Abstract
Background
Use of several immunomodulatory agents has been associated with reduced cardiovascular (CV) events in epidemiologic studies of rheumatoid arthritis (RA). However, it is unknown whether time-averaged disease activity in RA correlates with CV events.
Methods
We studied patients with RA followed in a longitudinal US-based registry. Time-averaged disease activity was assessed using the area under the curve of the Clinical Disease Activity Index, a validated measure of rheumatoid arthritis disease activity, assessed during follow-up. Age, gender, diabetes, hypertension, hyperlipidemia, body mass index, family history of myocardial infarction (MI), aspirin use, NSAID use presence of CV disease, and baseline immunomodulator use were assessed at baseline. Cox proportional hazards regression models were examined to determine the risk of a composite CV endpoint that included MI, stroke, and CV death.
Results
24,989 subjects followed for a median of 2.7 years were included in these analyses. During follow-up, we observed 422 confirmed CV endpoints for an incidence rate of 9.08 (95% confidence interval, CI, 7.90 – 10.26) per 1,000 person-years. In models adjusting for variables noted above, a 10-point reduction in time-averaged Clinical Disease Activity Index was associated with a 26% reduction in CV risk (95% confidence interval 17-34%). These results were robust in subgroup analyses stratified by presence of CV disease, use of corticosteroids, use of non-steroidal anti-inflammatory drugs or selective COX-2 inhibitors, change in RA treatment, and also when restricted to events adjudicated as definite or probable.
Conclusions
Reduced time-averaged disease activity in RA is associated with fewer CV events.
Keywords: rheumatoid arthritis, cardiovascular disease, epidemiology
INTRODUCTION
Rheumatoid arthritis (RA) patients suffer from an increased risk in cardiovascular (CV) events.(1, 2) This risk correlates with both traditional CV risk factors, as well as markers of inflammation, such as the erythrocyte sedimentation rate.(3, 4) Additionally, epidemiologic studies suggest that several disease-modifying anti-rheumatic drug (DMARD) treatments for RA associate with a reduced risk of CV events, including methotrexate and TNF antagonists.(5, 6) Several prior studies have demonstrated a cross-sectional association between atherosclerosis and disease activity.(3, 7) However, it is unknown whether time-averaged RA disease activity or reductions in disease activity, regardless of treatment, correlate with CV risk. This information carries potential importance for disease management recommendations.
Treatment recommendations for RA are based on evidence regarding improvements in pain and function, as well as the relative safety of drugs.(8, 9) Current CV management guidelines in RA focus on management of lipids and other risk factors, giving little guidance about the role of immunomodulators aimed at reducing systemic inflammation.(10) However, several recent trends make it imperative to better understand if reducing disease activity correlates with improved CV risk. First, the treatment paradigm in RA is being accelerated by an enthusiasm for “treat to target” in RA, whereby low disease activity or remission becomes the management goal.(11) Second, several secondary CV prevention trials of different immunomodulatory agents are being conducted in patients without a defined inflammatory condition.(12, 13) With this as background, we examined whether RA disease activity measured over a prolonged period of follow-up predicts CV risk. We hypothesized that patients who had lower time-averaged RA disease activity would suffer fewer CV events, regardless of which immunomodulatory treatments they had received.
METHODS
Study design and population
We undertook this study in a large registry of RA patients from the US, the Consortium of Rheumatology Researchers of North America (CORRONA). The methods of this registry have been well described.(14) Briefly, 268 rheumatologists from 103 sites in 35 US states contribute data approximately every four months using a structured case report form. At each visit, rheumatologists assess subject’s level of RA disease activity using the standardized Clinical Disease Activity Index (see below for details). In addition, data are collected regarding comorbidities and co-medications, including traditional CV risk factors described below (see Covariates Section).
Only those subjects in the registry diagnosed with RA by their treating rheumatologist through December 31, 2011 who had a Clinical Disease Activity Index measured at their first (baseline) visit were included. We excluded RA subjects in the registry who also carried the diagnosis of psoriatic arthritis, as well as those who were not using a DMARD at baseline or follow-up. The primary study cohort included all subjects in the registry with RA, and the first registry visit was considered baseline.
The cohort was followed from their baseline visit until the first of any of the following: death, loss to follow-up, December 30, 2011, or the first CV event (see below for CV event confirmation). Subjects with two consecutive visits with missing Clinical Disease Activity Index values were censored at the time of the second visit (see below for imputation methods).
All study activities have been approved by the responsible Institutional Review Board.
Disease activity
As noted, the Clinical Disease Activity Index was used to determine disease activity. This measure includes four elements: the physician’s report of the number of tender and swollen joints, the physician assessment of global arthritis activity, and the patient assessment of global arthritis activity.(15) It does not include an acute phase reactant, such as the C-reactive protein. However, this measure correlates well with the Disease Activity Score, which does include the C-reactive protein.(15) It is more widely used in typical clinical practice where a laboratory measure may not be available in a timely manner to make routine management decisions. The Clinical Disease Activity Index ranges in value from 0-76 and was examined as a continuous variable in our main analysis. We used the standard categories of Clinical Disease Activity Index as a secondary definition, where remission ≥ 2.8, low 2.9 – 10.0, moderate 10.1 – 22.0, and high > 22.0.(15)
The Clinical Disease Activity Index was measured at 98% of registry visits, allowing one to create an area under the curve for the longitudinal measurement. The area under the curve for Clinical Disease Activity Index was calculated at 6-month intervals with the geometric mean used to interpolate between visits if not occurring at the 6-month interval. From the area under the curve, a time-averaged Clinical Disease Activity Index was calculated and updated for each 6-month interval.
Missing Clinical Disease Activity Index values were imputed based on the following assumptions: when one of the four components of the Clinical Disease Activity Index was missing but it was available at the prior visit, the last observation of this component was used to impute the Clinical Disease Activity Index at the next visit; this was the case at 1% of visits. However, if two consecutive Clinical Disease Activity Index values were missing, subjects were censored at the second visit with missing data (0.4% of visits). If more than one component was missing at any visit, subjects were censored (1.4% of visits).
Cardiovascular outcomes
At each registry study visit, physicians report whether interval adverse events have occurred, including incident myocardial infarction (MI), stroke or CV death, among other adverse events. All physician-reported CV events prompt a second questionnaire to the rheumatologist to confirm the CV event, and request additional information including medical records from the treating acute care hospital for adjudication. All medical records were reviewed by an adjudication committee comprised of two cardiologists and a neurologist using adjudication methods established by the FDA.(16) Of the 422 rheumatologist-confirmed CV events, 147 (35%) had records available for adjudication; 93% of these were adjudicated as definite or probable, 3% as possible, and 4% as non-events. The primary analysis used a composite endpoint of the first confirmed MI, stroke, or CV death event and a secondary analysis focused on only those events that were adjudicated as definite or probable. We chose not to include heart failure as part of the study outcome because of its multi-factorial nature, especially in patients with RA, where the use of non-steroidal anti-inflammatory drugs and corticosteroids is so common.(17)
Covariates
We considered several variables in adjusted analyses. These included age at baseline, gender, as well as various CV risk factors. These risk factors were also assessed at baseline and not updated because of concerns regarding introducing bias through adjusting for mediators of the effect of time-averaged CDAI on the outcome of interest. They included body mass index, family history of MI, tobacco use, aspirin use, history of hypertension or use of medications for elevated blood pressure, history of diabetes, and a history of hyperlipidemia or use of medications for lipid abnormalities. We allowed patients with known CV disease in the primary analyses; an additional secondary analysis excluded them (see below). The duration of RA and typical RA treatments at baseline were assessed; these included DMARDs, corticosteroids, and non-steroidal anti-inflammatory drugs, including selective COX-2 inhibitors. Covariates were not updated in a time-varying fashion to avoid controlling for causal intermediates and ‘overadjust’ for factors that may mediate changes in RA disease activity.
Statistical analyses
The baseline characteristics were assessed using means, medians or categories for the primary cohort. As well, they were compared across Clinical Disease Activity Index categories assessed at the index visit. Incidence rates for the primary composite endpoint, as well as the secondary components were calculated. Person year rates were estimated based on the time from the index date to the censoring event with 95% confidence intervals (CIs) estimated assuming a binomial proportion.
Cox proportional hazard regression models were used to estimate the relative risk of CV events. The primary analysis examined the time-averaged Clinical Disease Activity Index from the area under the curve as a continuous variable; secondary analysis treated Clinical Disease Activity Index as a categorical variable (see above for definition of categories). Age and gender were included in all models, with traditional CV risk factors added in as well. Fully adjusted multivariable models also included baseline RA medication use and disease duration.
A series of secondary stratified analyses were pursued to assess the robustness of our primary findings. These included stratifying the cohort according to the following: 1) preexisting or known CV disease; 2) use of corticosteroids at baseline; and 3) use of nonsteroidal anti-inflammatory drugs or selective COX-2 inhibitors at baseline.
The proportional hazards assumptions were tested by examining the Kolmogorov-type supremum test(18) and there was no evidence that the proportionality assumption was violoated (p = 0.25) for the primary analysis. All analyses were run using SAS (version 9.2, Cary, North Carolina).
RESULTS
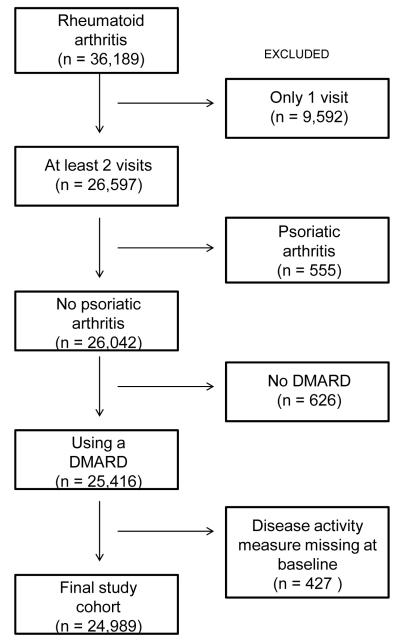
The cohort assembly is described in Figure 1. The exclusion criteria were applied sequentially to the 36,189 subjects with a diagnosis of RA enrolled as of December 31, 2011 in CORRONA. From the potentially eligible cohort, 11,200 (30.9%) were excluded leaving 24,989 subjects followed for a median of 2.7 years.
Figure 1.
This figure shows the assembly of the study cohort from the CORRONA registry. DMARD, disease modifying anti-rheumatic drug.
The baseline characteristics of the included subjects are listed in Table 1. The mean (standard deviation) age was 59 (13) years, 76% were women, and 89% were white. Coronary artery disease was present in 17%, hypertension in 29%, diabetes in 8%, hyperlipidemia in 25%, current tobacco use in 19%, and family history of MI in 3%. The mean (standard deviation) duration of RA was 10 (10) years, 77% were seropositive (either rheumatoid factor or anti-CCP), 61% used NSAIDs or selective COX-2 inhibitors, 84% used methotrexate, and 48% a TNF antagonist. Characteristics of the subjects categorized by baseline Clinical Disease Activity Index showed differences in demographics, cardiovascular risk factors, RA disease activity, as well as medication use.
Table 1.
Baseline characteristics of CORRONA subjects with rheumatoid arthritis
| Total cohort (n = 24,989) |
Clinical Disease Activity Index at baseline |
||||
|---|---|---|---|---|---|
| Remission (n = 5,511) |
Low (n = 8,411) |
Moderate (n = 6,638) |
High (n = 4,429) |
||
|
|
|||||
| Percentages unless otherwise noted | |||||
| Female gender | 77 | 72.6 | 75.2 | 78.7 | 79.2 |
| Age, mean (± SD) (years) | 58.9 (±13.4) | 58.1 (±13.8) | 59.5 (±13.5) | 59.2 (±13.2) | 58.3 (±13.0) |
| Age, <45 years | 14 | 16.6 | 14.0 | 13.0 | 14.0 |
| 45-64 years | 51 | 50.0 | 49.3 | 52.3 | 54.8 |
| 65+ years | 35 | 33.5 | 36.8 | 34.7 | 31.3 |
| Race, White | 89 | 90.4 | 89.4 | 88.0 | 87.2 |
| Black | 7 | 6.0 | 6.6 | 7.9 | 7.5 |
| Other | 4 | 3.6 | 4.0 | 4.1 | 5.4 |
| College education | 55 | 61.7 | 57.0 | 50.9 | 47.4 |
| Tobacco use, None | 66 | 68.2 | 67.5 | 65.0 | 62.5 |
| Previously | 15 | 12.1 | 13.5 | 16.3 | 18.7 |
| Currently | 19 | 19.6 | 19.0 | 18.7 | 18.9 |
| BMI, mean (±SD) (kg/m2) | 29.2 (±7.1) | 28.1 (±6.3) | 29.9 (±6.88) | 29.8 (±7.42) | 30.3 (±7.7) |
| Alcohol use (Y/N) | 43 | 51.4 | 44.6 | 39.0 | 36.0 |
| Alcohol use, None | 57 | 48.7 | 55.4 | 61.2 | 64.1 |
| Rarely | 21 | 23.7 | 21.5 | 20.3 | 18.4 |
| 1-3 drinks weekly | 17 | 21.6 | 18.2 | 14.4 | 14.0 |
| 7+ weekly | 21 | 6.0 | 4.9 | 4.2 | 3.5 |
| Cardiovascular risk factors | |||||
| Prior MI | 3 | 2.4 | 3.2 | 3.3 | 3.6 |
| Presence of CAD | 17 | 16.3 | 16.5 | 17.0 | 17.8 |
| Diabetes | 8 | 5.9 | 7.1 | 8.0 | 9.7 |
| Hypertension | 29 | 25.8 | 29.9 | 31.4 | 29.2 |
| Hyperlipidemia | 25 | 23.7 | 25.5 | 24.5 | 25.2 |
| Family history of MI | 3 | 2.6 | 2.8 | 2.8 | 3.2 |
| Physical activity*, none | 32 | 25.1 | 30.4 | 35.1 | 39.2 |
| 1-2 | 30 | 30.5 | 31.2 | 29.7 | 29.8 |
| 3-4 | 21 | 26.2 | 22.1 | 19.1 | 15.8 |
| 5-6 | 6 | 8.5 | 6.5 | 5.4 | 3.7 |
| 7 + | 10 | 9.7 | 9.8 | 10.7 | 11.5 |
| Aspirin use, yes/no | 29 | 25.3 | 27.8 | 28.0 | 27.9 |
| RA characteristics | |||||
| Duration of RA (years) | 10.1 (±9.8) | 8.7 (±8.6) | 10.3 (±9.8) | 10.8 (±10.4) | 10.4 (±10.1) |
| Seropositive# | 77 | 75.3 | 77.9 | 78.6 | 76.5 |
| CDAI | 12.3 (±11.9) | 1.2 (±0.8) | 6.1 (±2.1) | 15.3 (±3.4) | 33.0 (±10.9) |
| Modified HAQ | 0.4 (±0.5) | 0.1 (±0.2) | 0.3 (±0.4) | 0.5 (±0.5) | 0.7 (±0.6) |
| DAS-28 | 3.4 (±1.6) | 1.8 (±0.8) | 2.8 (±0.8) | 4.0 (±0.9) | 5.6 (±1.1) |
| Baseline medication use | |||||
| Prednisone, no use | 66 | 78.8 | 67.4 | 59.4 | 57.7 |
| 1-4 mg | 10 | 9.0 | 11.5 | 10.9 | 8.6 |
| 5-9 mg | 14 | 7.5 | 13.3 | 17.6 | 17.8 |
| 10+ mg | 6 | 2.4 | 4.8 | 7.8 | 11.3 |
| NSAID or coxib | 61 | 55.1 | 61.6 | 63.6 | 62.7 |
| Methotrexate | 84 | 82.5 | 85.0 | 84.8 | 85.1 |
| TNF antagonist | 49 | 42.8 | 46.8 | 51.3 | 54.7 |
| Rituximab | 3 | 1.1 | 1.8 | 3.2 | 4.8 |
| Abatacept | 4 | 2.2 | 3.4 | 4.9 | 6.7 |
| Tocilizumab | 0.4 | 0.1 | 0.2 | 0.5 | 0.7 |
Physical activity in number of exercise sessions per week. # Seropositive is based on rheumatoid factor or anti-CCP.
Abbreviations: RA, rheumatoid arthritis; CDAI, clinical disease activity index; HAQ, modified health assessment questionnaire; DAS, disease activity score-28; MI, myocardial infarction; CAD, coronary artery disease; BMI, body mass index
Missing data by variable: gender, n = 98; age, n = 157; education, n = 1,301; tobacco use, n = 6,838; BMI, n = 393; alcohol use, n = 1,002; duration of RA, n = 153; seropositive, n = 9,877; modified HAQ, n = 457; DAS28, n = 14,278; prednisone use, n = 870; physical activity, n = 1,405; and aspirin use, n = 434. Percentages are calculated based on the total number of subjects, including those with missing values.
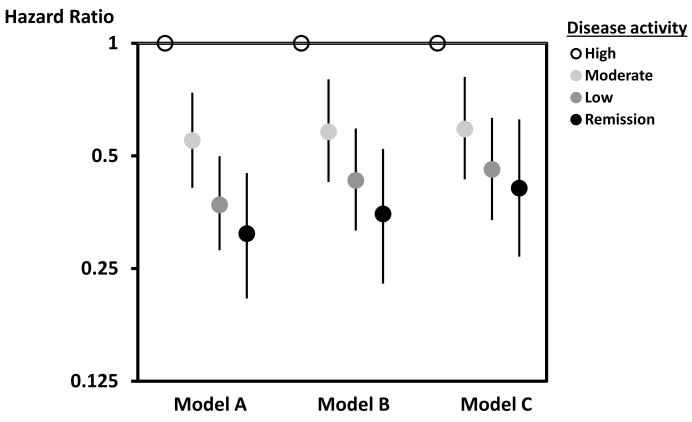
Table 2 shows the incidence rates for the composite CV outcome as well as the components. The incidence rate for the primary composite outcome was 7.79 (95% CI 6,70-8.88) per 1,000 person-years. In models adjusted for age, gender, traditional CV risk factors, as well as RA treatments at baseline, the risk of the primary CV outcome was reduced by 21% (95% CI 13-29%) per 10-point reduction in time-averaged Clinical Disease Activity Index. Figure 2 shows the reduction in risk associated with lower disease activity. In fully adjusted models (see Statistical Analysis section for covariates), there was a 53% (95% CI 30-68%) reduction in risk from high disease activity to remission.
Table 2.
Incidence rate per 1,000 person-years for cardiovascular events among CORRONA patients with rheumatoid arthritis
| Primary study cohort (n = 24,989) |
|||
|---|---|---|---|
| Events | Person-years | Incidence rate (95% CI) | |
| Composite cardiovascular events | 534 | 68,576 | 7.8 (6.7 – 8.9) |
| Myocardial infarction | 217 | 68,873 | 3.2 (2.5 – 3.9) |
| Stroke | 233 | 68,856 | 3.4 (2.7 – 4.1) |
| Cardiovascular death | 96 | 69,273 | 1.4 (0.9 – 1.9) |
Notes: Primary study cohort includes subjects with rheumatoid arthritis in CORRONA.
Figure 2.
This figure shows the hazard ratios for the primary analysis with the reference being high disease activity as measured by the Clinical Disease Activity Index. Model A is adjusted for age and gender only. Model B is adjusted for age, gender, age*gender interaction, and cardiovascular risk factors (prior MI, presence of CAD, diabetes, hypertension, hyperlipidemia, smoking, BMI (continuous), family history of MI, and aspirin use. Model C is adjusted for all variables in Model B + RA disease duration and baseline use of NSAIDs or selective COX-2 inhibitors, corticosteroids, disease modifying anti-rheumatic drugs, and biologic drugs.
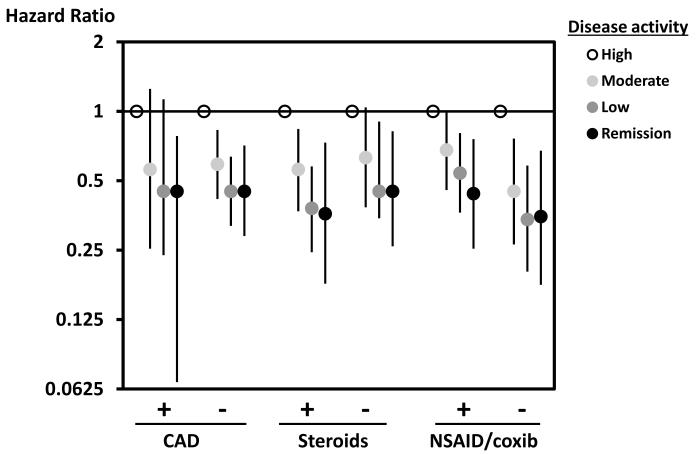
Sensitivity analyses performed in a series of subgroups are shown in Figure 3. The reduction in risk across decreasing Clinical Disease Activity Index levels was observed in all subgroups, including those with and without known CV disease, users and non-users of corticosteroids, and users and non-users of non-steroidal anti-inflammatory drugs or selective COX-2 inhibitors. Fully adjusted Cox regression analyses that only considered definite and probable adjudicated cases also showed similar trends to the primary analysis. Compared with high disease activity, moderate disease activity had a 35% reduction in CV outcomes (95% CI 0% - 60%), low disease activity had a 58% reduction (95% CI 32% – 74%), and remission a 60% reduction (95% CI 23% – 80%).
Figure 3.
This figure shows the hazard ratios for the subgroup analyses with the reference being high disease activity as measured by the Clinical Disease Activity Index. All hazard ratios are from Cox proportional hazard Model C which is adjusted for age, gender, age*gender interaction, and cardiovascular risk factors (prior MI, presence of CAD, diabetes, hypertension, hyperlipidemia, smoking, BMI (continuous), family history of MI, aspirin use, RA disease duration, and baseline use of NSAIDs or selective COX-2 inhibitors, corticosteroids, disease modifying anti-rheumatic drugs, and biologic drugs.
DISCUSSION
Persons with RA suffer from a higher risk of CV disease than those without RA.(19) Cardiovascular disease in RA also potentially serves as a relevant example of the benefits of inflammation control on CV risk. With this in mind, we examined the relationship between RA disease activity measured longitudinally and the risk of CV events. Among a large cohort of patients with RA followed for a median of 2.7 years, we found a significant trend towards a reduced risk of CV events with improved disease activity: a 21% reduction in CV risk for each 10 point lowering of the CDAI and a 53% reduction from high disease activity to remission. These results add significant new information regarding the importance of sustained control of RA disease activity, not only for improvement in pain and function, but also for reduced CV risk.
Strengths of the analysis include its large size, relatively long follow-up, and inclusion of RA patients followed in typical real-world clinical practice. In addition, we included information on body mass index, tobacco use, and family history, CV risk factors unavailable in many similar analyses. The findings were robust within multiple relevant subgroups – no prior CV disease, no prior corticosteroid use, no prior non-steroidal anti-inflammatory drug or selective COX-2 inhibitor use and subjects who changed RA treatment during follow-up. However, limitations of the analysis are also important to consider. We could not obtain medical records on all confirmed CV events. However, in events with records, 93% were adjudicated as probable or definite. We did not have a measured blood pressure, glycohemoglobin levels, or lipid panel results for subjects. Instead, we relied on the diagnoses and/or the presence of medications used for these conditions. This method has been used in similar previous analyses.(20) Most patients were white and thus, our findings may not apply to all RA populations. As well, residual confounding is always a concern in epidemiologic studies.
These findings fit into a series of prior studies that suggest the importance of RA disease activity. Del Rincon and colleagues demonstrated that the erythrocyte sedimentation rate was an independent predictor of subclinical CV disease in RA, as measured by the carotid intima medial thickness.(3) In a prior study, we demonstrated that baseline RA characteristics independently predicted future CV events.(21) But, unlike the current study, prior analyses have not examined longitudinal changes in disease activity. One prior study that included longitudinal disease activity, but was much smaller, suggested a significant elevation in CV risk with increased disease activity, but no significant elevation by disease category.(22) An observational study of TNF antagonists suggested that patients who respond to these agents experienced a lower risk of CV events than non-responders, suggesting that reducing disease activity is associated with improved CV risk.(23)
Several potential implications of the current study can be considered. First, RA disease activity during follow-up was associated with CV events, even after adjusting for the use of immunomodulatory treatments. This suggests that controlling disease activity may be a more important management strategy than use of a given immunomodulator, at least in the context of preventing CV events. This is a hypothesis worth testing, especially as enthusiasm grows for use of combination synthetic DMARDs. Second, the adoption of a treat to target strategy in RA (11) may be beneficial not only because of the observed improvement in pain and function, but also because of a reduction in CV risk. Finally, while these results were generated among a cohort of patients with RA, they do suggest the possibility that immunomodulatory strategies may improve CV outcomes in other populations. Such hypotheses are being tested in ongoing trials among non-rheumatic disease populations.(12, 13)
In conclusion, we examined a large RA cohort to determine the effect of time-averaged disease activity on CV risk. The results demonstrate a clear “dose-response” effect with reductions in disease activity associated with reduced CV risk, independent of immunomodulatory treatments. While these findings should not be interpreted to mean that traditional risk factors are not important, they do support the current RA recommendations for treating to low disease activity or remission.(8)
Acknowledgments
Support: This study was supported by CORRONA. In the last 2 years, CORRONA has received subscription fees from Abbvie, Amgen, AstraZeneca, Genentech, Horizon, Eli Lilly, Novartis, Pfizer, Vertex and UCB, but none related specifically to this study.
Footnotes
Disclosures: Dr. Solomon receives research support through grants to Brigham and Women’s Hospital from Amgen, Lilly, and Pfizer (none related to the current work). He also serves in unpaid roles on a trial sponsored by Pfizer. Dr Kremer receives salary support and owns stock in Corrona. He has received consulting fees and research support from Abbvie, Amgen, BMS, Genentech, Lilly, Pfizer and UCB. Dr. Curtis has received research grants and consulting for Genentech/Roche, Amgen, Abbvie, and Pfizer (none related to the current work) and receives support from AHRQ (R01 HS018517) and NIH (AR064172). Dr. Farkouh served on epidemiology advisory board for Genentech. Dr. Harrold receives research support through a grant from CORRONA to the University of Massachusetts Medical School. Dr. Hochberg is a consultant for Iroko Pharmaceuticals, Novartis AG and Pfizer Inc. Dr. Greenberg has received consulting fees from AstraZeneca and Pfizer.
REFERENCES
- 1.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis.[see comment] Circulation. 2003;107(9):1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 3.Del Rincon IWK, Stern MP, Freeman GL, O’Leary DH. Escalante A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2002;48:1833–1840. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 4.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70(3):482–7. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108(9):1362–70. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63(4):522–9. doi: 10.1002/acr.20371. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg JD, Kremer JM, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(4):576–82. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 8.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(5):625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–31. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 11.Haraoui B, Smolen JS, Aletaha D, Breedveld FC, Burmester G, Codreanu C, et al. Treating Rheumatoid Arthritis to Target: multinational recommendations assessment questionnaire. Ann Rheum Dis. 2011;70(11):1999–2002. doi: 10.1136/ard.2011.154179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162(4):597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(2):199–207. e15. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremer JM. The CORRONA database. Autoimmun Rev. 2006;5(1):46–54. doi: 10.1016/j.autrev.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–8. [PubMed] [Google Scholar]
- 16.Administration FaD Health and Human Services FaDAC, editor. Guidance for Industry, Diabetes Mellitus -- Evaluating Cardiovascular Risk. 2008.
- 17.Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000;102(20 Suppl 4):IV14–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 19.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis & Rheumatism. 2005;52(3):722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 20.Arts EE, Popa C, Den Broeder AA, Semb AG, Toms T, Kitas GD, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204024. [DOI] [PubMed] [Google Scholar]
- 21.Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69(11):1920–5. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arts EE, Fransen J, den Broeder AA, Popa CD, van Riel PL. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204531. [DOI] [PubMed] [Google Scholar]
- 23.Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56(9):2905–12. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]