Abstract
Six cephalosporin antibiotics were administered subcutaneously to mice at a level of 20 mg/kg. The serum levels of each were determined at five time intervals ranging from 5 to 120 min after dosing. Urinary recovery and the presence of active metabolites in mouse urine were determined. The peak serum levels and serum half-lives in mice were found to be positively correlated with the mean effective dose values obtained after lethal challenge with Escherichia coli. The administration of cefazolin and cephanone resulted in the highest serum level and the best protection. Good protection was obtained with cephaloridine despite somewhat lower serum levels. The cephalosporins with the acetoxy side chain (cephalothin, cephapirin, and cephacetrile) showed lower serum levels and the poorest protection. Cefazolin, cephaloridine, and cephalothin serum levels were also determined in dogs, squirrel monkeys, and rabbits. A mixed response was obtained in these species, with cefazolin peak serum levels being highest in rabbits and cephaloridine peak highest in dogs.
Full text
PDF
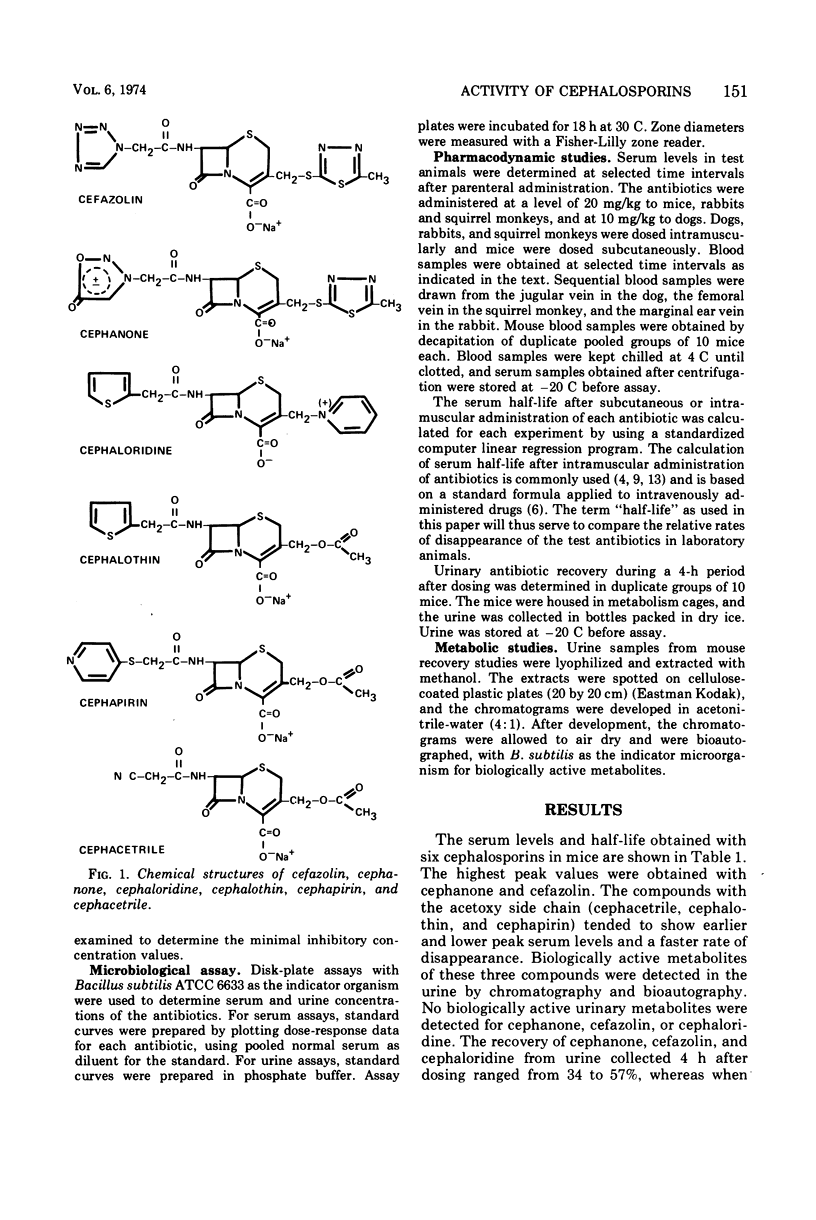
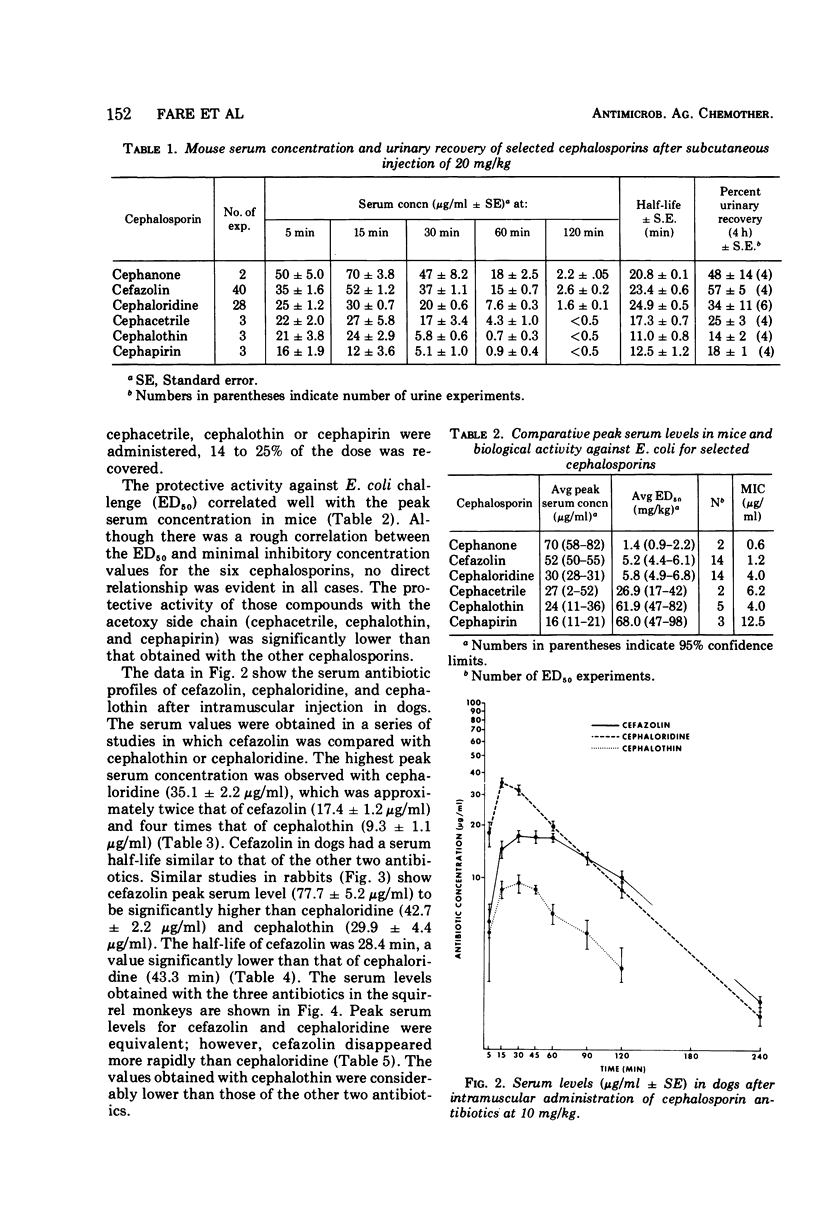
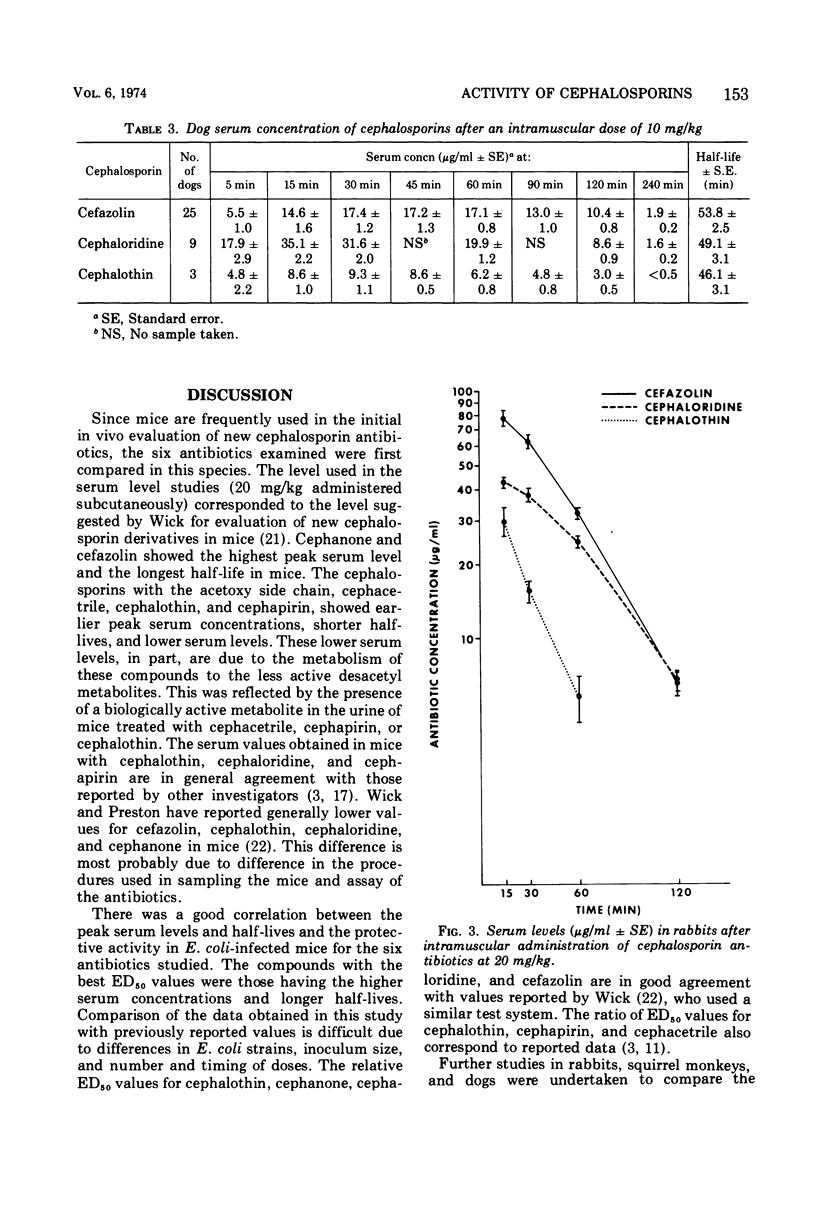
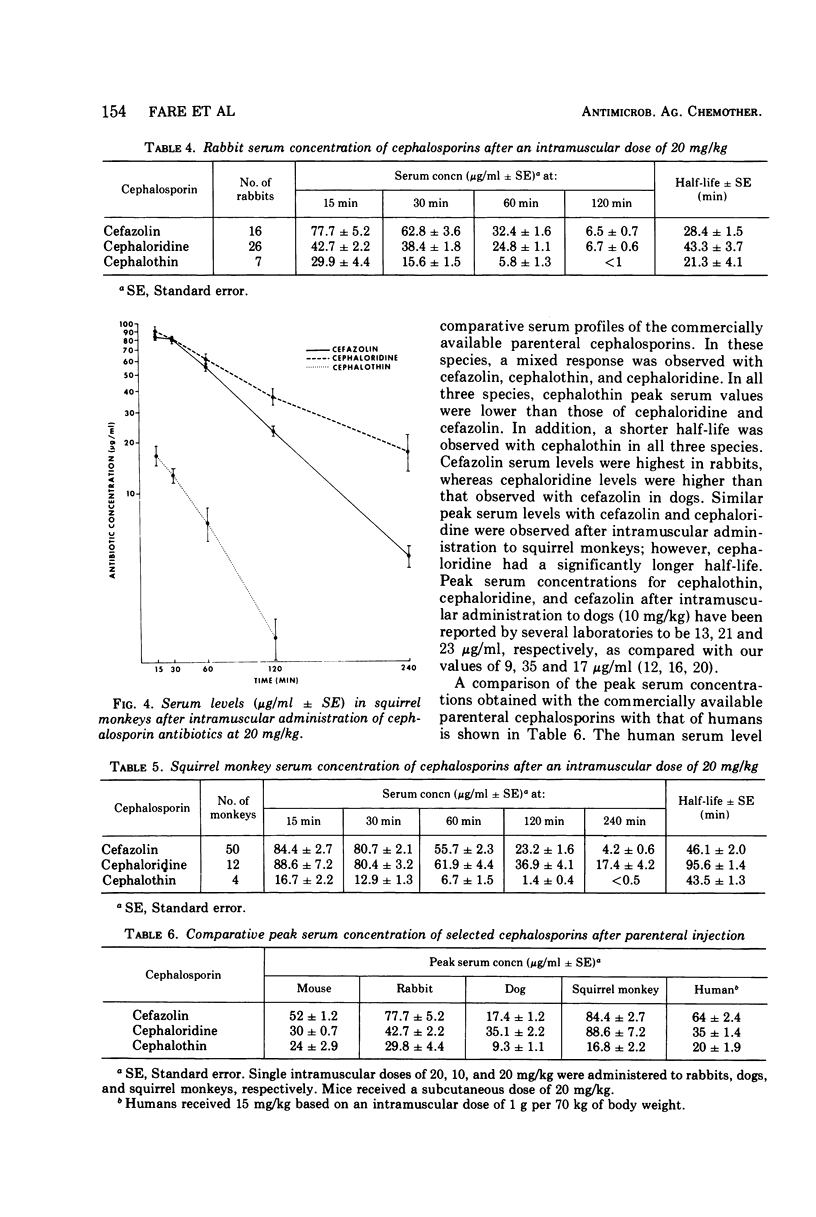

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J., Meyers B. R., Hirschman S. Z. Cephapirin: pharmacology in normal human volunteers. J Clin Pharmacol New Drugs. 1972 Feb-Mar;12(2):84–88. doi: 10.1002/j.1552-4604.1972.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Cahn M. M., Levy E. J., Actor P., Pauls J. F. Comparative serum levels and urinary recovery of cefazolin, cephaloridine, and cephalothin in man. J Clin Pharmacol. 1974 Jan;14(1):61–66. doi: 10.1002/j.1552-4604.1974.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Chisholm D. R., Leitner F., Misiek M., Wright G. E., Price K. E. Laboratory studies with a new cephalosporanic acid derivative. Antimicrob Agents Chemother (Bethesda) 1969;9:244–246. [PubMed] [Google Scholar]
- Craig W. A., Welling P. G., Jackson T. C., Kunin C. M. Pharmacology of cefazolin and other cephalosporins in patients with renal insufficiency. J Infect Dis. 1973 Oct;128(Suppl):S347–S345. doi: 10.1093/infdis/128.supplement_2.s347. [DOI] [PubMed] [Google Scholar]
- Gold J. A., McKee J. J., Ziv D. S. Experience with cefazolin: an overall summary of pharmacologic and clinical trials in man. J Infect Dis. 1973 Oct;128(Suppl):S415–S412. doi: 10.1093/infdis/128.supplement_2.s415. [DOI] [PubMed] [Google Scholar]
- Griffith R. S., Black H. R. Blood, urine and tissue concentrations of the cephalosporin antibiotics in normal subjects. Postgrad Med J. 1971 Feb;47(Suppl):32–40. [PubMed] [Google Scholar]
- Hodges G. R., Scholand J. F., Perkins R. L. Cephacetrile: clinical evaluation in 27 patients. Antimicrob Agents Chemother. 1973 Feb;3(2):228–234. doi: 10.1128/aac.3.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby W. M., Regamey C. Pharmacokinetics of cefazolin compared with four other cephalosporins. J Infect Dis. 1973 Oct;128(Suppl):S341–S346. doi: 10.1093/infdis/128.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- Knüsel F., Konopka E. A., Gelzer J., Rosselet A. Antimicrobial studies in vitro with CIBA 36278-Ba, a new cephalosporin derivative. Antimicrob Agents Chemother (Bethesda) 1970;10:140–149. [PubMed] [Google Scholar]
- Kradolfer F., Sackmann W., Zak O., Brunner H., Hess R., Konopka E. A., Gelzer J. CIBA 36278-Ba" chemotherapy and toxicology in laboratory animals. Antimicrob Agents Chemother (Bethesda) 1970;10:150–155. [PubMed] [Google Scholar]
- Levison M. E., Levison S. P., Ries K., Kaye D. Pharmacology of cefazolin in patients with normal and abnormal renal function. J Infect Dis. 1973 Oct;128(Suppl):S354–S357. doi: 10.1093/infdis/128.supplement_2.s354. [DOI] [PubMed] [Google Scholar]
- Meyers B. R., Hirschman S. Z., Nicholas P. Cephanone: in vitro antibacterial activity and pharmacology in normal human volunteers. Antimicrob Agents Chemother. 1972 Oct;2(4):250–254. doi: 10.1128/aac.2.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia G. J., Renz K. J., Gadebusch H. H. Comparison of the chemotherapeutic and pharmacodynamic activities of cephradine, cephalothin, and cephaloridine in mice. Antimicrob Agents Chemother. 1973 Feb;3(2):270–273. doi: 10.1128/aac.3.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y., Kuwahara S., Goto S. In vitro and in vivo evaluation of cefazolin, a new cephalosporin C derivative. Antimicrob Agents Chemother (Bethesda) 1969;9:236–243. [PubMed] [Google Scholar]
- Welles J. S., Gibson W. R., Harris P. N., Small P. M., Anderson R. C. Toxicity, distribution, and excretion of cephaloridine in laboratory animals. Antimicrob Agents Chemother (Bethesda) 1965;5:863–869. [PubMed] [Google Scholar]
- Wick W. E., Preston D. A. Biological properties of three 3-heterocyclic-thiomethyl cephalosporin antibiotics. Antimicrob Agents Chemother. 1972 Mar;1(3):221–234. doi: 10.1128/aac.1.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner P., MacGregor R., Bear D., Berman S., Holmes K., Turck M. Evaluation of a new cephalosporin antibiotic, cephapirin. Antimicrob Agents Chemother. 1972 Apr;1(4):303–309. doi: 10.1128/aac.1.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]



