow the progression of heart failure. More important, there must be a significant increase in the study of the problem, which for now focuses on myocardial fibrosis. Larger multicenter prospective trials are needed to clarify the role, if any, of pharmacotherapy. Pilot studies using surrogate endpoints would help justify needed trials, and research-funding agencies must recognize the need to support such trials. While it is reassuring to know of ongoing efforts made in these areas, clearly much more needs to be done before the “myocardial factor” can be attenuated.
Highlights.
Myocardial function (ie the “myocardial factor”) has historically been noted as a significant determinant of long-term prognosis of individuals with congenital heart disease.
Congenital heart patients today have many features of clinical heart failure.
Myocardial fibrosis is an important final common pathway in the development of myocardial dysfunction.
Methods for detecting and quantifying myocardial fibrosis are becoming more sophisticated, providing better tools for the study and treatment of myocardial fibrosis.
Acknowledgments
Dr. Broberg is supported by the United States National Heart Lung and Blood Institute and the American Heart Association.
Footnotes
Disclosures
Neither author has any conflicts of interest related to this topic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task force 1: The changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. doi: 10.1016/s0735-1097(01)01272-4. [DOI] [PubMed] [Google Scholar]
- 2.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 3.Blalock A. Cardiovascular surgery, past and present. J Thorac Cardiovasc Surg. 1966;51:153–167. [PubMed] [Google Scholar]
- 4.McIntosh HD, Cohen AI. Pulmonary stenosis; the importance of the myocardial factor in determining the clinical course and surgical results. Am Heart J. 1963;65:715–716. [Google Scholar]
- 5.Goldberg SJ, Weiss R, Adams FH. A comparison of the maximal endurance of normal children and patients with congenital cardiac disease. The Journal of pediatrics. 1966;69:46–55. doi: 10.1016/s0022-3476(66)80360-8. [DOI] [PubMed] [Google Scholar]
- 6.Epstein SE, Beiser GD, Goldstein RE, Rosing DR, Redwood DR, Morrow AG. Hemodynamic abnormalities in response to mild and intense upright exercise following operative correction of an atrial septal defect or tetralogy of fallot. Circulation. 1973;47:1065–1075. doi: 10.1161/01.cir.47.5.1065. [DOI] [PubMed] [Google Scholar]
- 7.Jarmakani JM, Graham TP, Jr, Canent RV, Jr, Jewett PH. Left heart function in children with tetralogy of fallot before and after palliative or corrective surgery. Circulation. 1972;46:478–490. doi: 10.1161/01.cir.46.3.478. [DOI] [PubMed] [Google Scholar]
- 8.Jones M, Ferrans VJ. Myocardial degeneration in congenital heart disease. Comparison of morphologic findings in young and old patients with congenital heart disease associated with muscular obstruction to right ventricular outflow. Am J Cardiol. 1977;39:1051–1063. doi: 10.1016/s0002-9149(77)80221-x. [DOI] [PubMed] [Google Scholar]
- 9.Jones M, Ferrans VJ, Morrow AG, Roberts WC. Ultrastructure of crista supraventricularis muscle in patients with congenital heart diseases associated with right ventricular outflow tract obstruction. Circulation. 1975;51:39–67. doi: 10.1161/01.cir.51.1.39. [DOI] [PubMed] [Google Scholar]
- 10.Bolger AP, Sharma R, Li W, Leenarts M, Kalra PR, Kemp M, Coats AJ, Anker SD, Gatzoulis MA. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation. 2002;106:92–99. doi: 10.1161/01.cir.0000020009.30736.3f. [DOI] [PubMed] [Google Scholar]
- 11.Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA, Diller GP. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life--single centre experience and review of published data. European heart journal. 2012;33:1386–1396. doi: 10.1093/eurheartj/ehr461. [DOI] [PubMed] [Google Scholar]
- 12.Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: The original heart failure syndrome. Eur Heart J. 2003;24:970–976. doi: 10.1016/s0195-668x(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 13.Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS, Johansson B, Bouzas B, Mullen MJ, Poole-Wilson PA, Francis DP, Gatzoulis MA. Exercise intolerance in adult congenital heart disease: Comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 14.Broberg CS, Aboulhosn J, Mongeon FP, Kay J, Valente AM, Khairy P, Earing MG, Opotowsky AR, Lui G, Gersony DR, Cook S, Ting JG, Webb G, Gurvitz MZ Alliance for Adult Research in Congenital C. Prevalence of left ventricular systolic dysfunction in adults with repaired tetralogy of fallot. Am J Cardiol. 2011;107:1215–1220. doi: 10.1016/j.amjcard.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Book WM, Hott BJ, McConnell M. B-type natriuretic peptide levels in adults with congenital heart disease and right ventricular failure. Am J Cardiol. 2005;95:545–546. doi: 10.1016/j.amjcard.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Chow PC, Cheung EW, Chong CY, Lun KS, Yung TC, Wong KT, Chau AK, Cheung YF. Brain natriuretic peptide as a biomarker of systemic right ventricular function in patients with transposition of great arteries after atrial switch operation. Int J Cardiol. 2008;127:192–197. doi: 10.1016/j.ijcard.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Giannakoulas G, Dimopoulos K, Bolger AP, Tay EL, Inuzuka R, Bedard E, Davos C, Swan L, Gatzoulis MA. Usefulness of natriuretic peptide levels to predict mortality in adults with congenital heart disease. Am J Cardiol. 105:869–873. doi: 10.1016/j.amjcard.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins WE, Chen Z, Fukagawa NK, Hall C, Knot HJ, LeWinter MM. Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease: Enhanced understanding of the relationship between hypoxia and natriuretic peptide secretion. Circulation. 2004;109:2872–2877. doi: 10.1161/01.CIR.0000129305.25115.80. [DOI] [PubMed] [Google Scholar]
- 19.Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon FP, Kay J, Valente AM, Earing MG, Lui G, Gersony DR, Cook S, Ting JG, Nickolaus MJ, Webb G, Landzberg MJ, Broberg CS Alliance for Adult Research in Congenital C. Arrhythmia burden in adults with surgically repaired tetralogy of fallot: A multi-institutional study. Circulation. 2010;122:868–875. doi: 10.1161/CIRCULATIONAHA.109.928481. [DOI] [PubMed] [Google Scholar]
- 20.Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 21.Khairy P, Harris L, Landzberg MJ, Viswanathan S, Barlow A, Gatzoulis MA, Fernandes SM, Beauchesne L, Therrien J, Chetaille P, Gordon E, Vonder Muhll I, Cecchin F. Implantable cardioverter-defibrillators in tetralogy of fallot. Circulation. 2008;117:363–370. doi: 10.1161/CIRCULATIONAHA.107.726372. [DOI] [PubMed] [Google Scholar]
- 22.Aboulhosn JA, Lluri G, Gurvitz MZ, Khairy P, Mongeon FP, Kay J, Valente AM, Earing MG, Opotowsky AR, Lui G, Gersony DR, Cook S, Child J, Ting J, Webb G, Landzberg M, Broberg CS Alliance for Adult Research in Congenital C. Left and right ventricular diastolic function in adults with surgically repaired tetralogy of fallot: A multi-institutional study. Can J Cardiol. 2013;29:866–872. doi: 10.1016/j.cjca.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Babu-Narayan SV, Goktekin O, Moon JC, Broberg CS, Pantely GA, Pennell DJ, Gatzoulis MA, Kilner PJ. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111:2091–2098. doi: 10.1161/01.CIR.0000162463.61626.3B. [DOI] [PubMed] [Google Scholar]
- 24.Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: A population-based study. J Am Coll Cardiol. 2007;50:1263–1271. doi: 10.1016/j.jacc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 25.Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000;86:1111–1116. doi: 10.1016/s0002-9149(00)01169-3. [DOI] [PubMed] [Google Scholar]
- 26.Delcayre C, Swynghedauw B. Molecular mechanisms of myocardial remodeling. The role of aldosterone. J Mol Cell Cardiol. 2002;34:1577–1584. doi: 10.1006/jmcc.2002.2088. [DOI] [PubMed] [Google Scholar]
- 27.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 28.Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: A pilot study. Circulation. 2005;112:2940–2945. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 29.Cohn JN. Myocardial structural effects of aldosterone receptor antagonism in heart failure. J Am Coll Cardiol. 2007;50:597–599. doi: 10.1016/j.jacc.2007.04.063. [DOI] [PubMed] [Google Scholar]
- 30.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O’Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. Jama. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 31.Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, Tokuda M, Daly CA, Tedrow UB, Stevenson WG, Jerosch-Herold M, Ghoshhajra BB, Kwong RY. Cmr quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC. Cardiovascular imaging. 2013;6:944–954. doi: 10.1016/j.jcmg.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki G, Morita H, Mishima T, Sharov VG, Todor A, Tanhehco EJ, Rudolph AE, McMahon EG, Goldstein S, Sabbah HN. Effects of long-term monotherapy with eplerenone, a novel aldosterone blocker, on progression of left ventricular dysfunction and remodeling in dogs with heart failure. Circulation. 2002;106:2967–2972. doi: 10.1161/01.cir.0000039104.56479.42. [DOI] [PubMed] [Google Scholar]
- 33.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: Insights from the randomized aldactone evaluation study (rales). Rales investigators. Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 34.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 35.Chan AK, Sanderson JE, Wang T, Lam W, Yip G, Wang M, Lam YY, Zhang Y, Yeung L, Wu EB, Chan WW, Wong JT, So N, Yu CM. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007;50:591–596. doi: 10.1016/j.jacc.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 36.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Marino P, Zardini P. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:304–310. doi: 10.1016/s0735-1097(02)01965-4. [DOI] [PubMed] [Google Scholar]
- 37.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 38.Anand K, Mooss AN, Mohiuddin SM. Aldosterone inhibition reduces the risk of sudden cardiac death in patients with heart failure. J Renin Angiotensin Aldosterone Syst. 2006;7:15–19. doi: 10.3317/jraas.2006.001. [DOI] [PubMed] [Google Scholar]
- 39.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riesenkampff E, Messroghli DR, Redington AN, Grosse-Wortmann L. Myocardial t1 mapping in pediatric and congenital heart disease. Circ Cardiovasc Imaging. 2015;8:e002504. doi: 10.1161/CIRCIMAGING.114.002504. [DOI] [PubMed] [Google Scholar]
- 41.Jones M, Ferrans VJ. Myocardial ultrastructure in children and adults with congenital heart disease. Cardiovasc Clin. 1979;10:501–530. [PubMed] [Google Scholar]
- 42.Chowdhury UK, Sathia S, Ray R, Singh R, Pradeep KK, Venugopal P. Histopathology of the right ventricular outflow tract and its relationship to clinical outcomes and arrhythmias in patients with tetralogy of fallot. J Thorac Cardiovasc Surg. 2006;132:270–277. doi: 10.1016/j.jtcvs.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, Khan M, Ho SY, Pennell DJ, Gatzoulis MA. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–413. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 44.Rathod RH, Prakash A, Powell AJ, Geva T. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after fontan operation. J Am Coll Cardiol. 2010;55:1721–1728. doi: 10.1016/j.jacc.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broberg CS, Prasad SK, Carr C, Babu-Narayan SV, Dimopoulos K, Gatzoulis MA. Myocardial fibrosis in eisenmenger syndrome: A descriptive cohort study exploring associations of late gadolinium enhancement with clinical status and survival. J Cardiovasc Magn Reson. 2014;16:32. doi: 10.1186/1532-429X-16-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diller GP, Dimopoulos K, Okonko D, Uebing A, Broberg CS, Babu-Narayan S, Bayne S, Poole-Wilson PA, Sutton R, Francis DP, Gatzoulis MA. Heart rate response during exercise predicts survival in adults with congenital heart disease. J Am Coll Cardiol. 2006;48:1250–1256. doi: 10.1016/j.jacc.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 47.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 48.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koopmann M, Hong K, Kholmovski EG, Huang EC, Hu N, Ying J, Levenson R, Vijayakumar S, Dosdall DJ, Ranjan R, Kim D. Post-contrast myocardial t(1) and ecv disagree in a longitudinal canine study. NMR in biomedicine. 2014;27:988–995. doi: 10.1002/nbm.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta S, Kaye D, Taylor A. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced t1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 51.Ling LH, Kistler PM, Ellims AH, Iles LM, Lee G, Hughes GL, Kalman JM, Kaye DM, Taylor AJ. Diffuse ventricular fibrosis in atrial fibrillation: Noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol. 2012;60:2402–2408. doi: 10.1016/j.jacc.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 52.Brouwer WP, Baars EN, Germans T, de Boer K, Beek AM, van der Velden J, van Rossum AC, Hofman MB. In-vivo t1 cardiovascular magnetic resonance study of diffuse myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2014;16:28. doi: 10.1186/1532-429X-16-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Florian A, Ludwig A, Engelen M, Waltenberger J, Rosch S, Sechtem U, Yilmaz A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson. 2014;16:81. doi: 10.1186/s12968-014-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aus dem Siepen F, Buss SJ, Messroghli D, Andre F, Lossnitzer D, Seitz S, Keller M, Schnabel PA, Giannitsis E, Korosoglou G, Katus HA, Steen H. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: Quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. European heart journal cardiovascular Imaging. 2014 doi: 10.1093/ehjci/jeu183. [DOI] [PubMed] [Google Scholar]
- 55.Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, Taylor AJ. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–828. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 56.Dusenbery SM, Jerosch-Herold M, Rickers C, Colan SD, Geva T, Newburger JW, Powell AJ. Myocardial extracellular remodeling is associated with ventricular diastolic dysfunction in children and young adults with congenital aortic stenosis. J Am Coll Cardiol. 2014;63:1778–1785. doi: 10.1016/j.jacc.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 57.Plymen CM, Sado DM, Taylor AM, Bolger AP, Lambiase PD, Hughes M, Moon JC. Diffuse myocardial fibrosis in the systemic right ventricle of patients late after mustard or senning surgery: An equilibrium contrast cardiovascular magnetic resonance study. European heart journal cardiovascular Imaging. 2013;14:963–968. doi: 10.1093/ehjci/jet014. [DOI] [PubMed] [Google Scholar]
- 58.Maestrini V, Treibel TA, White SK, Fontana M, Moon JC. T1 mapping for characterization of intracellular and extracellular myocardial diseases in heart failure. Current cardiovascular imaging reports. 2014;7:9287. doi: 10.1007/s12410-014-9287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hechter SJ, Fredriksen PM, Liu P, Veldtman G, Merchant N, Freeman M, Therrien J, Benson L, Siu S, Webb G. Angiotensin-converting enzyme inhibitors in adults after the mustard procedure. Am J Cardiol. 2001;87:660–663. A611. doi: 10.1016/s0002-9149(00)01452-1. [DOI] [PubMed] [Google Scholar]
- 60.Robinson B, Heise CT, Moore JW, Anella J, Sokoloski M, Eshaghpour E. Afterload reduction therapy in patients following intraatrial baffle operation for transposition of the great arteries. Pediatr Cardiol. 2002;23:618–623. doi: 10.1007/s00246-002-0046-2. [DOI] [PubMed] [Google Scholar]
- 61.Therrien J, Provost Y, Harrison J, Connelly M, Kaemmerer H, Webb GD. Effect of angiotensin receptor blockade on systemic right ventricular function and size: A small, randomized, placebo-controlled study. Int J Cardiol. 2008;129:187–192. doi: 10.1016/j.ijcard.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 62.Lester SJ, McElhinney DB, Viloria E, Reddy GP, Ryan E, Tworetzky W, Schiller NB, Foster E. Effects of losartan in patients with a systemically functioning morphologic right ventricle after atrial repair of transposition of the great arteries. Am J Cardiol. 2001;88:1314–1316. doi: 10.1016/s0002-9149(01)02098-7. [DOI] [PubMed] [Google Scholar]
- 63.Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, Marcotte F, Mercier LA. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: A multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–2416. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- 64.van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos-Hesslink JW, Zwinderman AH, Mulder BJ. Rationale and design of a trial on the effect of angiotensin ii receptor blockers on the function of the systemic right ventricle. Am Heart J. 2010;160:812–818. doi: 10.1016/j.ahj.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Babu-Narayan SV, Uebing A, Davlouros PA, Kemp M, Davidson S, Dimopoulos K, Bayne S, Pennell DJ, Gibson DG, Flather M, Kilner PJ, Li W, Gatzoulis MA. Randomised trial of ramipril in repaired tetralogy of fallot and pulmonary regurgitation: The appropriate study (ace inhibitors for potential prevention of the deleterious effects of pulmonary regurgitation in adults with repaired tetralogy of fallot) Int J Cardiol. 2012;154:299–305. doi: 10.1016/j.ijcard.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 66.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, Altmann K, Ghanayem NS, Margossian R, Chung WK, Border WL, Pearson GD, Stylianou MP, Mital S. Enalapril in infants with single ventricle: Results of a multicenter randomized trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kouatli AA, Garcia JA, Zellers TM, Weinstein EM, Mahony L. Enalapril does not enhance exercise capacity in patients after fontan procedure. Circulation. 1997;96:1507–1512. doi: 10.1161/01.cir.96.5.1507. [DOI] [PubMed] [Google Scholar]
- 68.Ishibashi N, Park IS, Waragai T, Yoshikawa T, Murakami Y, Mori K, Mimori S, Ando M, Takahashi Y, Doi S, Mizutani S, Nakanishi T. Effect of carvedilol on heart failure in patients with a functionally univentricular heart. Circ J. 2011;75:1394–1399. doi: 10.1253/circj.cj-10-0845. [DOI] [PubMed] [Google Scholar]
- 69.Josephson CB, Howlett JG, Jackson SD, Finley J, Kells CM. A case series of systemic right ventricular dysfunction post atrial switch for simple d-transposition of the great arteries: The impact of beta-blockade. Can J Cardiol. 2006;22:769–772. doi: 10.1016/s0828-282x(06)70293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giardini A, Lovato L, Donti A, Formigari R, Gargiulo G, Picchio FM, Fattori R. A pilot study on the effects of carvedilol on right ventricular remodelling and exercise tolerance in patients with systemic right ventricle. Int J Cardiol. 2006 doi: 10.1016/j.ijcard.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 71.Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol xl) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol. 2007;99:704–706. doi: 10.1016/j.amjcard.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 72.Lindenfeld J, Keller K, Campbell DN, Wolfe RR, Quaife RA. Improved systemic ventricular function after carvedilol administration in a patient with congenitally corrected transposition of the great arteries. J Heart Lung Transplant. 2003;22:198–201. doi: 10.1016/s1053-2498(02)00656-3. [DOI] [PubMed] [Google Scholar]
- 73.Khairy P, Harris L, Landzberg MJ, Fernandes SM, Barlow A, Mercier LA, Viswanathan S, Chetaille P, Gordon E, Dore A, Cecchin F. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: A multicenter study. Circulation. Arrhythmia and electrophysiology. 2008;1:250–257. doi: 10.1161/CIRCEP.108.776120. [DOI] [PubMed] [Google Scholar]
- 74.Norozi K, Bahlmann J, Raab B, Alpers V, Arnhold JO, Kuehne T, Klimes K, Zoege M, Geyer S, Wessel A, Buchhorn R. A prospective, randomized, double-blind, placebo controlled trial of beta-blockade in patients who have undergone surgical correction of tetralogy of fallot. Cardiol Young. 2007;17:372–379. doi: 10.1017/S1047951107000844. [DOI] [PubMed] [Google Scholar]