Abstract
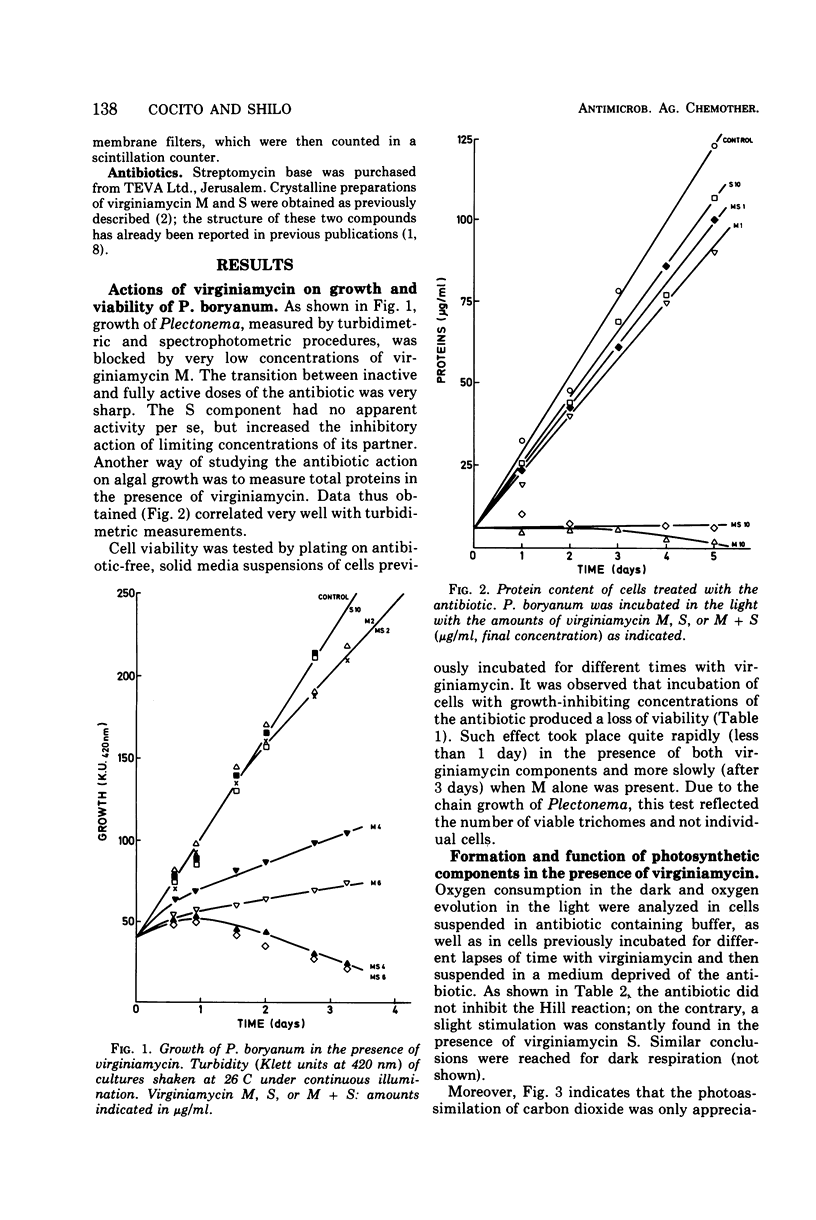
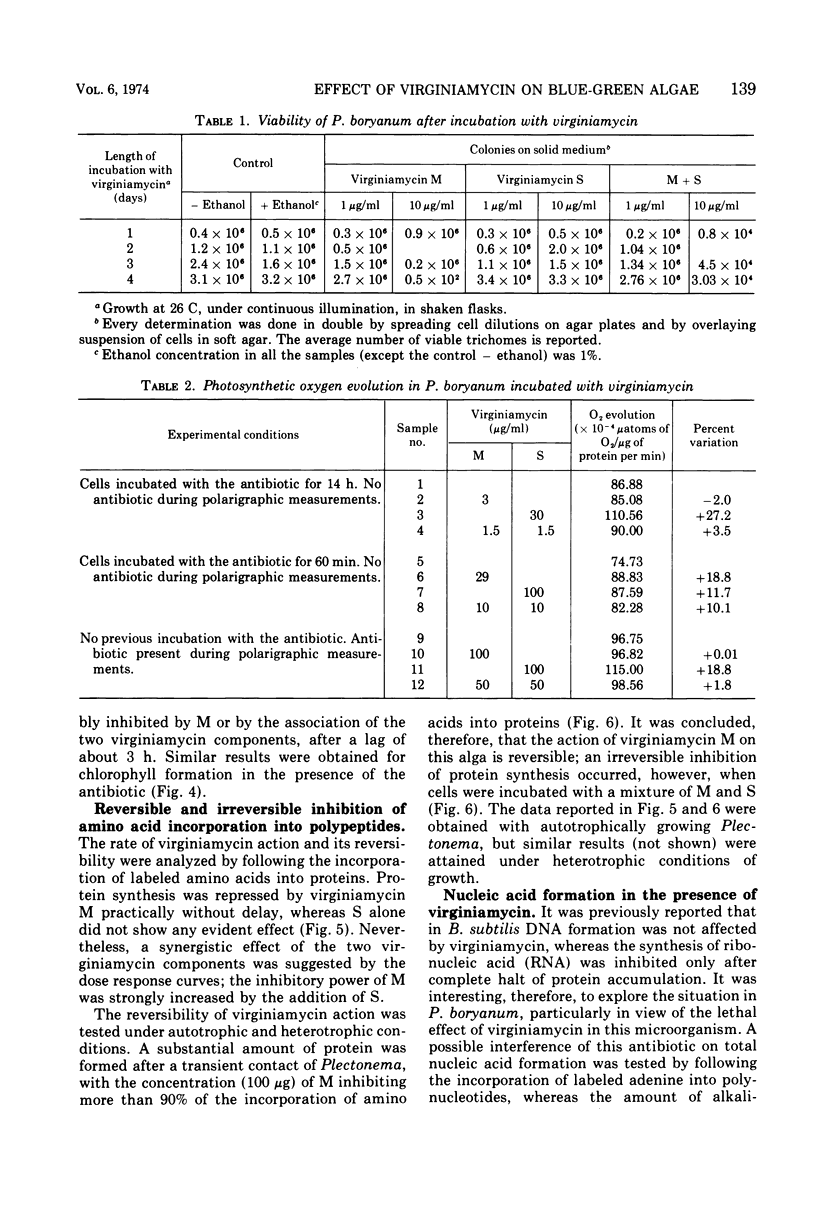
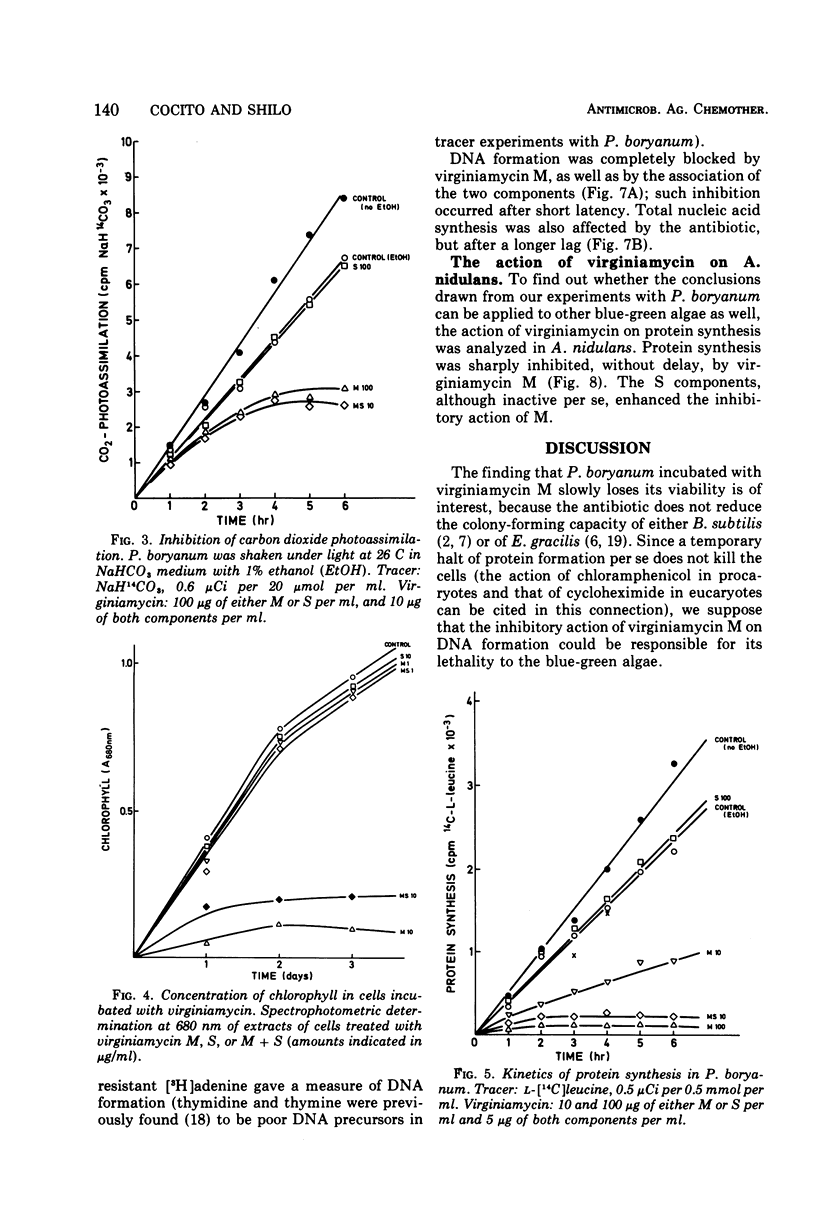
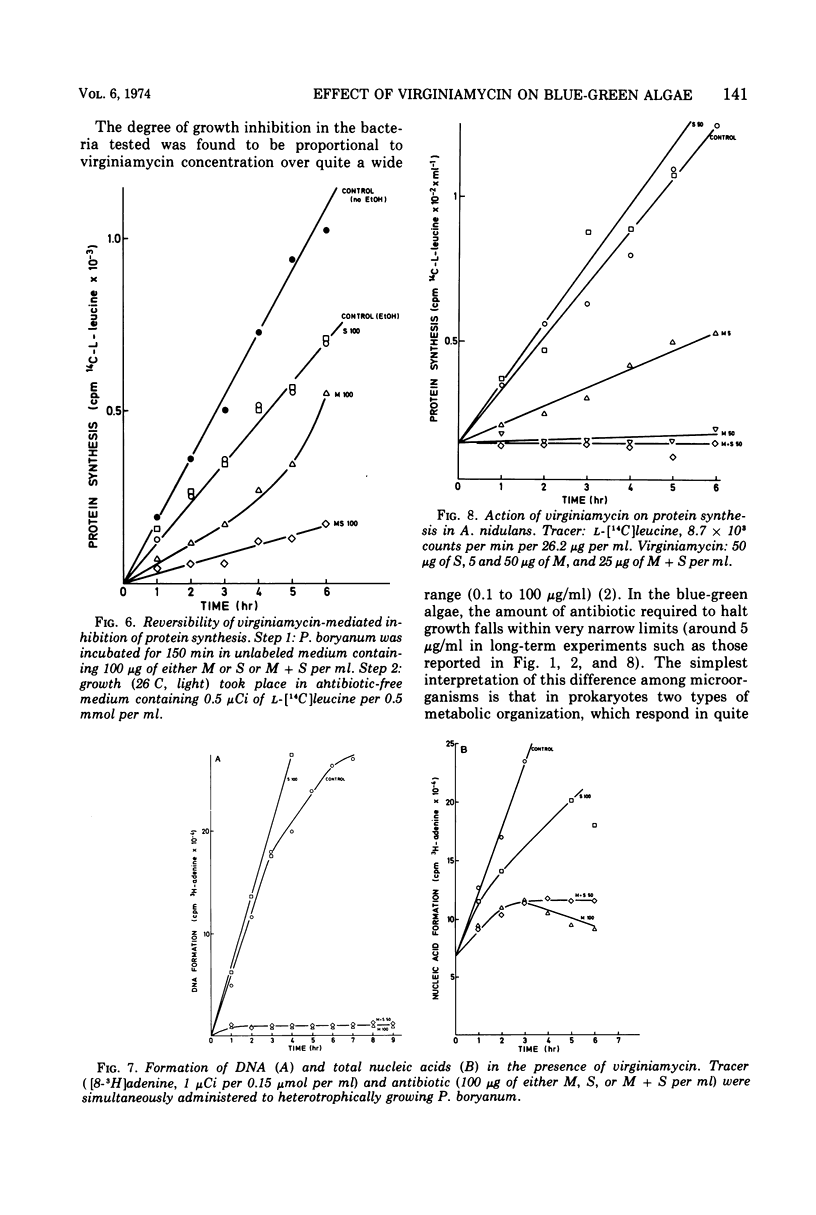
The M component of virginiamycin inhibited growth of Plectonema boryanum under both photoautotrophic and heterotrophic conditions. Though the S component of this antibiotic had no apparent activity per se, it enhanced the inhibitory action of its partner. Cells incubated with suitable concentrations of either M or M + S stopped growing and lysed. Loss of the colony-forming capacity occurred quickly in the presence of M + S and slowly in the presence of M alone. Virginiamycin M inhibited protein synthesis in autotrophically and heterotrophically growing Plectonema. This effect was very rapid and could be reversed by removing the antibiotic. The S component did not block the incorporation of amino acids into proteins, but prevented the reversibility of the inhibitory effect of M. Virginiamycin M or S did not affect the photosynthetic oxygen development (Hill's reaction) in Plectonema. Moreover, carbon dioxide photoassimilation and formation of chlorophyll were inhibited only after an appreciable lag. Deoxyribonucleic acid synthesis was blocked virtually without delay by virginiamycin M. Since virginiamycin inhibited protein synthesis in a similar fashion in the unicellular Anacystis nidulans, as well as in the filamentous P. boryanum, the mechanism of action of this antibiotic is probably the same in all blue-green algae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972 Sep;111(3):682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocito C. G., Bronchart R., Van Pel B. Phenotypic and genotypic changes induced in eucaryotic cells by protein inhibitors. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1688–1694. doi: 10.1016/0006-291x(72)90804-2. [DOI] [PubMed] [Google Scholar]
- Cocito C. Formation and decay of polyribosomes and ribosomes during the inhibition of protein synthesis and recovery. Biochimie. 1971;53(9):987–1000. doi: 10.1016/s0300-9084(71)80067-6. [DOI] [PubMed] [Google Scholar]
- Cocito C. Formation of ribosomal particles in virginiamycin sensitive and resistant mutants of Bacillus subtilis. Biochimie. 1973;55(2):153–161. doi: 10.1016/s0300-9084(73)80387-6. [DOI] [PubMed] [Google Scholar]
- Cocito C., Fraselle G. The properties of virginiamycin-resistant mutants of Bacillus subtilis. J Gen Microbiol. 1973 May;76(1):115–125. doi: 10.1099/00221287-76-1-115. [DOI] [PubMed] [Google Scholar]
- Cocito C., Kaji A. Virginiamycin M, a specific inhibitor of the acceptor site of ribosomes. Biochimie. 1971;53(6):763–770. doi: 10.1016/s0300-9084(71)80117-7. [DOI] [PubMed] [Google Scholar]
- Cocito C. Metabolism of macromolecules in bacteria treated with virginiamycin. J Gen Microbiol. 1969 Aug;57(2):179–194. doi: 10.1099/00221287-57-2-179. [DOI] [PubMed] [Google Scholar]
- Cocito C. The ribosomal cycle in bacteria treated with an inhibitor of protein synthesis. Biochimie. 1973;55(3):309–316. doi: 10.1016/s0300-9084(73)80130-0. [DOI] [PubMed] [Google Scholar]
- Cocito C., Voorma H. O., Bosch L. Interference of virginiamycin M with the initiation and the elongation of peptide chains in cell-free systems. Biochim Biophys Acta. 1974 Mar 27;340(3):285–298. doi: 10.1016/0005-2787(74)90274-3. [DOI] [PubMed] [Google Scholar]
- DE D. N., GHOSH S. N. CYTOCHEMICAL EVIDENCE FOR THE APPARENT ABSENCE OF HISTONE IN THE CELLS OF CYANOPHYCEAE. J Histochem Cytochem. 1965 Apr;13:298–298. doi: 10.1177/13.4.298. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madan V., Kumar H. D. Action of nalidixic acid and hydroxyurea on two blue-green algae. Z Allg Mikrobiol. 1971;11(6):495–499. doi: 10.1002/jobm.3630110605. [DOI] [PubMed] [Google Scholar]
- Makino F., Tsuzuki J. Absence of histone in the blue-green alga Anabaena cylindrica. Nature. 1971 Jun 18;231(5303):446–447. doi: 10.1038/231446a0. [DOI] [PubMed] [Google Scholar]
- NIKLOWITZ W., DREWS G. Beiträge zur Cytologie der Blaualgen. IV. Vergleichende elektronenmikroskopische Untersuchungen zur Substruktur einiger Hormogonales. Arch Mikrobiol. 1957;27(2):150–165. [PubMed] [Google Scholar]
- Padan E., Shilo M. Cyanophages-viruses attacking blue-green algae. Bacteriol Rev. 1973 Sep;37(3):343–370. doi: 10.1128/br.37.3.343-370.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safferman R. S., Morris M. E. Observations on the Occurrence, Distribution, and Seasonal Incidence of Blue-green Algal Viruses. Appl Microbiol. 1967 Sep;15(5):1219–1222. doi: 10.1128/am.15.5.1219-1222.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Haselkorn R. LPP-1 infection of the blue-green alga Plectonema boryanum. II. Viral deoxyribonucleic acid synthesis and host deoxyribonucleic acid breakdown. J Virol. 1970 Dec;6(6):834–840. doi: 10.1128/jvi.6.6.834-840.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pel B., Bronchart R., Kebers F., Cocito C. Structure and function of cytoplasmic organelles in transiently and permanently bleached Euglena. Exp Cell Res. 1973 Mar 30;78(1):103–110. doi: 10.1016/0014-4827(73)90043-8. [DOI] [PubMed] [Google Scholar]
- Van Pel B., Cocito C. Formation of chloroplast ribosomes and ribosomal RNA in Euglena incubated with protein inhibitors. Exp Cell Res. 1973 Mar 30;78(1):111–117. doi: 10.1016/0014-4827(73)90044-x. [DOI] [PubMed] [Google Scholar]
- ZEHNDER A., GORHAM P. R. Factors influencing the growth of Microcystis aeruginosa Kutz, emend, Elenkin. Can J Microbiol. 1960 Dec;6:645–660. doi: 10.1139/m60-077. [DOI] [PubMed] [Google Scholar]



