Abstract
Background
Human immunodeficiency virus (HIV) infection is associated with increased cardiovascular risk, and this risk correlates with markers of monocyte activation. We have shown that HIV is associated with a prothrombotic monocyte phenotype, which can be partially mitigated by statin therapy. We therefore explored the relationship between oxidized LDL particles and monocyte activation.
Methods
We performed phenotypic analysis of monocytes using flow cytometry on fresh whole blood in 54 patients with HIV and 24 controls without HIV. Plasma levels of oxLDL, soluble CD14, IL-6, soluble CD163 were measured by ELISA. In vitro experiments were performed using flow cytometry.
Results
Plasma levels of oxLDL were significantly increased in HIV-infection compared to controls (60.1 units vs 32.1 units, p<0.001). Monocyte expression of the oxLDL receptors, CD36 and Toll-like receptor 4, were also increased in HIV. OxLDL levels correlated with markers of monocyte activation, including soluble CD14, TF expression on inflammatory monocytes, and CD36. In vitro, stimulation with oxLDL, but not to LDL, resulted in expansion of inflammatory monocytes and increased monocyte expression of TF, recapitulating the monocyte profile we find in HIV disease.
Conclusions
OxLDL may contribute to monocyte activation and further study in the context of HIV disease is warranted.
Keywords: HIV, monocytes, oxidized LDL, tissue factor
Introduction
Immune activation and inflammation are hallmarks of chronic HIV infection;1,2 and even in the setting of combination antiretroviral therapy (ART) and well-controlled viremia, markers of inflammation and immune activation often remain elevated2–4. Numerous studies have demonstrated that markers of inflammation1,5,6, coagulation1,7,8 and indices of T cell and monocyte activation2,9 are independently associated with morbidity and mortality in HIV-infected patients.
Inflammation and leukocyte activation are important contributors to cardiovascular disease (CVD) and thrombotic risk in the general population10–12. Increased inflammation may contribute to greater risk for both venous13,14 and arterial15 thromboembolic events in HIV-infection. We, and others, have found increased expression of the procoagulant tissue factor (TF) on the surfaces of monocytes16,17 and platelets18 and within the circulation as microparticles4,19. Among HIV-infected patients, D-dimer levels correlate with proportions of TF+ monocytes 16 and with plasma levels of sCD1417, suggesting a link between monocyte activation and coagulation.
We have previously reported that a similar profile of monocyte activation is observed in HIV-uninfected patients with acute coronary syndromes (ACS)17. Monocyte subsets, including the CD14DimCD16+ patrolling monocytes, and CD14+CD16+ inflammatory monocytes, are enriched in HIV disease and in ACS; these cells express high levels of TF and CX3CR1 (the fractalkine receptor)17. As their eponym implies, CD14DimCD16+ monocytes appear equipped to “patrol” the vessel wall20, tethering to sites of activated endothelium expressing fractalkine, where they may mediate thrombosis via the expression of TF. Inflammatory monocytes also express high levels of pro-inflammatory cytokines in response to microbial products20, and proportions of these cells are independent predictors of cardiovascular events in HIV-uninfected subjects21.
Enrichment of these monocyte populations have been described in inflammatory conditions including: chronic kidney disease, atherosclerosis, sepsis, rheumatoid arthritis, and HIV disease21–25. What remains to be elucidated in each of these disease states, given distinct pathologic pathways, are the mechanisms driving monocyte activation and differentiation. A number of potential drivers of immune activation have been suggested in treated HIV infection, including low level HIV replication, systemic translocation of bacterial products from the damaged gastrointestinal tract, the presence of copathogens, and homeostatic responses to cytopenia3. As many HIV-infected patients also have altered lipid and metabolic profiles 26,27, we sought to explore the role of inflammatory lipids in the activation of monocytes in HIV disease.
Methods
Patients
These studies were performed in compliance with the Institutional Review Board at Case Western Reserve University/University Hospitals. Blood samples were obtained following informed consent. Healthy donors not known to be HIV-1 infected (N=24) were recruited from the general Case Western Reserve University staff population. HIV-infected donors (N=54) were recruited from the Special Immunology Unit of University Hospitals/Case Medical Center. None of the donors were fasting and all donors were selected by convenience sampling. Framingham-based risk score was calculated using the online calculator at www.mdcalc.com and is expressed as the risk of incident coronary heart disease within a 10 year period 28.
Cell preparation and incubations
Blood was drawn into EDTA coated tubes (ThermoFisher Scientific, Waltham, MA). Whole blood (500μl) was incubated in 15 ml polypropylene Falcon tubes (Becton Dickinson Labware, Franklin Lakes, NJ) for 3 hours with or without lipopolysaccharide (LPS, from E. Coli, 20ng/mL, Invivogen, San Diego, CA), low-density lipoprotein (LDL 50μg/mL) or oxidized LDL (oxLDL 50 μg/mL, Biomedical Technologies Incorporated, Ward Hill, MA).
Flow Cytometry
Monocytes subsets were identified by size, granularity, and by expression levels of CD14 and CD16. Fluorescence minus 1 and isotype gating strategies were used to identify expression of surface markers as previously reported17.
Cell surface molecule expression was monitored using the following fluorochrome-labeled antibodies: anti-Tissue Factor fluorescein isothiocyanate (FITC) (American Diagnostica, Stamford, CT), anti-CD14 Pacific Blue, anti-CD16 phycoerythrin (PE), anti-CD36 allophycocyanin (APC), (BD Pharmingen, San Diego, CA), and anti-HLA-DR APC-cy7 (BD Biosciences, San Jose, CA), anti-Toll-like receptor (TLR) 2 APC, anti-TLR4 PEcy7, and anti-TLR-6 biotin (eBioscience, San Diego, CA), streptavidin APC-cy7 (BD Bioscience).
Whole blood samples were incubated for 15 minutes on ice with FACS Lyse buffer (BD Biosciences) and then washed in buffer (phosphate buffered saline with 1% bovine serum albumin and 0.1% sodium azide). Cells were then stained for 30 minutes in the dark on ice and then washed in buffer, fixed in 1% formaldehyde, and analyzed using a Miltenyi MACS Quant flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany). MACS Quant software (version 2.21031.1, Miltenyi Biotec), and Prism 5.0 Graphpad software (La Jolla, CA) were used to organize and analyze the data.
Measurement of ox-LDL, IL-6, sCD14, and sCD163
Fresh whole blood samples were collected in EDTA containing tubes and were centrifuged for 15 minutes at 495× g. Plasma was removed and frozen at −80°C until thawed once and analyzed in batch. Levels of soluble CD14, soluble CD163, and IL-6 were measured using Quantikine ELISA kits (R&D Systems Minneapolis MN). The inter-assay variability ranged 0.4–8.6%, 0.7–18.3%, and 2.02–15.36%, respectively. Levels of oxLDL were also measured by ELISA (Mercodia Uppsala, Sweden); the intra-assay variability ranged 7.4–8.3%.
Statistical Methods
We used conventional measures of central location and dispersion to describe the data. We compared group medians using Mann-Whitney’s U test or one-way ANOVA with repeated measures where appropriate. We evaluated correlations between pairs of continuous variables using Spearman’s rank correlation. All comparisons are two-sided without formal correction for multiple comparisons, and p values <0.05 were considered statistically significant. Differences in gender and race between the populations were compared using a Chi-square test.
Results
Initial studies measuring plasma markers of inflammation and oxidized LDL were performed on samples from 54 HIV-infected patients and 24 healthy donors not known to be HIV-infected. Demographic information is displayed in Table 1; in brief, 26% of the HIV-infected patients were female, 34% were Caucasian. Their median age was 44 years, 91% were receiving ART; median CD4+ T cell count and plasma HIV-1 RNA level were 534 cells/μl and 48 copies/mL, respectively. Among the uninfected donors, 67% were female, 75% were Caucasian, and the median age was 38 years. Compared to the HIV-infected population, the uninfected donors were slightly younger (p=0.04), more often female (p=0.002), and more often Caucasian (p=0.025).
Table 1.
Demographic information for HIV-1 uninfected controls and HIV-1 infected patients.
| HIV-1 uninfected Controls N=24 |
HIV-1 infected patients N=54 |
|
|---|---|---|
| Age | Median =38 years | Median 44 years |
| Range=23–64 | Range= 23–73 | |
| Gender (%Female) | 67% | 26% |
| CD4+ T cell Count | * N.R. | Median= 534 cells/μL |
| Range= 15–1343 | ||
| Plasma HIV-1 RNA | † N.A. | Median = 48copies/mL |
| Range=48–578,997 copies/mL | ||
| On ART (%) | N.A. | 91% |
| Hepatitis Coinfection | N.R. | 13% |
| Diabetes | N.R. | 6% |
| Diagnosed Hypertension | N.R | 37% |
| Current Smoker (%) | 38% | 52% |
| Aspirin Use | N.R. | 2% |
| Statin Use | N.R. | 28% |
| Total Cholesterol | N.R | 158 |
| LDL | N.R. | Median= 92 |
| Range= 33–189 | ||
| HDL | N.R. | Median= 43 |
| Range = 16–86 | ||
| Triglycerides | N.R. | Median = 116 |
| Range = 43–1056 | ||
| Framingham Risk Score | N.R. | Median = 5.9 |
| Range = 0–35.7 |
Information reported is from available patient information.
N.A. = not applicable
N.R. = not reported
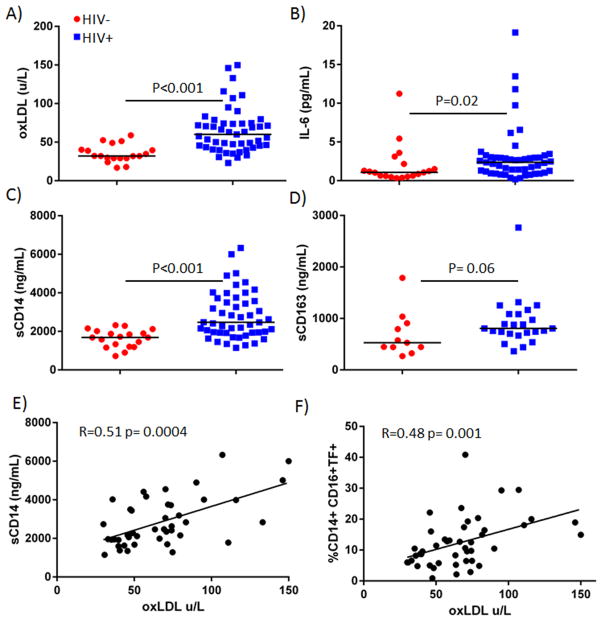
The median plasma levels of oxLDL were increased in HIV-infected patients (60.1 units/L, N=49) compared to levels among uninfected donors (32.1 units/L, N=19, p<0.001, Figure 1A). Among the HIV-infected donors, oxLDL levels correlated with levels of LDL (r=0.66, p<0.001) and total cholesterol (r= 0.45, p=0.005), but not with levels of triglycerides (r=0.22, p=0.15) or high density lipoproteins (HDL, r=−0.23, p=0.64).
Figure 1. Plasma levels of oxLDL are increased in HIV-1 infected patients and correlate with levels of sCD14 and tissue factor expression on inflammatory monocytes.
Plasma samples from all donors were thawed and levels A) oxidized LDL B) IL-6, C) sCD14, and D) sCD163 were measured by ELISA. There is a direct correlation between plasma levels of oxLDL and E) sCD14 and oxLDL and F) the proportion of inflammatory (CD14+CD16+) monocytes in HIV disease. Monocytes were assessed by flow cytometry.
We also measured plasma levels of IL-6 and sCD14, as these markers have been associated with all-cause mortality 1,2, and cardiovascular events 29 in HIV-infected patients. Not surprisingly, levels of IL-6 (Figure 1B) and sCD14 (Figure 1C) were both increased in HIV-infected patients (p=0.02, and p<0.001). We also measured sCD163, as it has been associated with non-calcified coronary artery plaques 30, with perivascular fat31, and with arterial inflammation in HIV-infected subjects32. Levels of sCD163 also tended to be increased in patients, but this increase did not reach significance (p=0.06, Figure 1D). Among HIV-infected patients, plasma levels of oxLDL and sCD14 were related (r=0.51, p=0.0004, Figure 1E) more closely than were levels of LDL and sCD14 (r=0.31, p=0.06), suggesting the possibility that oxLDL, more so than total LDL, may activate monocytes. There was no correlation between levels of oxLDL and IL-6 (r= 0.15, p=0.3) or sCD163 (r=0.1, p=0.65). Plasma oxLDL levels were also related to Framingham Risk Score (FRS) among patients with HIV disease (r=0.48, p=0.009). Plasma levels of LDL were also related to FRS (r= 0.495, p=0.006).
We analyzed the proportional representation and activation phenotype of monocyte subsets in whole blood samples from our donor groups. Flow cytometry data, including monocyte subset proportions and TF expression, have been published previously on 32 of the 54 patients 17. Among the 22 HIV+ patients not previously reported, we found an expansion of inflammatory (28% vs. 17%) and patrolling (12.2% vs. 8.7%) monocytes in HIV+ donors compared to these proportions in uninfected donors (p=0.001 and 0.01, respectively), confirming our previous findings17. We also confirmed that in HIV disease, inflammatory (10.5%) and patrolling (18.2%) monocytes are enriched for cells that express TF, when compared to these proportions in uninfected donors (5% and 8.8%, p=0.0004 and p<0.0001, respectively). This trend is also seen when analyzing all HIV+ patients; proportions of inflammatory and patrolling monocytes are enriched in HIV-infected patients (32.5% and 14.1%, p<0.0001 and p=0.001), and so are proportions of inflammatory and patrolling monocytes that express TF (17.1% and 23.6%, p<0.0001). Among all HIV-infected patients, plasma oxLDL levels are related to the proportion of TF+ inflammatory monocytes (r= 0.483, p=0.001, Figure 1F), providing another link between oxLDL levels and monocyte activation. There was no relationship between plasma levels of LDL and TF expression on any monocyte subset (%TF+ traditional, r= 0.09, p=0.64, %TF+ inflammatory, r= 0.26, p=0.18, and %TF+ patrolling, r=−0.04, p=0.83) among the HIV-infected population.
We next compared surface expression levels of several putative receptors for oxLDL: CD36, TLR4, TLR633, and TLR2 34 on blood monocytes of 22 HIV-infected patients and 24 uninfected donors. Expression of TLR2 was similar on monocyte subsets from HIV+ and HIV-donors (not shown). The proportion of each monocyte subset expressing TLR4 is increased among patients (p<0.03 for all, Figure 2A). Among both donor groups, the proportion of monocytes expressing CD36 is lowest among the patrolling monocyte subset, however, for each monocyte subset, the proportion of CD36+ cells is greater in the HIV+ subjects (p<0.04 for all, Figure 2B). Measurement of TLR6 was not reproducible. Plasma levels of oxLDL were directly related to the proportion of monocytes that expressed CD36 in patient samples (for %CD36+CD14+CD16-r=0.51, p=0.04, CD36+CD14+CD16+ r=0.56, p=0.03, CD36+CD14Dim CD16+ r=0.45, p=0.08, not shown) but only among patrolling monocytes in uninfected donors (r=0.72, p=0.03, not shown), consistent with an earlier report that oxLDL can induce CD36 expression35. Among patient samples, plasma LDL levels were related to %CD36+CD14+CD16-r=0.64, p=0.01, CD36+CD14+CD16+ r=0.61, p=0.02).
Figure 2. Expression of TLR4 and CD36 are increased on monocyte subsets from HIV-1 infected patients.
Whole blood samples were obtained from 54 HIV-1 infected donors and 24 healthy donors and the relative proportions of monocyte subsets were analyzed by flow cytometry. Three monocyte subsets were identified by size and granularity and by CD14 and CD16 expression. Representative histograms and summary data showing expression of A) Toll-Like Receptor 4 or B) CD36 on monocyte subsets from uninfected donors and HIV-1 infected patients are displayed.
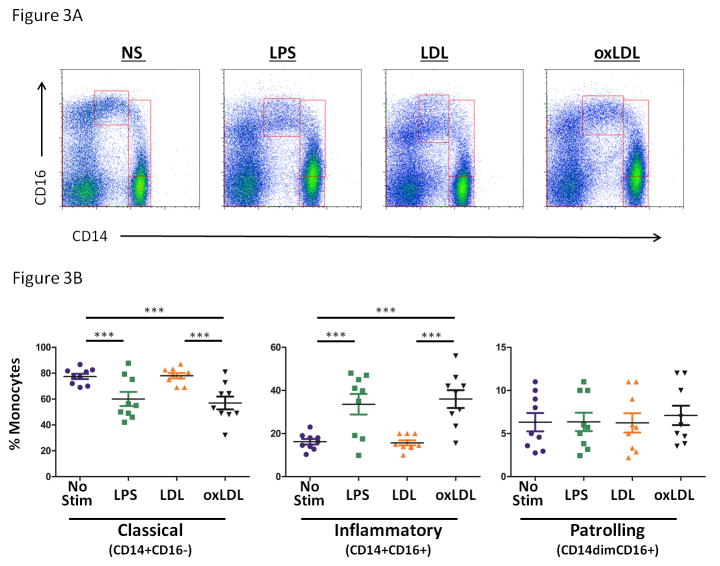
To explore further the role of oxLDL in activation of monocytes, we performed ex vivo stimulation assays, where whole blood samples from uninfected donors were left unstimulated, or were exposed to LPS (20ng/ml), naïve LDL (50ug/ml) or oxLDL (50ug/ml) for three hours. Exposure to LPS and oxLDL, but not LDL results in an increase in the mean proportion of inflammatory monocytes (No stim: 16.2 +/− 1.3 %, LPS= 33.6 +/− 4.8 %, LDL= 15.7 +/− 1.2 %, oxLDL=36.0+/− 4.1 %, Figure 3A and 3B). There was no change in the proportions of patrolling monocytes.
Figure 3. Exposure of whole blood samples to oxLDL, but not LDL, results in proportional increases in inflammatory (CD14+CD16+) monocytes.
Whole blood was obtained from HIV-1 uninfected subjects and was exposed to lipopolysaccharide (LPS, 20 ng/mL), LDL (50 μg/mL), or oxLDL (50 μg/mL) for 3 hours. Surface expression of CD14 and CD16 was measured on monocyte subsets by flow cytometry. Exposure to LPS or oxLDL, but not LDL resulted in an increase in the proportional representation of inflammatory monocytes. A) Representative dot plots B) Summary data. (repeated measures ANOVA, bonfierroni post tests; *** p<0.001)
Next, we measured induction of TF expression on monocyte subsets (Figure 4). Tissue factor expression was increased in response to stimulation with oxidized LDL, but not native LDL on classical monocytes (No stim: 2.7 +/− 0.7 %, LPS= 6.5 +/− 1.8 %, LDL= 2.7 +/− 0.6 %, oxLDL= 13.0 +/− 3.0 %). Similarly, only oxidized LDL stimulation increased TF expression by inflammatory monocytes (No stim: 9.3 +/− 0.6 %, LPS= 16.3 +/− 3.2 %, LDL= 9.6 +/− 1.1 %, oxLDL= 33.0 +/− 3.8 %); LPS induced TF expression on inflammatory monocytes, but the differences between unstimulated and LPS stimulated samples did not reach significance (p=0.07). Additional TF expression by patrolling monocytes was not consistently observed after stimulation with LPS, LDL or oxidized LDL.
Figure 4. Exposure of whole blood samples to oxLDL, but not LDL, results in increased surface expression of TF on classical and inflammatory monocyte subsets.
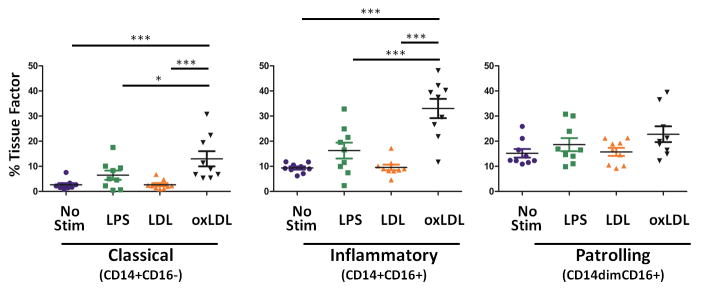
Whole blood was obtained from HIV-1 uninfected subjects and was exposed to lipopolysaccharide (LPS, 20 ng/mL), LDL (50 μg/mL), or oxLDL (50 μg/mL) for 3 hours. Surface expression tissue factor was measured on monocyte subsets by flow cytometry. OxLDL induces TF expression on classical and inflammatory monocytes. (repeated measures ANOVA, bonfierroni post tests; * p<0.05, *** p<0.001)
Discussion
Markers of monocyte activation2, inflammation, and coagulation1 are predictive of all-cause mortality in HIV-infected patients. The underlying mechanism(s) that may contribute to monocyte activation, inflammation, and coagulation in this setting have been incompletely described3. Recently, Piconi et al demonstrated that low-density LDL and apolipoprotein A were better correlated with cardiovascular risk in ART-treated, HIV+ patients than were inflammatory markers36. We propose that oxidized low-density lipoprotein may play a role in monocyte activation in HIV disease.
We report here that HIV-infected patients have increased plasma levels of oxLDL confirming a previous report37. A relationship between plasma levels of oxLDL and carotid intima-media thickness (CIMT) has also been reported in HIV disease38. Increased levels of oxLDL have also been observed in HIV-uninfected patients with acute coronary syndromes (ACS) or with stable coronary artery disease (CAD)39. Oxidized LDL uptake by macrophages within unstable coronary plaques has been found in persons with acute myocardial infarction 40. We have reported previously that in HIV-infection and among uninfected persons with ACS there are alterations of monocyte subsets and a procoagulant phenotype16,17. Thus, elevated levels of oxLDL in both patient populations may be a driving force that contributes to the shared monocyte activation profiles in these distinct conditions. The mechanisms behind the increased levels of oxLDL that we and others have reported in HIV-infected patients and patients with CVD are likely complex. Increased circulating levels of LDL in these patients may be related to multiple factors, including genetic factors, lifestyle, and in the case of HIV disease, antiretroviral therapy. The persistent inflammatory environment reported in HIV disease3, including high levels of inflammatory cytokines and abetted by activation of oxidases released from macrophages and neutrophils, may promote pro-inflammatory alterations of lipid particles.
Oxidized LDL is a proinflammatory form of LDL that is often found within atheromatous plaques and may contribute to smooth muscle cell and endothelial cell activation, and to the generation of foam cells41. Several receptors have been proposed for oxLDL, including the heterotrimer of CD36, TLR4, and TLR633, or the dimer of CD36 and TLR2 34. Activation of the heterotrimer CD36:TLR4:TLR6 on myeloid cells by oxLDL activates the inflammasome and generation of IL-1β 33,42. Previous studies found that oxLDL can induce TF expression on endothelial cells 43 and monocytes 44, increasing procoagulant activity. Here, we confirm that TF expression is increased on monocytes following exposure to oxLDL. We show for the first time, that exposure of monocytes to oxLDL results in an increased proportion of CD14+CD16+ cells, a cell type that is proportionally enriched in HIV-infected patients17 and is predictive of cardiovascular events in HIV-uninfected patients21. Baker et al recently found that the proportion of CD16+monocytes predicted coronary artery calcium progression in HIV-infected persons45.
We report that CD36, TLR4, and TLR2 are expressed on all three monocyte subsets 20 and that expression of CD36 and TLR4 is increased on monocytes among HIV-infected patients; and that plasma levels of oxLDL also appear to be directly related to expression of CD36 on monocytes in HIV-infected patients. Plasma levels of oxLDL were also directly related to sCD14, a marker of monocyte activation that has been linked to mortality in HIV disease2. This novel finding may provide insights into the drivers of sCD14 expression and provide targets for therapeutic intervention.
Statins have previously been associated with anti-inflammatory effects including the reduction of CRP46 and diminished monocyte TLR4 expression47. We recently demonstrated that rosuvastatin treatment reduces sCD14 levels and monocyte TF expression in ART-treated HIV+ patients48. Statins have been associated with reductions in oxLDL in HIV-uninfected populations49; thus, it is tempting to speculate that the anti-inflammatory action of statins may operate, in part, through the attenuation of oxLDL-mediated monocyte activation.
Our current study has limitations; the cross-sectional nature does not enable us to measure changes in oxLDL levels over time or during modifications to ART. The donor populations are not ideally matched, with more women and Caucasians among the uninfected donors than among the patients, however, when we compared levels of oxLDL, IL-6, sCD14, and sCD163 between women donors in the HIV infected (N=14) and uninfected groups (N=14), levels of these markers remained increased among HIV+ donors (P=0.0004, 0.07, 0.008, and 0.04, respectively). We also did not measure lipid profiles in our uninfected donors and cannot comment on the similarities and differences for these indices between the groups. While we measured significant increases in oxLDL in HIV-infected patients, there are likely multiple modified lipids and lipid subclasses that may be altered in HIV disease and some of these molecules may be pro-inflammatory.
This study does provide evidence for altered proinflammatory lipid levels in HIV-infected patients and a relationship between these levels and monocyte activation. Antiretroviral therapy is becoming more effective, yet markers of immune activation and coagulation remain elevated in treated HIV-infected patients50. During treated HIV disease there are likely multi-directional relationships between coagulation, inflammation, lipid profiles, and metabolism, with alterations in any likely affecting the others. Here, we demonstrate that pro-inflammatory oxLDL levels are related to indices of monocyte activation and confirm that this lipid species can induce in vitro a monocyte phenotype that is linked to morbidity; identifying another potential target for future interventional studies to prevent cardiovascular disease in patients living with HIV.
Acknowledgments
The authors would like to thank the Bad Boys of Cleveland, the Cleveland Immunopathogenesis Consortium, and Daniel I. Simon for helpful discussions related to this project.
Footnotes
Meetings where this data has been presented:
N. Funderburg, D. Zidar, M.M. Lederman. Monocyte subset activation by HIV, LPS, and OxLDL. Keystone Conference on Immune Activation in HIV Infection: Basic Mechanisms and Clinical Implications. Breckenridge CO. April 2013
N. Funderburg Oxidized Low Density Lipoprotein: Yet Another Contributor to Monocyte Activation in HIV-disease? AIDS Clinical Trials Group Meeting, End Organ Disease and Inflammation TSG Think Tank, Invited National Talk, Washington D.C. July 31, 2013
Author Contributions and Competing Interests
N.T.F. S.J. B.C. B.F. C.S. J.M. H.A.P-C performed experiments. B.R. and M.L. obtained patient samples. All authors contributed to experimental design, data analysis, and writing of the manuscript.
Dr McComsey has served as consultant, speaker, or received research grants from BMS, Pfizer, Merck, Gilead, and GSK Bristol-Myers Squibb, Pfizer, Merk, Gilead, and GlaxoSmithKline. All other authors declare no conflicts of interest.
Financial Disclosures
This work was supported by grants from the National Institutes of Health AI-07164, AI-67039, AI-68636,1K99HL108743-01, R00HL108743, R56HL126563, (Bethesda, MD), the Fasenmyer Foundation (Cleveland, OH), the Center for AIDS Research at Case Western Reserve University AI 36219 and the Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases. 2011 Mar 15;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Advances in immunology. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker JV, Huppler Hullsiek K, Bradford RL, Prosser R, Tracy RP, Key NS. Circulating Levels of Tissue Factor Microparticle Procoagulant Activity Are Reduced With Antiretroviral Therapy and Are Associated With Persistent Inflammation and Coagulation Activation Among HIV-Positive Patients. Journal of acquired immune deficiency syndromes (1999) 2013 Jul 1;63(3):367–371. doi: 10.1097/QAI.0b013e3182910121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. Journal of acquired immune deficiency syndromes (1999) 2009 Jul 1;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. The Journal of infectious diseases. 2010 Jun 15;201(12):1796–1805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012 Apr;54(7):984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. Journal of acquired immune deficiency syndromes (1999) 2010 Nov;55(3):316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of infectious diseases. 1999 Apr;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 10.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. The New England journal of medicine. 2005 Apr 21;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 11.Liuzzo G, Goronzy JJ, Yang H, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000 Jun 27;101(25):2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 12.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010 Feb;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crum-Cianflone NF, Weekes J, Bavaro M. Thromboses among HIV-Infected Patients during the Highly Active Antiretroviral Therapy Era. AIDS patient care and STDs. 2008 Sep 10; doi: 10.1089/apc.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fultz SL, McGinnis KA, Skanderson M, Ragni MV, Justice AC. Association of venous thromboembolism with human immunodeficiency virus and mortality in veterans. Am J Med. 2004 Mar 15;116(6):420–423. doi: 10.1016/j.amjmed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010 Jan 14;115(2):161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndromes. Blood. 2012 Oct 11; doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayne E, Funderburg NT, Sieg SF, et al. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. Journal of acquired immune deficiency syndromes (1999) 2012 Apr 1;59(4):340–346. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musselwhite LW, Sheikh V, Norton TD, et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS (London, England) 2011 Mar 27;25(6):787–795. doi: 10.1097/QAD.0b013e3283453fcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010 Sep 24;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogacev KS, Cremers B, Zawada AM, et al. CD14++CD16+ Monocytes Independently Predict Cardiovascular Events: A Cohort Study of 951 Patients Referred for Elective Coronary Angiography. Journal of the American College of Cardiology. 2012 Oct 16;60(16):1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993 Nov 15;82(10):3170–3176. [PubMed] [Google Scholar]
- 23.Kawanaka N, Yamamura M, Aita T, et al. CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002 Oct;46(10):2578–2586. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- 24.Rogacev KS, Seiler S, Zawada AM, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. European heart journal. 2011 Jan;32(1):84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Wang B, Han N, et al. CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. Journal of acquired immune deficiency syndromes (1999) 2009 Dec;52(5):553–559. doi: 10.1097/qai.0b013e3181c1d4fe. [DOI] [PubMed] [Google Scholar]
- 26.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. The New England journal of medicine. 2005 Jan 6;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 27.Rose H, Hoy J, Woolley I, et al. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008 Jul;199(1):79–86. doi: 10.1016/j.atherosclerosis.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May 12;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 29.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PloS one. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. The Journal of infectious diseases. 2011 Oct 15;204(8):1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longenecker CT, Jiang Y, Yun CH, et al. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. International journal of cardiology. 2013 Oct 9;168(4):4039–4045. doi: 10.1016/j.ijcard.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. Jama. 2012 Jul 25;308(4):379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature immunology. 2010 Feb;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjorkbacka H. Multiple roles of Toll-like receptor signaling in atherosclerosis. Curr Opin Lipidol. 2006 Oct;17(5):527–533. doi: 10.1097/01.mol.0000245258.25387.ec. [DOI] [PubMed] [Google Scholar]
- 35.Feng J, Han J, Pearce SF, et al. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. Journal of lipid research. 2000 May;41(5):688–696. [PubMed] [Google Scholar]
- 36.Piconi S, Parisotto S, Rizzardini G, et al. Atherosclerosis is associated with multiple pathogenic mechanisms in HIV-infected antiretroviral-naive or treated individuals. AIDS (London, England) 2013 Jan 28;27(3):381–389. doi: 10.1097/QAD.0b013e32835abcc9. [DOI] [PubMed] [Google Scholar]
- 37.Duong M, Petit JM, Martha B, et al. Concentration of circulating oxidized LDL in HIV-infected patients treated with antiretroviral agents: relation to HIV-related lipodystrophy. HIV clinical trials. 2006 Mar-Apr;7(2):41–47. doi: 10.1310/7381-m1yd-rtv5-4ryt. [DOI] [PubMed] [Google Scholar]
- 38.Parra S, Coll B, Aragones G, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010 Apr;11(4):225–231. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 39.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998 Oct 13;98(15):1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 40.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001 Apr 17;103(15):1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 41.Heery JM, Kozak M, Stafforini DM, et al. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. The Journal of clinical investigation. 1995 Nov;96(5):2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology. 2013 Aug;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bochkov VN, Mechtcheriakova D, Lucerna M, et al. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002 Jan 1;99(1):199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- 44.Owens AP, 3rd, Passam FH, Antoniak S, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. The Journal of clinical investigation. 2012 Feb 1;122(2):558–568. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker JV, Hullsiek KH, Singh A, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS (London, England) 2014 Mar 27;28(6):831–840. doi: 10.1097/QAD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. New England Journal of Medicine. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 47.Methe H, Kim J-O, Kofler S, Nabauer M, Weis M. Statins Decrease Toll-Like Receptor 4 Expression and Downstream Signaling in Human CD14+ Monocytes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005 Jul 1;25(7):1439–1445. doi: 10.1161/01.ATV.0000168410.44722.86. [DOI] [PubMed] [Google Scholar]
- 48.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin Treatment Reduces Markers of Monocyte Activation in HIV-Infected Subjects on Antiretroviral Therapy. Clin Infect Dis. 2014 Feb;58(4):588–595. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenson RS, Wolff D, Tangney CC. Statins reduce oxidized low-density lipoprotein levels, but do not alter soluble intercellular cell-adhesion molecule-1 and vascular cell-adhesion molecule-1 levels in subjects with hypercholesterolaemia. Clin Sci. 2004 Feb;106(2):215–217. doi: 10.1042/CS20030291. [DOI] [PubMed] [Google Scholar]
- 50.Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Curr Opin HIV AIDS. 2013 Nov 26; doi: 10.1097/COH.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]