Abstract
Estrogen metabolites are important biomarkers to evaluate cancer risks and metabolic diseases. Due to their low physiological levels, a sensitive and accurate method is required, especially for the quantitation of unconjugated forms of endogenous steroids and their metabolites in humans. Here, we evaluated various derivatives of estrogens for improved analysis by orbitrap LC/MS in human serum samples. A new chemical derivatization reagent was applied modifying phenolic steroids to form 1-methylimidazole-2-sulfonyl adducts. The method significantly improves the sensitivity 2–100 fold by full scan MS and targeted selected ion monitoring MS over other derivatization methods including, dansyl, picolinoyl, and pyridine-3-sulfonyl products.
Keywords: Estrogen metabolites, Orbitrap MS, Dansyl chloride, 1-Methylimidazole-2-sulfonyl chloride
1. Introduction
Circulating sex steroids, including endogenous estrogens and androgens, exert strong biological effects at very low concentrations. Estrogens and their catechol metabolites have been linked to DNA adduct formation and the development of different types of cancer including reproductive cancers [1,2]. The metabolism of estrone (E1) and estradiol (E2) is of particular interest since products of the 2-, 4-, and 16-hydroxylation pathway may indicate risk for breast and other cancers [3]. Additional steroid hormones, including testosterone (T) and progesterone (Prog) are also important markers of cancer risk or modulators of membrane receptors and can indicate metabolic disorders [4–6]. A highly sensitive and specific method for the determination of these hormones is therefore required for disease diagnosis and prevention as well as for cancer epidemiology studies.
Challenges in measuring endogenous hormones from biological fluids include their low physiologic concentrations and interference from complex matrices. In urine and plasma, steroids are found mostly conjugated as glucuronides, sulfates, and/or mixtures thereof, and if unconjugated then occur mostly bound to carrier proteins while unbound or “free” concentrations are extremely low. The analysis of unconjugated and particularly unbound estrogens in biological fluids is therefore quite difficult. Traditionally, endogenous steroids were measured by gas chromatography-mass spectrometry (GC/MS) [7]. Although sensitive, GC/MS requires large sample volumes and labor-intensive sample preparation procedures. The widely used immunoassays provide a simple and fast alternative, but the lack of desired specificity and sensitivity hinders their applications [8]. Currently, liquid chromatography-mass spectrometry (LC/MS) methods are preferred for steroid analyses due to vast improvements in sensitivity and selectivity achieved by newer mass spectrometers and by chemical derivatization that leads to better sensitivity compared to underivatized analytes [9,10]. Numerous methods have been developed to chemically modify steroids with an easily ionizable or pre-charged tag [11] to produce a form of the analyte that increases the signal/noise ratios during MS analysis. Many derivatizing reagents have been used in different laboratories to improve detection sensitivity, including dansyl [10,12], picolinoyl ester [13], pyridine sulfonyl [14], and N-methyl-nicotinyl derivatives [15,16].
In order to further evaluate the role of unconjugated sex steroids on cancer risk in postmenopausal women, we sought to develop a more sensitive, specific, simple and efficient method for their measurement. To this end, we tried to improve the sensitivity of our current steroid method by applying LC/MS using a recently introduced high-resolution orbitrap-based mass spectrometer (model Q Exactive) and the application of various derivatization methods for the quantitation of 18 sex steroids and their metabolites.
2. Experimental
2.1. Materials
Steroids standards estrone (E1), estradiol (E2), estriol (E3), 16α-hydroxyestrone (16αOH-E1), 16-epiestriol (16epi-E3), 17-epiestriol (17epi-E3), 16-ketoestradiol (16keto-E2), 2-hydroxyestrone (2OH-E1), 2-methoxyestrone (2MeO-E1), 2-methoxyestradiol (2MeO-E2), 2-hydroxy-3-O-methylestrone (2OH-3MeO-E1), 4-hydroxyestrone (4OH-E1), 4-hydroxyestradiol (4OH-E2), 2-hydroxyestradiol (2OH-E2), 4-methoxyestrone (4MeO-E1), 4-methoxyestradiol (4MeO-E2) were purchased from Steraloids (Newport, Rhode Island). Testosterone (T), and progesterone (Prog) were purchased from Sigma–Aldrich (St. Louis, MO). Deuterated internal standard: E1-d4, E2-d5, 16α-OHE1-d3, 4OH-E2-d5, 2MeO-E2-d5, T-d3, E3-d4, 2OHE1-13C6, 2MeOE1-13C6, 4OHE1-13C6, 2OHE2-13C6, and Prog-13C2, were purchased from Steraloids (Newport, Rhode Island). Dansyl chloride, 2-methyl-6-nitrobenzoic anhydride, 4-dimethylaminopyridine, picolinic acid, anhydrous pyridine, 1-methylimidazole-2-sulfonyl chloride, 2-(1H-1,2,4-triazol-1-yl)-3-pyridinesulfonyl chloride were purchased from Sigma–Aldrich. Pyridine-3-sulfonyl chloride was obtained from Matrix Scientific (Columbus SC). All solvents of LC/MS grade were purchased from Fisher Scientific (Pittsburgh, PA).
2.2. Stock and working standard solutions
Standard stock solutions were prepared in methanol containing the 18 compounds at a concentration of 10 ng/ml, including, T, Prog, E1, 2OH-3MeO-E1, 2MeO-E1, 4MeO-E1, 4OH-E1, 2OH-E1, 16αOH-E1, E2, 16keto-E2, 2MeO-E2, 4MeO-E2, 2OH-E2, 4OH-E2, E3, 16epi-E3, 17epi-E3. Internal standard solutions were prepared in methanol at 20 ng/ml containing 12 isotope-labeled compounds, including E1-d4, E2-d5, 4OHE2-d5, 2MeoE2-d5, 16α-OH-E1-d3, T-d3, E3-d4, 2OHE1-13C6, 2MeoE1-13C6, 4OHE1-13C6, 2OHE2-13C6, Prog-13C2. An eight-point working standard was made by serial dilution from stock solutions in methanol covering a range from 0.1 pg/mL to 3000 pg/mL. 150 μL of calibration standard and 10 μL of internal standard solution were combined, dried under nitrogen, and subjected to derivatization.
2.3. Serum sample preparation (unconjugated forms)
Commercial pooled normal human serum (Innovative, Novi, MI) was used as quality control samples. No estrogen metabolites were detected in this serum except E1 and 2OHE1 at low levels (data not shown). The serum samples were prepared to identify unconjugated forms of steroids therefore no enzymatic hydrolysis was applied [17]. 100 μL of quality control serum were mixed with 10 μL of internal standard mixture. Spiked serum was prepared by adding 15 μL of 100 pg/mL and 1000 pg/mL standard mixtures to arrive at a final concentration of 10 pg/mL and 100 pg/mL, respectively in the derivatized mixture. The unconjugated steroids were extracted from serum with 1.5 mL methyl tert-butyl ether (MtBE) by vortexing at 1800 rpm for 2 min. The upper organic layers were transferred to HPLC vials after centrifugation (2500 rpm, 5 min), followed by evaporation under nitrogen and chemical derivatization.
2.4. Derivatization with dansyl chloride (DSCl)
Dansyl derivatization was carried out according to our previous method [17,18]. To the dried serum sample or standards, 75 μL of aq. sodium bicarbonate (100 mM, pH 9) and 75 μL of dansyl chloride (3 mg/mL in acetone) were added. This mixture was capped and incubated at 65 °C for 15 min. After cooling the reaction mixture was transferred to HPLC inserts and subjected to LC/MS analysis.
2.5. Derivatization with picolinic acid (P)
Picolinoyl derivatization was carried out either using picolinoyl chloride (PCl) or a picolinic acid condensation mixture (Scheme 1) [13,19]. We applied the latter since the use of a suspension of PCl was difficult to aliquot. The chemical reaction was carried out according to a previous method [13,19] with modifications. We compared liquid–liquid extraction with solid phase extraction during sample cleanup, and found better recovery with liquid–liquid extraction. In brief, to the dried sample, we added 100 μL of freshly prepared condensation mixture (containing 100 mg 2-methyl-6-nitrobenzoic anhydride, 30 mg 4-dimethylaminopyridine, and 80 mg picolinic acid in 1.5 mL anhydrous pyridine) and 20 μL of triethylamine. The mixture was heated at 80 °C for 30 min. After incubation, the reaction was stopped by adding 300 μL of 2% aq. HCl, and extracted with 1.5 mL MtBE. The upper organic layer was separated, dried and redissolved in 150 μL of 0.1% formic acid in MeOH/H2O (1/1).
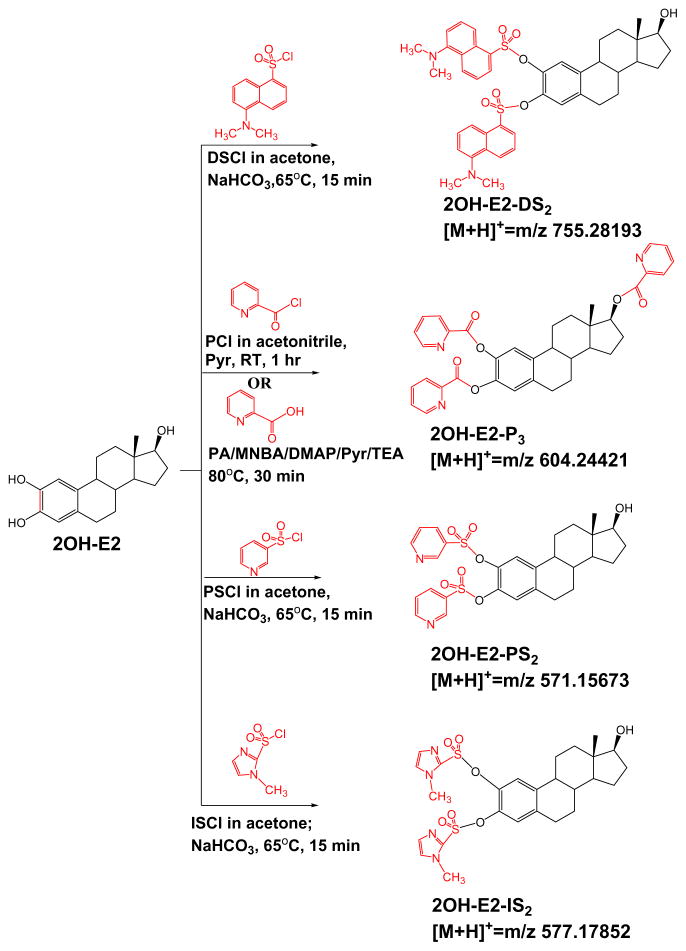
Scheme 1.
Schematic showing the derivatization reactions of 2OH-E2 with dansyl chloride (DSCl), picolinoyl chloride (PCl) or picolinic acid (PA) condensation mixture, pyridine-3-sulfonyl chloride (PSCl), and 1-methylimidazole-2-sulfonyl chloride (ISCl) DSCl: dansyl chloride; PCl: picolinoyl chloride; RT: room temperature; PA: picolinic acid; MNBA: 2-methyl-6-nitrobenzoic anhydride; DMAP: 4-dimethylaminopyridine; Pyr: pyridine; TEA: triethylamine; PSCl: pyridine-3-sulfonyl chloride; ISCl: 1-methylimidazole-2-sulfonyl chloride.
2.6. Derivatization with pyridine-3-sulfonyl chloride (PS)
Pyridine-3-sulfonyl derivatization was carried out in the same fashion as the dansyl derivatization. To the dried sample or working standards, we added 75 μL aq. sodium bicarbonate (100 mM, pH 9) and 75 μL of pyridine-3-sulfonyl chloride (3 mg/mL in acetone). The mixture was capped and incubated at 65 °C for 15 min. After cooling, the reaction mixture was transferred to HPLC inserts and subjected to LC/MS analysis.
2.7. Derivatization with 1-methylimidazole-2-sulfonyl chloride (IS)
Derivatization with 1-methylimidazole-2-sulfonyl chloride was carried out in the same fashion as dansyl chloride. To the dried sample or working standards, we added 75 μL of aq. sodium bicarbonate (100 mM, pH 9) and 75 μL of 1-methylimidazole-2-sulfonyl chloride (3 mg/mL in acetone). The mixture was capped and incubated at 65 °C for 15 min. After cooling the reaction mixture was transferred to HPLC inserts and subjected to LC/MS analysis.
2.8. Liquid chromatography and mass spectrometry
The analysis was carried out on a Q Exactive orbitrap mass spectrometer coupled to a model Accela ultra-HPLC system (both from Thermo Electron, Waltham, MA). 25 μL of the derivatized product were injected with a HTC Pal autosampler (Leap Technologies, Carrboro, NC) onto a Ascentis Express C18 column (3.0 × 150 mm, 2.7 μm, Supelco) using a mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). For the separation of dansyl derivatives, the following linear gradient A/B (v/v) was used: 60/40–20/80 from 0 to 20 min, hold at 20/80 for 10 min, then back to initial conditions 60/40 for 5 min at a flow rate of 800 μL/min (total run time of 35 min). The analysis of picolinoyl derivatives was carried out at a flow rate of 500 μL/min using a linear gradient of 60/40 (A/B) to 20/80 (A/B) in 15 min, hold at 20/80 for 5 min then back to initial conditions (60/40) in 5 min and equilibrate for 5 min (total run time of 30 min.). Pyridine sulfonyl derivatives were eluted at a flow rate of 500 μL/min with the following gradient; 70/30–40/60 from 0 to 10 min, hold at 40/60 for 10 min, then back to initial conditions 70/30 for 5 min (total run time of 25 min.). 1-Methylimidazole-2-sulfonyl derivatives were eluted at a flow rate of 500 μL/min with the following gradient; 65/35–30/70 from 0 to 15 min then back to initial 65/35 and equilibrate for 5 min (total run time of 20 min.).
Mass detection was carried out in a positive electrospray ionization (ESI) mode. The settings of the mass spectrometer were as follows: spray voltage 4.5 kV, capillary temperature 350 °C, maximum injection time 100 ms and scan range 150–1000. Nitrogen was used as sheath gas (pressure 30 units) and auxiliary gas (pressure 5 units). The in-source collision induced dissociation energy (CID) was set at 5 eV to dissociate dimers or sodium adducts, and the automatic gain control (AGC) was set at 1e6. MS analysis were performed by either full scan or targeted selected ion monitoring (tSIM) mode. tSIM MS scans the targeted masses in different time segments, which was divided by the retention times of the analytes. Data acquisition and analysis were performed using Thermo Electron Xcalibur software. Detection of the analyte was set within 5 ppm of the theoretical mass.
3. Results and discussion
Several derivatization methods have been previously reported to improve LC/MS analysis of estrogens. Among these methods, dansyl (DS) derivatization is widely used and results in limits of detection (LOD) of 8 pg/mL for E2 and E1 by ESI using SRM with a TSQ triple quadrupole MS [17]. This method follows the initial report by Nelson et al. [20] and is simple, fast and quantitative, and only phenolic alcohols react to a product containing the easily ionizable DS tag. Since these DS derivatives are produced under mild reaction conditions, other molecules containing the sulfonyl chloride moiety and a proton-affinity group were investigated. Previously other heterocycle-containing sulfonyl chlorides including pydrine-3-sulfonyl chloride, 1,2-dimethylimidazole-4-sulfonyl chloride, and 4-(1H-pyrazol-1-yl)benzenesulfonyl chloride were applied for derivatizing estrogens. Among them, the derivatives of pyridine-3-sulfonyl chloride appears to be more diagnostically valuable by showing estrogen-specific fragmentations by ESI MRM using triple quadrupole MS with a LOD of 10 pg/mL for E2 [14]. A more sensitive picolinoyl derivatization approach with a claimed LOD of 0.5 pg/mL and 1.0 pg/mL for E2 and E1, respectively, was proposed using ESI-SRM [13,21]. We followed up on these leads by performing them and other chemical modifications as shown in Scheme 1 for 2OHE2 as starting material including the resulting product ions for MS analysis.
For the quantification of unconjugated steroids in serum in postmenopausal women we intended to find a more sensitive and specific method because of their extremely low levels. To this end, we evaluated the current methods with a total of 18 steroids including 16 estrogen moieties and metabolites as well as the non-phenolic testosterone and progesterone, and 12 isotope-labeled internal standards, and compared the sensitivity of dansyl (DS), pyridine-3-sulfonyl (PS), and picolinoyl (P) derivatives in standards using a high-resolution quadrupole-orbitrap MS. To our knowledge, this is the first report of a direct comparison of these methods in the same laboratory. The chemical modifications by dansyl and pyridine-3-sulfonyl chloride were carried out according to previous reports [17,18,20]. Dried extracts were treated with sulfonyl chloride and sodium bicarbonate in a simple one-step reaction, and the resulting reaction mixture was directly subjected to LC/MS analysis without further purification. The derivatization with the picolinoyl reagent, on the other hand, involves more steps, and requires the use of freshly prepared and highly concentrated derivatizing reagents in strictly anhydrous solvents [13]. A purification step is also needed in order to remove the excess of reagents that makes this approach more complicated. Moreover, both phenolic and aliphatic alcohols are modified using this method, which leads to several reaction products that complicate data quantification.
Mass spectrometry analysis was carried out under positive ESI full scan (FS) mode. The most sensitive quantification was achieved using exact masses of the protonated [M+H]+ ions for DS, P, and PS derivatives. Since all tags contain proton-affine groups, the use of 0.1% formic acid in the mobile phase facilitates the formation of pre-charged [M+H]+ ions and hence improves sensitivity. In fact, we found the [M+H]+ ion to be more sensitive than the pre-formed tertiary nitrogen ions in our previous report [22]. Table 1 shows the detailed parameters used for the 18 steroids and 12 internal standards. The calibration curve was linear (R2 > 0.99) in the experimental concentration range from 1 pg/mL to 3000 pg/mL. In addition to full scan (FS), we also examined the measurement in the targeted selected ion monitoring (tSIM) mode to only scan the targeted masses in segments divided by retention times. This provides more specificity and selectivity over the full scan mode. Table 2 shows the results comparing limits of detection (LOD, S/N = 3) of each method using standard solutions. Our results showed that the LODs of DS products were in a range of 2–50 pg/mL by FS MS and 0.5–25 pg/mL by tSIM MS. PS products showed a similar range with 1.9–30 pg/mL by FS and 0.2–15 pg/mL by tSIM. Surprisingly, the LODs of P derivatization were 3–200 pg/mL by FS and significantly higher than the DS and PS products; tSIM for P products were only carried out for selected estrogen metabolites and showed a LOD range of 3–25 pg/mL, 2–20 times higher than DS products. The reason for the low sensitivity using P derivatization may be due to the loss of materials during sample workup procedures to remove excess reagent after derivatization, and it may also be due to incomplete conversion of the starting material especially for compounds that contain multiple phenolic groups. DS products showed better chromatographic separation than PS products, the analytes were eluted between 3 and 24 min, whereas for PS products elution was in a narrow range between 11 and 20 min. The wide range of retention times were more preferable for tSIM MS, as specific scans were conducted in time segments divided by retention times.
Table 1.
Parameters of dansyl (DS), picolinoyl (P), pyridine-3-sulfonyl (PS), and 1-methylimidazole-2-sulfonyl (IS) adducts of steroids using orbitrap mass spectrometry.
| Dansyl (DS)
|
Picolinoyl (P)
|
Pyridine-3-sulfonyl (PS)
|
1-Methylimidazole-2-sulfonyl (IS)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tag | Formula | [M+H]+ m/z | Tag | Formula | [M+H]+ m/z | Tag | Formula | [M+H]+ m/z | Tag | Formula | [M+H]+ m/z | |
| Test | C19H28O2 | 289.21621 | ||||||||||
| Prog | C21H30O2 | 315.23186 | ||||||||||
| E3 | DS | C30H35O5NS | 522.23087 | P3 | C36H33O6N3 | 604.24421 | PS | C23H27O5NS | 430.16827 | IS | C22H28O5N2S | 433.17917 |
| 16epi-E3 | DS | C30H35O5NS | 522.23087 | P3 | C36H33O6N3 | 604.24421 | PS | C23H27O5NS | 430.16827 | IS | C22H28O5N2S | 433.17917 |
| 17epi-E3 | DS | C30H35O5NS | 522.23087 | P3 | C36H33O6N3 | 604.24421 | PS | C23H27O5NS | 430.16827 | IS | C22H28O5N2S | 433.17917 |
| 16ketoE2 | DS | C30H33N5OS | 520.21522 | P2 | C30H28O5N2 | 497.20710 | PS | C23H25O5NS | 428.15262 | IS | C22H26O5N2S | 431.16352 |
| 16α-OHE1 | DS | C30H33N5OS | 520.21522 | P2 | C30H28O5N2 | 497.20710 | PS | C23H25O5NS | 428.15262 | IS | C22H26O5N2S | 431.16352 |
| 2MeOE2 | DS | C31H37O5NS | 536.24652 | P2 | C31H32O5N2 | 513.23840 | PS | C24H29O5NS | 444.18392 | IS | C23H30O5N2S | 447.19482 |
| 4MeOE2 | DS | C31H37O5NS | 536.24652 | P2 | C31H32O5N2 | 513.23840 | PS | C24H29O5NS | 444.18392 | IS | C23H30O5N2S | 447.19482 |
| E2 | DS | C30H35O4NS | 506.23596 | P2 | C30H30O4N2 | 483.22783 | PS | C23H27O4NS | 414.17336 | IS | C22H28O4N2S | 417.18426 |
| E1 | DS | C30H33O4NS | 504.22031 | P | C24H25O3N1 | 376.19072 | PS | C23H25O4NS | 412.15771 | IS | C22H26O4N2S | 415.16861 |
| 3OMeE1 | DS | C31H35O5NS | 534.23087 | P | C25H27O4N | 406.20129 | PS | C24H27O5NS | 442.16827 | IS | C23H28O5N2S | 445.17917 |
| 2MeOE1 | DS | C31H35O5NS | 534.23087 | P | C25H27O4N | 406.20129 | PS | C24H27O5NS | 442.16827 | IS | C23H28O5N2S | 445.17917 |
| 4OMeE1 | DS | C31H35O5NS | 534.23087 | P | C25H27O4N | 406.20129 | PS | C24H27O5N1S1 | 442.16827 | IS | C23H28O5N2S | 445.17917 |
| 2OH-E2 | DS2 | C42H46O7N2S2 | 755.28193 | P3 | C36H33O6N3 | 604.24421 | PS2 | C28H30O7N2S2 | 571.15673 | IS2 | C26H32O7N4S2 | 577.17852 |
| 4OH-E1 | DS2 | C42H44O7N2S2 | 753.26628 | P2 | C30H28O5N2 | 497.20710 | PS2 | C28H28O7N2S2 | 569.14108 | IS2 | C26H30O7N4S2 | 575.16287 |
| 2OH-E1 | DS2 | C42H44O7N2S2 | 753.26628 | P2 | C30H28O5N2 | 497.20710 | PS2 | C28H28O7N2S2 | 569.14108 | IS2 | C26H30O7N4S2 | 575.16287 |
| Test-d3 | C19H25O2-d3 | 292.23504 | ||||||||||
| Prog-13C2 | C19H30O2-13C2 | 317.23856 | ||||||||||
| E3-d4 | DS | C30H31O5NS-d4 | 526.25598 | P3 | C36H29O6N3-d4 | 608.26932 | PS | C23H23O5NS-d4 | 434.19338 | IS | C22H24O5N2S-d4 | 437.20428 |
| 16α-OHE1-d3 | DS | C30H30N5OS-d3 | 523.23405 | P2 | C30H25O5N2-d3 | 500.22593 | PS | C23H22O5NS-d3 | 431.17145 | IS | C22H23O5N2S-d3 | 434.18235 |
| 2-OMe-E2-d5 | DS | C31H32O5NS-d5 | 541.27791 | P2 | C31H27O5N2-d5 | 518.26978 | PS | C24H24O5NS-d5 | 449.21531 | IS | C23H25O5N2S-d5 | 452.22621 |
| E2-d5 | DS | C30H30O4NS-d5 | 511.26734 | P2 | C30H25O4N2-d5 | 488.25922 | PS | C23H22O4NS-d5 | 419.20474 | IS | C22H23O4N2S-d5 | 422.21564 |
| E1-d4 | DS | C30H29O4NS-d4 | 508.24542 | P | C24H21O3N1-d4 | 380.21583 | PS | C23H21O4NS-d4 | 416.18282 | IS | C22H22O4N2S-d4 | 419.19371 |
| 2MeOE1-13C6 | DS | C25H35O5NS-13C6 | 540.25100 | P | C19H27O4N-13C6 | 412.22141 | PS | C18H27O5NS-13C6 | 448.18840 | IS | C17H28O5N2S-13C6 | 451.19930 |
| 2OH-E2-13C6 | DS2 | C36H46O7N2S2-13C6 | 761.30205 | P3 | C30H33O6N3-13C6 | 610.26434 | PS2 | C22H30O7N2S2-13C6 | 577.17685 | IS2 | C20H32O7N4S2-13C6 | 583.19865 |
| 4-OH-E2-d5 | DS2 | C42H41O7N2S2-d5 | 760.31331 | P3 | C36H28O6N3-d5 | 609.27560 | PS2 | C28H25O7N2S2-d5 | 576.18811 | IS2 | C26H27O7N4S2-d5 | 582.20991 |
| 4OH-E1-13C6 | DS2 | C36H44O7N2S2-13C6 | 759.28640 | P2 | C24H28O5N2-13C6 | 503.22722 | PS2 | C22H28O7N2S2-13C6 | 575.16120 | IS2 | C20H30O7N4S2-13C6 | 581.18300 |
| 2OH-E1-13C6 | DS2 | C36H44O7N2S2-13C6 | 759.28640 | P2 | C24H28O5N2-13C6 | 503.22722 | PS2 | C22H28O7N2S2-13C6 | 575.16120 | IS2 | C20H30O7N4S2-13C6 | 581.18300 |
Table 2.
Limit of detection (LOD) of dansyl (DS), picolinoyl (P), pyridine-3-sulfonyl (PS), and 1-methylimidazole-2-sulfonyl (IS) derivatives by orbitrap LC/MS in full scan (FS) and targeted selected ion monitoring (tSIM) mode.
|
DS
|
P
|
PS
|
IS
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tR (min) | LOD (pg/ml) | tR (min) | LOD (pg/ml) | tR (min) | LOD (pg/ml) | tR (min) | LOD (pg/ml) | |||||
| FS | tSIM | FS | tSIM | FS | tSIM | FS | tSIM | |||||
| Test | 3.8 | 13 | 22 | 14.4 | 65 | ND | 11.5 | 3 | 10 | 7.5 | 6.4 | 12 |
| Prog | 9.1 | 26 | 15 | 14.8 | 200 | 18.1 | 11 | 6.6 | 13.8 | 4.5 | 11 | |
| E3 | 10.7 | 3.6 | 2.3 | 13.5 | 6.3 | 5 | 10.8 | 2.9 | 0.2 | 5.2 | 1.9 | 0.8 |
| 16epi-E3 | 13.8 | 2.7 | 1.8 | 13.3 | 7.5 | 4.0 | 12.4 | 2.5 | 0.2 | 7.1 | 1.2 | 0.6 |
| 17epi-E3 | 14.5 | 6.1 | 2.4 | 13.4 | 23 | 5 | 12.9 | 5.3 | 0.2 | 7.7 | 1.8 | 0.9 |
| 16ketoE2 | 13.6 | 3.7 | 2.1 | 12.8 | 46 | 17 | 12.3 | 11 | 0.6 | 7.1 | 1.4 | 0.3 |
| 16α-OHE1 | 13.9 | 6.0 | 3.1 | 13.1 | 78 | 23 | 12.6 | 13 | 0.9 | 7.4 | 2 | 0.4 |
| 2MeOE2 | 17.7 | 8.1 | 0.6 | 16.5 | 16 | ND | 16.7 | 4 | 3 | 11.1 | 1.4 | 0.1 |
| 4MeOE2 | 18.0 | 2.0 | 0.5 | 16.3 | 85 | ND | 16.2 | 20 | 2.4 | 10.5 | 1.6 | 0.1 |
| E2 | 18.2 | 2.0 | 0.6 | 16.5 | 5 | 16 | 16.4 | 6.2 | 11 | 10.7 | 0.9 | 0.4 |
| E1 | 19.8 | 2.8 | 6.4 | 14.0 | 5.3 | 3 | 19.3 | 1.9 | 4.4 | 12.3 | 0.7 | 0.1 |
| 3OMeE1 | 18.3 | 2.4 | 7.5 | 13.8 | 3.2 | ND | 18.2 | 3.3 | 6.4 | 12.3 | ND | ND |
| 2MeOE1 | 19.2 | 13 | 8.6 | 14.1 | 20 | ND | 19.5 | 5.6 | 12 | 12.8 | 2.6 | 0.7 |
| 4OMeE1 | 19.7 | 3.7 | 7.7 | 13.9 | 9.5 | ND | 19.3 | 20 | 15 | 12.3 | ND | ND |
| 4OH-E2 | 21.8 | 49 | 10 | ND | ND | ND | ND | 8.3 | 5.7 | 4.5 | ||
| 2OH-E2 | 22.9 | 15 | 3.0 | 14.8 | 200 | ND | 19.9 | 21 | 6 | 9.2 | 1.4 | 1 |
| 4OH-E1 | 24.2 | 19 | 1.7 | 12.0 | ND | ND | 16.0 | 30 | 5.1 | 10.0 | 1.5 | 0.03 |
| 2OH-E1 | 24.5 | 20 | 1.5 | 12.5 | 13 | 11 | 16.9 | 27 | 4 | 10.6 | 1.2 | 0.02 |
LODs were determined using signal to noise ratio (S/N = 3).
ND = not determined.
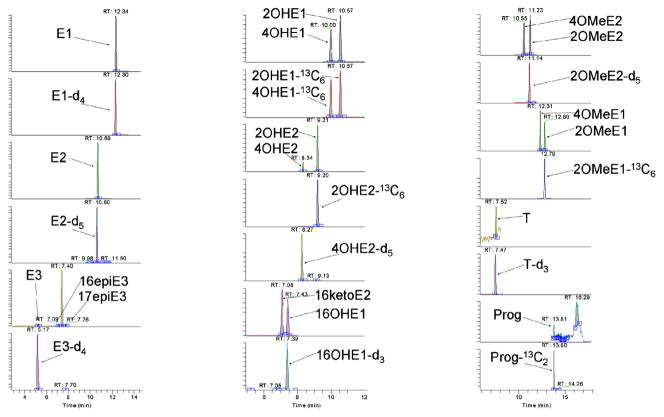
In our search to improve the sensitivity of DS products, we considered reagents containing a sulfonyl chloride group since this group readily and quantitatively reacts with phenolic moieties in mild conditions, and contains a proton-affine group to assure easy ionization for sensitive MS detection. Hence, the commercially available 1-methylimidazole-2-sulfonyl chloride (ISCl) and 2-(1H-1,2,4-triazol-1-yl)-3-pyridinesulfonyl chloride were selected to derivatize steroids. These two compounds contain the desired proton-affine imidazole and triazole groups to improve + ESI sensitivity. Structurally similar imidazole tags (1,2-dimethylimidazole-4-sulfonyl chloride) have been used previously to derivatize estrogens but their less desirable chromatographic properties and non-specific fragmentation ions under SRM have hindered their application [14]. Since orbitrap MS uses exact masses of parent ions for analyte quantitation, fragmentation is not needed. The sensitivity of the assay depends solely on the measurement of the parent ion molecule which is fundamentally different to SRM with tandem mass spectrometers. The derivatization reaction with 1-methylimidazole-2-sulfonyl chloride (ISCl) was carried out in mild conditions similar to the dansylation reaction in the presence of sodium bicarbonate (Scheme 1). However, the reaction with 2-(1H-1,2,4-triazol-1-yl)-3-pyridinesulfonyl chloride failed due to impurities of the commercial compound (data not shown). The reaction products with ISCl were separated using an Ascentis C18 column with 0.1% formic acid in water and 0.1% formic acid in acetonitrile as mobile phase, similar to the chromatographic conditions of dansyl derivatives. Since the imidazole is smaller than the dansyl moiety and less lipophilic, all compounds eluted between 5 and 14 min (Fig. 1) and the total run time was shorter (20 min) than that of the DS derivatives (35 min) and requires less organic solvent (underivatized Prog RT = 9 min, 2nd peak in DS; and RT = 13 min, last peak in IS). MS analysis was carried out in positive ESI mode by full scan and targeted SIM (tSIM) in segments. Table 1 shows the detailed parameters for quantification of the 18 steroids and 12 internal standards. Table 2 shows the LODs of IS derivatives by FS and tSIM obtained using standard solutions. Our results indicate that IS products in general show better sensitivity than DS products: the LODs are in a range of 0.7–7 pg/mL by FS MS, which is 2–10 times more sensitive than the DS products; in contrast, applying tSIM, the LODs are at a much lower range from 0.02–12 pg/mL. The LODs are especially lower for the poly-phenolic estrogens which exhibited an LOD 2–75 times lower than DS derivatives. The calibration curve was linear (R2 > 0.99) in the experimental concentration range of 1–3000 pg/mL. One drawback of the IS method, however, is the co-elution of 3OMeE1 and 4OMeE1. Since these two compounds were not of major interest in our current study, we did not investigate further the chromatographic conditions to separate them. Other columns and mobile phases and gradients can probably be found to separate these compounds.
Fig. 1.
LC/MS analysis of standards (25 pg/mL) in tSIM MS mode after IS derivatization.
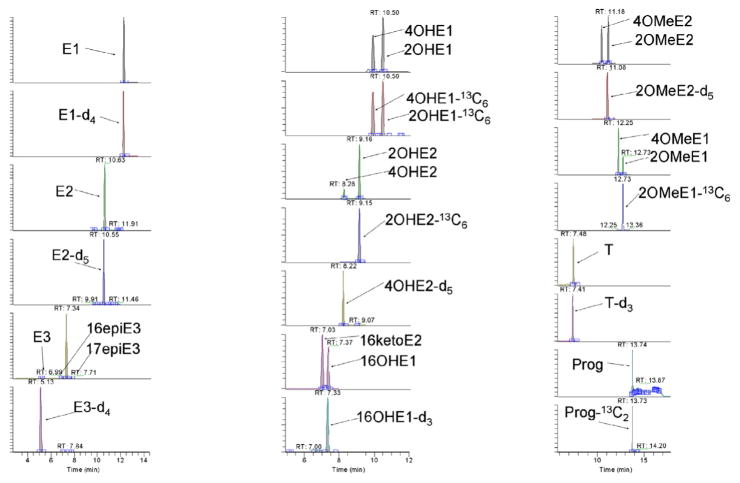
The IS method was then validated using commercially available serum with undetectable steroid levels except for E1 and 2OHE1 (data not shown). Table 3 shows the comparison of the limits of quantitation (LOQ) defined by a S/N = 10 in spiked serum samples of the DS and IS products. The values are higher than those of the standard LODs (Table 2) due to serum matrix effects and partial recoveries by liquid–liquid extractions (data not shown). Our results showed that tSIM MS is generally more sensitive than FS MS for both IS and DS. Under tSIM, the LOQs of IS derivatives are in a range of 0.2–2.2 pg/mL whereas DS method showed a range of 1.7–100 pg/mL. The LOQs of the major estrogens (E1, E2, E3) are 2–10 times more sensitive with the IS method. Interestingly, compounds with two phenolic groups (OHE1, OHE2) showed even greater improvement (3–80 times) using the IS method. This is probably because the double tagged products have more proton affinity. Precision was performed over three days (inter-day CV, n = 3) using serum spiked with 10 pg/mL and 100 pg/mL standards in duplicate (intra-day CV, n = 2) (Table 4). 100 μL of a commercially available pooled serum was used that was treated by the IS method and measured by tSIM MS. The intra-day CV of serum spiked with 10 pg/mL standard showed a mean intra-day CV of 13% and a mean inter-day CV of 13%; and serum spiked with 100 pg/mL of standards showed a mean intra-day CV of 8% and a mean inter-day CV of 8%. The native serum showed detectable E1 at 7.3 pg/mL (intra-day CV 25%; inter-day CV 14%); but 2OHE1 exhibited a higher CV due to a level close to its LOQ (Fig. 2).
Table 3.
Limits of quantitation (LOQ, S/N = 10) of DS and IS products.
| DS LOQ (pg/ml)
|
IS LOQ (pg/ml)
|
|||
|---|---|---|---|---|
| FS | tSIM | FS | tSIM | |
| E1 | 5.6 | 4.4 | 1.2 | 0.4 |
| E2 | 2.5 | 1.7 | 0.4 | 0.03 |
| E3 | 3.8 | 4.3 | 2.8 | 1.2 |
| 16epi-E3 | 5.5 | 4.4 | 2.7 | 1.4 |
| 17epi-E3 | 4.4 | 4 | 3.1 | 1.4 |
| 16OH-E1 | 8 | 4.9 | 4.7 | 0.6 |
| 16keto-E2 | 6 | 4.4 | 3.2 | 0.4 |
| 2OH-E1 | 100 | 2.9 | 3.2 | 0.7 |
| 4OH-E1 | 100 | 2.5 | 3.4 | 0.9 |
| 2OMe-E1 | 7.4 | 4.9 | 3.5 | 2.1 |
| 2OH-E2 | 50 | 50 | 1.7 | 0.6 |
| 4OH-E2 | 50 | 100 | 7.6 | 2.2 |
| 2OMe-E2 | 4.4 | 1.7 | 1.3 | 0.2 |
| 4OMe-E2 | 6 | 2.1 | 2.2 | 0.2 |
ND: not determined.
Table 4.
Precision of IS products by tSIM MS in serum.
| Serum 1
|
Serum 2
|
|||||
|---|---|---|---|---|---|---|
| Calc added Amt (pg/ml) Ave | CV
|
Calc added Amt (pg/ml) Ave | CV
|
|||
| Intra-day (%) | Inter-day (%) | Intra-day (%) | Inter-day (%) | |||
| E1 | 11.6 | 21 | 13 | 102.7 | 6 | 7 |
| E2 | 16.3 | 8 | 1 | 108.1 | 8 | 2 |
| E3 | 8.6 | 9 | 11 | 99.7 | 7 | 11 |
| 16epiE3 | 8.7 | 13 | ND | 61.3 | 26 | 18 |
| 17epiE3 | 8 | 14 | 16 | 97.9 | 2 | 8 |
| 16ketoE2 | 10.3 | 5 | 24 | 103.8 | 10 | 8 |
| 16OHE1 | 10.4 | 8 | 9 | 97.8 | 7 | 7 |
| 2OHE1 | 7.9 | 11 | 15 | 93.7 | 4 | 4 |
| 4OHE1 | 8.2 | 16 | 22 | 95.7 | 8 | 4 |
| 2OHE2 | 11.4 | 5 | 17 | 107.1 | 7 | 13 |
| 4OHE2 | 5.6 | 31 | 19 | 90.1 | 9 | 8 |
| 2OMeE1 | 12.7 | 9 | 14 | 105.5 | 5 | 10 |
| 2OMeE2 | 8.9 | 11 | 10 | 98.3 | 8 | 8 |
| 4OMeE2 | 10.9 | 7 | 7 | 99.1 | 5 | 7 |
Fig. 2.
LC/MS analysis of Serum 1 in tSIM MS mode after IS derivatization. For absolute levels see Table 4.
4. Conclusion
In this study, we applied different derivatization methods to improve the speed and sensitivity of profiling 18 steroids by orbitrap LC/MS. For our products, we found that the targeted selected ion monitoring (tSIM) mode is in general more sensitive than the full scan MS mode (Table 2). Using tSIM, we found sensitivities of IS (0.02–12 pg/mL) > DS (0.5–22 pg/mL) > PS (0.2–15 pg/mL) > P (3–23 pg/mL, partial set of analytes). To our knowledge, this is the first report of sensitivity comparisons among steroid derivatives performed in the same laboratory. This should be of great value for epidemiologic and other research studies that require steroid detection at extremely low levels.
References
- 1.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci USA. 1997;94:10937–42. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol. 2008;630:148–65. [PubMed] [Google Scholar]
- 3.Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, Hankinson SE. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2-to 3-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18:2860–8. doi: 10.1158/1055-9965.EPI-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Key T, Appleby P, Barnes I, Reeves G, Endogenous H Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 5.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 6.Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2011;108:13728–33. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–71. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ. Limitations of direct estradiol and testosterone immunoassay kits. Steroids. 2003;68:1173–8. doi: 10.1016/j.steroids.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Keefer LK, Waterhouse DJ, Saavedra JE, Veenstra TD, Ziegler RG. Measuring seven endogenous ketolic estrogens simultaneously in human urine by high-performance liquid chromatography–mass spectrometry. Anal Chem. 2004;76:5829–36. doi: 10.1021/ac049405i. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography–tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004 Feb;50:373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 11.Higashi T, Yamauchi A, Shimada K. 2-Hydrazino-1-methylpyridine: a highly sensitive derivatization reagent for oxosteroids in liquid chromatography–electrospray ionization-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2005;825:214–22. doi: 10.1016/j.jchromb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 2008;129:530–9. doi: 10.1309/LC03BHQ5XJPJYEKG. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids. 2007;72:819–27. doi: 10.1016/j.steroids.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Spink DC. Analysis of steroidal estrogens as pyridine-3-sulfonyl derivatives by liquid chromatography electrospray tandem mass spectrometry. Anal Biochem. 2008;375:105–14. doi: 10.1016/j.ab.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang WC, Regnier FE, Sliva D, Adamec J. Stable isotope-coded quaternization for comparative quantification of estrogen metabolites by high-performance liquid chromatography–electrospray ionization mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2008;870:233–40. doi: 10.1016/j.jchromb.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: past present, and future. Steroids. 2010;75:297–306. doi: 10.1016/j.steroids.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography–tandem mass spectrometry. Anal Chem (Washington, DC, US) 2009;79:7813–21. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 18.Franke AA, Custer LJ, Morimoto Y, Nordt FJ, Maskarinec G. Analysis of urinary estrogens, their oxidized metabolites, and other endogenous steroids by benchtop orbitrap LCMS versus traditional quadrupole GCMS. Anal Bioanal Chem. 2011;401:1319–30. doi: 10.1007/s00216-011-5164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda A, Yamashita K, Hara T, Ikegami T, Miyazaki T, Shirai M, et al. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC–ESI–MS/MS. J Lipid Res. 2009;50:350–7. doi: 10.1194/jlr.D800040-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Nelson RE, Grebe SK, OK DJ, Singh RJ. Liquid chromatography–tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita K, Takahashi M, Tsukamoto S, Numazawa M, Okuyama M, Honma S. Use of novel picolinoyl derivatization for simultaneous quantification of six corticosteroids by liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr A. 2007;1173:120–8. doi: 10.1016/j.chroma.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Franke AA. Improved LC–MS method for the determination of fatty acids in red blood cells by LC-orbitrap MS. Anal Chem. 2011;83:3192–8. doi: 10.1021/ac103093w. [DOI] [PMC free article] [PubMed] [Google Scholar]