Abstract
Background & Aims
Celiac disease (CeD) is a prevalent autoimmune condition. Recurrent signs and symptoms are common despite treatment with a gluten free diet (GFD), yet no approved or proven non-dietary treatment is available.
Methods
In this multicenter randomized, double-blind, placebo-controlled study, we assessed larazotide acetate 0.5, 1, or 2 mg three times daily to relieve ongoing symptoms in 342 adults with CeD who had been on a GFD for ≥12 months and maintained their current GFD during the study. The study included a 4-week placebo run-in, 12-week treatment, and 4-week placebo run-out phase. The primary endpoint was the difference in average on-treatment Celiac Disease Gastrointestinal Symptom Rating Scale score (CeD-GSRS).
Results
The primary endpoint was met at the 0.5 mg dose of larazotide acetate with fewer symptoms compared with placebo by Modified Intention to Treat (n=340) (ANCOVA p=0.022, MMRM p=0.005). The 0.5mg dose showed effect on exploratory endpoints including, 26% decrease in Celiac Disease Patient Reported Outcome Symptomatic Days (p=0.017); 31% increase in Improved Symptom Days (p=0.034); ≥50% reduction from baseline of weekly average Abdominal Pain Score for ≥6 out of 12 weeks of treatment (p=0.022); and a decrease in Non-GI symptoms of headache and tiredness (p=0.010). The 1 and 2 mg doses were no different than placebo for any endpoint. Safety was comparable to placebo.
Conclusions
Larazotide acetate 0.5 mg reduced signs and symptoms in CeD patients on a GFD better than a GFD alone. While results were mixed, this study represents the first successful trial of a novel therapeutic agent targeting Tight Junction regulation in patients with CeD who are symptomatic despite a GFD. Clinicaltrials.gov, NCT01396213
Keywords: Celiac disease, gluten, therapeutic, tight junction
INTRODUCTION
Celiac disease (CeD), a genetic autoimmune condition, affects ~1% of the western population.1, 2 CeD is triggered by ingestion of gluten-containing foods and managed by a gluten-free diet (GFD).3, 4 Recurrent CeD signs and symptoms due to inadvertent or deliberate gluten exposure have been reported in approximately 70% of CeD patients on a GFD.5, 6 While persistent symptoms may have a variety of causes, one potential source is sporadic gluten exposure,7 which may contribute to persistent enteropathy, continued symptoms, and reduced quality of life.
In CeD, paracellular permeability is increased by an inflammatory response to gluten entry into the intestinal mucosa.8 Increased permeability promotes gluten peptide transport to gut-associated lymphoid tissue, initiating inflammatory cytokine release and T-cell recruitment.8–10 An intestinal permeability-inflammation loop is established, leading to a multitude of gastrointestinal and systemic manifestations.11
Larazotide acetate is a novel, locally acting non-systemic, synthetic 8-amino acid oral peptide, discovered during functional screening of synthetic Vibrio cholera related peptides. Larazotide acetate is a first-in-class tight junction (TJ) regulator under development as an adjunct to a GFD. Larazotide acetate appears to prevent opening of intestinal TJs by promoting TJ assembly and actin filament rearrangement, which prevents gluten from reaching the intestinal submucosa and triggering an inflammatory response (Supplementary Figure S-1).8, 12
Nonclinical studies with larazotide acetate have demonstrated proof-of-concept of TJ regulation including the inhibition of gliadin-induced TJ alteration, macrophage recruitment and increases in intestinal permeability.8, 12, 13 In four prior clinical trials, larazotide acetate demonstrated a safety profile comparable to placebo.14–16 In initial clinical trials utilizing gluten challenge, larazotide acetate prevented gluten-induced symptoms and blunted increases in anti-tTG antibodies, INF-gamma, and intestinal permeability.14–16 These results provided the foundation for the current Phase IIb study.
Despite being a common condition that often responds incompletely to GFD, there is currently no approved non-dietary treatment for CeD.17, 18 This multicenter, randomized, placebo-controlled trial is the largest reported clinical trial conducted in CeD and was designed to assess efficacy and safety of larazotide acetate as an adjunct to a GFD in adult patients with persistent symptoms despite maintenance of a long term GFD. A secondary objective was to validate the Celiac Disease Patient Reported Outcome (CeD PRO) instrument as a daily measure of therapeutic effects.
METHODS
The protocol was approved by relevant institutional review boards. Patients provided written informed consent, the study was conducted according to Good Clinical Practice and registered on Clinicaltrials.gov (NCT01396213).
Patients
Entry criteria were: age 18–75 years, BMI 16–45 kg/m2, CeD confirmed by intestinal biopsy or capsule endoscopy (capsule endoscopy was the entry criteria for 7 of 342 patients randomized) plus positive serology 12 months before study entry, maintenance of a GFD for ≥12 consecutive months before screening, and adherence to current GFD on study. Underweight patients (BMI 16–18.5) were included as these patients were felt by the investigators to reflect patients who were underweight due to active celiac disease and thus, would be most likely to benefit from therapy but yet not at significantly increased risk due to severe malabsorption or other conditions. All celiac serologies were performed centrally using the INOVA Quanta-Flash assay at Mayo Clinical Laboratories. The cutoff for levels for positive for tTG IgA and IgG were 4.0 and 6.0, respectively. The cutoff levels for positive Deamidated Gliadin Peptide (DGP) IgA and IgG were 20. In order to evaluate study-related changes in serologic titers, patients with undetectable anti-tTG and anti-DGP antibody levels were excluded. Patients experienced at least one gluten-related symptom (diarrhea, abdominal pain, bloating, nausea) in the month before screening, and at screening, were required to have a qualifying score of ≥2, reflecting ‘mild discomfort’ on the CeD domains of the Gastrointestinal Symptom Rating Scale19 (CeD-GSRS). The GSRS and CeD-GSRS, which contains 10 items from the GSRS, Diarrhea, Abdominal Pain, and Indigestion domains (Supplementary Appendix A), have been used in multiple trials of CeD14, 20–22 and other GI disorders.23, 24 All survey data was collected daily from patients using an electronic clinical outcome assessment data collection device (Bracket Global, Wayne, PA,).
Exclusion criteria included refractory CeD, severe CeD complications (e.g., enteropathy-associated T-cell lymphoma), other chronic inflammatory GI disease (e.g., inflammatory bowel disease), diabetes, or autoimmune, psychiatric, or neurological disease that could interfere with assessments. Smoking, pregnancy or breastfeeding, previous exposure to larazotide acetate, concomitant use of systemic or intestinal immune suppressants, continuous antibiotics, non-steroidal anti-inflammatory drugs, and medications that alter gastric pH or intestinal permeability were prohibited.
Study Design and Procedures
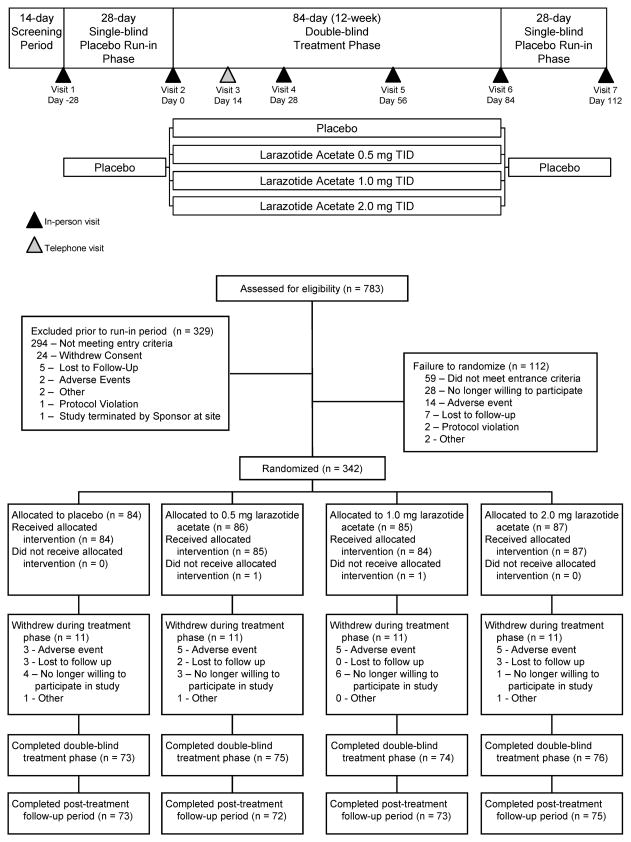
This 20-week study was conducted in three phases: a 4-week single-blind placebo run-in phase, a 12-week double-blind treatment phase, and a 4-week placebo run-out phase (Figure 2). A qualifying score of ≥2 on the CeD-GSRS (Supplementary Appendix A) was required for randomization.
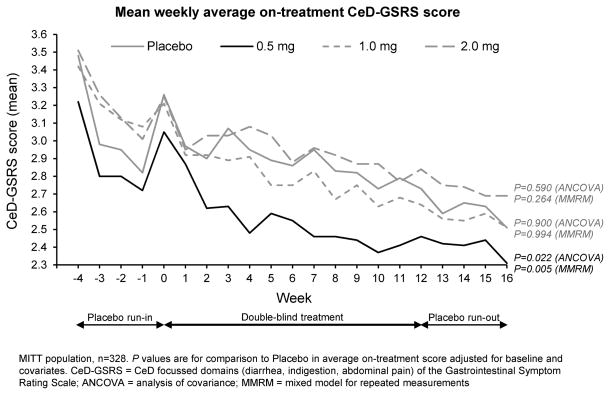
Figure 2. Primary Endpoint: Average on-treatment scores on the CeD-GSRS.
The 0·5 mg larazotide acetate dose met the primary endpoint.
Randomization and masking
Patients were stratified into four groups according to gender (85%:15%, female: male) and baseline CeD-GSRS scores (<3 or ≥3). Randomization was performed using permuted-block randomization and was kept confidential until the study was unblinded. Randomization was 1:1:1:1 to larazotide acetate 0·5mg, 1mg, or 2mg capsules, or placebo in identical capsules.
Following the 4-week placebo run-in, study drugs were self-administered three times daily (TID), 15 minutes before meals. Patients returned unused capsules for drug compliance assessment.
Blood was collected at visits 2, 4, 6, and 7 or early termination for chemistry, hematology, anti-tTG and anti-DGP antibodies.
Patients rated CeD symptoms daily on the CeD PRO and weekly with the CeD-GSRS. The CeD PRO developed by the Sponsor in accordance with the US Food and Drug Administration’s SEALD guidelines25 was initially validated in this trial (Supplementary Appendix B). Patients completed the CeD PRO using an electronic clinical outcome assessment data collection device. Responses were scored on an 11-point (0–10) Likert scale with higher scores indicating greater symptom severity. Results represent averages for each item in a domain or subdomain, aggregated over a 7-day period.
Endpoint Measures
The primary endpoint was the difference in average weekly on-treatment CeD-GSRS score for each dose versus placebo, over the 12-week active treatment period. The CeD-GSRS captures treatment effect over time in this disease characterized by chronic and variable flares with episodic symptoms.14, 20, 26 Symptom improvement was chosen as a primary endpoint in accordance with regulatory guidance.25 Secondary endpoints were change from baseline in CeD-GSRS score, average weekly on-treatment score, and change from baseline in both the CeD PRO GI and Abdominal domain scores.
Exploratory endpoints included average weekly on-treatment differences in total and individual GSRS domain scores; number of patients experiencing ≥50% reduction from baseline weekly average CeD PRO Abdominal domain scores for ≥6 of 12 weeks; average on-treatment weekly number of bowel movements and stool consistency measured using the Bristol Stool Form Scale; average on-treatment weekly number of CeD PRO GI Symptomatic Days, defined as mean CeD PRO Abdominal domain scores ≥3; or Diarrhea and Loose Stool domain score ≥3; average on-treatment weekly number of Improved Symptom Days, defined as mean CeD PRO Abdominal domain scores ≤ 1·5; and Diarrhea and Loose Stool domain score ≤1·5; CeD PRO Non-GI domain (headache and tiredness) scores; and changes in anti-tTG and anti-DGP antibody levels over the treatment phase.
GFD compliance was assessed using the Gluten-Free Diet Compliance Questionnaire (GFDCQ; Supplementary Appendix C), administered at week 16 and assessed voluntary and/or inadvertently ingested gluten on-study.
Safety assessments included frequency and severity of treatment-emergent AEs (TEAEs), serious AEs (SAEs), clinical laboratory parameters (hematology, chemistry, urinalysis, see also table S-2), ECGs, and vital signs.
Statistical Analyses
Estimates for treatment effect and variability of changes from baseline in CeD-GSRS scores in the CLIN1001-006 study14 were used to determine sample size. Based on a standard deviation of σ =0·548 and type-1 error rate of α =0·05, and assuming a 14% drop-out rate, 80 subjects per treatment group (320 total) would provide 80% power to detect a 0·3-point change from baseline difference in CeD-GSRS score between larazotide acetate doses and placebo.
Efficacy analyses included all patients receiving ≥1 dose of study drug during double-blind treatment and had ≥1 post-baseline assessment (Modified Intent-to-Treat [MITT] population). Efficacy results for larazotide acetate doses were independently compared with placebo. An analysis of covariance (ANCOVA) was used for the primary, secondary, and exploratory endpoints related to GSRS, CeD-GSRS, and CeD PRO scores, with treatment, gender, baseline CeD-GSRS randomization stratum, and randomization cohort as fixed effects, and baseline score as a covariate. Sensitivity analyses were conducted using Mixed Model for Repeated Measures (MMRM). MMRM analyses included treatment and study week as main effects, gender, baseline CeD-GSRS randomization stratum, randomization cohort, and baseline score as covariates, and weekly CeD PRO scores as repeated measures. Baseline score was the last non-missing observation before the first dose of investigational drug. Proportion of patients with ≥50% reduction from baseline CeD PRO Abdominal domain scores for ≥6 of 12 weeks was assessed using a Cochrane-Mantel-Haenszel test for between-treatment comparison, stratified by gender, baseline CeD-GSRS, randomization stratum, and randomization cohort.
Safety assessments were performed for all patients who received ≥1 dose of study drug.
Role of the Funding Source
The principal investigator and leading co-investigators designed the study in collaboration with Alba Therapeutics Corporation and Cephalon/Teva, conducted the study, and provided oversight for data collection. Statistical analysis including sensitivity analysis was jointly designed a priori by the authors and Alba Therapeutics. Data analysis was performed by Chao Wang, PhD and John Han, PhD of PharmaData Associates, funded by Alba and Cephalon/Teva. All authors contributed to data interpretation and writing and editing the manuscript. Drs. Leffler and Murray had full access to all study data, contributed equally to manuscript preparation, and had final responsibility for the publication.
RESULTS
Study Population
This study was conducted at 74 North American sites. A total of 783 patients were screened and 454 participants entered the placebo run-in phase (Figure 1). At the end of placebo run-in 342 patients were randomized to receive placebo (n=84), or larazotide acetate 0·5mg (n=86), 1mg (n=85), or 2mg (n=87) TID. Two randomized patients were lost-to-follow-up before receiving drug, leaving 340 patients for MITT. Discontinuation rate during double-blind treatment was consistent across treatment groups (11 patients per arm), most frequently due to AEs (n=18, Supplementary Table S-1) or unwillingness to participate (n=14). Mean (SD) treatment duration for all patients was 80 (15·5) days. Patient characteristics were similar across treatment groups. (Table 1).
Figure 1.
Study Design and Patient Disposition
Table 1.
Patient Demographics and Disease Characteristics
| Larazotide Acetate (n=258) | |||||
|---|---|---|---|---|---|
|
| |||||
| Placebo (n=84) | 0·5 mg TID (n=86) | 1·0 mg TID (n=85) | 2·0 mg TID (n=87) | Total (N=342) | |
|
| |||||
| General characteristics | |||||
| Age (years), mean [SD] | 45·5 [14·6] | 44·2 [14·4] | 46·2 [15·0] | 44·7 [16·0] | 45·2 [15·0] |
| Female, n (%) | 69 (82·1) | 73 (84·9) | 71 (83·5) | 72 (82·8) | 285 (83·3) |
| White, n (%) | 83 (98·8) | 84 (97·7) | 85 (100) | 86 (98·9) | 338 (98·8) |
| Weight (kg), mean [SD] | 74·3 [16·8] | 75·5 [16·0] | 72·2 [15·4] | 73·4 [15·3] | 73·9 [15·8] |
| Height (cm), mean [SD] | 167·3 [9·6] | 165·4 [8·9] | 166·2 [8·6] | 167·4 [8·5] | 166·5 [8·9] |
| Body Mass Index (kg/m2), mean [SD] | 26·6 [5·4] | 27·6 [5·3] | 26·0 [4·6] | 26·1 [4·6] | 26·6 [5·0] |
|
| |||||
| Clinical characteristics | |||||
| Time since CeD symptom onset (months), mean [SD] | 145·5 [141·1] | 149·2 [144·8]a | 162·9 [150·6]a | 174·6 [174·7] | 158·1 [158·3]a |
| Time since CeD diagnosis* (months), mean [SD] | 60·3 [51·3] | 58·0 [50·1]b | 66·7 [68·2]b | 71·5 [65·07]b | 64·1 [59·2]b |
| Time on most recent GFD* (months), mean [SD] | 62·1 [58·7] | 60·3 [60·1] | 71·4 [86·2] | 70·9 [63·7] | 66·2 [68·0] |
|
| |||||
| Symptoms at diagnosis, n (%) | |||||
| Diarrhea | 67 (79·8) | 68 (79·1) | 69 (81·2) | 64 (73·6) | 268 (78·4) |
| Bloating | 75 (89·3) | 77 (89·5) | 78 (91·8) | 78 (89·7) | 308 (90·1) |
| Abdominal distention/ Stomach swelling | 64 (76·2) | 62 (72·1) | 68 (80·0) | 68 (78·2) | 262 (76·6) |
| Recurrent abdominal pain | 72 (85·7) | 78 (90·7) | 76 (89·4) | 75 (86·2) | 301 (88·0) |
|
| |||||
| Scores at baseline, mean [SD] | |||||
| CeD-GSRS | 3·26 [0·92] | 3·05 [0·84] | 3·21 [0·77] | 3·25 [0·96] | Not Calculated |
| Total GSRS | 3·03 [0·82] | 2·89 [0·80] | 3·03 [0·75] | 3·09 [0·89] | |
| CeD PRO Abdominal Domain | 2·56 [1·71] | 2·19 [1·34] | 2·63 [1·59] | 2·90 [1·58] | |
| CeD PRO GI Domain | 2·27 [1·46] | 1·86 [1·10] | 2·23 [1·29] | 2·35 [1·29] | |
| Average weekly number of bowel movements† | 9·00 [7·11] | 7·59 [4·62] | 7·25 [4·78] | 7·95 [5·45] | |
Assessed at screening visit
As recorded in the Bristol Stool Form Scale (BSFS) daily diary
Data missing for one patient each in the 0·5 mg and 1·0 mg TID group
Data missing for one patient in each of the three larazotide acetate treatment groups
CeD = Celiac Disease; GFD = gluten-free diet; GSRS = Gastrointestinal Symptom Rating Scale; CeD-GSRS = Celiac Disease focused domains of the Gastrointestinal Symptom Rating Scale; CeD PRO = Celiac Disease Patient Reported Outcome; BSFS = Bristol Stool Form Scale
There were no significant differences between cohorts in any measure
Patients reported multiple CeD symptoms during the placebo run-in: 97% gas, 92% bloating, 79% abdominal cramping, 80% pain, and 67% loose stools. Constipation, nausea, diarrhea, and vomiting were reported by 57%, 50%, 44%, and 4% of patients, respectively. Non-GI symptoms, headache and tiredness, were each reported by 70% of patients.
Efficacy
Primary endpoint
The primary endpoint, improved average on-treatment CeD-GSRS score versus placebo was met at the 0·5mg larazotide acetate dose (ANCOVA p=0·022, MMRM p=0·005) but not for the 1mg or 2mg doses. (Table 2) Consistent with prior studies,14 mean CeD-GSRS scores trended down during the placebo run-in, then increased at week 0 when patients not meeting the mean weekly CeD-GSRS score eligibility threshold were discontinued (Figure 2). Symptomatic improvement with larazotide acetate 0·5mg was evident by treatment week 2 and was sustained over the 12-week treatment period. Higher larazotide acetate doses were not significantly different from placebo. Per-protocol results were similar for the primary endpoints with improved average on-treatment CeD-GSRS score versus placebo at the 0·5mg larazotide acetate dose (ANCOVA p=0·007, MMRM p=0·001). Similarly, results remained significant after adjustment for both age (ANCOVA 0.020, MMRM 0.005) and BMI (ANCOVA 0.017, MMRM 0.004).
Table 2.
Efficacy Endpoints in the MITT Population
| Placebo (n=84) | 0·5 mg TID (n=86) | 1·0 mg TID (n=85) | 2·0 mg TID (n=87) | |
|---|---|---|---|---|
|
| ||||
| PRIMARY ENDPOINT | ||||
|
| ||||
| Average on-treatment CeD-GSRS score, mean [SD] | 2·88 [0·72] | 2·59 [0·7] | 2·84 [0· 8] | 2·92 [0·8] |
| P value vs. placebo - ANCOVA, MMRM | - | p=0·022, p=0·005 | NS, NS | NS, NS |
|
| ||||
| SECONDARY ENDPOINTS | ||||
|
| ||||
| CeD-GSRS score change from baseline to end of treatment, mean [SD] | −0·50 [1·0] | −0·54 [0·9] | −0·40 [1·0] | −0·37 [1·0] |
| P value vs. placebo - ANCOVA, MMRM | NS, p=0·041 | NS, NS | NS, NS | |
| Average on-treatment score - CeD PRO Abdominal Domain, mean [SD] | 2·27 [1·3] | 2·04 [1·3] | 2·59 [1·6] | 2·77 [1·5] |
| P value vs. placebo - ANCOVA, MMRM | NS, NS | NS, p=0·036 | NS, NS | |
| Average on-treatment score – CeD PRO GI Domain, mean [SD] | 2·05 [1·19] | 1·69 [1·01] | 2·21 [1·33] | 2·31 [1·26] |
| P value vs. placebo - ANCOVA, MMRM | NS, NS | NS, NS | NS, NS | |
| Change from baseline to end treatment - CeD PRO Abdominal Domain, mean [SD] | −0·43 [1·73] | −0·26 [1·47] | −0·18 [1·27] | −0·16 [1·32] |
| P value vs. placebo - ANCOVA, MMRM | NS, NS | NS, NS | 0·023, NS | |
| Change from baseline to end treatment - CeD PRO GI Domain, mean [SD] | −0·38 [1·45] | −0·28 [1·24] | −0·13 [1·00] | 0·00 [1·23] |
| P value vs. placebo - ANCOVA, MMRM | NS, NS | NS, NS | 0·013, 0·028 | |
|
| ||||
| EXPLORATORY ENDPOINTS | ||||
|
| ||||
| Average on-treatment total GSRS score, mean [SD] | 2·70 [0· 7] | 2·47 [0·7] | 2·70 [0·8] | 2·76 [0·8] |
| P value vs. placebo - ANCOVA, MMRM | p=0·017, p=0·004 | NS, NS | NS, NS | |
| Average on-treatment scores of individual GSRS Domains, mean [SD] | ||||
| ANCOVA p value vs. placebo | ||||
| Diarrhea | 2·77 [1·2] | 2·40 [1·1], NS | 2·61 [1·1], NS | 2·74 [1·21], NS |
| Indigestion | 3·18 [0·8] | 2·87 [0·8] p=0·029 | 3·18 [1·0], NS | 3·23 [0·88], NS |
| Constipation | 2·58 [1·1] | 2·45 [1·0], p=0·050 | 2·64 [1·1], NS | 2·61 [1·14], NS |
| Abdominal pain | 2·58 [0·9] | 2·42 [0·8], p=0·024 | 2·62 [0·8], NS | 2·68 [0·94], NS |
| Reflux | 2·00 [1·1] - |
1·87 [0·9], NS | 2·08 [1·1], NS | 2·19 [1·07], NS |
| ≥50% reduction from baseline in weekly average score for ≥6 weeks, n (%), CMH p value vs. placebo | ||||
| CeD-GSRS | 1 (1·2) | 6 (7·2), NS | 1 (1·2), NS | 1 (1·2), NS |
| CeD PRO Abdominal Domain | 12 (14·3) | 24 (28·6), p=0·022 | 12 (14·3), NS | 17 (19·5), NS |
| CeD PRO GI Domain | 14 (16·7) | 29 (34·5) p=0·002 | 15 (17·9), NS | 15 (17·2), NS |
| Average weekly number of bowel movements (BSFS)‡, mean [SD] | 8·96 [7·0] | 6·90 [4·2] | 7·20 [4·2] | 8·72 [5·9] |
| ANCOVA p value vs. placebo* | NS | NS | NS | |
| Average on-treatment weekly number of CeD PRO Symptomatic Days | 2·38 [2·01] | 1·73 [1·7] | 2·48 [2·0] | 2·63 [1·8] |
| ANCOVA p value vs. placebo | p=0·017 | NS | NS | |
| Average on-treatment weekly number of CeD PRO Improved Symptom Days | 1·99 [2·0] | 2·51 [1·9] | 1·80 [1·9] | 1·46 [1·6] |
| ANCOVA p value vs. placebo | p=0·034 | NS | NS | |
| Average on-treatment CeD PRO Non-GI Domain score, mean [SD] | 2·75 [1·55] | 2·44 [1·4] | 2·73 [1·4] | 3·11 [1·8] |
| ANCOVA, MMRM p value vs. placebo | - | NS, p=0·010 | NS, NS | NS, NS |
As recorded in the Bristol Stool Form Scale (BSFS) daily diary
CeD-GSRS = Celiac Disease focused domains of the Gastrointestinal Symptom Rating Scale; CeD PRO = Celiac Disease Patient Reported Outcome; ANCOVA = Analysis of covariance; MMRM = Mixed Model for Repeated Measures; CMH = Cochrane-Mantel-Haenszel; NS = not statistically significant (p>0·05)
All statistical analyses include baseline score, gender, baseline GSRS Stratum, and randomization cohort as covariates.
Secondary endpoints
Numeric differences in favor of larazotide acetate 0·5mg were observed in average on-treatment scores although not all achieved statistical significance (Table 2). Improvement from baseline in mean CeD-GSRS score was greater in the larazotide acetate 0·5mg group (MMRM p=0·041). Average on-treatment scores and changes from baseline scores in the CeD PRO Abdominal and GI domains favored the 0·5mg treatment arm but were not statistically significant. No significant improvements were noted for any secondary endpoint at higher larazotide acetate doses.
Exploratory Endpoints
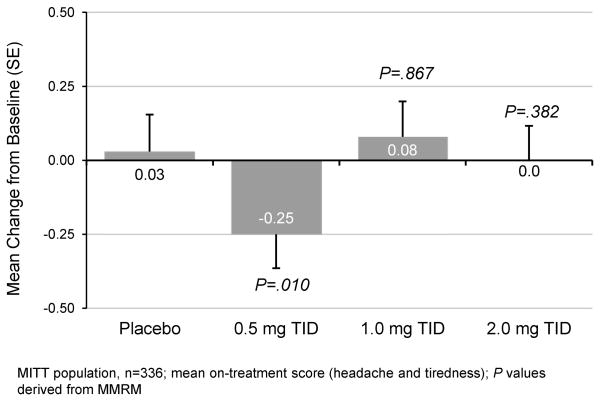
Exploratory endpoints also supported the effectiveness of 0·5mg TID larazotide acetate (Table 2). Average on-treatment total GSRS score decreased in the 0·5mg larazotide acetate group versus placebo (ANCOVA p=0·017; MMRM p=0·004). Average on-treatment CeD PRO Non-GI domain scores for headache and tiredness were also lower in the 0·5 mg larazotide acetate group (ANCOVA NS, MMRM p=0·010) (Supplementary Figure S-2).
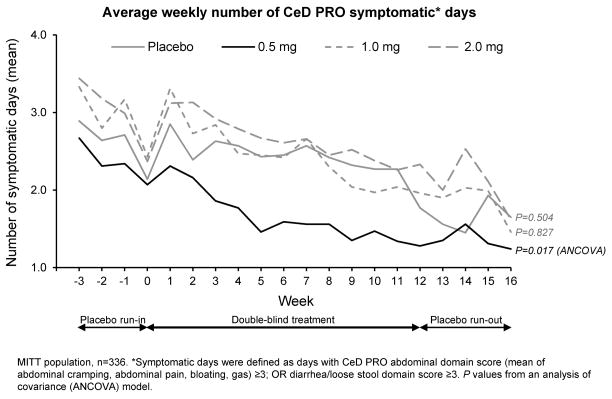
Patients receiving 0·5mg larazotide acetate had 26% fewer CeD PRO GI Symptomatic Days (defined a priori as a day with a mean score of ≥3 on either the abdominal symptom or diarrhea/loose stool domains) than patients receiving placebo. Average on-treatment weekly number of CeD PRO GI Symptomatic Days was 1·73 for 0·5mg larazotide acetate versus 2·38 for placebo ANCOVA p=0·017; Figure 3), indicating reductions of 0·56 days/week, and an overall reduction of 6·72 symptomatic days over the treatment period versus placebo. This finding was accentuated in subanalysis of patients with ≥3 symptomatic days per week at baseline (n=60). In this cohort, there was a reduction of 1.89 days per week in the 0.5mg arm compared to 0.58 days per week in the placebo arm, a net decrease of 15.72 fewer symptomatic days during the 12 weeks of treatment.
Figure 3.
Larazotide Acetate 0·5 mg TID Reduced Average On-Treatment Weekly Number of CeD PRO Symptomatic* Days
Similarly, in the 0·5mg larazotide arm there was a 31% increase in average on-treatment weekly number of CeD PRO Improved Symptom Days (2·51 versus 1·99 with placebo, ANCOVA p=0·034), an increase of 0·49 days/week or 5·88 days over the treatment period. Weekly average CeD PRO Abdominal domain scores were reduced ≥50% from baseline for ≥6 weeks in the larazotide acetate 0·5mg arm (ANCOVA p=0·022).
In the subset of patients reporting the highest number of GI Symptomatic Days scores (5–7/week), there was a median reduction of 2·21 GI Symptomatic Days per week with 0·5mg larazotide acetate, versus a median increase of 0·08 days/week with placebo. There was no change or worsening from baseline anti-tTG or anti-DGP antibody titers in any treatment group (Supplementary Figure S-3). On the Gluten-Free Diet Compliance Questionnaire, 35% and 59% of patients in the larazotide acetate 0·5mg group reported having voluntary or accidental gluten exposure, respectively, compared with 27% and 52% in the placebo group.
Safety and Tolerability
Larazotide acetate safety and tolerability were comparable with placebo at all dose levels (Supplementary Tables S-2 and S-3). No significant changes were noted in vital signs, laboratory measures, or ECGs at any larazotide acetate dose. The most frequent TEAEs by system organ class were GI disorders and were equally frequent in all treatment groups. No significant change in iron status was noted with treatment in any of the treatment groups. There were no drug-related SAEs.
DISCUSSION
Larazotide acetate 0·5mg TID improved signs and symptoms of CeD among patients with persistent symptoms despite a GFD. Conversely, higher treatment doses showed no effect. This is the largest randomized controlled trial in CeD and the first and only trial of a novel TJ therapeutic agent for CeD to meet its primary endpoint. Larazotide acetate reduced GI and non-GI symptoms of CeD, decreased the weekly number of Ced PRO GI Symptomatic Days, increased number of Improved Symptom Days, and reduced Abdominal domain symptom severity scores by ≥50% for at least half of the active treatment period.
In the 0·5mg larazotide acetate group, the subset of patients reporting the highest number of GI Symptomatic Days scores (5–7)/week at baseline experienced approximately 30 fewer GI Symptomatic Days in contrast to an increase of approximately 10 GI Symptomatic Days in the placebo group over the 12-week treatment period. This level of improvement is similar to what is regarded as clinically meaningful in other conditions with episodic symptoms.27–29
Results of this trial are consistent with previous studies, which demonstrated reduction in gluten-induced signs and symptoms during a gluten challenge.14–16 An effective adjunct to the GFD has the potential to transform CeD treatment and improve the lives of patients. Practicing a strict GFD is a continuous burden and often an unsuccessful struggle for many people with CeD.30–32 Gluten exposure is one of the common causes of ongoing or recurrent symptoms in patients with celiac disease on a GFD. The prevalence of persistent symptoms suggests that there is a substantial unmet medical need for pharmacological approaches that can improve CeD signs and symptoms beyond what is possible with the GFD alone.30–32
CeD PRO results demonstrate that moderate-to-severe CeD symptoms are common despite a GFD. In the 4-week placebo-run in phase, GI symptoms were reported by more than 90% of patients, and over two-thirds of patients reported tiredness and headache, suggesting that extra-intestinal symptoms have significant impact on wellbeing in patients with CeD. The placebo run-in demonstrated substantial day to day variability in symptoms. This is not well described in the literature but it is not surprising as symptoms may be linked to gluten exposure, which is highly variable and intermittent for patients attempting a GFD. While the observed reduction of GI symptoms may reflect non-specific effects, this is consistent with the proposed mechanism of action of larazotide acetate of limiting gluten entry into the lamina propria by preventing intestinal TJ opening. Although larazotide acetate is a locally acting peptide restricted to the luminal surface of the small intestine,16 the 0·5mg dose also reduced tiredness and headache. This suggests larazotide acetate might reduce extraintestinal symptoms, potentially through reduced local inflammation with subsequent reduction in cytokine release.
While we feel that this study supports the safety and efficacy of TJ modulation as a therapeutic modality in celiac disease, we do recognize potential limitations. First, direct comparison between the present study and the prior gluten challenge studies is difficult, due to the lack of a dose response and the different doses used between studies. In the first study published, doses of 0.25mg, 1mg, 4mg and 8mg t.i.d. were administered and both the 0.25mg and 4mg doses prevented symptoms recorded by the GSRS; only the 0.25mg dose prevented symptoms recorded by the CeD-GSRS.15 In the second gluten challenge study, 1mg, 4mg and 8mg t.i.d. were administered and only the 1mg group was effective in ameliorating symptoms induced by gluten challenge measured by the CeD-GSRS.14, 15 While the results of this study overall are consistent with prior studies, we acknowledge that the dose range chosen was based on earlier gluten challenge data, and for this reason, the optimal dose to study during a ‘real life’ study may not be fully understood. This inverse-dose effect is not unique to larazotide acetate and has been seen for other minimally or non-absorbed oral peptides.33 Why higher doses appear to be less effective is unclear, but may involve peptide aggregation at higher doses, reducing activity in vivo.
Second, while prevention of elevation of celiac antibody titers despite reported gluten exposure is reassuring, we did not document a reduction in serologic titers in any treatment arm. This is likely because the majority of participants entered the study with serologic titers in the normal range, and thus were not expected to be responsive to change over the course of the ‘Real Life’ study. Larazotide acetate did prevent the increase of anti-tTG antibody titers during gluten challenge suggesting disease modification.14 Whether larazotide acetate may result in reduction of serologic titers over a longer time period in individuals with persistently high serologic titers will be evaluated in future studies. It is also possible that larazotide acetate may non-specifically alleviate symptoms, as celiac disease may co-exist with other common conditions including irritable bowel syndrome. Although efficacy during gluten challenge is suggestive of a mechanism of action relevant to celiac disease, the specificity of larazotide acetate for celiac disease and its utility in other conditions remains to be determined. Additionally, there were few participants enrolled over the age of 65, so efficacy in this age group cannot be inferred from the current study. Finally, histology was not an endpoint evaluated in this study. While the role of histology for celiac diagnosis is clear, its utility in monitoring is controversial as there is poor correlation between symptoms, serology, histology and quality of life in treated patients, and recent data suggests that ongoing intestinal inflammation is not associated with significant long term complications.34, 35 Further, while the kinetics of histologic deterioration during gluten challenge are well understood, the degree, timing and clinical significance of improvements with adjunctive therapy in treated patients is unknown. Due to these limitations, histology may not be an appropriate primary endpoint for clinical trials designed to improve symptoms in patients with CeD on the GFD.
Regulating TJ activity represents a potential novel modality for treatment of diseases that involve epithelial barriers. Altered intestinal permeability is associated with many autoimmune disorders including Crohn’s disease, multiple sclerosis, and Type 1 diabetes.36 The potential therapeutic activity of larazotide acetate in other disorders associated with TJ dysregulation should be further explored.
In summary, larazotide acetate 0·5mg reduced GI and non-GI symptoms, resulting in fewer GI Symptomatic Days. Further, in studies now including a total of 828 subjects, larazotide acetate has not been associated with safety concerns. This study represents the first therapeutic trial in CeD to meet a primary endpoint of reducing symptoms in patients attempting to maintain a GFD. Larazotide acetate, the first of a novel class of agents targeting TJ regulation, may thus represent an important therapeutic option for CeD patients with persistent symptoms although the overall efficacy and risk/benefit ratio of this therapy remain to be fully assessed. Our results contribute to a growing body of evidence of the safety and efficacy of larazotide acetate and support further investigation.
Supplementary Material
Acknowledgments
The authors acknowledge Sheila Truten, BS of MC2 for editorial support, funded by Alba Therapeutics.
Abbreviations
- CeD
Celiac disease
- GSRS
Gastrointestinal Symptom Rating Scale
- CeD PRO
Celiac Disease Patient Reported Outcome
- GI
Gastrointestinal
- tTG
Tissue transglutaminase
- DGP
Deamidated gliadin peptide
Footnotes
First draft of manuscript was prepared by Daniel Leffler with writing assistance from Beth Llewellyn, BA and Kate Huber, MBA
Author Contributions: Daniel Leffler: Study design, patient recruitment, data analysis, manuscript preparation
Ciaran Kelly: Study design, patient recruitment, data analysis, manuscript preparation
Peter Green: Study design, patient recruitment, data analysis, manuscript preparation
Richard Fedorak: Study design, patient recruitment, manuscript preparation
Anthony DiMarino: Study design, patient recruitment, manuscript preparation
Wendy Perrow: Study design, manuscript preparation
Henrik Rasmussen: Study design, data analysis, manuscript preparation
Chao Wang: Statistical analysis
Premysl Bercik: patient recruitment, data analysis, manuscript preparation
Natalie Bachir: patient recruitment, manuscript preparation
Joseph Murray: Study design, patient recruitment, data analysis, manuscript preparation
Disclosures:
Daniel Leffler: consultant for/research support from: Alba Therapeutics, Alvine Pharmaceuticals, INOVA diagnostics, Genzyme, Coronado Biosciences, Sidney Frank Foundation, Shire Pharmaceuticals, Ironwood Pharmaceuticals, GI Supply.
Ciaran Kelly: consultant and scientific advisor related to Celiac disease for Alba, Alvine, ImmunosanT and Pfizer
Peter Green: Scientific Advisory Board: Alba Therapeutics, Alvine Pharmaceuticals, ImmunsanT
Richard Fedorak: Consultant/Advisory Board Member: Abbvie, Ferring, Janssen, Shire, VSL#3, Celltrion. Recipient of Clinical/Basic Research Grants: Abbvie, Alba Therapeutics, Bristol Myers Squibb, Centocor, GSK, Genentec, Janssen, Merck, Millennium, Novartis, Pfizer, Proctor & Gamble, Roche, VSL#3, Celltrion, owner/shareholder Metablolomic Technologies Inc. (www.metabolomictechnologies.ca)
Anthony DiMarino: Grant/research support Alba Therapeutics
Wendy Perrow: CEO and employee of Alba Therapeutics
Henrik Rasmussen: Consultant for Alba Therapeutics
Chao Wang: Consultant for Alba Therapeutics
Premysl Bercik: Research grant support from Nestle Switzerland, Advisory Board Janssen Canada, Advisory Board Forest Laboratories Canada
Natalie Bachir: none
Joseph Murray: Research support: Alba Therapeutics (>$50,000), Alvine Pharmaceuticals, Inc. NIH (past grant support)
The principal investigator and leading co-investigators designed the study in collaboration with Alba Therapeutics Corporation and Cephalon/Teva, conducted the study, and provided oversight for data collection. Data analysis was performed by Chao Wang, PhD and John Han, PhD of PharmaData Associates, funded by Alba and Cephalon/Teva. All authors contributed to data interpretation and writing and editing the manuscript. Drs. Leffler and Murray had full access to all study data, contributed equally to manuscript preparation, and had final responsibility for the publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mooney PD, Hadjivassiliou M, Sanders DS. Coeliac disease. BMJ. 2014;348:g1561. doi: 10.1136/bmj.g1561. [DOI] [PubMed] [Google Scholar]
- 2.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–28. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leffler DA, Dennis M, Hyett B, et al. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5:445–50. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Hall NJ, Rubin GP, Charnock A. Intentional and inadvertent non-adherence in adult coeliac disease. A cross-sectional survey Appetite. 2013;68:56–62. doi: 10.1016/j.appet.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007;85:160–6. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 8.Gopalakrishnan S, Durai M, Kitchens K, et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides. 2012 doi: 10.1016/j.peptides.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. e3. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–9. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matysiak-Budnik T, Candalh C, Dugave C, et al. Alterations of the intestinal transport and processing of gliadin peptides in celiac disease. Gastroenterology. 2003;125:696–707. doi: 10.1016/s0016-5085(03)01049-7. [DOI] [PubMed] [Google Scholar]
- 12.Gopalakrishnan S, Tripathi A, Tamiz AP, et al. Larazotide acetate promotes tight junction assembly in epithelial cells. Peptides. 2012 doi: 10.1016/j.peptides.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J Immunol. 2002;169:5595–600. doi: 10.4049/jimmunol.169.10.5595. [DOI] [PubMed] [Google Scholar]
- 14.Kelly CP, Green PH, Murray JA, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37:252–62. doi: 10.1111/apt.12147. [DOI] [PubMed] [Google Scholar]
- 15.Leffler DA, Kelly CP, Abdallah HZ, et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol. 2012;107:1554–62. doi: 10.1038/ajg.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paterson BM, Lammers KM, Arrieta MC, et al. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–66. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 17.Crowe SE. Management of celiac disease: beyond the gluten-free diet. Gastroenterology. 2014;146:1594–6. doi: 10.1053/j.gastro.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–33. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Svedlund J, Sjodin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–34. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 20.Mustalahti K, Lohiniemi S, Collin P, et al. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract. 2002;5:105–13. [PubMed] [Google Scholar]
- 21.Lohiniemi S, Maki M, Kaukinen K, et al. Gastrointestinal symptoms rating scale in coeliac disease patients on wheat starch-based gluten-free diets. Scand J Gastroenterol. 2000;35:947–9. doi: 10.1080/003655200750023002. [DOI] [PubMed] [Google Scholar]
- 22.Leffler D, Schuppan D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2012 doi: 10.1136/gutjnl-2012-302196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulich KR, Madisch A, Pacini F, et al. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study. Health Qual Life Outcomes. 2008;6:12. doi: 10.1186/1477-7525-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma S, Giaffer MH. Helicobacter pylori eradication ameliorates symptoms and improves quality of life in patients on long-term acid suppression. A large prospective study in primary care. Dig Dis Sci. 2002;47:1567–74. doi: 10.1023/a:1015823320831. [DOI] [PubMed] [Google Scholar]
- 25.McLeod LD, Coon CD, Martin SA, et al. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11:163–9. doi: 10.1586/erp.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurppa K, Paavola A, Collin P, et al. Benefits of a Gluten-free diet for Asymptomatic Patients with Serologic Markers of Celiac Disease. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–73. doi: 10.1111/j.1526-4610.2011.01990.x. [DOI] [PubMed] [Google Scholar]
- 28.Macdougall JE, Johnston JM, Lavins BJ, et al. An evaluation of the FDA responder endpoint for IBS-C clinical trials: analysis of data from linaclotide Phase 3 clinical trials. Neurogastroenterol Motil. 2013;25:481–6. doi: 10.1111/nmo.12089. [DOI] [PubMed] [Google Scholar]
- 29.Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527–36. doi: 10.1056/NEJMoa1010863. [DOI] [PubMed] [Google Scholar]
- 30.Shah S, Akbari M, Vanga R, et al. Patient Perception of Treatment Burden Is High in Celiac Disease Compared With Other Common Conditions. Am J Gastroenterol. 2014 doi: 10.1038/ajg.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barratt SM, Leeds JS, Sanders DS. Quality of life in Coeliac Disease is determined by perceived degree of difficulty adhering to a gluten-free diet, not the level of dietary adherence ultimately achieved. J Gastrointestin Liver Dis. 2011;20:241–5. [PubMed] [Google Scholar]
- 32.Aziz I, Evans KE, Papageorgiou V, et al. Are patients with coeliac disease seeking alternative therapies to a gluten-free diet? J Gastrointestin Liver Dis. 2011;20:27–31. [PubMed] [Google Scholar]
- 33.Brenneman DE, Spong CY, Hauser JM, et al. Protective peptides that are orally active and mechanistically nonchiral. J Pharmacol Exp Ther. 2004;309:1190–7. doi: 10.1124/jpet.103.063891. [DOI] [PubMed] [Google Scholar]
- 34.Rubio-Tapia A, Rahim MW, See JA, et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412–20. doi: 10.1038/ajg.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and mortality in coeliac disease. Aliment Pharmacol Ther. 2013;37:332–9. doi: 10.1111/apt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–20. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.