Abstract
Mutanolysin partially purified from the culture filtrate of Streptomyces globisporus 1829 consists of two main lytic enzymes with an isoelectric point near pH 8.5 and 10, respectively, and proteolytic enzyme is associated with the latter lytic enzyme. Mutanolysin exhibited maximal lytic activity at 60 C in the pH range 6.5 to 7.0 and was stable at 50 C in the acid range. N-bromosuccinimide caused complete inhibition of lytic activity at 1 mM, whereas calcium and magnesium ions at the same concentration caused activation. Mutanolysin had lytic or bactericidal activity against the living cells of Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis, Lactobacillus acidophilus, and Actinomyces viscosus, which are considered to be etiologic agents of dental caries, but had no activity against S. aureus and all gram-negative strains tested. The lytic activity was well retained in human saliva. Digestion of the cell walls of S. mutans BHT by mutanolysin was accompanied by the liberation of free amino groups and reducing sugars. Mutanolysin may be expected to be a useful agent for dental caries control.
Full text
PDF


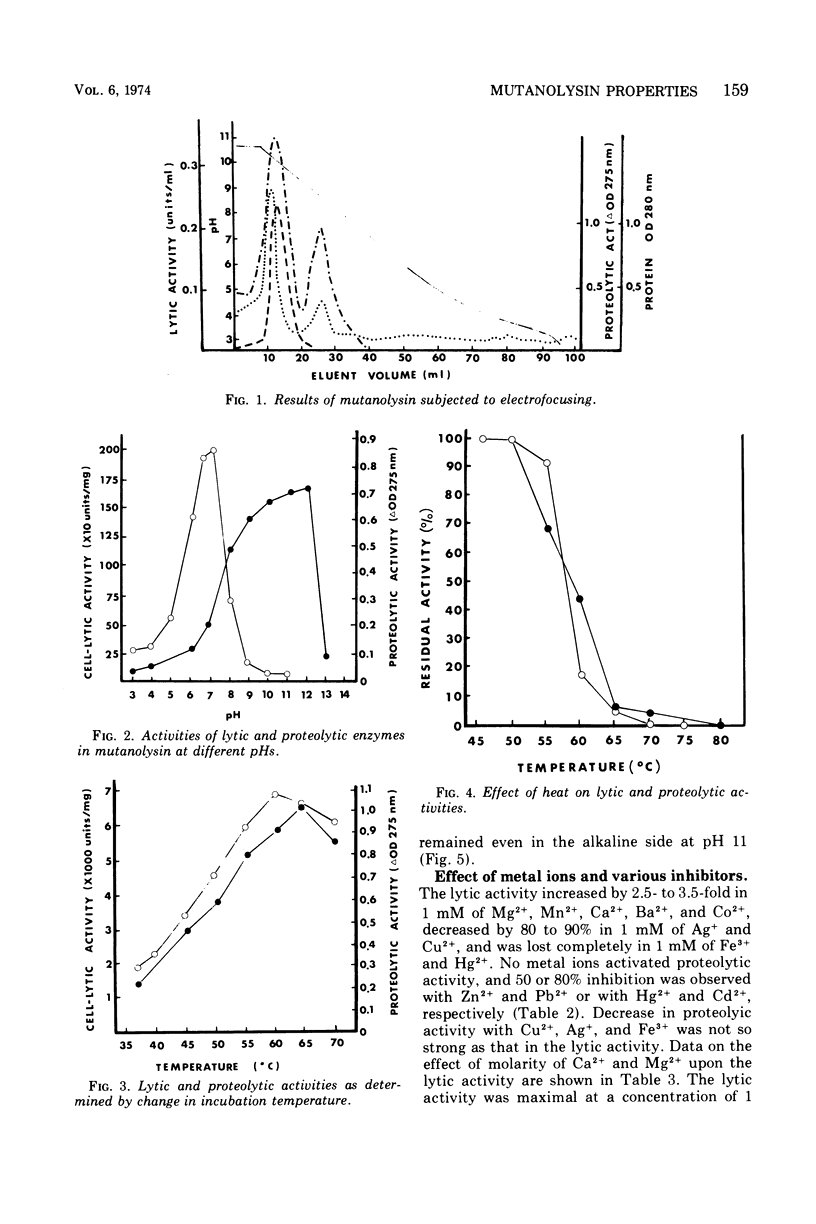
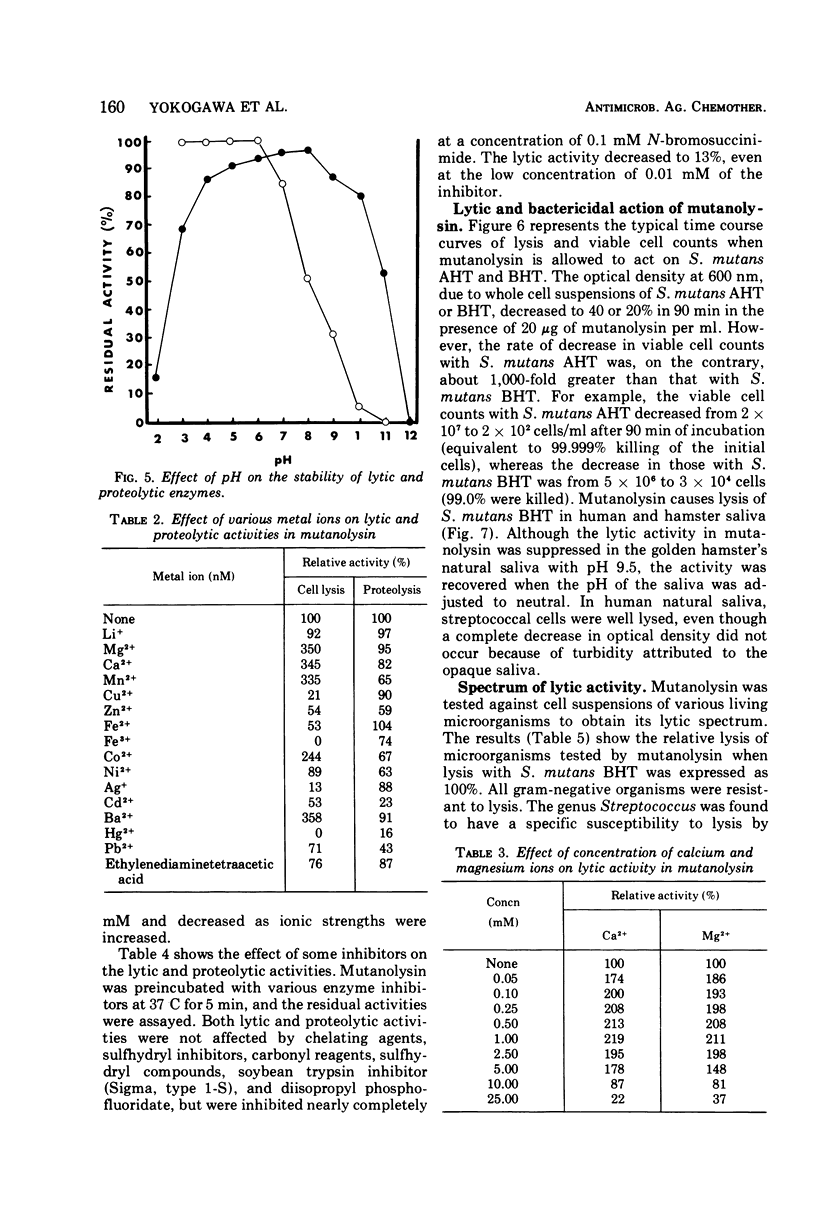
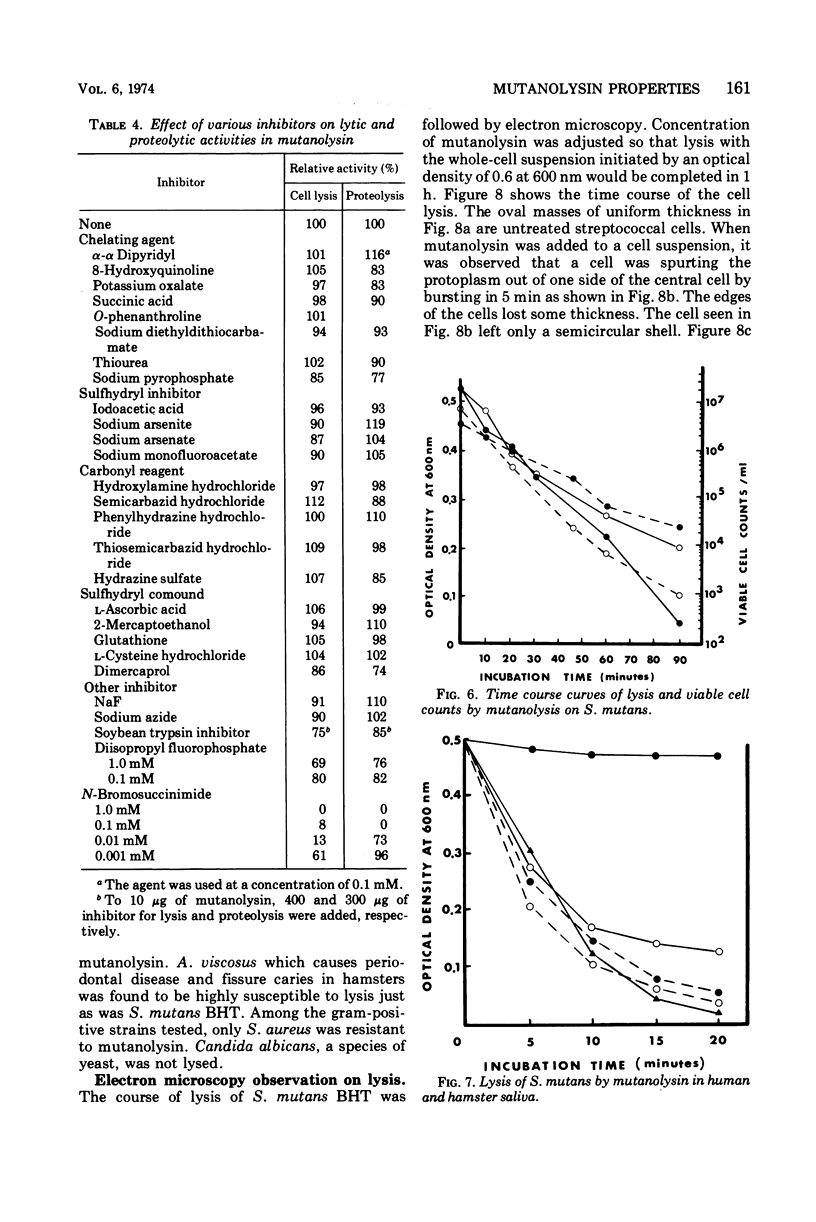



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKULIS S. S., SMITH C., BOLTRALIK J. J., HEYMANN H. STRUCTURE OF STREPTOCOCCAL CELL WALLS. IV. PURIFICATION AND PROPERTIES OF STREPTOCOCCAL PHAGE MURALYSIN. J Biol Chem. 1964 Dec;239:4027–4033. [PubMed] [Google Scholar]
- Charlton G., Fitzgerald R. J., Keyes P. H. Determination of saliva and dental plaque pH in hamsters with glass micro-electrodes. Arch Oral Biol. 1971 Jun;16(6):649–654. doi: 10.1016/0003-9969(71)90068-9. [DOI] [PubMed] [Google Scholar]
- Dixon R. E., Goodman J. S., Koenig M. G. Lysostaphin: an enzymatic approach to staphylococcal disease. 3. Combined lysostaphin-methicillin therapy of established staphylococcal abscesses in mice. Yale J Biol Med. 1968 Aug;41(1):62–68. [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Ecologic factors in dental caries. The fate of antibiotic-resistant cariogenic streptococci in hamsters. Am J Pathol. 1963 Jun;42:759–772. [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald R. J., Jordan H. V., Archard H. O. Dental caries in gnotobiotic rats infected with a variety of Lactobacillus acidophilus. Arch Oral Biol. 1966 May;11(5):473–476. doi: 10.1016/0003-9969(66)90153-1. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., König K. G., Mühlemann H. R., Regolati B. Effect of dextranase on caries in rats harbouring an indigenous cariogenic bacterial flora. Arch Oral Biol. 1969 May;14(5):555–558. doi: 10.1016/0003-9969(69)90149-6. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Imoto T., Funatsu G., Funatsu M. The position of the active tryptophan residue in lysozyme. J Biochem. 1965 Sep;58(3):227–235. doi: 10.1093/oxfordjournals.jbchem.a128190. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- Jablon J. M., Zinner D. D. Differentiation of cariogenic streptococci by fluorescent antibody. J Bacteriol. 1966 Dec;92(6):1590–1596. doi: 10.1128/jb.92.6.1590-1596.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M. SYMPOSIUM ON RELATIONSHIP OF STRUCTURE OF MICROORGANISMS TO THEIR IMMUNOLOGICAL PROPERTIES. IV. ANTIGENIC AND BIOCHEMICAL COMPOSITION OF HEMOLYTIC STREPTOCOCCAL CELL WALLS. Bacteriol Rev. 1963 Dec;27:369–380. doi: 10.1128/br.27.4.369-380.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B., Carlsson J. Various types of streptococci and experimental caries in hamsters. Arch Oral Biol. 1970 Jan;15(1):25–32. doi: 10.1016/0003-9969(70)90142-1. [DOI] [PubMed] [Google Scholar]
- König K. G., Guggenheim B. In-vivo effects of dextranase on plaque and caries. Helv Odontol Acta. 1968 Oct;12(2):48–55. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J., Dziewiatkowski D. D. The in-vitro effect of fungal dextranase on human dental plaque. Arch Oral Biol. 1972 Jan;17(1):35–42. doi: 10.1016/0003-9969(72)90131-8. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Quickel K. E., Jr, Selden R., Caldwell J. R., Nora N. F., Schaffner W. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl Microbiol. 1971 Sep;22(3):446–450. doi: 10.1128/am.22.3.446-450.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S., Dworkin M. Bacteriolytic enzymes produced by Myxococcus xanthus. J Bacteriol. 1972 Apr;110(1):236–245. doi: 10.1128/jb.110.1.236-245.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]