Abstract
Objective
To evaluate the quality of weight change (change in fat mass vs. fat-free mass [FFM]), changes in cardiorespiratory fitness (CRF), and frequencies of metabolic risk factors in adolescent females with obesity that either lost weight or gained weight following lifestyle treatment.
Study design
Fifty-eight girls (mean age = 13.0 ± 1.6 yrs; 77% black; mean body mass index (BMI) = 36.5 ± 4.5 kg/m2) completed a 6-month lifestyle intervention combining dietary and behavioral counseling with aerobic and resistance exercise training. We examined baseline to 6-month differences in weight (kg), body composition, CRF, and frequencies of metabolic risk factors between weight loss and weight gain groups.
Results
In the weight loss group, body weight (-4.50 ± 3.53 kg, p < 0.001), fat mass (-4.50 ± 2.20 kg, p < 0.001), and body fat percentage ([BF%] -2.97% ± 1.45%, p < 0.001) decreased, and FFM was unchanged at 6 months. In the weight gain group, body weight (4.50 ± 2.20 kg, p < 0.001), fat mass (1.52 ± 3.16 kg, p < 0.024), and FFM (2.99 ± 2.45 kg, p < 0.001) increased, and BF% was unchanged. Both groups improved CRF (p < 0.05). Frequencies of metabolic risk factors were reduced across all participants after the 6-month treatment.
Conclusion
Participation in a weight management program might elicit health improvements in obese adolescent females who increase weight and fat mass, provided that FFM gains are sufficient to negate increases in body fat percentage.
Keywords: Adolescents, body composition, weight management program
The long-standing position that weight loss is requisite for health improvements in obese adults has been challenged by a new paradigm that emphasizes health promotion and the adoption of healthy lifestyle behaviors over weight loss as a primary goal of interventions (4). There is evidence that high levels of physical activity and/or cardiorespiratory fitness (CRF) attenuate the health risks associated with obesity in adults (5, 6). Short- and long-term improvements in body composition, CRF and metabolic risk factors have been reported in adolescents, independent of weight loss (7-12). In the absence of weight loss, reduced frequencies of metabolic risk factors were coincident with the preservation of fat-free mass (FFM) (7, 8, 10, 11). Fat mass, however, did not increase in any of the aforementioned studies, and, importantly, the association between CRF and metabolic syndrome (MetS) appears to be mediated by adiposity (15). The inability to distinguish the respective effects of body fatness, FFM, and CRF on MetS in obese adolescents precludes any clear interpretation as to which outcomes should be of most concern to clinicians.
It is unclear whether improvements in CRF and metabolic risk factors might be realized in adolescents whose body weight and fat mass increase during the treatment period. Because body weight is positively associated with metabolic disease risk in adolescents (18), the risk of transitioning from the metabolically healthy to the metabolically unhealthy phenotype with weight gain is also of concern. Therefore, the aim of this study was to compare the quality of weight change (change in fat mass vs. FFM) and changes in CRF and frequencies of metabolic risk factors in obese female adolescents who either lost weight or gained weight following participation in a multidisciplinary weight management program.
Methods
This study evaluated outcomes from obese adolescent females aged 11-18 years enrolled in the Teaching, Encouragement, Exercise, Nutrition, and Support (T.E.E.N.S.) weight management program at Children's Hospital of Richmond at Virginia Commonwealth University (VCU). In order to enroll in the T.E.E.N.S. program, participants were between 11 and 17 years of age, had a BMI ≥85th percentile, weighed less than 182 kg (400 lbs), were free of any medical conditions that preclude weight loss, and had a primary care physician. In the current study, we chose to evaluate only females to control for the potential influence of sex on the outcome measures of interest (19, 20). Given the challenges associated with attrition in this population (21) and the intent of this report to examine the influence of body weight and composition changes on cardiometabolic disease risk factors, only those who completed 6 months of the intervention were included in the analyses. All measures were recorded at baseline and after 6 months of program participation. Participants were retrospectively assigned to either the weight gain or weight loss group, depending on whether the 6-month weight change was positive or negative. The VCU Institutional Review Board approved all study procedures.
Body weight and height were measured by trained research staff using a digital scale and stadiometer to the nearest 0.1 kg and cm, respectively, and BMI was calculated as weight (kg) divided by height (m2). BMI percentiles and Z-scores were determined using the 2000 Centers for Disease Control and Prevention growth charts (22). Waist circumference was measured at the umbilicus and recorded to the nearest 0.1 cm using an anthropometric measuring tape. Pubertal status was classified by study physicians according to Tanner Staging (23). Body composition was assessed via dual energy x-ray absorptiometry ([DXA], Hologic D4500a/Discovery).
Resting blood pressure (BP) was assessed in triplicate with the participant in a seated position using an automated blood pressure analyzer (Connex Vital Signs Monitor 6000 Series, Welch Allyn, Inc., New York). Elevated BP was defined as either systolic or diastolic BP >90th percentile for age, sex and height (24). Blood samples were collected from an antecubital vein after an overnight 12-hour fast. An automated clinical chemistry analyzer (Advia 1800 Chemistry System, Siemens Healthcare, Pennsylvania) was used to measure total cholesterol (TC), high-density lipoprotein (HDL), and triglycerides (TG). Glucose oxidase methodology was used to determine plasma glucose concentrations (2300 Stat Plus, YSI Life Sciences, Ohio). We also calculated the TG-to-HDL ratio (TG:HDL), as an elevated TG:HDL is associated with an increased risk of cardiometabolic disease in adolescents (25, 26).
Our classification of MetS in obese adolescents was contingent upon the participant's exhibiting ≥3 of the following risk factors: moderate obesity (BMI >97th percentile for age and sex); TG ≥110 mg/dL; HDL-C ≤40 mg/dL; systolic or diastolic BP >90th percentile for age, sex, and height; and fasting glucose ≥100 mg/dL (27). In the current study, BMI percentiles were converted to Z-scores and moderate obesity was defined as having a BMI Z-score >2.0 in order to standardize for age and sex (28).
After excluding BMI Z-score from the classification criteria, participants who were absent of any remaining MetS risk factors were identified as having the MHO phenotype (18, 29). Alternatively, participants with 1 MetS risk factor other than BMI Z-score >2.0 were considered metabolically unhealthy obese.
Peak oxygen consumption (VO2peak), a measure of CRF, was assessed using a metabolic cart (Vmax Encore, Sensormedics, Yorba Linda, CA) during a graded treadmill test to volitional fatigue. The treadmill protocol consisted of an initial 4-minute stage at 2.5 mph, followed by a 2-minute stage at 3.0 mph. During subsequent 2-minute stages, speed was held constant, and grade was increased to 2%, 5%, 8%, 11%, 14%, and 17%. The final 2 stages were performed at a treadmill speed of 3.5 mph and grades of 17% and 20%. Heart rate was monitored throughout the test with a heart rate monitor (E600, Polar Electro, Lake Success, NY). To account for the influence of body weight and composition on CRF, VO2peak was assessed in absolute terms (L O2·min-1) as well as in relation to body weight (ml O2·kg-1·min-1) and FFM (ml O2·kg FFM-1·min-1).
The T.E.E.N.S. weight management intervention has been described in detail elsewhere (27, 30, 31). In brief, participants attended weekly nutritional education or behavioral support sessions and engaged in supervised aerobic and resistance training three days per week. The nutrition education sessions followed a standardized lesson plan (30) targeting high risk eating behaviors (e.g., sugared beverage intake and breakfast consumption) associated with obesity. Behavioral support sessions (on alternating weeks as nutrition education sessions) used a behavior therapy approach to change, including goal-setting, stimulus control, social support, reinforcement, and examination of barriers and facilitators to program participation. The aerobic exercise component included 30 minutes of continuous activity performed at a heart rate ≥150 beats per minute, and the resistance training component included 2-3 sets of 12-15 repetitions of at least 10 resistance exercises. Two additional days of physical activity outside of the structured sessions were encouraged, and family YMCA memberships were provided to participants. After the first 3 months, participants were only required to attend supervised exercise sessions twice per week, although additional exercise sessions were encouraged.
Statistical Analyses
All statistical analyses were performed using SPSS version 22 for Windows (SPSS, Inc., Chicago, IL). Paired samples t-tests were used to determine if changes in continuous outcome variables were significant across all subjects and within the weight gain and weight loss groups. One-way analysis of variance (ANOVA) was used to determine whether six-month changes in anthropometric, and VO2peak measures differed between the weight gain and weight loss groups. Fisher's exact tests were used to determine if pubertal status, race, and frequencies of MetS risk factors differed between weight change groups. The prevalence of MetS and metabolic risk factors was compared across time points using McNemar's procedure. We also evaluated whether the number of participants demonstrating the metabolically healthy and unhealthy phenotypes differed with respect to whether the six-month weight change was positive or negative. All p-values were 2-tailed and deemed statistically significant if p<0.05.
Results
Baseline and six-month data were available for 58 subjects. Forty-four (75.9%) participants were black, 10 (17.2%) white, 3 (5.2%) Hispanic, and 1 (1.7%) did not report race. Tanner Staging was available for 49 (84.5%) participants at baseline and 38 (65.5%) participants at 6 months. Only 1 participant was identified as pre-pubertal (Tanner Stage 1) at baseline. There were no anthropometric differences at baseline or in the change from baseline to six months between those with and without Tanner stage data (p>0.05). Complete metabolic risk factor data were available for 57 (98.3%) of the 58 participants.
Of the 58 participants, 33 (57%) lost weight following the 6-month intervention (Table). At baseline, BF% was higher in the weight gain group compared with the weight loss group (43.8 ± 4.1% vs. 40.5 ± 3.5, p=0.002). No other between-group differences in anthropometrics, CRF, or metabolic risk factors were observed at baseline. Additionally, ANOVA revealed no significant differences between weight loss and weight gain groups in race, and Tanner Stage. At 6 months, height increased significantly in the weight gain group. However, all other differences between weight gain and weight loss groups at 6 months remained significant after controlling for change in height. Groups did not differ with respect to the number of exercise sessions attended during the intervention (p=0.557). Therefore, all analyses are unadjusted unless otherwise noted.
Table 1. Changes in anthropometric measures, fitness, and cardiometabolic health in obese adolescent females who either gained or lost weight during treatment.
| Weight Loss Group (N=33) | Weight Gain Group (N=25) | |||||
|---|---|---|---|---|---|---|
| Baseline M (SD) | 6 months M (SD) | p | Baseline M (SD) | 6 months M (SD) | p | |
| Anthropometrics | ||||||
| Weight (kg) | 97.3 (15.1) | 92.8 (14.7) | <0.001 | 96.0 (15.8) | 100.5 (16.5)‡ | <0.001 |
| Height (cm) | 163.2 (7.6) | 163.6 (7.4) | 0.115 | 161.2 (7.0) | 162.6 (6.2)‡ | <0.001 |
| BMI (kg/m2) | 36.5 (4.6) | 34.6 (4.5) | <0.001 | 36.7 (4.6) | 37.7 (4.6)*‡ | 0.017 |
| BMI percentile | 98.8 (1.4) | 98.1 (2.4) | 0.001 | 99.1 (0.7) | 99.1 (0.6)*‡ | 0.490 |
| BMI Z-score | 2.4 (0.3) | 2.2 (0.3) | <0.001 | 2.4 (0.3) | 2.4 (0.2)*‡ | 0.829 |
| Body fat percentage | 40.5 (3.5) | 37.5 (3.9) | <0.001 | 43.8 (4.1)* | 43.3 (4.0)†‡ | 0.180 |
| Fat mass (kg) | 39.5 (8.1) | 35.0 (8.0) | <0.001 | 42.3 (9.5) | 43.8 (9.8)†‡ | 0.024 |
| Fat-free mass (kg) | 57.7 (8.6) | 57.6 (8.1) | 0.780 | 53.6 (7.6) | 56.6 (7.8)‡ | <0.001 |
| WC (cm) | 97.9 (10.4) | 93.8 (9.6) | <0.001 | 97.8 (8.5) | 98.2 (9.0)‡ | 0.620 |
| Cardiorespiratory Fitness | ||||||
| VO2peak (L/min) | 2.3 (0.4) | 2.6 (0.4) | <0.001 | 2.3 (0.3) | 2.5 (0.3) | 0.001 |
| VO2peak (ml/kg/min) | 24.6 (4.7) | 28.2 (4.6) | <0.001 | 24.7 (4.6) | 26.1 (5.6)‡ | 0.047 |
| VO2peak (ml/kg FFM/min) | 41.0 (7.1) | 45.3 (6.5) | <0.001 | 43.5 (6.2) | 45.3 (8.0) | 0.145 |
| Cardiometabolic Disease (N = 57) | ||||||
| Systolic blood pressure | 119.2 (10.1) | 115.9 (9.3) | 0.080 | 117.1 (8.5) | 115.8 (9.4) | 0.488 |
| Diastolic blood pressure | 66.9 (4.7) | 65.1 (8.1) | 0.162 | 64.6 (6.8) | 61.9 (7.6) | 0.062 |
| Total cholesterol | 149.9 (25.9) | 144.8 (22.4) | 0.075 | 157.2 (30.3) | 155.3 (27.8) | 0.607 |
| LDL-C | 94.3 (22.5) | 88.5 (21.9) | 0.034 | 99.2 (29.2) | 97.3 (25.5) | 0.609 |
| HDL-C | 42.9 (9.7) | 42.5 (8.0) | 0.758 | 41.3 (8.0) | 42.0 (7.4) | 0.552 |
| Triglycerides | 72.0 (27.6) | 69.2 (35.0) | 0.717 | 77.2 (33.7) | 80.1 (31.0) | 0.664 |
| Triglycerides:HDL-C | 1.8 (0.8) | 1.8 (1.3) | 0.911 | 2.0 (1.0) | 2.0 (1.0) | 0.766 |
| Fasting glucose | 84.6 (7.7) | 82.1 (6.0) | 0.031 | 84.6 (9.5) | 89.6 (28.4) | 0.833‡ |
| Metabolic Syndrome§ | 5 (15.2%) | 4 (12.1%) | 0.500 | 3 (12.5%) | 5 (20.8%) | 0.099 |
| MHO phenotypeδ | 13 (39.4%) | 19 (57.6%) | <0.001 | 9 (37.5%) | 11 (45.8%) ‡ | 0.675 |
Abbreviations: BMI, body mass index; WC, waist circumference; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; MHO, metabolically healthy obese
Significantly different from WL at the same time point (p<0.05).
Significantly different from WL at the same time point (p<0.01).
Change from baseline to 6 months was significantly different from WL.
Metabolic syndrome was diagnosed if ≥3 of the following were present: elevated blood pressure - systolic or diastolic blood pressure ≥90th percentile for age, sex, and height; HDL-C - ≤40 mg/dL; triglycerides - ≥110 mg/dL; fasting glucose - ≥100 mg/dL; BMI z-score - >2.0.
MHO phenotype was diagnosed if no other risk factors other than BMI z-score >2.0 were present.
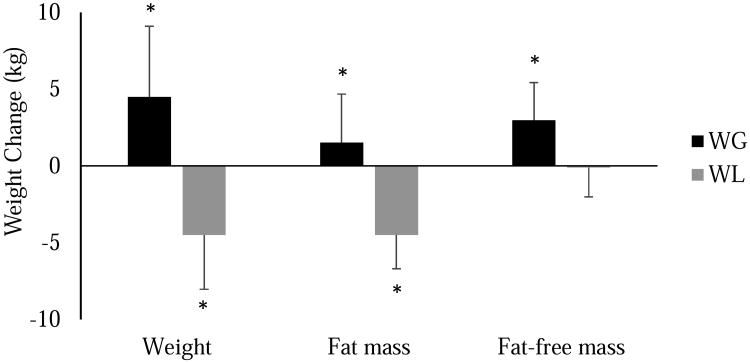
Anthropometric and CRF measures, with the exception of FFM, were significantly improved in the weight loss group at 6 months (Table). As expected, 6-month anthropometric changes were significantly different between the weight gain and weight loss groups (Table and Figure 1). Fat mass decreased by 4.5 ± 2.2 kg (p<0.001) in the weight loss group, and, given the preservation of FFM, both weight (-4.5 ± 3.5 kg, p<0.001) and BF% (-3.0 ± 1.5%, p<0.001) were also reduced. In contrast, weight increased by 4.5 ± 4.6 kg (p<0.001) in the weight gain group, with approximately two-thirds of the observed weight gain attributed to gains in FFM. All measures of CRF were significantly increased in the weight loss group. In the weight gain group, absolute and relative VO2peak, but not VO2peak relative to FFM, were improved at 6 months.
Figure 1. Body weight and composition changes in female adolescents who either lost or gained weight following participation in a six-month behavioral weight loss intervention.
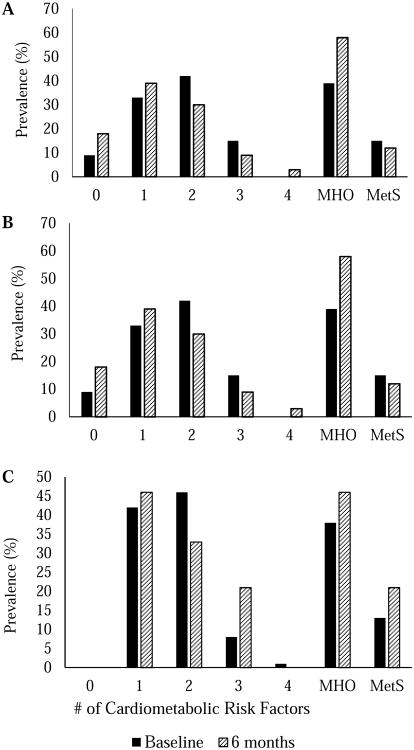
Figure 2 shows the percentage of all participants and within the weight gain and weight loss groups exhibiting 0, 1, 2, 3, and 4 cardiometabolic risk factors, as well as MetS and the MHO phenotype, before and after treatment. In the weight loss group, LDL-C and fasting glucose decreased following treatment (p < 0.05), and no changes in cardiometabolic disease risk factors were observed in the weight gain group. The prevalence of MetS at baseline was relatively low at baseline and slightly decreased and increased in the weight loss (15.2% to 12.1%) or weight gain (12.5% to 20.8%) groups, respectively, with no difference between groups (p = 0.620). In contrast, prevalence of the MHO phenotype differed between weight change groups following treatment. The percentage of participants who were classified as MHO increased in both the weight loss (+18.2%) and weight gain (+8.3%) groups following 6 months of program participation. Alternatively, 16.7% of participants in the weight gain group compared with none in the weight loss group became metabolically unhealthy after exhibiting the MHO phenotype at baseline (p=0.029).
Figure 2. Percentages of A, all participants, B, those who lost weight, and C, those who gained weight with 0, 1, 2, 3, and 4 cardiometabolic risk factors, the metabolically healthy obese (MHO) phenotype, and the metabolic syndrome (MetS) prior to and after 6 months of participation in a behavioral weight management program.
Discussion
The weight loss and weight gain groups in the current study demonstrated increases in CRF; however, only the weight loss group improved body composition. Our finding that, of those who had the MHO phenotype at baseline, only participants in the weight gain group became metabolically unhealthy at 6 months supports previous reports' that adiposity mediates the relation between CRF and MetS in adolescents (15, 32). Although the degree of weight loss is a determinant of the magnitude of improvement in metabolic health (16), reductions in risk factors were previously reported in adolescents who gained weight but lost fat mass (8). The transition from metabolically healthy to metabolically unhealthy obese in some participants who gained weight but none who lost weight suggests that body composition might also mediate any relation between improvements in FFM and MetS. However, the prevalence of the metabolically unhealthy phenotype was reduced in both weight change groups, even though obesity (BMI ≥95th percentile) was maintained in all but one participant. Clearly, further investigation of the relationship between body composition, CRF, and cardiometabolic health is needed in the obese adolescent population.
The generalizability of the current study's results is limited to predominately black females, and the lower prevalence of MetS in black compared with white (33) and female compared with male (18) adolescents may explain the relatively healthy metabolic profile observed in our participants. In turn, low baseline frequencies of individual risk factors reduced the likelihood of observing differences in improvements between weight loss and weight gain groups. Additionally, we assigned participants to either the weight loss or weight gain groups based on whether weight change was positive or negative, and the inclusion of participants with clinically negligible changes in body weight could have also limited our ability to detect differences in cardiometabolic risk factors and CRF. Lastly, conclusions cannot be drawn as to whether between-group differences in caloric intake or participant effort during the exercise sessions could further explain the group differences. However, each participant received the same nutritional education and participated in a standardized exercise training protocol. Further, exercise attendance did not differ between groups.
The wide variability in individual responses to weight management interventions often makes the evaluation of overall program efficacy challenging. After grouping our program participants according to whether weight was lost or gained, we observed dissimilar patterns of body composition change. Although the magnitude of improvement in metabolic health appeared to be larger with weight loss, obese adolescent females who gained body weight and fat mass still improved their CRF following participation in an intensive weight management program. Of note, two-thirds of the weight gain in the weight gain group was attributed to increased FFM, with a concomitant preservation of body composition. It is currently unclear whether the health benefits associated with increases in FFM, without the loss of fat mass, are maintained over the long-term. Further exploration of the associations among weight, body composition, fitness, and cardiometabolic risk factors is needed to establish dose-response relationships among treatment strategies, weight loss outcomes, and health measurements to improve upon current treatment strategies.
Acknowledgments
Funded by the National Institutes of Health Clinical and Translational Science Awards (UL1TR000058 [to VCU] and K23HD053742 [to E.W.]), Virginia Premier Health Plan, Inc, and Children's Hospital Foundation.
Clinical trials number: NCT00167830
Abbreviations
- CRF
cardiorespiratory fitness
- FFM
fat-free mass
- MetS
metabolic syndrome
- MHO
metabolically healthy obese
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock EP, O'Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125:e396–418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 3.Rolland-Cachera MF, Thibault H, Souberbielle JC, Soulie D, Carbonel P, Deheeger M, et al. Massive obesity in adolescents: dietary interventions and behaviours associated with weight regain at 2 y follow-up. Int J Obes Relat Metab Disord. 2004;28:514–9. doi: 10.1038/sj.ijo.0802605. [DOI] [PubMed] [Google Scholar]
- 4.Bacon L, Aphramor L. Weight science: evaluating the evidence for a paradigm shift. Nutr J. 2011;10:9. doi: 10.1186/1475-2891-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan GE. The “fit but fat” concept revisited: population-based estimates using NHANES. Int J Behav Nutr Phys Act. 2010;7:47. doi: 10.1186/1479-5868-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S646–62. doi: 10.1097/00005768-199911001-00025. [DOI] [PubMed] [Google Scholar]
- 7.Balagopal P, George D, Patton N, Yarandi H, Roberts WL, Bayne E, et al. Lifestyle-only intervention attenuates the inflammatory state associated with obesity: a randomized controlled study in adolescents. J Pediatr. 2005;146:342–8. doi: 10.1016/j.jpeds.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Marcano H, Fernandez M, Paoli M, Santomauro M, Camacho N, Cichetti R, et al. Limited Weight Loss or Simply No Weight Gain following Lifestyle-Only Intervention Tends to Redistribute Body Fat, to Decrease Lipid Concentrations, and to Improve Parameters of Insulin Sensitivity in Obese Children. Int J Pediatr Endocrinol. 2011;2011:241703. doi: 10.1155/2011/241703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjonna AE, Stolen TO, Bye A, Volden M, Slordahl SA, Odegard R, et al. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond) 2009;116:317–26. doi: 10.1042/CS20080249. [DOI] [PubMed] [Google Scholar]
- 10.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472–9. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Bianchini JA, da Silva DF, Nardo CC, Carolino ID, Hernandes F, Nardo N., Jr Multidisciplinary therapy reduces risk factors for metabolic syndrome in obese adolescents. Eur J Pediatr. 2013;172:215–21. doi: 10.1007/s00431-012-1865-7. [DOI] [PubMed] [Google Scholar]
- 12.Caranti DA, de Mello MT, Prado WL, Tock L, Siqueira KO, de Piano A, et al. Short- and long-term beneficial effects of a multidisciplinary therapy for the control of metabolic syndrome in obese adolescents. Metabolism. 2007;56:1293–300. doi: 10.1016/j.metabol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Laurson KR, Welk GJ, Eisenmann JC. Diagnostic performance of BMI percentiles to identify adolescents with metabolic syndrome. Pediatrics. 2014;133:e330–8. doi: 10.1542/peds.2013-1308. [DOI] [PubMed] [Google Scholar]
- 15.Eisenmann JC. Aerobic fitness, fatness and the metabolic syndrome in children and adolescents. Acta Paediatr. 2007;96:1723–9. doi: 10.1111/j.1651-2227.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 16.Reinehr T, Kleber M, Toschke AM. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis. 2009;207:174–80. doi: 10.1016/j.atherosclerosis.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 17.Senechal M, Wicklow B, Wittmeier K, Hay J, MacIntosh AC, Eskicioglu P, et al. Cardiorespiratory fitness and adiposity in metabolically healthy overweight and obese youth. Pediatrics. 2013;132:e85–92. doi: 10.1542/peds.2013-0296. [DOI] [PubMed] [Google Scholar]
- 18.Camhi SM, Katzmarzyk PT. Prevalence of cardiometabolic risk factor clustering and body mass index in adolescents. J Pediatr. 2011;159:303–7. doi: 10.1016/j.jpeds.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10–5. doi: 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- 20.Pate RR, Wang CY, Dowda M, Farrell SW, O'Neill JR. Cardiorespiratory fitness levels among US youth 12 to 19 years of age: findings from the 1999-2002 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2006;160:1005–12. doi: 10.1001/archpedi.160.10.1005. [DOI] [PubMed] [Google Scholar]
- 21.Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev. 2011;12:e273–81. doi: 10.1111/j.1467-789X.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab. 1986;15:411–51. doi: 10.1016/s0300-595x(86)80005-6. [DOI] [PubMed] [Google Scholar]
- 24.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr. 2003;142:368–72. doi: 10.1067/mpd.2003.205. [DOI] [PubMed] [Google Scholar]
- 25.Bailey DP, Savory LA, Denton SJ, Davies BR, Kerr CJ. The triglyceride to high-density lipoprotein ratio identifies children who may be at risk of developing cardiometabolic disease. Acta Paediatr. 2014;103:e349–53. doi: 10.1111/apa.12677. [DOI] [PubMed] [Google Scholar]
- 26.Pacifico L, Bonci E, Andreoli G, Romaggioli S, Di Miscio R, Lombardo CV, et al. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr Metab Cardiovasc Dis. 2014;24:737–43. doi: 10.1016/j.numecd.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Wickham EP, Stern M, Evans RK, Bryan DL, Moskowitz WB, Clore JN, et al. Prevalence of the metabolic syndrome among obese adolescents enrolled in a multidisciplinary weight management program: clinical correlates and response to treatment. Metab Syndr Relat Disord. 2009;7:179–86. doi: 10.1089/met.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 29.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 30.Bean MK, Mazzeo SE, Stern M, Evans RK, Bryan D, Ning Y, et al. Six-month dietary changes in ethnically diverse, obese adolescents participating in a multidisciplinary weight management program. Clin Pediatr (Phila) 2011;50:408–16. doi: 10.1177/0009922810393497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans RK, Franco RL, Stern M, Wickham EP, Bryan DL, Herrick JE, et al. Evaluation of a 6-month multi-disciplinary healthy weight management program targeting urban, overweight adolescents: effects on physical fitness, physical activity, and blood lipid profiles. Int J Pediatr Obes. 2009;4:130–3. doi: 10.1080/17477160802314997. [DOI] [PubMed] [Google Scholar]
- 32.Rizzo NS, Ruiz JR, Hurtig-Wennlof A, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: the European youth heart study. J Pediatr. 2007;150:388–94. doi: 10.1016/j.jpeds.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Srinivasan SR, Berenson GS. Path analysis of metabolic syndrome components in black versus white children, adolescents, and adults: the Bogalusa Heart Study. Ann Epidemiol. 2008;18:85–91. doi: 10.1016/j.annepidem.2007.07.090. [DOI] [PubMed] [Google Scholar]