Abstract
The processes underlying action planning are fundamental to adaptive behavior and can be influenced by recent motor experience. Here, we used a novel fMRI Repetition Suppression (RS) design to test the hypotheses that action planning unfolds more efficiently for successive actions made with the same hand. More efficient processing was predicted to correspond with both faster response times (RTs) to initiate actions and reduced fMRI activity levels – RS. Consistent with these predictions, we detected faster RTs for actions made with the same hand and accompanying fMRI-RS within bilateral posterior parietal cortex and right-lateralized parietal operculum. Within posterior parietal cortex, these RS effects were localized to intraparietal and superior parietal cortices. These same areas were more strongly activated for actions involving the contralateral hand. The findings provide compelling new evidence for the specification of action plans in hand-specific terms, and indicate that these processes are sensitive to recent motor history. Consistent with computational efficiency accounts of motor history effects, the findings are interpreted as evidence for comparatively more efficient processing underlying action planning when successive actions involve the same versus opposite hand.
Keywords: action planning, recent motor experience, grasping, sensorimotor control, fMRI repetition suppression, action priming
1. Introduction
Human behavioral evidence suggests that the mechanisms underlying action planning are sensitive to recent movement history. For example, the ways that objects are grasped partly reflect recent grasp history (Cohen and Rosenbaum, 2004, 2011; Dixon et al., 2012; Kelso et al., 1994; Rosenbaum and Jorgensen, 1992; Schutz et al., 2011; Short and Cauraugh, 1997). Similar effects of recent motor history have been shown for the spatial paths of arm movements during successive reaching actions (Jax and Rosenbaum, 2007), the coordinated patterns of bimanual rhythmic finger movements (Kelso, 1981, as cited in Weiss and Wark 2009), and the movement characteristics of paddle swings during table-tennis (Sorensen et al., 2001). According to some accounts, motor history effects reflect more efficient planning when recently executed motor programs are reused as opposed to newly specified (Rosenbaum et al., 2012). Here, we refer to this hypothesis as the planning efficiency account of recent motor history effects, and define better efficiency as faster planning associated with reduced neural processing costs when recently specified sensorimotor parameters can be reused.
We recently provided additional support for this account (Valyear and Frey, 2014). We showed that response times (RTs) to initiate successive actions are faster when the same versus alternate hand is used, even though those actions involved distinct grasps and object placement movements to distinct locations. These findings provide critical support for the planning efficiency account; in particular, since prior evidence reveals that actions are (at least partly) planned in advance of movement onsets (Klatzky et al., 1995; Pellegrino et al., 1989; Stelmach et al., 1994; Sternberg et al., 1978). In line with this framework, we interpreted our results as arising from repetition-related computational gains in the processes that underlie hand-specific planning.
Consistent with the hypothesis that repeated elements of successive actions are planned more efficiently, the above behavioral findings parallel newer evidence showing reduced fMRI signal levels for repeated hand actions within parietofrontal areas governing action planning. These effects, known as fMRI repetition suppression (fMRI-RS), have been shown for repeated grasping (Kroliczak et al., 2008; Monaco et al., 2011; Monaco et al., 2014) and manual gestures (Chouinard and Goodale, 2009; Dinstein et al., 2007; Hamilton and Grafton, 2009). Critically, fMRI-RS has been linked to more efficient neuronal-level processing (Grill-Spector et al., 2006; James and Gauthier, 2006; Wiggs and Martin, 1998), and thus, these prior results are consistent with planning efficiency accounts of behavioral effects of recent motor experience.
Repetition-related decreases in the firing durations of neurons encoding action plans could explain both fMRI-RS and decreased response times to initiate actions. For example, if planning mechanisms operate in an activity-threshold-dependent manner (Cisek, 2007; Hanks et al., 2006), then changes in baseline activity levels according to recent motor history could account for faster planning and shorter durations of neural firing. Motor history can modulate baseline activity in neurons underlying the control of saccadic eye movements and these changes correlate with saccadic reaction times (Fecteau and Munoz, 2003).
The purpose of the current study was to provide evidence for concurrent repetition-related decreases in response times to initiate actions and fMRI-RS within areas underlying action planning. Despite the relative prevalence of evidence for both behavioral motor history effects and fMRI-RS for repeated actions, to our knowledge, no study to date has demonstrated both effects concurrently.
Specifically, our primary aim was to provide a critical test of the planning efficiency account of our prior behavioral results showing RT differences according to recent hand-use history (Valyear and Frey, 2014). The efficiency hypothesis predicts that these RT effects will be accompanied by fMRI-RS within areas implicated in action planning.
The anatomical specificity of our predictions should be clear, and is worth emphasis. If faster RTs for repeated use of the same hand reflect more efficient planning, then fMRI-RS effects should be localized to those brain areas underlying action planning. Our task involves reaching, grasping, and object manipulation. As such, predicted areas correspond with those that have been implicated in reach, grasp, and manual object manipulation planning – bilateral posterior parietal and frontal premotor areas, including anterior/posterior intraparietal, superior/inferior parietal, and dorsal/ventral premotor cortices (Astafiev et al., 2003; Beurze et al., 2007, 2009; Gallivan et al., 2011; Jacobs et al., 2010; Marangon et al., 2011).
A second major aim of this study was to investigate the potential specificity of fMRI-RS for actions made with the same versus alternate limb, and in turn, the potential for across-limb RS effects. Prior research in this area has been limited to the study of repeated (versus non-repeated) elements of actions involving the same limb. To test for possible limb-specific RS effects, conditions involving successive actions with the same versus different limbs must be compared. This was a second new and important contribution of the current study.
2. Materials and Methods
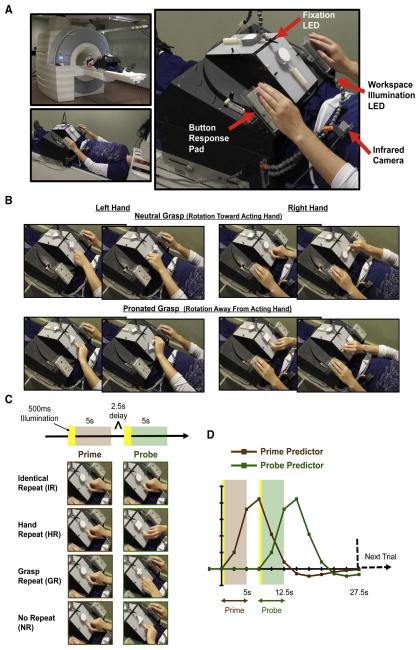
On each trial, participants performed pairs of successive actions – a prime and probe – involving unimanual object rotation movements with either hand (Fig. 1). Which hand was to be used and which direction objects were to be rotated depended on a set of arbitrary rules defined by the shape of objects. Four conditions were defined by the relationship between prime and probe events: either the same actions were repeated (Identical Repeat, IR), hand was repeated but grasp posture was changed (Hand Repeat, HR), grasp posture was repeated but hand was changed (Grasp Repeat, GR), or neither hand nor grasp posture were repeated (No Repeat, NR) (Fig. 1C).
Figure 1. Methods.
(A) Apparatus used to present objects. (B) Complete set of object rotation actions required, for either hand. (C) Experimental conditions and the timing of events within trials. Prime and probe events were defined as 5s periods, each beginning with 500ms illumination of objects and the workspace followed by task performance in the dark. Task performance involved reaching, grasping and rotating objects, which took approximately 2–3s to complete. An additional 2.5s delay period separated prime-probe events. (D) Prime and probe predictors used for the general linear model for the main analysis.
Conditions involving the repeated (IR and HR) versus non-repeated (GR and NR) hands were predicted to result in more efficient planning, as evidenced by 1) faster response times (RTs) to initiate actions, and 2) fMRI-RS within parietofrontal brain areas known to underlie action planning.
In principle, more efficient neural processing may have also been detected for repeated grasps for successive actions involving alternate hands – i.e. for the GR condition. We addressed this possibility with the contrast: NR > GR. Such results would have provided evidence for effector-independent levels of grasp planning, shared across hands during successive actions.
2.1. Subjects
Twenty-one healthy individuals participated in the study. Data from one participant was excluded due to non-compliance with the task (i.e. video data showed a high percentage of trials where bimanual actions were used to manipulate objects). The remaining twenty (6 female) participants were between 19–54 years of age (mean age = 28 +/− 8.5 years). All participants were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971), and provided informed consent in accordance with the local IRB and the Declaration of Helsinki. One participant had vision problems in one eye; estimated 10% vision available in the affected eye, due to a welding accident at the age of 18. He was 35 years of age at the time of testing. All other participants had normal or corrected-to-normal vision. None of the participants had any prior history of psychiatric illness, and all participants were naïve to the goals of the study. The experiment took approximately three hours to complete (including pre-scan training), and participants received financial compensation for their participation.
2.2. Stimuli and presentation setup
Four novel objects were used, made up of simple 3D shapes – sphere, cube, triangle, plus-sign – affixed to 6.5 (length) x 2.5 (width) x 1.7 (depth) cm handles (Fig 1B/C). Use of the same handle dimensions for all objects ensured that differences in hand configurations used to manipulate objects were not related to differences in the physical properties of their handles. Duplicates of each object were included in the set so that even when identity was repeated within trials, the experimenter exchanged objects and the turntable was rotated.
Objects were presented using the platform and turntable apparatus shown in Figure 1 (revised from Valyear et al., 2012). There were two sides to the apparatus to allow for independent presentation of prime and probe events. Each side comprised a workspace where objects were attached centrally and could be rotated clockwise or counterclockwise. The platform was specifically adjusted for each individual so that objects and the workspace could be comfortably viewed through mirrors, and so that objects could be manipulated with minimal movement of the arm. Specifically, the setup allowed participants to reorient objects without the need to move their upper arm or shoulder. Performing hand actions without shoulder movement effectively minimizes potential for movement-related artifacts (Culham, 2004). Response pads were fitted into plastic casings mounted to the apparatus, positioned to the left and right of the workspace. The distance from left/right response pads to objects was ~19cm, on center. In the rest position, participants lightly pressed on the top surface of each response pad with their left/right hands (Fig. 1A). Button releases provided measures of response times to initiate movements, and were used to identify error trials where both hands were moved (see 2.7.2. Videos).
Participants were instructed to fixate a small light source from a light-emitting diode (LED) transmitted via a single optical fiber attached to an adjustable plastic stalk positioned directly (~2cm) above where objects were presented (Fig. 1A). For both prime and probe events, objects were made visible by brief (500ms) illumination of a super-bright white LED transmitted via a bundle of five optical fibers attached to a second adjustable stalk. The experiment was otherwise carried out in complete darkness. An MRI-compatible infrared-sensitive camera (MRC Systems GmbH) was used to record participant’s hand actions.
With the participant in position (with their head localized to the isocenter of the magnetic field), the apparatus remained outside the scanner bore. An experimenter stood next to the bore and manually replaced objects and rotated the turntable according to auditory cues conveyed through MRI-compatible headphones. The signal to replace prime objects and rotate the turntable occurred 2.5s prior to the onset of prime events. Replacement of probe objects and rotation of the turntable for a second time then occurred during the 2.5s delay period between prime and probe events. Although movements of masses within an MRI scanner’s magnetic field can cause artifacts with echo-planar imaging (Barry et al., 2010), the experimenter’s movements occurred outside (or nearly outside) the scanner’s magnetic field and thus were not expected to result in any such artifacts. Also, experimenter movements were present for all trials; any potential artifacts would have affected all conditions similarly.
2.3. Procedure
Objects were presented with their handles oriented vertically, viewed by participants through the use of mirrors while they lay supine in the MRI scanner (Fig. 1). According to arbitrary rules defined by the shape of objects, participants used either their left or right hands to reorient objects so that their handles faced either to the left or right. Participants’ actions were minimal-amplitude movements, involving mainly the wrist, fingers and thumb, and were approximately 2–3s in duration (see 2.4. Pre-scan training). The physical constraints of the setup were such that distinct hand configurations – grasp postures – were elicited depending on which direction objects were to be rotated and which hand was used to perform the manipulations (Fig. 1B). Most importantly, the setup resulted in the performance of actions involving the same hand but with distinct movements and object manipulations. Individual trials comprised two actions – a prime and a probe – separated by a 2.5s delay interval. Prime and probe events were defined as 5s periods, each beginning with a brief (500ms) illumination of objects and the workspace followed by task performance in the dark (Fig. 1C). To facilitate object identification, participants were instructed to view objects directly during 500ms illumination periods. They were then instructed to return to and to maintain their gaze on the fixation LED during the performance of actions, and throughout the rest of the experiment. Eye movement data were not acquired. From the offset of probe events, trials were separated by 15s intervals to allow for the fMRI signal to return to baseline levels.
Participants performed between 6–8 runs (with an average of 7.3 runs per subject). Each run lasted 7min and 35s, and comprised 16 trials involving all possible combinations of prime-probe pairings. Thus, each run included 4 trials per condition, and prime events had no predictive value.
A custom Matlab (R2011b) script was used to create 8 distinct run orders whereby trial history (N-1) was balanced for condition within runs, and prime events involving either the same grasp or the same hand as probe events of the preceding trial were evenly distributed across conditions. Run orders were randomized for presentation across individuals. Two coding schemes defined object-hand-rotation rules, counterbalanced across participants. Coding schemes were such that pairs of objects assigned to either the left or right hand, left or right direction of rotations, were switched for either scheme.
2.4. Pre-scan training
Prior to scanning (mean = 8 +/− 5.5 days, range = 1–20), participants took part in a behavioral training session to learn the task and to gain familiarity with the stimuli and materials, events and timing. Training was performed in a mock scanner designed to approximate the same physical constraints as the real MRI scanner but with no magnetic field. This allowed participants to practice the task in conditions comparable to the fMRI experiment. The same turntable apparatus and materials used in the real MRI scanner were used for training (Fig. 1).
Another important goal of the training session was to clearly specify and practice the particular actions that were to be performed in the scanner. The problems associated with movements of the head while in the scanner were thoroughly explained, and participants were told that their hand actions should not involve movements of the upper arm or shoulder, and that their head should be kept still at all times.
Object-hand-rotation rules were provided visually, using both a simple depiction of the setup showing the left/right hand x left/right target handle rotation rules for each object, as well as via explicit demonstration of each of the four actions by the experimenter. The experimenter demonstrated each action using the grasp postures depicted in Figure 1B. After first exposure to tasks and stimuli, participants performed 3 runs of the experiment. The first run was performed under dim lighting conditions to provide the participant with visual feedback of their actions, and so that the experimenter could more easily communicate with the participant, to point out errors or make suggestions about the particular mechanics of actions, if necessary. Subsequent runs were performed in the dark, comparable to the conditions experienced in the real MRI scanner.
During training it was recognized that for the majority of participants using a precision grasp involving opposition of the fingers and thumb to rotate objects was problematic with respect to keeping the shoulders and head still. This was particularly true for rotation tasks that required object handles to be reoriented away from the hand used for manipulation (i.e. “away from the acting hand”; see Fig. 1B), where a pronated grasp posture was required. To solve this problem, instead of grasping these participants used only their fingers to rotate object handles in position (Fig. 2). These manipulation strategies allowed participants to complete rotation tasks without moving their shoulder/head, which was more important than insisting that participants use a precision grasp to solve the tasks. Critically, regardless of the type of manipulation strategy used, distinct hand configurations were elicited depending on which direction objects were to be rotated. As such, the HR condition involved successive actions with the same (repeated) hand that were different (non-repeated) with respect to hand/arm movements, independent of particular manipulation strategy participants adopted (i.e. whether participants used grasps or non-grasps). Conversely, for the GR condition, the macroscopic movement features of successive actions were repeated (while hand was changed), independent of whether participants performed grasps or non-grasps. In other words, our a priori definitions of trial-by-trial conditions were unaffected by the kinds of manipulation strategies participants used – either hand and/or the gross movement features of actions were repeated or non-repeated, independent of the type of manipulation strategy employed. Nonetheless, manipulation strategies used during the fMRI experiment were carefully qualified offline via video data analysis for both prime and probe events on a trial-by-trial basis (see 2.7.2. Videos). The table embedded in Figure 2 shows the results of these observations, represented as the percentage of trials involving grasps per individual.
Figure 2. Non-grasp manipulation strategies.

Shown are examples of non-grasp manipulation strategies used to perform object-rotation tasks. Most importantly, the movement characteristics are distinct for rotations away-from versus toward the acting hand. As such, the HR condition comprised successive actions with the same hand that involved distinct movements and object manipulation goals. The table inset shows which manipulation strategy was preferred for actions involving object rotations toward versus away from the acting hand per subject, expressed as the percentage of trials involving grasps. Almost all participants used non-grasps for rotations away from the hand, and many also used non-grasps for rotations toward the acting hand.
2.5. Imaging parameters
Imaging was performed on a 3-Tesla Siemens TIM Trio MRI scanner. The T1-weighted anatomical images were collected using a multiplanar rapidly acquired gradient echo (MP-RAGE) pulse sequence: time to repetition (TR) = 1920ms; time to echo (TE) = 2.92ms; flip angle = 9°; matrix size = 256 × 256; field of view (FOV) = 256mm; 176 contiguous sagittal slices; slice thickness = 1mm; in-plane resolution = 1mm × 1mm. Auto Align Scout and True FISP sequences were executed before the start of each functional run to ensure that slices were prescribed in exactly the same positions across runs. Functional MRI volumes were collected using a T2*-weighted single-shot gradient-echo echo-planar imaging (EPI) acquisition sequence: TR = 2500ms; TE = 30ms; flip angle = 77°; matrix size = 64 × 64; FOV = 256mm; slice thickness = 4mm; in-plane resolution = 4mm × 4mm; acceleration factor (integrated parallel acquisition technologies, iPAT) = 2 with generalized auto-calibrating partially parallel acquisitions (GRAPPA) reconstruction. Each volume comprised 40 contiguous (no gap) axial-oblique slices spanning from the most superior point of cortex ventrally to include the entire cerebellum (i.e. whole-brain coverage).
2.6. fMRI data preprocessing
Imaging data were preprocessed and analyzed using Brain Voyager QX version 2.6.0.2288, 64-bit (Brain Innovation, Maastricht, The Netherlands). Each functional run was assessed for subject head motion by viewing cineloop animations and by examining Brain Voyager motion-detection parameter plots after running 3-D motion correction algorithms on the untransformed two-dimensional data. Motion correction was performed per individual using BV QX intra-session alignment options (involving resampling with sinc interpolation) with the reference volume taken as the closest volume to the T1-weighted anatomical scan. For one participant, motion correction outputs showed evidence of single abrupt head movements in 6/8 runs that were less than 3mm (translations) or 3° (rotations) in magnitude, and one run showed two such movements. A separate run from this same individual showed a gradual drift (in the z-dimension) of approximately 6mm. For all other participants, no evidence for abrupt head movements and no gradual deviations larger than 3 mm/° per run were observed.
To remove low-frequency trends, functional data were preprocessed with BVQX default temporal high-pass filter procedures using a GLM with Fourier basis set options approach, with two pairs of sines/cosines predictor functions specified (for complete details, see: http://support.brainvoyager.com/functional-analysis-preparation/27-pre-processing/73-users-guide-temporal-high-pass-filtering.html). Alignment of functional-to-anatomical volumes used both file-header information (initial registration) and gradient-based affine transformation (6–12 parameters) procedures (for complete details, see: http://support.brainvoyager.com/volume-space/28-coregistration-functional-anatomical/387-users-guide-fa-using-gradient-driven-affine-transformations.html). Following co-registration, data were then transformed to standard stereotaxic space (Talairach and Tournoux, 1988). Data were spatially smoothed for group analyses using a Gaussian kernel of 6 mm (full-width, at half-maximum).
2.7. Behavioral data analysis
2.7.1. Response times
The time from the onset of the illumination of objects for probe events until the release of (left/right hand) start buttons was used to calculate response times (RTs; i.e., times-to-movement onset). Outlier analyses involved removal of trials more than two standard deviations above or below the mean, performed separately for each individual. Data from prescan training trials were not included in the analyses. In the interest of preserving fMRI data, trials identified as outliers according to RT data were not excluded from fMRI analyses.
Probe RTs were entered into a three-factor Hand (two levels) x Grasp (two levels) x Condition (four levels) repeated measures analysis of variance (RM-ANOVA). Greenhouse-Geisser correction for violations of the sphericity assumption was applied, taken to be significant at p < 0.05. Post hoc follow-ups to significant main effects compared all possible pairwise comparisons of the most relevant factor. Bonferroni corrections for multiple comparisons were applied with a corrected p < 0.05 taken as significant.
Due to equipment failure, button response time data was not collected for one participant, and was missing for 1 run for a second participant.
2.7.2. Videos
Videos were observed offline by four independent raters who were asked to evaluate and score both prime and probe events per trial with respect to the following possibilities: (i) movements were initiated with the wrong hand, (ii) movements showed abrupt changes in hand postures during reaching, (iii) objects were rotated in the wrong direction, and/or (iv) the experimenter presented the wrong objects.
The following other types of occurrences were also noted, collectively referred to as (v) miscellaneous errors: no objects were presented, no responses were made, rotation of the turntable apparatus resulted in misorientation of objects prior to their presentation, the participant bumped the apparatus during reaching, and/or bimanual actions were performed.
Videos were scored by two independent raters (Rater 1 scored all videos for all participants, while Rater’s 2, 3, and 4 scored videos for nine, three, and eight participants, respectively). For each observation type (i-v, listed above), Cohen’s Kappa coefficients were computed as measures of inter-rater reliability. Rater 4 was the lead author, while Raters 1, 2, and 3 were naïve to the specific predictions and goals of the experiment.
A fifth rater naïve to the goals of the study was asked to observe videos for all trials for all participants to qualify the types of manipulations that were used. Specifically, for each trial, Rater 5 examined prime and probe events and classified actions as involving either grasps or non-grasps, with the later type defined as involving movements of the fingers only (see Fig. 2). Each of these manipulation-types were further characterized as involving object rotation movements away-from versus toward the acting hand. Importantly, as noted earlier, the arm/hand movements of away- versus toward-the-acting-hand rotations differed independent of whether participants used grasps or non-grasps.
Although not expected, it was possible for participants to change their response strategies for GR/IR condition trials, switching from grasp to non-grasp responses, or vice-versa. Recall that participants were free to choose how objects were manipulated. By design, some ways to manipulate objects were biomechanically difficult and/or impossible. This ensured that the movement characteristics for prime-probe events for HR/NR conditions differed, while at the same time forced participants to select movements naturally, according to anticipated task constraints. However, since it was possible to comfortably perform actions involving the same constraints using either grasps or non-grasps, participants could, in principle, switch response styles across prime-probe events for IR/GR conditions. In other words, although we predicted that when participants were faced with successive events involving identical constraints they would perform actions in the same ways, this was not necessarily the case. If evident, GR trials involving a change in manipulation strategy were redefined as NR trials (since both hand and manipulation-type would have been changed), while IR trials were redefined as error trials, excluded from RT analysis and assigned an error predictor of no-interest for fMRI analyses.
Trials that involved the following types of errors for either prime or probe events were excluded from RT analysis: i) when participants rotated objects incorrectly, ii) when no objects were presented, iii) when rotation of the turntable apparatus resulted in misoriented objects prior to their presentation, or iv) when participants performed bimanual actions to manipulate objects. For fMRI analyses, these trials were assigned a predictor function of no-interest (see 2.8. fMRI data analysis). Experimenter errors that involved presentation of the incorrect object were recoded accordingly and included in both the RT and fMRI analyses.
In addition, in a recent behavioral study involving a similar design we found that when participants made hand errors – initiating movements with the wrong hand – they invariably corrected their movements ‘in flight’, stopping their initial responses to switch hands, to complete the task with the correct hand. We reasoned that this kind of behavior might drive-up activity within brain areas important for the control of actions. Moreover, our previous results showed that although hand errors were altogether uncommon (< 2% of trials, at the group level), they nonetheless occurred more frequently for GR and NR conditions; trials involving hand switches. Thus, to avoid biasing the detection of greater activity for NR/GR versus the HR condition, we also assigned predictors of no-interest to trials with these error types. For this purpose, we relied on button release data (objective measures) to identify trials with incorrect hand movements.
Camera-related hardware issues resulted in the failure to collect video data for four participants for 0.5/8, 1/7, 3/8, and 4/8 runs, respectively.
2.8. fMRI data analysis
2.8.1. Whole-brain voxel-wise analyses
For each condition, two independent predictor functions were specified – the first aligned to the onset of the prime event and the second aligned to the onset of the probe event (Fig. 1D). Each predictor was modeled as a two-volume (5 s) boxcar function convolved with BVQX two-gamma function designed to estimate spatiotemporal characteristics of the BOLD response. Separation of prime and probe events with independent predictors allowed us to better target differences in fMRI activity attributable to probe events; contrasts used to reveal fMRI-RS involved condition-probe predictors only. Further, as part of our post-hoc, region-of-interest analyses (see 2.8.2. Region-of-interest (ROI) analyses), we directly tested for differences between prime condition activity levels within identified brain areas by comparing beta weights assigned to condition-prime predictors.
Since it was not of interest to disentangle prime and probe events for error trials, these were assigned a single predictor function of no-interest that included both prime and probe events separated by the 2.5s delay period, convolved with BVQX two-gamma function (resulting in a single function with two peaks).
All analyses were based on a group-level random-effects (RFX) general linear model (GLM) with nine predictors specified: IRPRIME, HRPRIME, GRPRIME, NRPRIME, IRPROBE, HRPROBE, GRPROBE, NRPROBE, and an error predictor. For runs without errors, “dummy” predictors (columns of all zeroes in the design matrix) were included. Each run was percent-transformed prior to GLM analysis. Resultant statistical activation maps were set to thresholds of t(19) = 2.35, p < 0.03 uncorrected, p < 0.05 cluster-size corrected for multiple comparisons. The Duvernoy (1999) anatomical atlas was used as a guide to identify and name active brain areas.
First, to identify areas showing task-related responses we performed the contrast: IRPRIME + HRPRIME + GRPRIME + NRPRIME > rest. These results were then used to define an inclusion mask to constrain subsequent contrasts. This method increases the sensitivity of subsequent statistical tests by reducing the number of voxels required for correction for multiple comparisons to those showing task-related activity increases. By using prime-condition predictors, this contrast is orthogonal to all subsequent contrasts involving probe-condition predictors.
Our main hypothesis was that areas underlying hand-specific planning mechanisms will show reduced fMRI activity levels (fMRI-RS) for successive actions made with the same (repeated) versus opposite (switched) hands. To address this hypothesis, we used a conjunction analysis. First, we performed the contrast: NRPROBE > IRPROBE. Since the IR condition involved repeated use of the same hand while the NR condition did not, this contrast was predicted to identify areas underlying hand-specific planning. At the same time, however, we understood that resultant activity defined by this contrast may reflect fMRI-RS effects for a variety of other reasons, including those attributable to the repetition of postural characteristics of actions, objects, and/or object-defined action rules (which were uniquely repeated for the IR condition).
To selectively identify areas underlying hand-specific planning, we performed the conjunction contrast: NRPROBE > IRPROBE ∧ NRPROBE > HRPROBE. Here, the second contrast of the conjunction – NRPROBE > HRPROBE – is equated for changes in actions, objects, and object-defined action rules. Resultant activations would not be attributable to the repetition of these factors, but rather would selectively reflect RS for repeated use of the same hand.
Similarly, to identify areas showing fMRI-RS for repeated grasps with opposite hands, we performed the conjunction contrast: NRPROBE > IRPROBE ∧ NRPROBE > GRPROBE. Activity revealed by this contrast would provide evidence of fMRI-RS for grasp planning across hands, and would suggest that grasp plans can be specified independent of and shared between hands.
If the contrast: NRPROBE > IRPROBE ∧ NRPROBE > GRPROBE was found to yield no significant activity, we planned to combine NR and GR conditions, both involving hand switches, to contrast with the HR condition to identify areas underlying hand-specific planning processes. In other words, if the brain was found to respond to NR and GR conditions similarly, we reasoned that by collapsing these conditions we would strengthen our estimate of responses for trials involving changed hands. As such, the conjunction contrast: NRPROBE > IRPROBE ∧ (NRPROBE + GRPROBE) > HRPROBE (balanced) was used to identify areas underlying hand-specific planning. Of course, activity defined by this contrast was predicted to closely overlap with activity defined by the contrast NRPROBE > IRPROBE ∧ NRPROBE > HRPROBE, only we reasoned that the strength of effects may be bolstered.
2.8.2. Region-of-interest (ROI) analyses
Two additional analyses were performed on each of the areas showing fMRI-RS for the HR condition as identified with the contrast: NRPROBE > IRPROBE ∧ (NRPROBE + GRPROBE) > HRPROBE.
The purpose of the first analysis was to evaluate whether the differences in activity between conditions were entirely attributable to probe and not prime events. Although the contrasts used to define areas were based on probe-condition predictors, differences between prime-conditions were nonetheless possible, and may have influenced activity attributed to probe events, and thus our results. To rule out this possibility, per area, beta weight values assigned to prime-condition predictors were entered into a single factor RM-ANOVA. To be clear, these analyses involve prime-condition predictors only, and are independent from the statistical contrasts used to define ROIs according to probe-condition predictors. Conversely, inclusion of beta weight values assigned to probe-condition predictors in these analyses would violate assumptions of statistical independence, and is thus inappropriate (Kriegeskorte et al., 2009).
A second, independent ROI analysis was carried out to determine if areas showing fMRI-RS for the HR condition also showed preferential activity for actions performed with the contralateral hand. For this analysis, a new RFX-GLM was computed whereby conditions were redefined as involving either two successive left- or right-handed actions (made up of IR and HR conditions from the original model). Errors and trials involving both hands (made up of GR and NR conditions from the original model) were specified as conditions of no-interest. For each area, beta weight values assigned to left- versus right-hand predictors were then compared using t-tests.
3. Results
3.1. Behavioral results
3.1.1. Response times
Three-factor RM-ANOVA revealed a significant main effect of Condition (F(2.5, 44.3) = 76, p < 1.0 × 10−7) and no significant main effects of Hand (F(1, 18) = 1.4, p = 0.25) or Grasp (F(1, 18) = 2.56, p = 0.13) (Fig. 3). No two-way (Condition x Hand, p = 0.64; Condition x Grasp, p = 0.0521; Hand x Grasp, p = 0.87) or three-way (p = 0.2) interactions were significant. Post-hoc pairwise comparisons revealed faster RTs for IR versus all other conditions (all p’s < 0.001). Critically, priming was evident as a RT advantage for HR versus NR conditions (p < 0.05; Fig. 3B). Participants were faster to initiate actions for probe events when they repeated the same hand used for prime events (even though hand movements and object manipulations changed), consistent with the hypothesis that hand-specific planning processes are sensitive to recent hand-use history. Faster RTs for HR relative to GR conditions also provided evidence for hand repetition priming (p < 0.05; Fig. 3B).
Figure 3. Probe response time results.
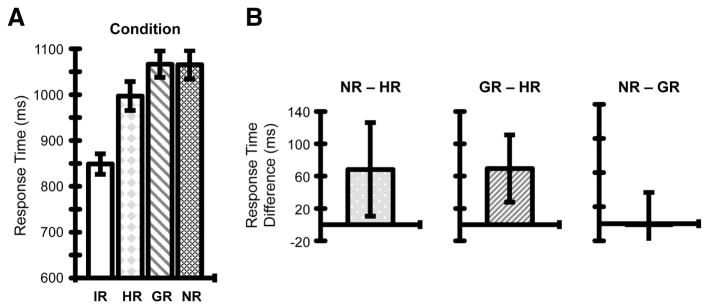
(A) Group mean RTs for probe events as a function of Condition. Error bars reflect mean standard errors. (B) Post-hoc pairwise comparisons revealed hand repetition priming, evident as the mean difference between RTs for NR – HR conditions (left), as well as GR – HR conditions (middle). Conversely, there was no reliable evidence for (hand-independent) grasp repetition priming, shown as the mean difference between RTs for NR – GR conditions (right), not statistically different from zero. For each of these post-hoc comparisons, error bars reflect 95% confidence intervals based on the standard errors of the respective mean difference scores across individuals, Bonferroni corrected.
In contrast to hand repetition priming effects, our findings revealed no evidence for the influence of recent motor history on the planning of grasp/manipulation-type independent of hand. RTs for GR and NR conditions were statistically comparable (p =1.0; Fig. 3B). This indicates that when successive actions involved changing hands there were no performance gains (or costs) associated with repeating (or changing) the type of hand manipulation used. This suggests that planning at the level of hand movements – grasp/manipulation type – is computed independently per hand, and supports the involvement of hand-specific levels of representation for grasp planning.
These results replicate our previous findings (Valyear and Frey, 2014), and indicate that hand-specific levels of action planning are sensitive to recent motor history.
3.1.2. Videos
The results of video coding analyses (Rater’s 1–4) revealed infrequent errors, overall, and good agreement between Raters (see Inline Supplementary Table 1).
Rater 5 classified the type of manipulation responses as either grasps (Fig. 1B) or non-grasps (Fig. 2), involving movements either toward or away from the acting hand. The table included in Figure 2 provides the results of these analyses per subject. Almost all subjects preferred to use a non-grasp manipulation strategy for object-rotations away from the acting hand, which allowed them to avoid the use of relatively pronated grasp postures. Many also used non-grasps manipulations to perform rotations toward the acting hand.
These analyses detected a small number of trials (a mere 29 trials across 12 subjects) originally defined as GR condition trials whereby participants unexpectedly changed their manipulation strategies from prime to probe events, switching from grasp to non-grasp responses, or vice-versa. These trials were redefined as NR condition trials. Similarly, 3 trials across 3 subjects originally defined as IR condition trials involved a change in manipulation strategies, and were redefined as error trials, excluded from both RT and fMRI analyses. Notably, however, while appropriate, these adjustments did not change the relevant statistical outcomes for either of the RT or fMRI results.
3.2. fMRI results
These analyses included 2193 trials (IR = 557; HR = 556; GR = 519; NR = 561).
The contrast IRPRIME + HRPRIME + GRPRIME + NRPRIME > rest was used to identify voxels significantly activated by the task (Inline Supplementary Fig. 1). As expected, this revealed widespread activation of sensorimotor areas, including bilateral primary motor and somatosensory, secondary somatosensory, dorsal and ventral premotor, posterior parietal and cingulate motor areas, as well as the thalamus, basal ganglia, and cerebellum. Bilateral dorsolateral prefrontal cortex, medial occipital and lateral occipito-temporal cortex were also activated by the task. These results were used to define an inclusion mask to constrain subsequent contrasts.
3.2.1. Repetition suppression for the IR condition
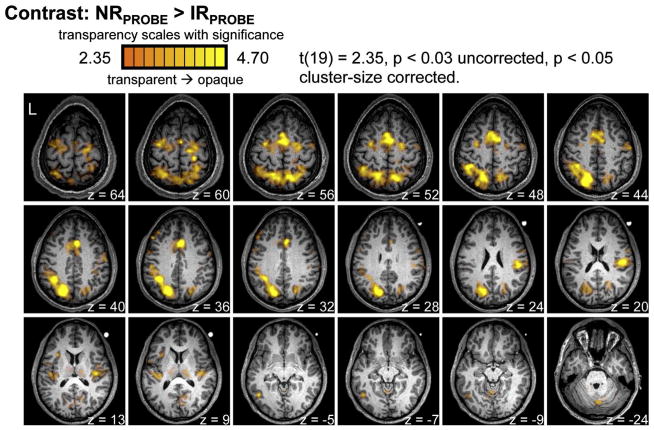
The contrast: NRPROBE > IRPROBE identified a network of parietofrontal areas previously implicated in the sensorimotor control of actions, including bilateral posterior parietal cortex (PPC), dorsal and ventral premotor cortex, medial frontal cortex (including cingulate, supplementary, and pre-supplementary motor areas), basal ganglia, and secondary somatosensory cortex within the parietal operculum (Fig. 4). PPC activity overlapped with superior parietal and parieto-occipital cortex, medially, as well as both posterior and anterior aspects of intraparietal cortex, laterally. The effects within parietal operculum were more robust in the right versus left hemisphere. Activity was also evident within left-lateralized dorsolateral prefrontal cortex, and occipito-temporal cortex overlapping with posterior middle temporal gyrus, as well as bilateral medial and right-hemisphere lateral cerebellum.
Figure 4. Repetition suppression for the IR condition.
The contrast NRPROBE > IRPROBE yielded activity in bilateral posterior parietal cortex, dorsal and ventral premotor cortex, medial frontal cortex (including cingulate, supplementary, and pre-supplementary motor areas), secondary somatosensory cortex within the parietal operculum, basal ganglia, and left-lateralized dorsal lateral prefrontal and occipito-temporal cortex.
The activity identified by this contrast comprised one large contiguous cluster of voxels spanning parietofrontal cortex, including parieto-occipital and medial occipital cortices, and four additional independent clusters: right parietal operculum, left dorsolateral prefrontal cortex, left occipito-temporal cortex, and bilateral cerebellum.
3.2.2. Repetition suppression for the HR condition
Brain areas involved in hand-specific levels of action planning were predicted to show fMRI-RS for trials involving successive actions with the same versus alternate hands. To test these predictions, we used the conjunction contrast: NRPROBE > IRPROBE ∧ NRPROBE > HRPROBE. This contrast failed to detect significant fMRI-RS effects that survived cluster-size correction for multiple comparisons (for uncorrected results, see Inline Supplementary Fig. 2). However, as detailed below (see 3.2.4. Repetition suppression for the HR condition ((NR + GR) > HR)), when NR and GR conditions were combined and contrasted against the HR condition, significant fMRI-RS effects for the HR condition were revealed.
3.2.3. Repetition suppression for the GR condition
Using the contrast: NRPROBE > IRPROBE ∧ NRPROBE > GRPROBE, we tested for evidence of fMRI-RS for repeated grasps with opposite hands. Notably, considering that the majority of participants used non-grasp strategies to manipulate objects (Fig. 2), it is more appropriate to think of this contrast as a test for possible effector-independent RS effects for repeated hand movements rather than grasps, per se. Such results would suggest that movement characteristics of hand actions are specified independent of and shared between hands, and that these representations are sensitive to recent history.
We found no evidence of this, however, as the contrast NRPROBE > IRPROBE ∧ NRPROBE > GRPROBE revealed no significant fMRI-RS effects that survived cluster-size correction for multiple comparisons (Inline Supplementary Fig. 3). Further, to increase power we combined NR and HR conditions and performed the contrast: NRPROBE > IRPROBE ∧ (NRPROBE + HRPROBE) > GRPROBE. Still, no areas of activity survived correction (Inline Supplementary Fig. 4).
We strongly urge cautious interpretation of these results, however. The activation thresholding methods used in the current study adhere to conventional standards. However, some authors have argued that these conventions may be overly conservative, and as a consequence, Type II error rates may be inflated (Lieberman and Cunningham, 2009). In brief, future experiments are necessary to more concretely determine whether fMRI-RS is possible for successive actions involving repeated movement features with opposite hands.
With these considerations in mind, the absence of fMRI-RS for repeated hand movements across hands is consistent with our behavioral results; RTs for GR and NR conditions were statistically similar (Fig. 3B). Both results suggest that planning successive actions made with opposite hands is uninfluenced by the similarity of the distal movement features of those actions.
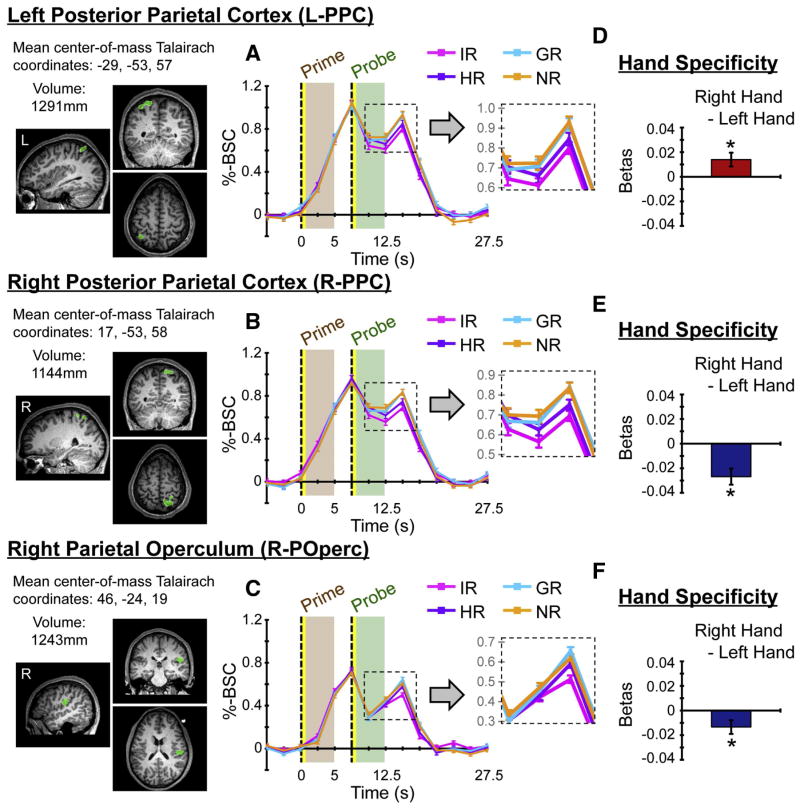
3.2.4. Repetition suppression for the HR condition ((NR + GR) > HR)
As planned, we combined the NR and GR conditions, both involving hand switches, to compare with the HR condition as follows: NRPROBE > IRPROBE ∧ (NRPROBE + GRPROBE) > HRPROBE. This contrast revealed significant fMRI-RS effects in three, independent clusters of activity: left and right posterior parietal cortex (L- and R-PPC) and right lateralized parietal operculum (R-POp) (Fig. 5). Bilateral PPC activity overlapped laterally with intraparietal cortex and medially with superior parietal cortex. At uncorrected threshold levels, fMRI-RS effects for the HR condition were also evident in left dorsal precentral and bilateral cingulate cortex (Inline Supplementary Fig. 5). At more liberal thresholds, effects within both the parietal operculum and dorsal premotor cortex were evident bilaterally.
Figure 5. Repetition suppression for HR condition.
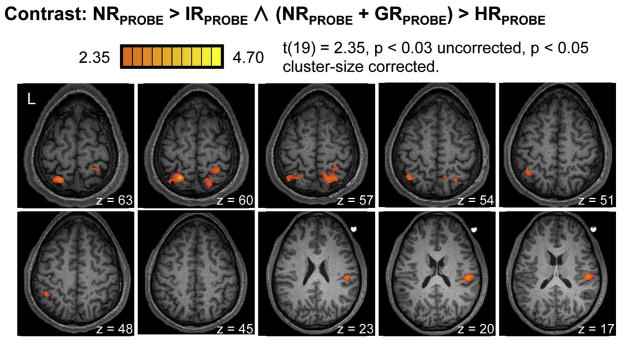
The contrast of NRPROBE > IRPROBE ∧ (NRPROBE + GRPROBE) > HRPROBE yielded significant fMRI-RS effects for the HR condition within left and right posterior parietal cortex and right-lateralized parietal operculum. At uncorrected thresholds, activity for this contrast was also evident in left dorsal precentral and bilateral cingulate cortices (Inline Supplementary Fig. 5). Similar results but at uncorrected thresholds were identified with the contrast NRPROBE > IRPROBE ∧ NRPROBE > HRPROBE (Inline Supplementary Fig. 2).
Similar results but at uncorrected thresholds were identified with the contrast NRPROBE > IRPROBE ∧ NRPROBE > HRPROBE (Inline Supplementary Fig. 2).
3.2.5. Region-of-interest (ROI) analyses
Two additional ROI analyses steps were performed on those areas showing significant fMRI-RS effects for the HR condition as identified with the contrast: NRPROBE > IRPROBE ∧ (NRPROBE + GRPROBE) > HRPROBE (Fig. 5).
First, it was important for us to directly test whether condition-specific changes in the fMRI signal within these areas were attributable to probe and not prime events. Figure 6 (A, B, C) shows the event-related averaged percent BOLD signal change (%-BSC) values per condition as a function of time from each of the areas that showed significant fMRI-RS for the HR condition. Notice that the signal strengths per condition corresponding in time with prime events tightly overlap, and only separate later, coincident with the timing of probe events. For each area, this was quantitatively supported by statistical comparisons of beta weights per condition-primes (Table 1).
Figure 6. ROI results.
Two additional analyses were performed on each of the areas showing fMRI-RS the HR condition as defined by the contrast: NRPROBE > IRPROBE ∧ (NRPROBE + GRPROBE) > HRPROBE (Fig. 5). (A, B, C) For each area, event-related averaged percent BOLD signal change (%-BSC) values per condition are shown as a function of time. Notice how signal changes attributable to prime events closely overlap across conditions. Differences between conditions arise later in time, attributable to probe events. These aspects of time-course data are also shown at finer scales (see insets), to better illustrate condition differences. Error bars reflect standard errors of the means at each time point. (D, E, F) For each area, the mean difference scores between beta weights for right- versus left-handed actions are indicated. Error bars reflect 95% confidence intervals. All three areas show evidence of contralateral effector-specificity (p’s < 0.001).
Table 1. Region-of-interest results.
Mean beta weights per condition are shown for both prime and probe predictors, extracted from each of the areas showing fMRI-RS for the HR condition as defined by the contrast NRPROBE > IRPROBE ∧ (NRPROBE + GRPROBE) > HRPROBE. Probe-condition beta values are shown for descriptive purposes only. Standard errors of means are indicated in brackets. Results of the single-factor RM-ANOVA revealed no significant differences in betas per condition for prime events. These results indicate that conditional differences in activity levels were entirely attributable to probe events.
| Area | Prime | RM-ANOVA | Probe | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IR | HR | GR | NR | IR | HR | GR | NR | ||
| L-PPC | 0.98 (0.12) | 1.02 (0.13) | 0.98 (0.13) | 0.99 (0.12) | F(2.3, 44.5) = 0.35, p = 0.74 | 0.78 (0.12) | 0.86 (0.12) | 0.94 (0.13) | 0.98 (0.14) |
| R-PPC | 0.93 (0.11) | 0.93 (0.11) | 0.88 (0.11) | 0.89 (0.10) | F(2.4, 46.5) = 1.27, p = 0.30 | 0.66 (0.09) | 0.74 (0.09) | 0.82 (0.11) | 0.85 (0.10) |
| R-POp | 0.71 (0.09) | 0.71 (0.10) | 0.67 (0.09) | 0.68 (0.10) | F(2.5, 47.6) = 1.61, p = 0.21 | 0.50 (0.07) | 0.56 (0.07) | 0.63 (0.08) | 0.62 (0.08) |
Second, we tested whether these same areas also showed preferential activity for right- versus left-handed actions, independent of RS effects. Conditions were redefined as involving either two successive left- or right-handed actions (made up of IR and HR conditions from the original model), and for each area, the beta weights assigned to these conditions were compared. The results revealed evidence of contralateral effector-specificity; each of the three areas identified showed stronger activity for actions involving the contralateral hand (Fig. 6D, E, F; all p’s < .001).
4. Discussion
The current study provides convergent behavioral and fMRI support for the hypothesis that action planning unfolds more efficiently when the same hand is used for successive actions. Participants are faster to initiate actions when hand is repeated, and these results are paralleled by fMRI-RS within bilateral PPC and right-lateralized POp. RS effects within PPC are localized to bilateral posterior intraparietal and superior parietal cortices, extending laterally and anteriorly within the left hemisphere, overlapping with anterior intraparietal cortex. These same areas show evidence of contralateral effector-specificity, activated more strongly for actions involving the contralateral hand.
We contend that these results reflect changes in the efficiency of neural response mechanisms important for planning goal-directed actions. Our interpretation is that relatively faster processing is possible when recently selected motor parameters can be reactivated rather than newly programmed. Accordingly, our findings provide evidence for the reactivation of limb-specific sensorimotor parameters within identified areas of bilateral PPC and right-hemisphere POp. This model not only accounts for our fMRI-RS results, but also the behavioral RT advantage we observe for performing successive actions with the same versus alternate hand (see also Valyear and Frey, 2014), as well as prior demonstrations of hand selection biases in favor of recent hand-use history (Rostoft et al., 2002; Weiss and Wark, 2009).
Several previous findings provide critical support for this interpretation. Repeated use of the same hand results in focal RS effects within brain areas – bilateral posterior intraparietal and superior parietal cortices, and left-lateralized anterior intraparietal cortex – previously implicated as important for grasp planning in the absence of overt movements (Jacobs et al., 2010; Johnson et al., 2002; Marangon et al., 2011). Notably, these prior imaging studies also consistently report strikingly similar patterns of left cerebral asymmetries in activation magnitudes and extents within anterior intraparietal and bilateral superior parietal cortices (see also Rushworth et al., 2003; Schluter et al., 2001). Consistently, other fMRI studies targeting preparatory activity prior to the performance of reaching/pointing (Astafiev et al., 2003; Beurze et al., 2007, 2009) and grasping (Gallivan et al., 2011) reliably identify similar areas. Beurze et al. (2007) showed that these areas maintain and integrate target with effector (left versus right hand) information prior to upcoming reaching movements (see also Beurze et al., 2009); entirely consistent with the current results showing hand-specific planning in these same areas. Also, prior fMRI-RS results suggest that anterior intraparietal cortex mediates the visual specification of action possibilities for object grasping and manipulation (Kroliczak et al., 2008; Valyear et al., 2012). Finally, TMS to PPC modulates hand selection (Oliveira et al., 2010). When reaching to targets with either hand, TMS to left (but not right) PPC was found to bias hand selection in favor of the ipsilateral hand. Altogether, this prior evidence is consistent with an interpretation of the current findings as more efficient processing within posterior parietal brain areas governing premovement planning when successive actions involve the same versus alternate hand.
Our results also implicate the POp, in particular within the right hemisphere (although bilateral effects were observed at reduced statistical thresholds). This activity closely corresponds with the expected location of secondary somatosensory cortex (SII) (Eickhoff et al., 2007). At least two previous fMRI studies reveal preferential involvement of SII for grasping versus reaching/pointing (Cavina-Pratesi et al., 2010; Frey et al., 2005). In monkeys, neural activity in SII not only reflects sensory discrimination but also response selection (Romo et al., 2002). We believe our results in POp reflect the influence of recent motor history on neural representations underlying high-level sensory information (e.g. proprioception). This information is important for action planning – e.g. to estimate both the current hand/body position and the anticipated sensory consequences of planned actions.
Although less robust, our results also reveal evidence for fMRI-RS for the HR condition within dorsal premotor and medial frontal cortex. These areas are densely interconnected with PPC (Wise et al., 1997), concurrently activated in prior studies of reach planning/control (Astafiev et al., 2003; Beurze et al., 2007; Prado et al., 2005), as well as implicit grasp planning (Jacobs et al., 2010; Johnson et al., 2002; Marangon et al., 2011). In monkeys, these areas are also known to underlie arm/hand selection and control (Cisek and Kalaska, 2005; Hoshi and Tanji, 2004, 2006). Additionally, previous imaging results implicate these areas as important for planning and hand selection during bimanual object manipulation (Theorin and Johansson, 2010).
The explanation for the comparatively weak fMRI-RS effects for hand repetition in dorsal premotor and medial frontal areas is unclear. It seems unlikely that this relates to differential sensitivity to repetition effects in premotor versus posterior parietal areas, in general; prior studies have shown fMRI-RS for repeated hand actions in premotor areas (Chouinard and Goodale, 2009; Dinstein et al., 2007; Kroliczak et al., 2008). One possibility is that the relatively weak RS effects observed in the current study relate to the means by which information about which hand to use was conveyed – i.e. via the visual features of target objects. This is speculative, however. Future studies are needed to further explore the potentially distinct levels of sensitivity to hand repetition across parietal-frontal areas, as the current results suggest.
4.1 The neural bases of hand-use history effects
How might the efficiency of planning processes change according to recent hand-use history? According to one prominent neurophysiological model known as the Affordance Competition Hypothesis (Cisek, 2007; Cisek and Kalaska, 2010), action planning involves resolving competition between concurrently activated neural populations within reciprocally connected parietofrontal circuits. These neural populations define the spatiotemporal parameters of potential actions and compete for selection through a complex interplay of excitation and inhibition (Cisek, 2006). Populations encoding similar metrics of possible actions excite one another, while populations encoding distinct metrics inhibit one another. When the activity of one population reaches a particular threshold, its activity levels further increase and competing populations are inhibited. The spatiotemporal metrics of the actions encoded by the suprathreshold-level population are selected, and this activity flows to primary motor cortex and ultimately to spinal cord machinery for the control of actions.
In keeping with this framework, persistent changes in baseline activity levels within neurons encoding hand-specific plans could account for the current fMRI-RS results. Baseline activity that remained elevated for recently selected (or suppressed for recently non-selected) populations would result in more (versus less) efficient planning for repeated versus non-repeated hand conditions. Differences in the durations of neural firing according to trial history can explain fMRI-RS (James and Gauthier, 2006).
Alternatively, if selection thresholds were lowered or raised for recently excited versus inhibited populations, or the accumulation rates of activity within these populations were modified according to recent hand-use history, this could account for relative changes in planning efficiency. Similarly, if the range of responsive populations were to be narrowed for successive actions involving the same hand, reduced fMRI activity levels would arise. This latter account coincides with previously proposed ‘sharpening’ models of priming and fMRI-RS (Wiggs and Martin, 1998). Notably, these possibilities are not mutually exclusive.
These accounts are speculative. However, trial history has been shown to modulate baseline activity in neurons governing the control of saccadic eye movements, and these changes correlate with reaction times to initiate saccades (Fecteau and Munoz, 2003). Other neural recording studies indicate that the threshold levels for saccade selection are modifiable according to task demands (Jantz et al., 2013). These data suggest that the above accounts are physiologically possible. Nonetheless, future neurophysiological investigations are required to substantiate these possibilities.
4.2. Online control
As an alternate interpretation, one could argue that our results also reflect repeated sensorimotor demands related to online limb control. While distal aspects of prime-probe actions – forearm, wrist, hand, and digit movements – for the HR condition were distinct, proximal control of the arm may have been similar. Accordingly, perhaps proximal control parameters were partly repeated, and this contributed to the RS effects observed.
While we accept that such an account is possible, for several reasons we consider our planning interpretation to be better justified. First, our fMRI-RS results are accompanied by behavioral evidence for more efficient premovement planning, reflected in reduced RTs to initiate actions when hand is repeated. While our planning interpretation accommodates both sets of results, a purely online control account does not. Second, both our behavioral and fMRI-RS results indicate limb-specific levels of processing separate from the encoding of distal elements of hand actions. While this response property is suitable for mediating planning processes – specifying which arm/hand to use for upcoming actions – it would seem to be of limited function for online control, where both proximal and distal movement features must be tightly coordinated. Relevant to this interpretation, neurophysiological evidence indicates areas within monkey PPC that show limb-specific planning (Cui and Andersen, 2007). Whereas cells within the parietal reach region are active for upcoming reach targets prior to effector selection, cells in superior parietal area 5d are responsive only after an effector choice is made (either autonomously or via instruction). Similar results were obtained for neurons within dorsal premotor cortex (Hoshi and Tanji, 2000). Here, some cells show preparatory-period activity for reach targets prior to instruction about which hand is to be used, while others are activated only after this information is specified. Thirdly, if fMRI-RS for the HR condition were to reflect more efficient processing underlying online sensorimotor control of the proximal segments of the arm, we would have also expected to detect effects within primary sensorimotor cortices, as shown previously for repeated actions with the same limb (Chouinard and Goodale, 2009; Hamilton and Grafton, 2009). For these reasons, our planning interpretation better accommodates our complete pattern of behavioral and fMRI results, is more parsimonious, and integrates more consistently with the existing literature.
4.3. Limitations and future directions
The current findings provide compelling new evidence for limb-specific levels of action planning in human PPC. Unfortunately, however, we are unable to determine whether the observed fMRI-RS effects are organized with respect to contra/ipsilateral limb planning. That is, it would be of value to determine if RS effects observed within left/right PPC show specificity for repeated actions with the contra- versus ipsilateral limb. However, the current design is insufficiently powered to enable separation of trials involving repeated left- versus right-handed actions. This presents an important avenue for future research.
Having noted this, the current data indicate that areas showing fMRI-RS for repeated use of the same hand are also more strongly activated for actions involving the contralateral hand (Fig. 7). In light of various previous findings, our results may be viewed as evidence for lateralized hand-specific action plans. In monkeys, neural activity levels within posterior parietal areas governing control of the arm for reaching correlate with RTs for actions made with the contra- but not ipsilateral arm (Chang et al., 2008), and inactivation of these areas selectively impairs control of the contralateral arm (Hwang et al., 2012). In humans, TMS to anterior intraparietal cortex results in grasping impairments specific to the contralateral hand (Rice et al., 2007), while stimulation applied to posterior-medial intraparietal areas impairs reaching with the contra- but not ipsilateral limb (Desmurget et al., 1999; although, see also Vesia et al., 2010, who showed evidence for more graded levels of contralateral specificity). Similarly, optic ataxic individuals with damage to PPC typically have more troubles reaching and grasping with the contralesional hand (in addition to field effects – i.e. worse performance for targets presented in the contralesional visual field) (Karnath and Perenin, 2005).
While our results indicate limb-specificity, prior imaging work targeting preparatory activity indicates a primarily left-lateralized network of parietofrontal areas underlying action planning independent of the hand used to perform those actions (Astafiev et al., 2003; Jacobs et al., 2010; Johnson et al., 2002; Schluter et al., 2001). As Jacobs et al. (2010) explain, however, overlapping activity for actions made with different effectors is necessary but not sufficient evidence for effector-independent representations. They show overlapping left-lateralized parietofrontal activations for grasping with the hand or a tool, regardless of whether the left or right limb was used. Importantly, the accompanying behavioral data clearly indicated reliance on effector-specific representations. Similarly, while the current results provide unequivocal evidence for limb-specific levels of processing, both left and right-handed action plans may be represented in these same areas. Indeed, recent findings with fMRI pattern analyses methods reveal both contra- and ipsilateral limb encoding for reaching/grasping within a number of parietofrontal areas (Gallivan et al., 2013). Again, this discussion underscores the importance of future experiments designed to test if RS effects show specificity for repeated actions with a particular hand.
4.4. Conclusions
The current investigation yields two important findings. First, our results suggest that recent hand-use history modulates the efficiency of neural events governing the planning of upcoming hand actions, localized within bilateral PPC and right POp. When successive actions involve the same hand planning processes unfold more efficiently, and as a result, both fMRI activity levels and behavioral response times to initiate actions are reduced. In turn, these results provide compelling new evidence for hand-specific levels of action planning within these brain areas. Gaining a better understanding of how the brain is organized with respect to hand-specific planning mechanisms may improve rehabilitation interventions for individuals suffering from movement problems. For example, application of neuromodulatory methods targeting posterior parietal brain areas governing hand-specific planning may promote the use and thus recovery of the affected limb of stroke survivors.
Supplementary Material
Highlights.
Participants use either hand to manipulate real objects in the MRI scanner.
Successive actions with the same hand are more efficiently planned.
Reduced response times and fMRI Repetition Suppression co-occur.
Hand-specific planning involves bilateral posterior parietal cortex.
Acknowledgments
This work was supported by a research grant from the National Institutes of Health to Scott Frey (NS053962) and a Postdoctoral Fellowship from the Natural Sciences and Engineering Council of Canada to Ken Valyear. We thank Ariel Shifter, Conan Zhu, Cortney Howard, Louie Markovitz, Ronny Wolfram, and Noah Marchal for their assistance with video analyses, data collection, and/or technical support. We also thank Delvin Mellerup for his support in developing the in-scanner grasping materials.
Footnotes
The near-significant Condition x Grasp interaction (p = 0.052) reflected a tendency for condition differences to be more pronounced for grasps/non-grasps involving rotation movements away from the acting hand.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23 (11):4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RL, Williams JM, Klassen LM, Gallivan JP, Culham JC, Menon RS. Evaluation of preprocessing steps to compensate for magnetic field distortions due to body movements in BOLD fMRI. Magnetic Resonance Imaging. 2010;28 (2):235–244. doi: 10.1016/j.mri.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. Journal of Neurophysiology. 2007;97 (1):188–199. doi: 10.1152/jn.00456.2006. [DOI] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP. Spatial and effector processing in the human parietofrontal network for reaches and saccades. Journal of Neurophysiology. 2009;101 (6):3053–3062. doi: 10.1152/jn.91194.2008. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Monaco S, Fattori P, Galletti C, McAdam TD, Quinlan DJ, Culham JC. Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. J Neurosci. 2010;30 (31):10306–10323. doi: 10.1523/JNEUROSCI.2023-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Dickinson AR, Snyder LH. Limb-specific representation for reaching in the posterior parietal cortex. J Neurosci. 2008;28 (24):6128–6140. doi: 10.1523/JNEUROSCI.1442-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Goodale MA. FMRI adaptation during performance of learned arbitrary visuomotor conditional associations. Neuroimage. 2009;48 (4):696–706. doi: 10.1016/j.neuroimage.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci. 2006;26 (38):9761–9770. doi: 10.1523/JNEUROSCI.5605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2007;362 (1485):1585–1599. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45 (5):801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Cohen RG, Rosenbaum DA. Where grasps are made reveals how grasps are planned: generation and recall of motor plans. Experimental Brain Research. 2004;157 (4):486–495. doi: 10.1007/s00221-004-1862-9. [DOI] [PubMed] [Google Scholar]
- Cohen RG, Rosenbaum DA. Prospective and retrospective effects in human motor control: planning grasps for object rotation and translation. Psychological Research. 2011;75 (4):341–349. doi: 10.1007/s00426-010-0311-6. [DOI] [PubMed] [Google Scholar]
- Cui H, Andersen RA. Posterior parietal cortex encodes autonomously selected motor plans. Neuron. 2007;56 (3):552–559. doi: 10.1016/j.neuron.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC. Human brain imaging reveals a parietal area specialized for grasping. In: Kanwisher N, Duncan J, editors. Attention and Performance XX: Functional Brain Imaging of Human Cognition. Oxford, U.K: Oxford University Press; 2004. [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nature Neuroscience. 1999;2 (6):563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. Journal of Neurophysiology. 2007;98 (3):1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon P, McAnsh S, Read L. Repetition effects in grasping. Canadian Journal of Experimental Psychology. 2012;66 (1):1–17. doi: 10.1037/a0026192. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply. 2. New York: Springer-Verlag Wien; 1999. completely revised and enlarged edition ed. [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cerebral Cortex. 2007;17 (8):1800–1811. doi: 10.1093/cercor/bhl090. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Exploring the consequences of the previous trial. Nat Rev Neurosci. 2003;4 (6):435–443. doi: 10.1038/nrn1114. [DOI] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Research Cognitive Brain Research. 2005;23 (2–3):397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Flanagan JR, Culham JC. Where one hand meets the other: limb-specific and action-dependent movement plans decoded from preparatory signals in single human frontoparietal brain areas. J Neurosci. 2013;33 (5):1991–2008. doi: 10.1523/JNEUROSCI.0541-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Valyear KF, Pettypiece CE, Culham JC. Decoding action intentions from preparatory brain activity in human parieto-frontal networks. J Neurosci. 2011;31 (26):9599–9610. doi: 10.1523/JNEUROSCI.0080-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10 (1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Repetition suppression for performed hand gestures revealed by fMRI. Human Brain Mapping. 2009;30 (9):2898–2906. doi: 10.1002/hbm.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks TD, Ditterich J, Shadlen MN. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nature Neuroscience. 2006;9 (5):682–689. doi: 10.1038/nn1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408 (6811):466–470. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential roles of neuronal activity in the supplementary and presupplementary motor areas: from information retrieval to motor planning and execution. Journal of Neurophysiology. 2004;92 (6):3482–3499. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. Journal of Neurophysiology. 2006;95 (6):3596–3616. doi: 10.1152/jn.01126.2005. [DOI] [PubMed] [Google Scholar]
- Hwang EJ, Hauschild M, Wilke M, Andersen RA. Inactivation of the parietal reach region causes optic ataxia, impairing reaches but not saccades. Neuron. 2012;76 (5):1021–1029. doi: 10.1016/j.neuron.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Danielmeier C, Frey SH. Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. Journal of Cognitive Neuroscience. 2010;22 (11):2594–2608. doi: 10.1162/jocn.2009.21372. [DOI] [PubMed] [Google Scholar]
- James TW, Gauthier I. Repetition-induced changes in BOLD response reflect accumulation of neural activity. Human Brain Mapping. 2006;27 (1):37–46. doi: 10.1002/hbm.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantz JJ, Watanabe M, Everling S, Munoz DP. Threshold mechanism for saccade initiation in frontal eye field and superior colliculus. Journal of Neurophysiology. 2013;109 (11):2767–2780. doi: 10.1152/jn.00611.2012. [DOI] [PubMed] [Google Scholar]
- Jax SA, Rosenbaum DA. Hand path priming in manual obstacle avoidance: evidence that the dorsal stream does not only control visually guided actions in real time. Journal of Experimental Psychology: Human Perception and Performance. 2007;33 (2):425–441. doi: 10.1037/0096-1523.33.2.425. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Heinze HJ. Selective activation of a parietofrontal circuit during implicitly imagined prehension. Neuroimage. 2002;17 (4):1693–1704. doi: 10.1006/nimg.2002.1265. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Perenin MT. Cortical control of visually guided reaching: evidence from patients with optic ataxia. Cerebral Cortex. 2005;15 (10):1561–1569. doi: 10.1093/cercor/bhi034. [DOI] [PubMed] [Google Scholar]
- Kelso JAS. On the oscillatory basis of movement. Bulletin of the Psychonomic Society. 1981;18(63) [Google Scholar]
- Kelso JAS, Buchanan JJ, Murata T. Multifunctionality and switching in the coordination dynamics of reaching and grasping. Human Movement Science. 1994;13 (1):63–94. [Google Scholar]
- Klatzky RL, Fikes TG, Pellegrino JW. Planning for hand shape and arm transport when reaching for objects. Acta Psychologica. 1995;88 (3):209–232. doi: 10.1016/0001-6918(93)e0068-d. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12 (5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroliczak G, McAdam TD, Quinlan DJ, Culham JC. The human dorsal stream adapts to real actions and 3D shape processing: a functional magnetic resonance imaging study. Journal of Neurophysiology. 2008;100 (5):2627–2639. doi: 10.1152/jn.01376.2007. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4 (4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangon M, Jacobs S, Frey SH. Evidence for context sensitivity of grasp representations in human parietal and premotor cortices. Journal of Neurophysiology. 2011;105 (5):2536–2546. doi: 10.1152/jn.00796.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco S, Cavina-Pratesi C, Sedda A, Fattori P, Galletti C, Culham JC. Functional magnetic resonance adaptation reveals the involvement of the dorsomedial stream in hand orientation for grasping. Journal of Neurophysiology. 2011;106 (5):2248–2263. doi: 10.1152/jn.01069.2010. [DOI] [PubMed] [Google Scholar]
- Monaco S, Chen Y, Medendorp WP, Crawford JD, Fiehler K, Henriques DY. Functional magnetic resonance imaging adaptation reveals the cortical networks for processing grasp-relevant object properties. Cerebral Cortex. 2014;24 (6):1540–1554. doi: 10.1093/cercor/bht006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9 (1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, Diedrichsen J, Verstynen T, Duque J, Ivry RB. Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107 (41):17751–17756. doi: 10.1073/pnas.1006223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino JW, Klatzky RL, McCloskey BP. Timecourse of preshaping for functional responses to objects. J Mot Behav. 1989;21 (3):307–316. doi: 10.1080/00222895.1989.10735484. [DOI] [PubMed] [Google Scholar]
- Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT. Two cortical systems for reaching in central and peripheral vision. Neuron. 2005;48 (5):849–858. doi: 10.1016/j.neuron.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Rice NJ, Tunik E, Cross ES, Grafton ST. On-line grasp control is mediated by the contralateral hemisphere. Brain Research. 2007;1175:76–84. doi: 10.1016/j.brainres.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, Lemus L, Brody CD. Neuronal correlates of decision-making in secondary somatosensory cortex. Nature Neuroscience. 2002;5 (11):1217–1225. doi: 10.1038/nn950. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Chapman KM, Weigelt M, Weiss DJ, van der Wel R. Cognition, action, and object manipulation. Psychological Bulletin. 2012;138 (5):924–946. doi: 10.1037/a0027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA, Jorgensen MJ. Planning macroscopic aspects of manual control. Human Movement Science. 1992;11:61–69. [Google Scholar]
- Rostoft MS, Sigmundsson H, Whiting HT, Ingvaldsen RP. Dynamics of hand preference in 4 year-old children. Behavioural Brain Research. 2002;132 (1):59–68. doi: 10.1016/s0166-4328(01)00415-6. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20(Suppl 1):S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39 (2):105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Schutz C, Weigelt M, Odekerken D, Klein-Soetebier T, Schack T. Motor control strategies in a continuous task space. Motor Control. 2011;15 (3):321–341. doi: 10.1123/mcj.15.3.321. [DOI] [PubMed] [Google Scholar]
- Short MW, Cauraugh JH. Planning macroscopic aspects of manual control: end-state comfort and point-of-change effects. Acta Psychologica. 1997;96 (1–2):133–147. doi: 10.1016/s0001-6918(97)00006-1. [DOI] [PubMed] [Google Scholar]
- Sorensen V, Ingvaldsen RP, Whiting HT. The application of co-ordination dynamics to the analysis of discrete movements using table-tennis as a paradigm skill. Biological Cybernetics. 2001;85 (1):27–38. doi: 10.1007/PL00007994. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Castiello U, Jeannerod M. Orienting the finger opposition space during prehension movements. J Mot Behav. 1994;26 (2):178–186. doi: 10.1080/00222895.1994.9941672. [DOI] [PubMed] [Google Scholar]
- Sternberg S, Monsell S, Knoll RL, Wright CE. The latency and duration of rapid movement sequences: Comparisons of speech and typewriting. In: Stelmach GE, editor. Information Processing in Motor Control and Learning. New York: Academic Press, Inc; 1978. pp. 117–152. [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Theorin A, Johansson RS. Selection of prime actor in humans during bimanual object manipulation. J Neurosci. 2010;30 (31):10448–10459. doi: 10.1523/JNEUROSCI.1624-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyear KF, Frey SH. Hand selection for object grasping is influenced by recent motor history. Psychonomic Bulletin & Review. 2014;21 (2):566–573. doi: 10.3758/s13423-013-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyear KF, Gallivan JP, McLean DA, Culham JC. fMRI repetition suppression for familiar but not arbitrary actions with tools. J Neurosci. 2012;32 (12):4247–4259. doi: 10.1523/JNEUROSCI.5270-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesia M, Prime SL, Yan X, Sergio LE, Crawford JD. Specificity of human parietal saccade and reach regions during transcranial magnetic stimulation. J Neurosci. 2010;30 (39):13053–13065. doi: 10.1523/JNEUROSCI.1644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ, Wark J. Hysteresis effects in a motor task with cotton-top tamarins (Sanguinus oedipus) Journal of Experimental Psychology: Animal Behavior Processes. 2009;35 (3):427–433. doi: 10.1037/a0013964. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8 (2):227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annual Review of Neuroscience. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.