Abstract
Objective
Obesity, a Sirtuin-1 (SIRT-1) deficient state, increases morbidity and resource utilization in critically ill patients. SIRT-1 deficiency increases microvascular inflammation and mortality in early sepsis. Our objective was to study the effect of resveratrol (RSV), a SIRT-1 activator, on microvascular inflammation in obese septic mice.
Methods and procedures
ob/ob and C57Bl/6 (WT) mice were pre-treated with RSV vs. DMSO (Vehicle) prior to cecal ligation and puncture (Sepsis). We studied 1) Leukocyte/Platelet adhesion 2) E-selectin, ICAM-1 and SIRT-1 expression in small intestines 3) 7-day survival. A group of RSV treated mice received SIRT-1 inhibitor (EX-527) with sepsis-induction and leukocyte/platelet adhesion and E-selectin/ICAM-1 expression were studied. We treated endothelial (HUVEC) cells with RSV to study E-selectin/ICAM-1 and p65-acetylation (AC-p65) in response to LPS.
Results
RSV treatment decreased leukocyte/platelet adhesion and E-selectin/ICAM-1 expression with increased SIRT1 expression in septic ob/ob and WT mice; decreased E-Selectin/ICAM-1 expression via increased SIRT-1 expression and decreased AC-p65 expression in HUVEC. EX-527 abolished RSV-induced attenuation of microvascular inflammation in ob/ob septic mice. Finally, ob/ob mice in Sepsis+RSV group had significantly increased 7-day survival vs. Sepsis+Vehicle group.
Conclusion
RSV increases SIRT-1 expression in ob/ob-septic mice to reduce microvascular inflammation and improves survival.
Keywords: Sepsis, Obesity, SIRT-1, Resveratrol, Adhesion Molecule, Leukocyte adhesion
INTRODUCTION
Severe sepsis and septic shock are the leading causes of death in critically ill patients in non-coronary intensive care units worldwide1,2. In the USA alone they cause over 200,000 deaths per year and costs $16 billion annually3. High mortality is generated both by the inciting infection and by host-derived inflammatory injury to multiple organs.
Microvasculature with its strategic place between systemic circulation and local tissue environment plays a central role in sepsis-induced inflammation with multiple organ failure. Circulating cell-endothelial cell interactions in the microcirculation are the rate determining factors in inflammatory response in various tissues in response to variety of stimuli4 including sepsis. Others and our previous work demonstrated increased leukocyte adhesion in post-capillary venules after induction of sepsis5-7.
Obesity, now recognized as a disease, affects more than one third of the adults in the USA. Estimates project obesity-related healthcare cost to increase by $48-66 billion/year in USA by year 20308. While controversy exists regarding the effect of obesity and overweight on mortality in critically ill patients9, literature suggests that morbid obesity is associated with increased resource utilization and cost of care in already expensive care of critically ill patients in the intensive care units10,11. We have used a mouse model to better understand the interaction of obesity and sepsis and found increased mortality and exaggerated microvascular inflammation during early sepsis6 but the mechanisms responsible are unclear.
Previously, we reported that NAD+ dependent activation of class III histone deacetylase sirtuin 1 (SIRT-1) coordinates the acute inflammatory response in sepsis. We found that SIRT-1 inhibition during early sepsis significantly exaggerates leukocyte adhesion and increases mortality12. Growing body of evidence supports that obesity with chronic inflammation is associated with low levels of NAD+ and SIRT-113. In contrast to the state of over nutrition of obesity, calorie restriction 14 and weight loss lead to increased SIRT-1 expression13 and reduce inflammation and imbalanced metabolism. The effects of low SIRT-1 expression during obesity on microvascular inflammation associated with sepsis are not well defined, nor is it known whether SIRT-1 activation might reduce microvascular inflammation and limit systemic injury due to sepsis.
Resveratrol (3, 5, 4′-trans-trihydroxystilbene; RSV), a potent activator of SIRT-115,16 present in grapes, berries, peanuts and red wine, is known for its anti-inflammatory and anti-cancer properties14,17-19. The anti-inflammatory effects of RSV, which also link to activation of AMP-activated protein kinase (AMPK), attenuate lung and kidney injury and decrease mortality in rodent sepsis18,20,21. While beneficial in lean mice with sepsis, the effect of RSV on sepsis with obesity, a state of reduced SIRT-1 expression, are unclear. Also, it is unknown whether the circulating cell-endothelial cell interactions in septic mice are modulated by RSV treatment. Here, we report the effect of increased SIRT-1 expression using RSV on circulating cell-endothelial cell interactions and survival in obese mice with sepsis.
MATERIAL AND METHODS
Animals
This study was approved by the Institutional Animal Care and Use Committee of the Wake Forest School of Medicine and was performed according to the NIH guidelines. The wild type (WT: C57Bl/6) and ob/ob (B6.Cg-Lepob/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA).
Mice were injected with RSV (RSV) 30mg/kg (4ml/kg) or equivalent volume of DMSO (Vehicle) (4ml/kg) intraperitoneally 18 hours pre-sepsis. This dose of RSV in mice was as per documented literature21. In one group of mice, RSV pre-treated mice received EX-527 (10 mg/kg intraperitoneally; 4ml/kg, Vehicle: DMSO) within 10 minutes of cecal ligation and puncture.
Cecal ligation and puncture (CLP)
Sepsis was induced using CLP as described in the literature12,22. Briefly, laparotomy incision (1-1.5 cm) was performed; cecum was identified, ligated and perforated two times with a 22-gauge needle. Contents were returned to abdominal cavity and incision was closed in two layers (peritoneum and skin), followed by fluid resuscitation (normal saline 1ml subcutaneously) given to each mouse. The Sham-operated mice underwent laparotomy and fluid resuscitation as described above, without cecal ligation or puncture. Mice with CLP (Sepsis) and Sham surgeries were used for tissue harvest (small intestinal tissue) or intravital fluorescent video microscopy 6 hours post-surgery.
Intravital fluorescent video microscopy (IVM)
Six hours after CLP/Sham surgery, the mice underwent carotid artery and jugular venous cannulations. The laparotomy incision was opened, small intestine (ilium) was exteriorized and care was taken to keep small intestine moist through the entire procedure. After allowing 30 minutes to stand, the small intestinal microcirculation was studied (n= 4-6 mice/group) using Nikon Eclipse FN1 microscope as described previously5,12. Mice were injected with Rhodamine G(Sigma-Aldrich; St Louis, MO, USA: 0.02% in 100 μL of PBS) for in vivo leukocyte visualization (labeled red) while platelets were labeled green ex vivo with carboxyfluorescein diacetate succinimidyl ester (CFSE: Sigma-Aldrich; St Louis, MO, USA; 90 μM). This allowed simultaneous monitoring of leukocytes and platelets in each venule.
The details of the platelet isolation technique are as outlined previously6. Leukocyte contamination of the platelet suspension was evaluated from a 25 μl sample to ensure that the leukocyte count of the infusate did not exceed 0.01%. The platelets (n=100 × 106) were infused intravenously over 5 min (yielding <5% of the total platelet count) and allowed to circulate for a period of 5 min before recording on a DVD. Literature suggests that these platelet isolation procedures have no significant effect on the activity or viability of isolated platelets23.
Post-capillary venules (three to five in number; 4-6 mice per group) in each mouse intestine were recorded to quantify leukocyte and platelet adhesion. Recordings were analyzed for one minute. A cell (leukocyte/platelet) was considered adherent if it remained stationary for at least 30 consecutive seconds of the one minute recording. The mean of the average values of leukocyte (LA) and platelet adhesion (PA) determined in each mouse were then used to generate a group mean.
Immunohistochemistry of small intestinal tissue
Small intestinal tissue was harvested and frozen using OTC medium. Acetone fixed frozen sections of tissue were stained using SIRT-1(Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA), E-selectin and ICAM-1 (BD biosciences, San Jose, CA, USA) and Von Willebrand factor (VWF, Abcam, Inc. Cambridge, MA, USA). Cy™3-conjugated labeled secondary antibodies for SIRT-1 and E-selectin and FITC conjugated secondary antibody for VWF were purchased from Jackson Immuno Research Laboratories, Inc. (West Grove, PA, USA).
Image acquisition
Virtual images were captured with an Olympus VS110 (20x/0.75 objective). Static images were captured utilizing OlyVIA (Olympus), at the designated magnification. We used Olympus XM10 camera for image acquisition.
Cell Culture
Human umbilical vein/vascular endothelium (HUVEC) cell line was obtained from American Type Culture Collection (CRL-1730) and maintained in F-12K medium containing 0.1mg/mL of heparin, 0.03-0.05mg/mL endothelial cell growth supplement, 100 units/ml penicillin, 100mg/ml streptomycin and 10% of heat inactivated FBS at 37 °C and 5% CO2. Early passage (2-4) cultures were used.
RNA extraction and RT-PCR
HUVEC were pre-treated with Vehicle (DMSO) or 10uM of RSV for 24 h prior to further stimulation with LPS (100ng/mL) for 4 h. RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA). The mRNA expression levels of target genes were quantified by quantitative real-time PCR with SensiFAST™ Probe Lo-ROX One-Step Kit (Bioline, BIO-78005) as described before12. All results were normalized to GAPDH RNA levels. Relative quantification was calculated using the ΔΔ comparative threshold formula. All samples were run in quadruplicates to calculate average and SE value. TaqMan primer/probes for GAPDH, E-Selectin and ICAM-1 were purchased from Invitrogen (Grand Island, NY, USA).
Western blot
HUVEC were seeded in 6-well plate (3×105 cells per well) the before the treatment and stimulation as described above. Total cell lysate was collected in lysis buffer (65.8 mM Tris-HCl, pH 6.8, 2.1% SDS, 26.3% glycerol, plus Complete mini Protease Inhibitor Cocktail from Roche) followed by the sonication for 10min. Cell debris were removed, protein concentration determined with Bio-Rad DC protein assay and adjusted into the same value and resolved by SDS-PAGE, Protein levels were measured by immnoblotting, using GAPDH as loading control. The membrane was blocked and then incubated with antibodies against SIRT-1 (sc-15404, Santa Cruz Biotechnology, USA), Ac-p65 (Abcam, ab19870, 1:300), total p65 (Santa Cruz Biotechnology, sc-372, 1:1000) and GAPDH (AM4300, 1:15000; Invitrogen, Carlsbad, CA, USA) overnight. The blots were incubated with Alexa Fluor 680-conjugated secondary antibodies (827-10080 and 827-10081, 1:10,000; LI-COR Biosciences, Lincoln, NE, USA) on day 2. The signal was developed and quantified with a Li-COR Odyssey Infrared Imager system (Li-COR Biosciences).
Statistics
All data were analyzed using Tukey-Kramer post-hoc analysis (Statview, SAS; Cary, NC, USA). A p<0.05 was designated as significant. Log rank test was used to compare survival between the groups in the Kaplan-Meier survival curves and a p<0.05 was designated as significant.
RESULTS
Mean arterial blood pressure, weights and leukocyte counts of mice in different groups
The mean arterial blood pressure (MAP) and weights of different groups of mice are shown in Table 1. As expected, weights of all ob/ob mice were significantly higher than the corresponding WT cohort. We did not find significant differences in the mean arterial blood pressure (MAP) between ob/ob or WT mice experimental groups.
Table 1. Mean arterial blood pressure (MAP), peripheral leukocyte/neutrophil count and weight in different groups.
| MAP (Mean ± SEM) |
Weight (Mean ± SEM) |
Total leukocytes/μl (Mean ± SEM) |
PMN/ μl (Mean ± SEM) |
|
|---|---|---|---|---|
| WT Sham + Vehicle | 68± 3 | 24.64±1.34 | 7687±187 | 3125±1000 |
| WT Sepsis + Vehicle | 66±7 | 29.03±0.48 | 3125±544# | 1154±624 |
| WT Sham + RSV | 77±3 | 24.13±1.49 | 6937±187 | 2625±375 |
| WT Sepsis + RSV | 73±3 | 26.69±0.83 | 6625±190 | 1750±144 |
| ob/ob Sham + Vehicle | 63±1 | 42.41±1.95* | 5750±250* | 2312±187 |
| ob/ob Sepsis + Vehicle | 64±2 | 40.70±3.04* | 3458±110 | 2582±397 |
| ob/ob Sham + RSV | 69±4 | 37.31±3.5* | 5750±375 | 1268±981 |
| ob/ob Sepsis + RSV | 73±3 | 38.30±2.19* | 5333±512 | 2833±480 |
p<0.05 vs. respective WT control group
p<0.05 vs. WT Sham+ Vehicle
We show that the total leukocyte counts of ob/ob Sham +Vehicle mice were significantly lower compared to WT counterpart, while no such differences were found in between Sham+RSV, Sepsis+Vehicle and Sepsis+RSV groups. Similarly, there were no statistically significant differences found in polymorphonuclear neutrophil counts (PMN) between different groups of mice.
Resveratrol decreases leukocyte and platelet adhesion in intestinal microcirculation in obese mice with sepsis
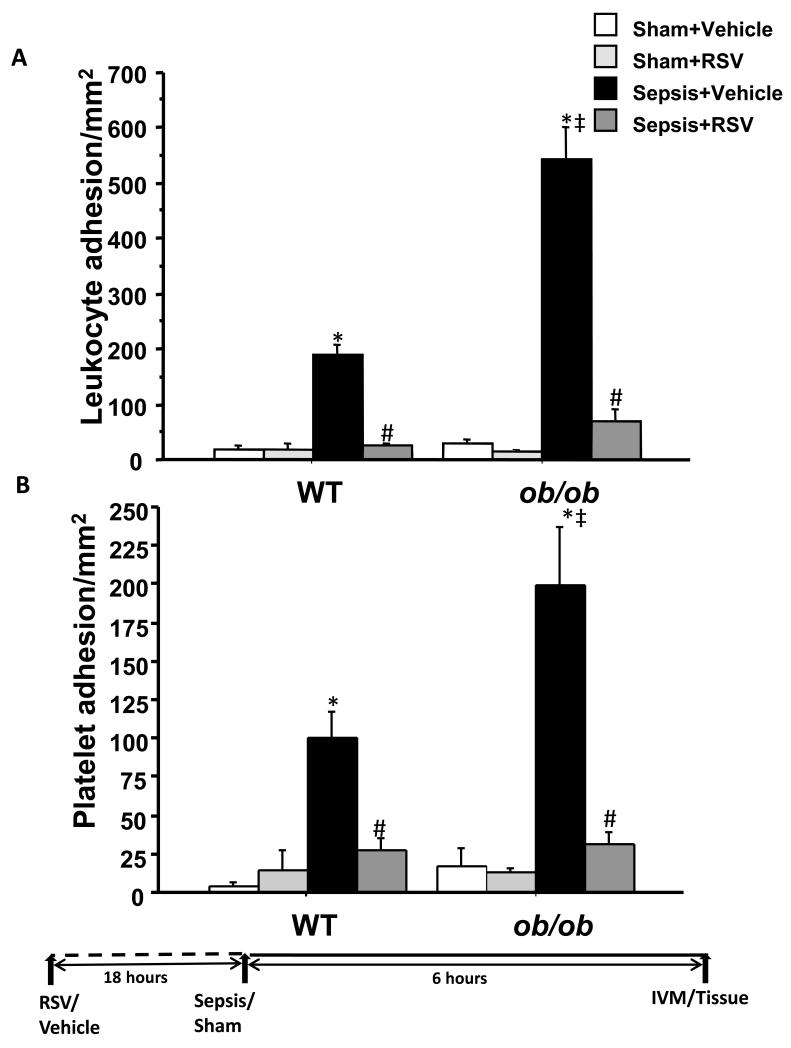
We have shown previously that leukocyte and platelet adhesion is increased in WT mice with polymicrobial sepsis; we further observed that ob/ ob mice6 and SIRT-1 deficient WT mice sepsis had further increase in leukocyte adhesion12. These observations supported that SIRT-1 deficiency participated in an exaggerated adhesion response and suggested that SIRT-1 activation may attenuate leukocyte adhesion during sepsis. We first tested this concept in WT sepsis and found that pre-treatment with RSV decreased leukocyte and platelet adhesion in early sepsis. We then tested the effect of RSV in ob/ob mice with and without sepsis. As depicted in Figure 1, we show that leukocyte and platelet adhesion in vehicle treated ob/ob mice were significantly higher compared to WT mice with sepsis, a finding consistent with our previous report6. More importantly, mice pre-treated with RSV showed significantly decreased leukocyte and platelet adhesion compared to vehicle treated mice in ob/ob septic mice. Leukocyte and platelet adhesion in intestinal microcirculation in ob/ob and WT mice were significantly increased compared to respective Sham controls and there were no significant differences leukocyte/platelet adhesions between different sham groups. These results demonstrate that RSV limits leukocyte and platelet adhesion in ob/ob and WT mice with sepsis.
Figure 1. RSV attenuates leukocyte and platelet adhesion in small intestinal microcirculation.
Leukocyte (Figure 1A) and platelet (Figure 1B) adhesion in small intestinal microcirculation (mice n=4-6/group) (mean ± SEM) were measured using intravital microscopy (IVM). Leukocyte and platelet adhesion in Sepsis + Vehicle groups (black bars) were significantly increased vs. Sham + Vehicle (white bars) groups in both, WT and ob/ob strains. Sepsis + Vehicle treated ob/ob mice had significantly higher leukocyte and platelet adhesion than WT mice. Sepsis + RSV (dark gray) groups had significantly attenuated leukocyte and platelet adhesion compared to Sepsis + Vehicle in both WT and ob/ob strains; n=4-6/group * p<0.05 vs. respective Sham + Vehicle group; # p<0.05 vs. respective Sepsis + Vehicle groups’ ‡ p<0.05 vs. WT Sepsis + Vehicle group using Tukey’s post-hoc analysis; error bars: s.e.m.
Resveratrol decreases E-selectin and ICAM-1 expression in intestinal tissue in obese mice with sepsis
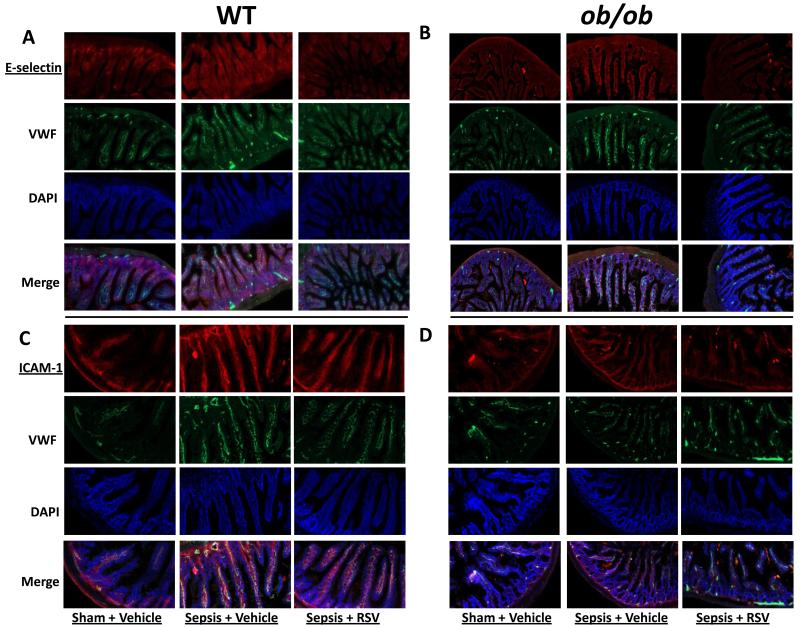
We and others4,12,24 have reported that the E-selectin and ICAM-1 expression modulate leukocyte adhesion in models of inflammation and sepsis12,24. Recently, we showed that SIRT-1 inhibition increases E-selectin/ICAM-1 expression during sepsis 12 and together with results in Figure 1, suggest an inverse relationship between SIRT-1 and E-selectin/ICAM-1 expression. To test this inverse relationship, we used immunohistochemistry (IHC) to observe the effect of RSV on E-selectin and ICAM-1 expression in small intestinal tissue in WT and ob/ob mice with sepsis. As we had previously found, E-selectin and ICAM-1 expression increased in small intestinal tissue of WT and ob/ob mice with sepsis (Figure 2 A/B and C/D, E-selectin and ICAM-1 respectively). We observed better co-localization between ICAM-1-vWF compared to E-selectin-vWF, probably due to non-specific staining pattern of either vWF or E-selectin. To resolve the issue of effect of RSV on endothelial-specific E-selectin expression, (see below).
Figure 2. RSV attenuates E-selectin and ICAM-1 expression in small intestinal tissue.
We show the E-selectin and ICAM-1 expression in small intestinal tissue sections were studied using immunohistochemistry (20× magnification) in WT and ob/ob mice. E-selectin expression in Sepsis + Vehicle group was significantly increased vs. Sham + Vehicle group in both, WT (2A) and ob/ob (2B) strains. Sepsis + RSV groups had significantly decreased E-selectin expression compared to Sepsis + Vehicle in both WT and ob/ob strains.
We show ICAM-1 expression in small intestinal tissue sections in WT (Figure 2C) and ob/ob (Figure 2D) mice. Similar to E-selectin expression, ICAM-1 expression in Sepsis + Vehicle group were significantly increased vs. Sham + Vehicle group in both, WT and ob/ob tissue sections. Sepsis + RSV groups had significantly decreased ICAM-1 expression compared to Sepsis + Vehicle in both WT and ob/ob strains.
Consistent with an inverse relationship hypothesis however, we found decreased E-selectin and ICAM-1 expression in RSV treated mice. Taken together, these results suggest that E-selectin and ICAM-1 expression correlate with leukocyte adhesion during sepsis. Moreover, we observed an inverse relationship between E-selectin/ ICAM-1 and SIRT-1.
Resveratrol decreases E-selectin and ICAM-1 expression via increased SIRT-1 in endothelial cells
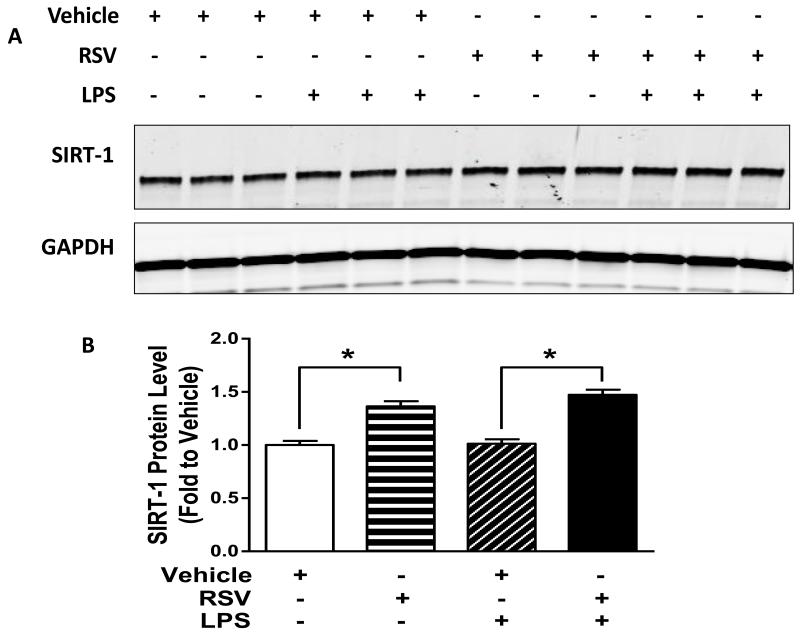
To examine the effect of RSV on endothelial cell SIRT-1 and adhesion molecule expression, we treated HUVEC with RSV overnight and stimulated with LPS. First we tested whether RSV treatment increased SIRT-1 expression in HUVEC. As shown in Figure 3 A and B we show that there was significantly increased expression of SIRT-1 in RSV treated HUVEC.
Figure 3. RSV increases SIRT-1 expression in HUVEC.
We next studied if RSV treatment increased SIRT-1 protein expression in HUVEC using Western Blot. Cells were pre-treated with RSV and stimulated with LPS as indicated. Representative WB and quantitative bar graph are shown in Figures 3A and B respectively. RSV treatment increased SIRT-1 protein expression significantly (* p<0.05 vs. Vehicle treatment).
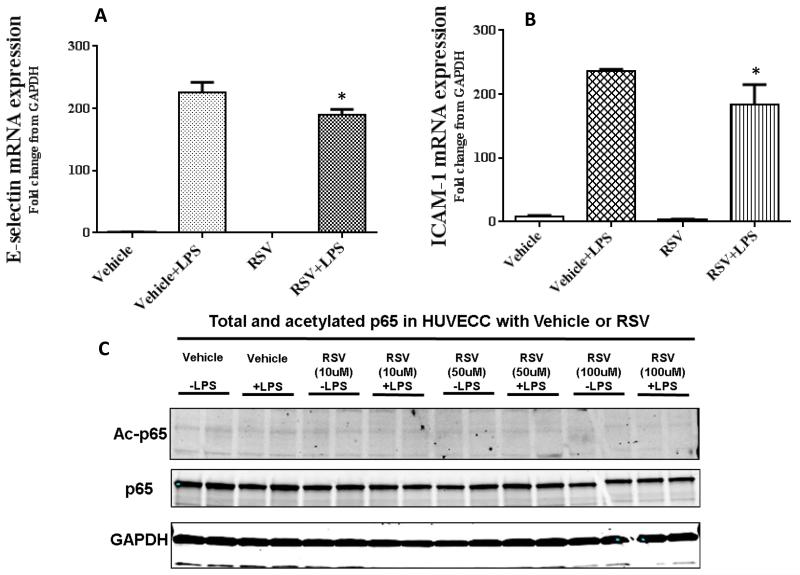
Next we tested whether the RSV treatment decreased adhesion molecule expression in HUVEC. As shown in Figure 4 A and B, there was a significant decrease in E-selectin and ICAM-1 mRNA expression in RSV treated HUVEC vs. Vehicle when stimulated with LPS.
Figure 4. Resveratrol attenuates E-selectin and ICAM-1 expression and decreases p65 acetylation.
We next examined the effect of RSV on E-selectin and ICAM-1 expression in human umbilical vein endothelial cells (HUVEC). Cells were pre-treated with RSV/Vehicle, stimulated with LPS and E-selectin and ICAM-1 mRNA (mean ± SEM) expression were studied. We show that RSV treatment attenuates E-selectin (Figure 4A) and ICAM-1 (Figure 4B) expression with LPS stimulus compared to Vehicle (* p<0.05 vs. Vehicle + LPS).
Next we studied the effect of RSV treatment on p65 acetylation in endothelial cells with and without LPS stimulus. As shown in the representative WB blots in Figure 4C, there was reduction in AC-p65 expression in HUVEC with RSV in a dose dependent manner.
SIRT-1 is known to deacetylate p6525. So we further tested whether RSV treatment in HUVEC modulates acetylated p65 expression. As shown in Figure 4C, there was a dose dependent decrease in Ac-p65 in HUVEC treated with RSV.
Resveratrol increases SIRT-1 expression in intestinal tissue in obese mice with sepsis
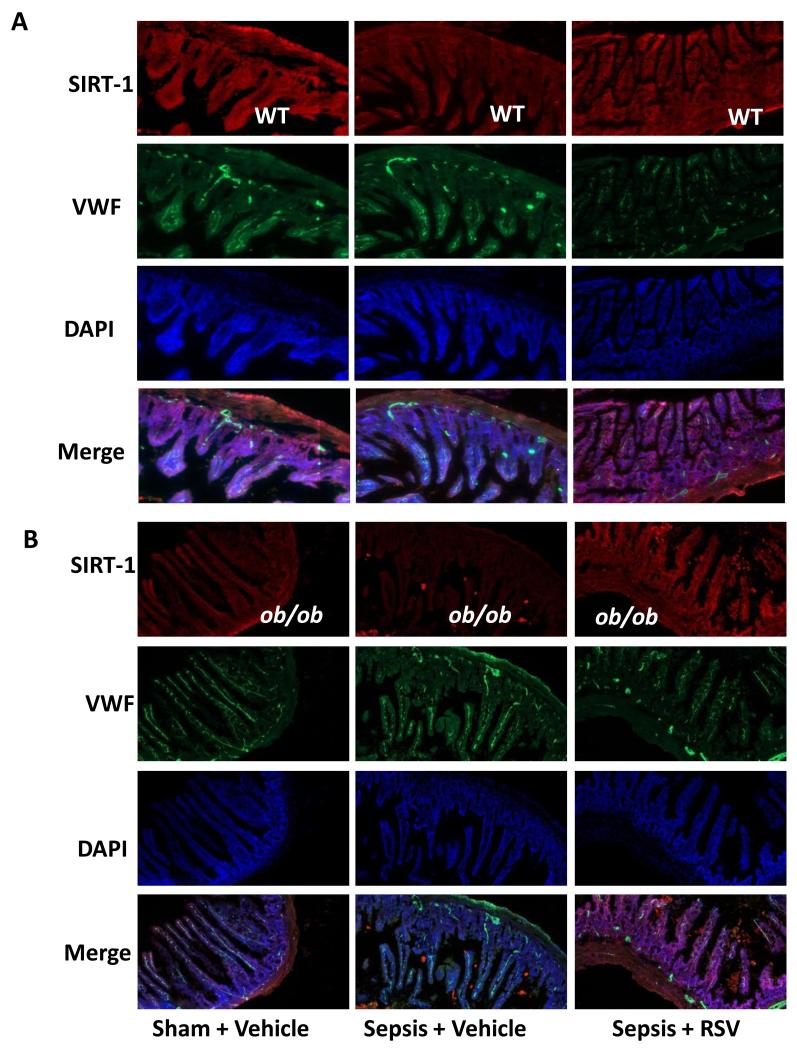
The beneficial effects of RSV are attributed to its ability to increase SIRT-1 expression in SIRT-1 deficient states such as aging and obesity14,25-27. The next experiments are designed to confirm (1) ob/ob as a SIRT-1 depleted state in mouse intestine and (2) the inverse relationship between SIRT-1 and E-selectin/ICAM-1 expression. We confirmed decreased SIRT-1 expression in the intestine of ob/ob mice compared to WT Sham and observed that SIRT-1 decreases with sepsis in both, WT (Figure 5A) and ob/ob mice (Figure 5B). Consistent with our results in HUVEC, we also found that RSV treated mice had increased SIRT-1 expression in the small intestine in both, ob/ob and WT mice with sepsis.
Figure 5. RSV increases SIRT-1 expression in small intestinal tissue.
SIRT-1 expression in small intestinal tissue sections was studied using immunohistochemistry (20× magnification) in WT (Figure 5A) and ob/ob (Figure 5B) mice. There was a lower SIRT-1 expression in ob/ob vs. WT Sham+Vehicle groups. SIRT-1 expression in Sepsis + Vehicle group was significantly decreased vs. Sham + Vehicle group in both, WT and ob/ob strains. Sepsis + RSV groups had significantly increased SIRT-1 expression compared to Sepsis + Vehicle in both WT and ob/ob strains.
Resveratrol decreases microvascular inflammation in obese septic mice via SIRT-1 modulation
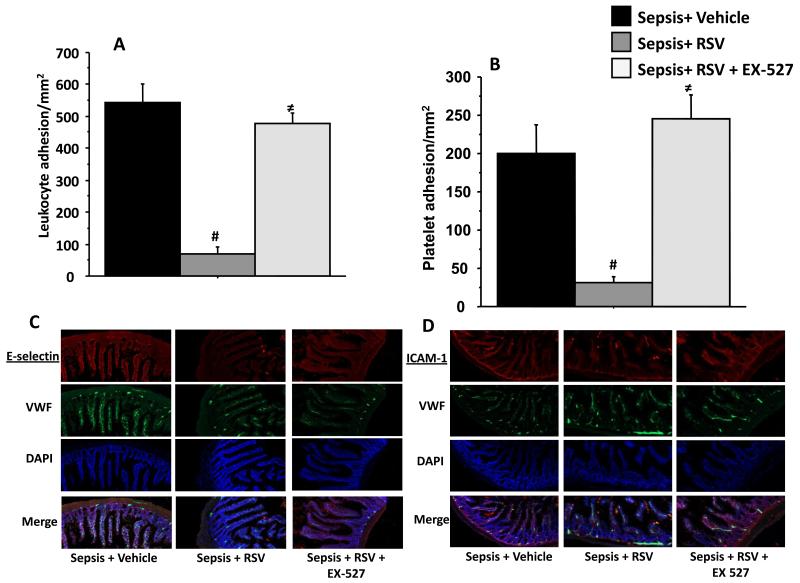
To confirm that decreased leukocyte adhesion and E-selection expression in ob/ob mice is dependent on increased SIRT-1 expression we treated ob/ob mice with both, RSV and a SIRT-1 specific inhibitor EX-527. As shown in Figure 6A and B, the attenuation of leukocyte and platelet adhesion seen in RSV treated ob/ob mice with sepsis was completely abolished by the addition of EX-527. This supports that RSV acts to decrease cell adhesion in ob/ob septic mice through increased SIRT-1 expression.
Figure 6. RSV decreases leukocyte adhesion in obese septic mice via SIRT-1 modulation.
RSV treated ob/ob mice with sepsis were treated with SIRT-1 specific inhibitor EX-527 and leukocyte and platelet adhesion were studied in small intestinal microcirculation using IVM and compared to Sepsis + Vehicle (black bars) and Sepsis + RSV (dark grey) treated groups. Leukocyte (Figure 6A) and platelet adhesion (Figure 6B) in Sepsis + RSV + EX-527 (light grey) groups were significantly increased compared to Sepsis + RSV group. There was no statistically significant difference in leukocyte and platelet adhesion between Sepsis + Vehicle vs. Sepsis +RSV + EX-527 group. # p<0.05 vs. Sepsis + Vehicle; ≠ p<0.05 vs. Sepsis + RSV. We next studied the E-selectin and ICAM-1 expression using immunohistochemistry (20× magnification) in Sepsis + Vehicle, Sepsis + RSV and Sepsis + RSV + EX-527 treated ob/ob mice with sepsis. As shown in Figure 6C and D, E-selectin and ICAM-1 expression in Sepsis + RSV + EX-527 treated mice were increased compared to Sepsis + RSV respectively.
We then determined the contribution of SIRT-1 dependent adhesion molecule expression to this increase in leukocyte/platelet adhesion in Sepsis + RSV + EX-527 compared to Sepsis + RSV. We studied E-selectin and ICAM-1 expression in the small intestinal tissue. As shown in Figure 6 C and D, we found that in ob/ob mice, the E-selectin and ICAM-1 expression in Sepsis + RSV + EX-527 were increased compared to Sepsis + RSV although not to the same extent as Sepsis + Vehicle groups. The data from Figure 6 suggest that RSV attenuates microvascular inflammation via modulation of SIRT-1.
Taken together, our data confirm and extend an observation that obesity is a SIRT-1 deficient state. We found that ob/ob mice have decreased intestinal SIRT-1 and increased microvascular inflammation compared to WT mice with sepsis. Conversely, increased SIRT-1 using RSV treatment attenuated microvascular inflammation via E-selection/ICAM-1 expression ob/ob-septic mice.
RSV improves survival in obese septic mice
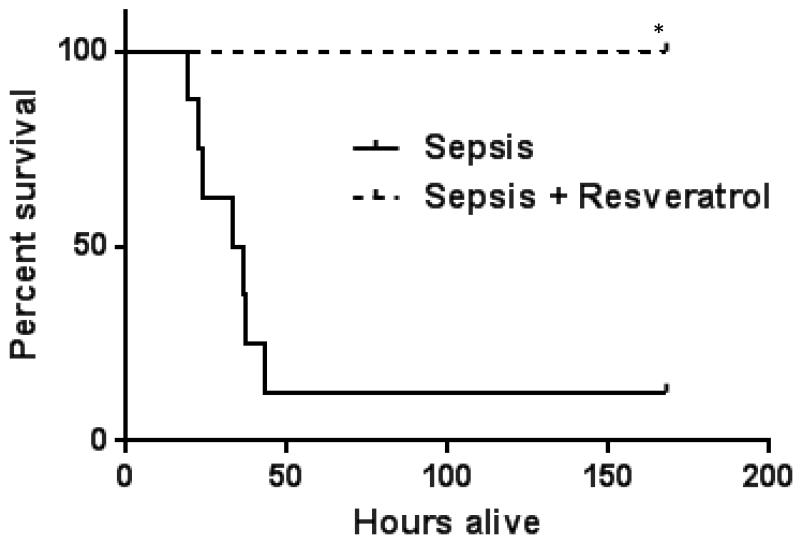
Given decreased adhesion/adhesion molecule expression and microvascular inflammation with RSV treatment, we then determined whether RSV pre-treatment would affect 7-day survival in ob/ob mice with sepsis. As shown in Figure 7, in ob/ob mice treated with RSV had 100% survival compared to 12.5% survival in vehicle treated mice.
Figure 7. RSV improves survival in obese septic mice.
We treated ob/ob septic mice with either Vehicle or RSV and studied 7-day survival. As shown in Figure 7, in ob/ob mice Sepsis+ RSV treated mice had significantly higher survival compared to Sepsis+ Vehicle group. * p<0.05 vs. Sepsis.
DISCUSSION
This study improves our understanding of the role of NAD+ sensor SIRT-1 in sepsis-induced inflammation, with focus on the microvasculature. The major findings include: 1) RSV acts by inducing SIRT-1 to limit the degree of leukocyte-platelet adhesion in the small intestine of septic ob/ob and WT mice without significant differences in the circulating leukocyte/PMN counts, 3) This microvascular response is associated with decreased expression of E-selectin and ICAM-1 via decreased Ac-p65 expression in endothelial cells 3) The effect of RSV in sepsis, at least in part, is by inducing SIRT-1 activity and 4) RSV pre-treatment markedly improves survival in ob/ob mice with sepsis.
Sepsis is the 11th leading cause of death in US with high cost of care in the non-coronary intensive care units28,29. Microvasculature with its strategic place at the interface of systemic circulation and local tissue environment is highly sensitive to changes in both, local and systemic inflammation. Leukocyte adhesion is a rate determining factor in inflammatory responses including sepsis. Not surprisingly, endothelial cell activation markers, especially adhesion molecule expression biomarkers, like E-selectin, directly correlate with mortality in sepsis30. We have studied leukocyte and platelet adhesion in early and late phases of sepsis and shown that increased leukocyte adhesion in early sepsis is followed by endotoxin tolerance in the microcirculation in late sepsis12. We have also shown that the leukocyte and platelet adhesion are exaggerated in obese compared to lean mice with early sepsis6. The results presented here suggest that leukocyte adhesion in the microvascular circulation can be modulated to improve sepsis outcomes.
Obesity increases resource utilization and cost of care in critically ill patients10,11. Obesity is a SIRT-1 deficient state14. Recently, we discovered that SIRT-1 genetic deficiency or pharmacologic inhibition SIRT-1 during early sepsis exaggerates leukocyte adhesion via increased E-selectin and ICAM-1 expression and decreases sepsis survival in mice12. We also have shown previously that the E-selectin and ICAM-1 antibody administration abolishes leukocyte adhesion in microcirculation in our previous work6,24. While RSV is shown to reduce chronic inflammatory phenotype in obesity17, the effect of RSV in acute inflammation in obese subjects is not very well known. Moreover, the effect of RSV on microvascular dysfunction in sepsis is also not very well studied. To begin to address this knowledge gap, we studied the effect of RSV induced increase in SIRT-1 expression in SIRT-1 deficient ob/ob mice with acute inflammation of sepsis. We show that RSV pre-treatment decreases circulating cell-endothelial cell interaction in the microcirculation via modulation of E-selectin and ICAM-1 expression.
To further examine the effect of RSV on endothelial cells specifically, we treated HUVEC with RSV and studied E-selectin, ICAM-1 and SIRT-1 expression in response to LPS stimulus. Similar to intestinal tissue, we show inverse relationship between RSV induced SIRT-1 and adhesion molecule expression in HUVEC.
The anti-inflammatory action of RSV in vivo may be due to either increased expression of SIRT-1 or AMPK. To further study the contribution of increased SIRT-1 in decreasing cell adhesion and adhesion molecule expression we inhibited SIRT-1 with EX-527 in RSV pre-treated ob/ob septic mice. EX-527 is known to be a selective sirtuin inhibitor31. We show that the attenuation of leukocyte and platelet adhesion seen in RSV treated ob/ob mice with sepsis were completely abolished by the addition of EX-527 via modulation of E-selectin and ICAM-1 expression (Figure 6). Together these data support the inverse relationship between SIRT-1 and E-selectin expression in ob/ob septic mice.
Sir2 gene was first linked to replicative lifespan of budding yeast as a nuclear regulator of heterochromatin, and it acts by sensing nicotinamide adenine dinucleotide (NAD+) and deacetylating nearby histones32. There are seven mammalian homologues of Sir2 proteins. Of these, SIRT-1 is studied most extensively. SIRT-1 is a critically important director of the innate immune response, as well as the metabolic shifts associated with acute and chronic inflammation33-35. As a chromatin modifier, SIRT-1 directs many cell and organism processes. One clearly established effect on inflammation is its connection with the NFkB, where it controls RelA transactivation properties by deacetylating lysine 310, thereby deactivating and degrading this critically important gene activator. Among the many genes whose transcription is supported by RelA and its control by SIRT-1 are proinflammatory cytokines like TNF-α and IL-1 ß, as well as adhesion regulators, including E-selectin12,36
The SIRT-1 concept also applies to human inflammation37,38. RSV, a known anti-inflammatory and anti-cancer agent increases SIRT-1 expression, which limits the transactivation of RelA (p65) 26,27,32. One plausible contributing mechanism for our observation that RSV protects microvascular proinflammatory adhesion responses in septic mice is by limiting RelA activity and reducing expression of E-selectin/ ICAM-1. Moreover, E-selectin/ ICAM-1 induction would be amplified in ob/ob mice with its association with low SIRT-1 activity and further decreases in early sepsis as noted in this study. We examined the mechanism responsible for the “anti-inflammatory” effect of RSV in endothelial (HUVEC) in this project. Specifically, we studied acetylated p65 expression in HUVEC in response to LPS and showed that RSV, in a dose-dependent manner, decreased Ac-p65 and adhesion molecule expression in HUVEC stimulated with LPS. Other mechanisms likely contribute, such as limiting the systemic inflammation induced by amplified expression of proinflammatory cytokines, like TNF-alpha-induced acetylation of NF-kappa B p6525 and increased AMPK activity15,39. Although p65 acetylation has an established role in acute inflammation, additional effects of RSV on histone and/or chromatin modification need further study.
Accumulating evidence supports SIRT-1 as a critically important regulator of immune and metabolic homeostasis40. In contrast to the effects of low SIRT-1 on early inflammation, as demonstrated by this study, late sepsis is associated with persistent elevations in NAD+ and SIRT-112,38. Sustained SIRT-1 activity due to unknown mechanisms contributes to the high mortality of late sepsis, as demonstrated by our recent report that inhibiting SIRT-1 by EX-527 reverses immune suppression and rescues septic mice from death12. More studies are needed to better understand how SIRT-1activity can be targeted to treat chronic and acute inflammatory diseases. In the case of sepsis, this is a critical need, since no such treatments are available and deaths are rising. This study supports a preventive approach to improving sepsis outcomes, especially in at risk obese or elderly patients, by modifying inflammation at the microvascular interface.
Acknowledgments
FUNDING SOURCES:
This work was supported by the following NIH grants: Vidula T. Vachharajani: 1) K08GM086470, 2) R01GM099807 Charles E McCall: 1) R01AI065791, 2) R01AI079144
Footnotes
CONFLICT OF INTEREST: The authors have no conflict of interest.
Author Contribution: Concept and design: VTV, BKY and CEM; Data generation: XW, NLB, VTV; Analysis and interpretation: XW, BKY, VTV, CEM; Generating the manuscript: XW, VTV, CEM and BKY.
REFERENCES
- 1.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Critical care medicine. 2007;35:1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 2.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood KA, Angus DC. Pharmacoeconomic implications of new therapies in sepsis. PharmacoEconomics. 2004;22:895–906. doi: 10.2165/00019053-200422140-00001. [DOI] [PubMed] [Google Scholar]
- 4.Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. The Journal of clinical investigation. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer G, Stokes KY, Terao S, Granger DN. Sepsis-induced intestinal microvascular and inflammatory responses in obese mice. Shock. 2009;31:275–279. doi: 10.1097/SHK.0b013e3181834ab3. [DOI] [PubMed] [Google Scholar]
- 6.Vachharajani V, Russell JM, Scott KL, et al. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12:183–194. doi: 10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- 7.Vachharajani V, Vital S, Russell J. Modulation of circulating cell-endothelial cell interaction by erythropoietin in lean and obese mice with cecal ligation and puncture. Pathophysiology: the official journal of the International Society for Pathophysiology /ISP. 2010;17:9–18. doi: 10.1016/j.pathophys.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 9.Arabi YM, Dara SI, Tamim HM, et al. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Critical care. 2013;17:R72. doi: 10.1186/cc12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prescott HC, Chang VW, O’Brien JM, Jr., Langa KM, Iwashyna T. Obesity and 1-Year Outcomes in Older Americans With Severe Sepsis. Critical care medicine. 2014 Apr 8; doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Critical care medicine. 2008;36:151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 12.Vachharajani VT, Fu Liu T, Brown CM, et al. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. Journal of leukocyte biology. 2014 Jul 7; doi: 10.1189/jlb.3MA0114-034RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moschen AR, Wieser V, Gerner RR, et al. Adipose tissue and liver expression of SIRT1, 3, and 6 increase after extensive weight loss in morbid obesity. Journal of hepatology. 2013;59:1315–1322. doi: 10.1016/j.jhep.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen MM, Jorgensen JO, Jessen N, Richelsen B, Pedersen SB. Resveratrol in metabolic health: an overview of the current evidence and perspectives. Annals of the New York Academy of Sciences. 2013;1290:74–82. doi: 10.1111/nyas.12141. [DOI] [PubMed] [Google Scholar]
- 15.Guo H, Chen Y, Liao L, Wu W. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2013;27:189–198. doi: 10.1007/s10557-013-6442-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu LQ, Fan ZQ, Tang YF, Ke ZJ. The resveratrol attenuates ethanol-induced hepatocyte apoptosis via inhibiting ER-related caspase-12 activation and PDE activity in vitro. Alcoholism, clinical and experimental research. 2014;38:683–693. doi: 10.1111/acer.12311. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Zorita S, Fernandez-Quintela A, Lasa A, Hijona E, Bujanda L, Portillo MP. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition. 2013;29:1374–1380. doi: 10.1016/j.nut.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Li T, Zhang J, Feng J, et al. Resveratrol reduces acute lung injury in a LPSinduced sepsis mouse model via activation of Sirt1. Molecular medicine reports. 2013;7:1889–1895. doi: 10.3892/mmr.2013.1444. [DOI] [PubMed] [Google Scholar]
- 19.Yang SJ, Lim Y. Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation. Metabolism: clinical and experimental. 2014 Feb 11; doi: 10.1016/j.metabol.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Holthoff JH, Wang Z, Seely KA, Gokden N, Mayeux PR. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney international. 2012;81:370–378. doi: 10.1038/ki.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolgazi M, Sener G, Cetinel S, Gedik N, Alican I. Resveratrol reduces renal and lung injury caused by sepsis in rats. The Journal of surgical research. 2006;134:315–321. doi: 10.1016/j.jss.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Does endotoxin play a major role in inducing the depression of macrophage function during polymicrobial sepsis? Archives of surgery. 1995;130:1178–1184. doi: 10.1001/archsurg.1995.01430110036007. discussion 1184-1175. [DOI] [PubMed] [Google Scholar]
- 23.Tailor A, Granger DN. Hypercholesterolemia promotes P-selectin-dependent platelet-endothelial cell adhesion in postcapillary venules. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:675–680. doi: 10.1161/01.ATV.0000056742.97580.79. [DOI] [PubMed] [Google Scholar]
- 24.Vachharajani V, Cunningham C, Yoza B, Carson J, Jr., Vachharajani TJ, McCall C. Adiponectin-deficiency exaggerates sepsis-induced microvascular dysfunction in the mouse brain. Obesity. 2012;20:498–504. doi: 10.1038/oby.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin QQ, Yan CF, Lin R, et al. SIRT1 regulates TNF-alpha-induced expression of CD40 in 3T3-L1 adipocytes via NF-kappaB pathway. Cytokine. 2012;60:447–455. doi: 10.1016/j.cyto.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Camins A, Sureda FX, Junyent F, et al. Sirtuin activators: designing molecules to extend life span. Biochimica et biophysica acta. 2010;1799:740–749. doi: 10.1016/j.bbagrm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Costa Cdos S, Rohden F, Hammes TO, et al. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARgamma1-3 mRNA expression in human visceral adipocytes. Obesity surgery. 2011;21:356–361. doi: 10.1007/s11695-010-0251-7. [DOI] [PubMed] [Google Scholar]
- 28.Hoyert DL, Xu J. Deaths: preliminary data for 2011. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012;61:1–51. [PubMed] [Google Scholar]
- 29.Eber MR, Laxminarayan R, Perencevich EN, Malani A. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Archives of internal medicine. 2010;170:347–353. doi: 10.1001/archinternmed.2009.509. [DOI] [PubMed] [Google Scholar]
- 30.Skibsted S, Jones AE, Puskarich MA, et al. Biomarkers of endothelial cell activation in early sepsis. Shock. 2013;39:427–432. doi: 10.1097/SHK.0b013e3182903f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gertz M, Fischer F, Nguyen GT, et al. Ex-527 inhibits Sirtuins by exploiting their unique NAD+-dependent deacetylation mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2772–2781. doi: 10.1073/pnas.1303628110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinclair DA, Guarente L. Small-molecule allosteric activators of sirtuins. Annual review of pharmacology and toxicology. 2014;54:363–380. doi: 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cellular signalling. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Liu TF, Brown CM, El Gazzar M, et al. Fueling the flame: bioenergy couples metabolism and inflammation. Journal of leukocyte biology. 2012;92:499–507. doi: 10.1189/jlb.0212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, Zhang W, Pan H, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PloS one. 2012;7:e46364. doi: 10.1371/journal.pone.0046364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceolotto G, De Kreutzenberg SV, Cattelan A, et al. Sirtuin 1 stabilization by HuR represses TNF-alpha- and glucose-induced E-selectin release and endothelial cell adhesiveness in vitro: relevance to human metabolic syndrome. Clinical science. 2014;127:449–461. doi: 10.1042/CS20130439. [DOI] [PubMed] [Google Scholar]
- 37.Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. The Journal of biological chemistry. 2012;287:25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. The Journal of biological chemistry. 2011;286:9856–9864. doi: 10.1074/jbc.M110.196790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubota S, Ozawa Y, Kurihara T, et al. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Investigative ophthalmology & visual science. 2011;52:9142–9148. doi: 10.1167/iovs.11-8041. [DOI] [PubMed] [Google Scholar]
- 40.Vachharajani V, Liu T, McCall CE. Epigenetic coordination of acute systemic inflammation: potential therapeutic targets. Expert review of clinical immunology. 2014 Aug 4;:1–10. doi: 10.1586/1744666X.2014.943192. [DOI] [PMC free article] [PubMed] [Google Scholar]